The authors devised a simple table to record in-process data on the preparation of mesenchymal stem/stromal cells (MSCs). The authors suggest that comparisons of data generated by different laboratories would be facilitated if similar in-process data, probably as supplemental materials, were included in publications using MSCs.
Keywords: Heterogeneity, Plasticity, Criteria
Abstract
There has been great interest in research and clinical trials with the adult stem/progenitor cells referred to as mesenchymal stem/stromal cells (MSCs). However, there are no definitive markers for the cells and no assays that would reflect the therapeutic efficacy of the cells in vivo. There are in effect no adequate release criteria that define the quality or efficacy of the cells. The problems are compounded by the fact that a variety of different protocols has been used to isolate the cells and expand them in culture. The result is that many publications have used MSCs with different properties, frequently without the investigators being aware of the differences. As a partial solution to these problems, we have devised a simple table to record in-process data on the preparation of MSCs. We suggest that comparisons of data generated by different laboratories would be facilitated if similar in-process data, probably as supplemental materials, were included in publications using MSCs.
Introduction
The cells referred to as mesenchymal stem/progenitor cells or multimodal stromal cells (MSCs) are the subject of extensive research and are being used in a large number of experimental and clinical trials for various diseases. There are more than 26,000 entries in PubMed under “mesenchymal stem cells,” more than 100 clinical trials have been registered (clinicaltrials.gov) with MSCs or related cells, and more than six biotech companies are in phase II and III trials in efforts to commercialize the cells [1]. The results from some of the trials are encouraging, but few have provided universally accepted data on efficacy [2]. More striking is that administration of MSCs has produced beneficial effects in a large series of animal models for human diseases, including models for multiple sclerosis, heart disease, diabetes, and some cancers. Progress has been made in the field in spite of two serious limitations: there are no definitive markers for the cells and no assays that would reflect the therapeutic efficacy of the cells in vivo. The problems are compounded by the fact that cultures of MSCs are heterogeneous even when isolated as single-cell-derived colonies. In addition, they are highly sensitive to the protocols used to isolate and expand the cells in culture [3–7]. The result is that different laboratories have used MSCs with strikingly different properties, frequently with the scientists being unaware of the differences. As a partial solution to these problems, we have prepared a simplified table for recording in-process data, similar to in-process data frequently used in the pharmaceutical industry, for the preparation of MSCs from aspirates of bone marrow with a protocol developed in our laboratory.
Results
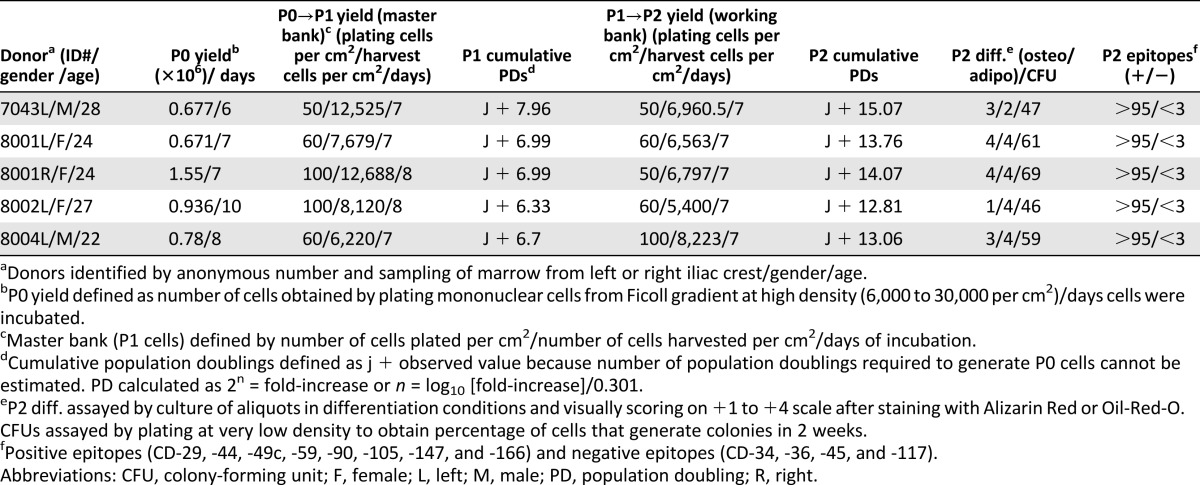
To help investigators address the variability among preparations of MSCs, we established a National Institutes of Health-sponsored center to provide investigators with MSCs from master banks prepared with a standardized protocol [7–11]. The aim of the center is to provide researchers with reference MSCs that could be compared in their experiments with MSCs prepared with other protocols. With continuing support from the National Institutes of Health, we have been distributing the MSCs from the master banks to more than 250 laboratories in this country and abroad for the past 10 years. We have devoted considerable time and effort to standardizing the protocols for preparing the master banks. We elected to use conditions under which the cells are expanded at low density to a limited number of population doublings so that the cultures are enriched for rapidly self-renewing, spindle-shaped MSCs that appear to be earlier progenitor cells than the larger and slowly replicating cells that predominate in confluent cultures [3, 7–10]. We have provided investigators who received MSCs from our master banks with detailed protocols for expanding the cells from master banks (referred to as P1 cells) to generate working banks. We have also provided them with specification sheets summarizing the data obtained in our own laboratory on the characteristics of MSCs expanded from the same master bank. However, the data we provided have proven too cumbersome and voluminous to include in publications in which the cells were used, including publications from our own laboratory. Therefore, we recently developed a simplified table to record in-process data on the preparation of each master bank and the characteristics of MSCs expanded from a working bank prepared from the same master bank (Table 1). In practice we have expanded three vials from the same master bank through three passages and not distributed cells from master banks of cells that do not maintain the same characteristics through all three passages. Also, we have encouraged recipients of the cells to contact us for advice if they do not obtain results in expanding the P1 cell from our master banks to produce working banks of P2 MSCs.
Table 1.
In-process data on master banks and working banks of human mesenchymal stem/stromal cells from bone marrow prepared with a protocol to keep the cultures enriched for rapidly self-renewing, progenitor-like cells
Discussion
There are multiple causes of the variability among different preparations of MSCs.
MSCs from rodents present a special series of problems. In the case of mouse MSCs, the initial isolates prepared by the classic technique of plating bone marrow on tissue culture plastic are heavily contaminated by macrophages [12]. In addition, the adherent MSCs are difficult to expand. They initially proliferate slowly and then undergo a crisis in which most of the cells undergo apoptosis. In a manner similar to mouse fibroblasts [13], the few cells that survive grow rapidly because they are transformed. And like many transformed cells, the transformed mouse MSCs continue to undergo further genomic changes as they are expanded so that they become tumorigenic [14]. A recent report indicates that the problems of expanding mouse MSCs are caused by their sensitivity to oxygen-induced p53-driven apoptosis [15]. Therefore, culturing mouse MSCs under hypoxic conditions is probably a means of surmounting some of the variability in the future [15]. Another source of variability among mouse MSCs is that the cells from different strains have different properties and requirements for optimal growth [10]. The variability of mouse MSCs has limited the extent to which the cells can be used to tap the power of transgenic mice. Rat MSCs from bone marrow have been studied less extensively but are also difficult to expand in culture and probably undergo spontaneous transformation [11, 16].
Surprisingly, human MSCs do not present the same problems. MSCs isolated by plating mononuclear cells from bone marrow on tissue culture plastic are free of hematopoietic cells after a single passage [7–9]. However, there is great variability in different preparations of the cells. In the case of MSCs obtained by aspirates of bone marrow, one source of the variability is that the blind aspirates of the 1 or 2 ml of marrow provide different yields of mononuclear cells, and the mononuclear cells provide different yields of cells that give rise to plastic-adherent cells (Table 2). The yields are higher in young children and may decline in donors of more than 60 years of age, but a large part of the variation is probably from the sampling of regions of the marrow that are rich in hematopoietic cells or peripheral blood, particularly if volumes of greater than approximately 2 ml are withdrawn in the aspirate. An additional and major source of variability in preparations of human MSCs derives from the inherent plasticity of the cells. The plasticity of MSCs is apparent in animal models in that they respond to different microenvironments created by injuries to tissues caused by dramatic changes that include major alterations in their transcriptomes, secretion of the paracrine factors including microvesicles containing microRNAs, and, surprisingly, transfer of mitochondria [2, 17–21]. A similar plasticity is apparent as MSCs are expanded in culture. If plated and cultured at high density, the cells expand slowly, become larger, and lose some of their progenitor-like properties [3]. If plated and expanded at low density, the cells tend to retain their spindle-like morphologies and progenitor-like properties. However, variability is apparent even if the cells are plated at clonal densities. Individual single-cell-generated colonies vary in size, density, cell morphologies, and differentiation potential [3]. Remarkably, as the colonies grow from single cells, the cells in the inner regions of the colonies become more differentiated than the cells on the periphery [22]. Replating cells from a single colony generates the same heterogeneity in colonies with the same variations in size, density, morphologies, differential potentials, and profiles of expressed genes. The process can be repeated through 20 or more population doublings until the MSCs become more like fibroblasts and gradually approach senescence.
Table 2.
Commonly used assays for preparations of MSCs from human bone marrow
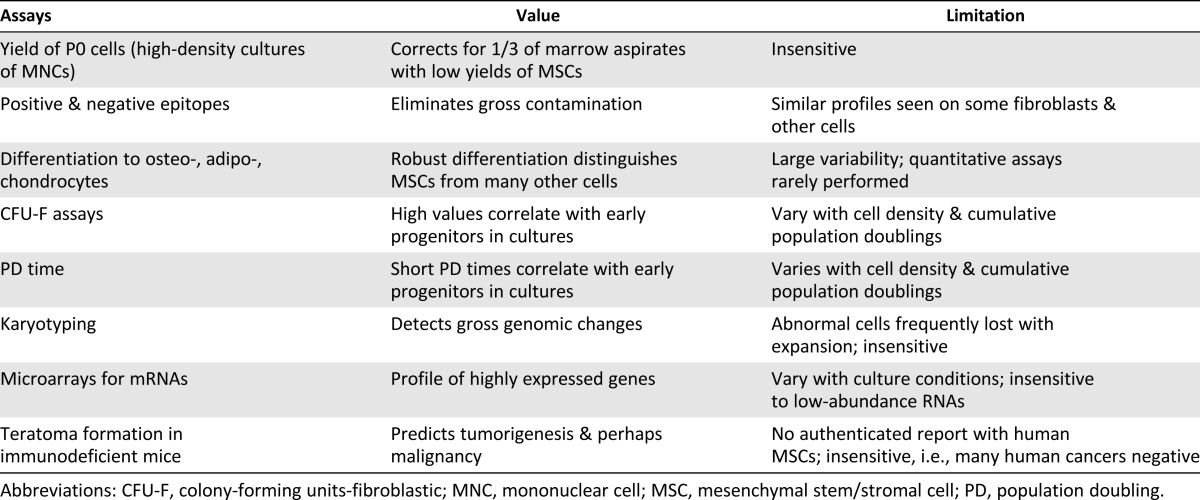
Several attempts have been made to overcome some of the variability in preparations of MSCs by suggesting criteria for the definition of the cells. In particular, a committee of the International Society for Cellular Therapy [23] suggested criteria for characterizing MSCs isolated from bone marrow by features such as adherence to tissue culture plastic, epitopes on surface proteins, and differentiation potential. More recently, a joint statement of the International Federation for Adipose Therapeutics and Science and the International Society for Cellular Therapy refined the criteria to distinguish adipose-derived MSCs from MSCs from other sources [24]. As is generally recognized, however, the criteria for MSCs have limitations (Table 2). In the case of bone marrow-derived MSCs, the profile of epitopes is not unique to MSCs and is expressed by some fibroblasts and other cells. The criterion of trilineage differentiation varies among preparations that meet the other criteria and is difficult to evaluate quantitatively. Criteria such as formation of colony-forming units-fibroblastic are highly dependent on the conditions used to culture the cells in that they are high in freshly plated cultures and decrease as the cultures expand [28]. In addition, karyotyping assays of mRNAs with microchips and assays for teratoma formation in mice have proven to be of limited value.
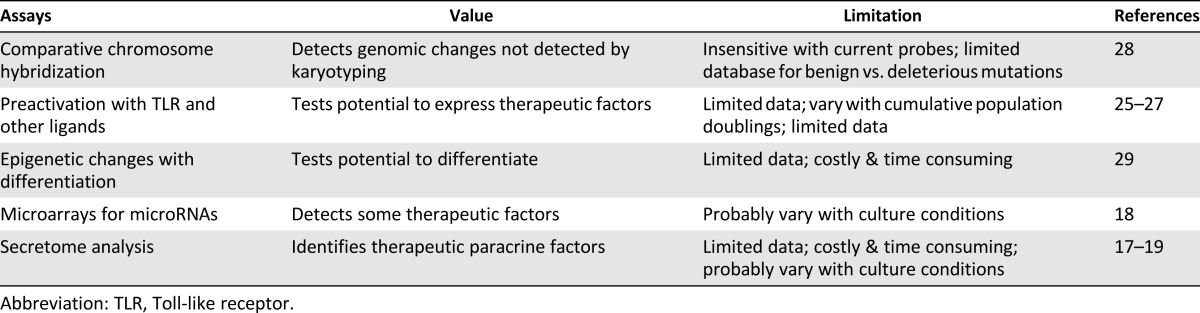
Several attempts have also been made recently to use novel and more informative technologies to assay MSCs (Table 3). Some strategies, particularly protocols to preactivate MSCs in culture with Toll-like receptor ligands or other stimulants, show great promise as efficacy tests for the cells [25–27]. New technologies for epigenetic analyses of cells could provide important information about MSCs, but the large amounts of data generated are still difficult to interpret [29]. A new algorithm for pluripotent cells [30] can serve as an important model of analyses of microarray data from MSCs, but it will require identification of a cluster of MSC-specific genes similar to the 450 genes that have been used to identify pluripotent cells. However, none of the new technologies have been fully tested, and some are currently too time consuming and costly to be used widely.
Table 3.
Recently developed assays for characterization of human mesenchymal stem/stromal cells
In effect, progress in research and clinical trials with MSCs is severely limited by the lack of definitive markers and a quantitative surrogate assay for the efficacy of the cells in vivo. In contrast to research on hematopoietic stem cells, there is no animal model comparable to the marrow ablated mouse that made it possible to assay the efficacy of candidate cells. Publications on MSCs have used a variety of protocols to prepare the cells, frequently without much detail about the protocols. We have received anecdotal reports that the cells from our master banks are more effective in some animal disease models than MSCs prepared with other protocols. However, neither we nor the scientists to whom we have provided MSCs have been able to make detailed comparisons among MSCs prepared with different protocols. More definitive assays for MSCs are likely to be developed in the future. In the interim, publication of in-process data should help in comparing data from different publications.
Table 1, presented in this work for summarizing in-process data for preparation of MSCs, is an initial draft that should be modified to fit individual needs of different investigators. Variations on it will probably be required over time, including simple means of incorporating additional data that affect the variability among preparations of MSCs, such as the medium and atmospheric gases used for expansion and the conditions for freezing and thawing the cells. The use of such summaries is likely to have a greater impact on the field if reviewers and leading journals required such information, probably as supplemental material, for publications on MSCs. There would also be a greater impact if a consortium of leading scientists in the field endorsed the suggestion.
Author Contributions
R.L.R.: conception and design, data analysis and interpretation, manuscript writing, final approval of the manuscript; D.J.P.: conception and design, data analysis and interpretation, manuscript writing, final approval of the manuscript, financial support.
Disclosure of Potential Conflicts of Interest
D.J.P. has uncompensated consultancy, ownership/stock options, and intellectual property rights.
References
- 1.Syed BA, Evans JB. Stem cell therapy market. Nat Rev Drug Discov. 2013;12:185–186. doi: 10.1038/nrd3953. [DOI] [PubMed] [Google Scholar]
- 2.Keating A. Mesenchymal stromal cells: New directions. Cell Stem Cell. 2012;10:709–716. doi: 10.1016/j.stem.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 3.Colter DC, Sekiya I, Prockop DJ. Identification of a subpopulation of rapidly self-renewing and multipotential adult stem cells in colonies of human marrow stromal cells. Proc Natl Acad Sci USA. 2001;98:7841–7845. doi: 10.1073/pnas.141221698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phinney DG. Biochemical heterogeneity of mesenchymal stem cell populations: Clues to their therapeutic efficacy. Cell Cycle. 2007;6:2884–2889. doi: 10.4161/cc.6.23.5095. [DOI] [PubMed] [Google Scholar]
- 5.Ho AD, Wagner W, Franke W. Heterogeneity of mesenchymal stromal cell preparations. Cytotherapy. 2008;10:320–330. doi: 10.1080/14653240802217011. [DOI] [PubMed] [Google Scholar]
- 6.Whitfield MJ, Lee WC, Van Vliet KJ. Onset of heterogeneity in culture-expanded bone marrow stromal cells. Stem Cell Res. 2013;11:1365–1377. doi: 10.1016/j.scr.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Sekiya I, Larson BL, Smith JR, et al. Expansion of human adult stem cells from bone marrow stroma: Conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells. 2002;20:530–541. doi: 10.1634/stemcells.20-6-530. [DOI] [PubMed] [Google Scholar]
- 8.Reger RL, Tucker AH, Wolfe MR. Differentiation and characterization of human MSCs. Methods Mol Biol. 2008;449:93–107. doi: 10.1007/978-1-60327-169-1_7. [DOI] [PubMed] [Google Scholar]
- 9.Reger RL, Wolfe MR. Freezing harvested hMSCs and recovery of hMSCs from frozen vials for subsequent expansion, analysis and experimentation. Methods Mol Biol. 2008;449:109–116. doi: 10.1007/978-1-60327-169-1_8. [DOI] [PubMed] [Google Scholar]
- 10.Peister A, Mellad JA, Larson BL, et al. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103:1662–1668. doi: 10.1182/blood-2003-09-3070. [DOI] [PubMed] [Google Scholar]
- 11.Javazon EH, Colter DC, Schwarz EJ, et al. Rat marrow stromal cells are more sensitive to plating density and expand more rapidly from single-cell-derived colonies than human marrow stromal cells. Stem Cells. 2001;19:219–225. doi: 10.1634/stemcells.19-3-219. [DOI] [PubMed] [Google Scholar]
- 12.Phinney DG. Isolation of mesenchymal stem cells from murine bone marrow by immunodepletion. Methods Mol Biol. 2008;449:171–186. doi: 10.1007/978-1-60327-169-1_12. [DOI] [PubMed] [Google Scholar]
- 13.Rubin H. Multistage carcinogenesis in cell culture. Dev Biol. 2001;106:61–66. discussion 67, 143–60. [PubMed] [Google Scholar]
- 14.Tolar J, Nauta AJ, Osborn MJ, et al. Sarcoma derived from cultured mesenchymal stem cells. Stem Cells. 2007;25:371–379. doi: 10.1634/stemcells.2005-0620. [DOI] [PubMed] [Google Scholar]
- 15.Boregowda SV, Krishnappa V, Chambers JW, et al. Atmospheric oxygen inhibits growth and differentiation of marrow-derived mouse mesenchymal stem cells via a p53-dependent mechanism: Implications for long-term culture expansion. Stem Cells. 2012;30:975–987. doi: 10.1002/stem.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cui X, Liu J, Bai L, et al. IL-6 induces malignant transformation of rat mesenchymal stem cells in association with enhanced signaling of signal transducer and activator of transcription 3. Cancer Sci. 2013 doi: 10.1111/cas.12313. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ranganath SH, Levy O, Inamdar MS, et al. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell. 2012;10:244–258. doi: 10.1016/j.stem.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fakhry M, Hamade E, Badran B, et al. Molecular mechanisms of mesenchymal stem cell differentiation towards osteoblasts. World J Stem Cells. 2013;5:136–148. doi: 10.4252/wjsc.v5.i4.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burrows GG, Van't Hof W, Newell LF, et al. Dissection of the human multipotent adult progenitor cell secretome by proteomic analysis. Stem Cells Transl Med. 2013;2:745–757. doi: 10.5966/sctm.2013-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spees JL, Olson SD, Whitney MJ, et al. Mitochondrial transfer between cells can rescue aerobic respiration. Proc Natl Acad Sci USA. 2006;103:1283–1288. doi: 10.1073/pnas.0510511103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Islam MN, Das SR, Emin MT, et al. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18:759–765. doi: 10.1038/nm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ylöstalo J, Bazhanov N, Prockop DJ. Reversible commitment to differentiation by human multipotent stromal cells in single-cell-derived colonies. Exp Hematol. 2008;36:1390–1402. doi: 10.1016/j.exphem.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells: The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 24.Bourin P, Bunnell BA, Casteilla L, et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT) Cytotherapy. 2013;15:641–648. doi: 10.1016/j.jcyt.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waterman RS, Tomchuck SL, Henkle SL, et al. A new mesenchymal stem cell (MSC) paradigm: Polarization into a pro-inflammatory MSC1 or an immunosuppressive MSC2 phenotype. PLoS One. 2010;5:e10088. doi: 10.1371/journal.pone.0010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee RH, Yoon N, Reneau JC, et al. Preactivation of human MSCs with TNF-α enhances tumor-suppressive activity. Cell Stem Cell. 2012;11:825–835. doi: 10.1016/j.stem.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 27.Bustos ML, Huleihel L, Meyer EM, et al. Activation of human mesenchymal stem cells impacts their therapeutic abilities in lung injury by increasing interleukin (IL)-10 and IL-1RN levels. Stem Cells Transl Med. 2013;2:884–895. doi: 10.5966/sctm.2013-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larson BL, Ylostalo J, Lee RH, et al. Sox11 is expressed in early progenitor human multipotent stromal cells and decreases with extensive expansion of the cells. Tissue Eng Part A. 2010;16:3385–3394. doi: 10.1089/ten.tea.2010.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herlofsen SR, Bryne JC, Høiby T, et al. Genome-wide map of quantified epigenetic changes during in vitro chondrogenic differentiation of primary human mesenchymal stem cells. BMC Genomics. 2013;14:105. doi: 10.1186/1471-2164-14-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Müller FJ, Schuldt BM, Williams R, et al. A bioinformatic assay for pluripotency in human cells. Nat Methods. 2011;8:315–317. doi: 10.1038/nmeth.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]


