Abstract
Objective
The most important cause of infant mortality during the first month of life is related to congenital abnormalities. Nevertheless, timely diagnosis of these diseases can reduce the severity of their effects. The present study aimed to investigate the cost-effectiveness of the neonatal screening program in Fars Province, Iran.
Methods
In this study, costs of executing the screening programs, treatment of the diagnosed cases, treatment of affected, non-screened individuals, quality of life, and incremental cost-effectiveness ratios were measured in two study groups.
Findings
Performing the screening programs for phenylketonuria, congenital hypothyroidism, galactosemia, and favism resulted in respectively $3386, $13078, $19641, and $1088 saving per patient. Overall, the study results revealed the cost-effectiveness of execution of the neonatal screening program.
Conclusion
Neonatal screening program is one of the health interventions which lead to long-term beneficial outcome for the patients, financial saving for the society, and improvement of the patients’ quantity as well as quality of life.
Keywords: Cost-utility Analysis, Screening, Economic Evaluation, Phenylketonuria, Congenital Hypothyroidism, Favism, Galactosemia
Introduction
Neonatal screening is a systematic public health program for screening infants in the first few days after birth. Genetic and metabolic disorders are among the major causes of mortality before birth and during infancy. More than half of the congenital abnormalities usually remain undetected and are only incidentally diagnosed later in life. In general, congenital disorders during the first month of life are the most important causes of infant mortality, as the infant gets older, the chance of detecting the congenital abnormalities increases[1].
Although some of the metabolic symptoms are detectable from the first days of life, sometimes these symptoms are weak and, consequently, diagnosis of these diseases can be delayed for months and even years[2]. Early diagnosis of metabolic diseases can reduce their effects. For each month of delay in diagnosis and treatment of certain metabolic conditions, the child's IQ can decrease significantly and corrective treatment becomes more difficult. These children may also be faced with severe brain damage, mental retardation, paresis, liver disorders, kidney stones, visual impairments, and heart diseases. The undesirable effects of these complications and the financial burden of providing these patients with healthcare services which is imposed on the society clearly show the necessity to investigate and take preventive measures toward such genetic disorders[3].
Identification of treatable hereditary metabolic diseases is quite important specially up to the age of 1 year, because early diagnosis can lead to the treatment of the disease and prevention of mental retardation, improvement of some symptoms and considerably prevent the progressive brain damage[4].
Today, almost 7.6 million infants with genetic or congenital abnormalities are annually born around the world and 90% of such births occur in low-income countries[5]. Although Iran's population is one fourth of the population of the USA, there is the same number of handicapped individuals in both countries[5].
In order to prevent such consequences, neonatal screening programs were being conducted in most developed countries and also expanded to developing countries including Iran[6]. The most important stage in prevention and treatment of the patients with metabolic disorders is a screening in which, the affected infant enters into the treatment cycle through a simple, inexpensive test[7].
Based on the above-mentioned necessities, program of neonatal screening was started in Iran in 2003, experimentally executed in 3 provinces in 2004 and gradually expanded through the country. Today, neonatal screening programs are being conducted for phenylketonuria, congenital hypothyroidism, glucose-6-phosphate dehydro-genase deficiency (G6PD), and galactosemia[8].
In addition to importance of identification and prevention of these diseases, particularly during the first years of life, limitation of the resources in the health department has caused the policymakers and planners of this department to pay more attention to their health costs.
Recently, considering the enormous increase in healthcare expenditures due to the development in health and treatment technologies as well as the numerous problems, the countries are facing for funding the health system, and health managers should utilize the available resources as efficiently as possible[1].
Thus, the present study aims to compute the costs of executing the neonatal screening programs, determine their utility against the costs of treating the patients with the 4 target diseases, and compare the results with those of not conducting the screening programs in Fars Province.
Subjects and Methods
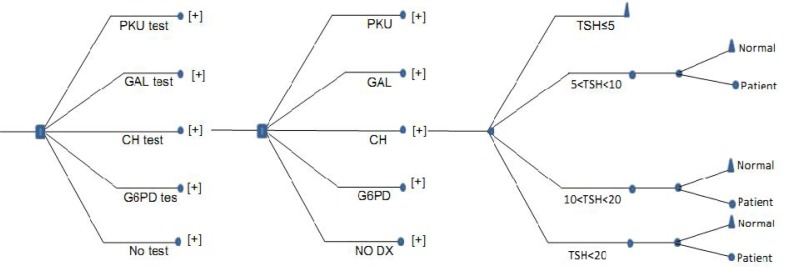
The present study is a descriptive-analytical one conducted in a cross-sectional manner. In this study, the direct medical costs were measured from a social perspective, we also used Decision Tree Module which is a decision approach that describes decision process that always starts with a question and focuses on questions in such a way that each possible answer to a question is followed by a new question or by a final decision (Fig. 1). The study population included 81837 newborns referred to Shiraz neonatal screening laboratory in 2010.
Fig. 1.
Decision Tree for Neonatal Screening Programme for Phenylketonuria (PKU), Congenital hypothyroidism (CH), Galactosemia (GAL), Favism (G6PDD), No diagnosis at birth (No Dx).
In the current study, computation of costs included investment costs such as facilities and equipment, and current patient/family expenses such as salaries, medications, transportation and medical equipment. Additionally, screening program costs included the specific costs for identifying each screened disease, with common program expenditures divided among the diseases according to the tariffs of the Ministry of Health.
In order to collect the data regarding the costs of caring for the unscreened patients who were affected with any of the diseases, first the list of the services they had received was extracted from their medical records. Then, three specialists who are still dealing with the treatment of this group of patients were interviewed and the number of hospitalizations in each year as well as the list of the services the patients had received were asked; and finally, the tariffs of the private sector (after subtracting the profit percentage) were used in order to compute total costs of each service package for the organization.
In order to estimate the utility, time trade off, which is a direct method for assessing the utility, was utilized. Using this method, the respondents were asked to choose between a special health status for a period of time and a complete health status for a shorter period of time. In fact, they were required to make a trade-off between the length of life and its quality[9].
In order to assess the quality of life and compute the utility, a questionnaire was prepared by the authors and the nurses who work in the centers providing services for these patients in Nemazi, Hafez, and Zeynabiyeh hospitals, were asked about their working experience and information about the patients’ status. In this part of the study, the statistical community included all the nurses and the target population included the nurses working in the centers which provided services for these 4 groups of patients. Besides, considering CI of 95%, power of 80%, SD of 3, and minimum margin of error of 2, a 36-subject sample size was calculated using the following equation:
N= (Z1-α/2 + Z1-β) 2 × S2 / d2 where, N= desired sample size, α = type I error, β= type II error, d= difference between population- and sample mean values
We selected and interviewed 18 male and the same number of female nurses using simple random sampling method from the list of the nurses working in the centers which provide services for this kind of patients. Two series of forms were prepared for each of the four diseases, one for screened and treated subjects and one for the affected ones. Each form included explanations about the disease, individual's status, treatment method, and issues they had to observe. Then, the interviewees had to answer whether they preferred to live with controlled mentioned diseases for maximal 10 years, or live without any diseases for less than 10 years. According to the responses, the utility of each status per year was measured using the following equation:
Utility in a specific health status per year = Length of living in complete health/Period of time spent in that specific condition.
For cost-utility analysis, comparison of different interventions, and easy decision making, Incremental Cost Effectiveness Ratio (ICER) was computed by dividing the difference between the two interventions’ costs by the difference of their outcomes [quality-adjusted life years (QALYs) measured for diagnosed and treated cases and QALYs estimated for the patients). The intervention with smaller ICER was considered as more cost-effective.
In the present study, new intervention refers to executing the screening program, while old intervention refers to not performing the program. After calculating the costs of executing the neonatal screening program, including current and investment costs, as well as costs of treating the individuals diagnosed through examinations, these two costs were added and subtracted from the costs related to the treatment of the patients who were infected by the four mentioned diseases. Then, the obtained measure was divided by the number obtained by subtracting QALYs from time trade off in both conditions and the obtained number showed ICER.
Since the advantages and outcomes of performing the neonatal screening program will be revealed over the time, in order to measure the present value of the screening outcomes; i.e., higher life expectancy and life quality, in the current study, the future costs of treating the individuals and the quality of the future life were discounted with a 3% discount rate.
The acceptable range of the study variables was determined in two ways. Regarding the variables of expenditure, discount rate, prevalence rate of the diseases, and life expectancy, first 20% of the values of the variables was added to and subtracted from each variable and higher and lower ends of the acceptable range were identified. Considering utility, CI was measured by 95% confidence coefficient and added to and subtracted from the mean. Then, the higher and lower ends of the acceptable range were determined. After identifying the higher and lower ends of the acceptable range of each variable, one-way as well as two-way sensitivity analyses were performed. In order to present the results of one-way and two-way sensitivity analyses, net monetary benefit index and worst-best analysis were used, respectively.
Findings
In order to determine the average of neonatal screening costs in the present study, costs of screening were estimated for 81837 newborns referred to Shiraz neonatal screening laboratory in 2010. Table 1 shows the results obtained from costs data, including the costs of executing the screening program, treating the patients with positive screening test results, and treating the unscreened patients.
Table 1.
Costs of screening and treating the newborns in Shiraz University of Medical Sciences, 2010
| Type of cost | PKU US$ | CH US$ | GAL US$ | G6PD US$ |
|---|---|---|---|---|
| Mean cost of performing the screening | 2.28 | 1.44 | 0.96 | 1.63 |
| Cost of early treatment of screened patients | 7037 | 1014 | 4243 | 3.2 |
| Cost of delayed treatment of unscreened patients | 9223 | 7548 | 12677 | 292 |
PKU: Phenylketonuria; CH: Congenital hypothyroidism; GAL: Galactosemia; G6PD: glucose-6-phosphate dehydro-genase deficiency
The study findings showed that in order to execute the screening program for the four mentioned diseases in Shiraz University of Medical Sciences in 2010, 64.846 Rials ($65,8) were spent for each patient. In addition, 100.000 Rials ($101,52) were obtained from each patient as the service tariff, which is more than the total cost of the examinations and according to the authorities of the non-communicable department in the deputy of health, the difference will be spent for treatment and follow-up of the patients.
After the screening examinations, the individuals with positive phenylketonuria and galactosemia test results were referred to Nader Kazemi Clinic and those with congenital hypothyroidism were treated in Imam Reza Clinic, Shiraz, Iran. Regarding the favism patients, the families were only advised to prevent the children from consuming broad beans and exposing to special chemicals and medications.
According to the results, cost of treating a newborn with positive phenylketonuria test results with ($73.274,04) was more than the other three diseases. Moreover, comparison of the costs imposed on the health system in neonatal screening programs showed that the highest treatment expenditure was related to the patients with galactosemia with $131.991,03. It should be noted that the mentioned costs are related to the patients’ one year of life and have been estimated after applying discount for the future years’ expenditure.
Table 2 shows the results of time trade off and computing the data related to utility for each disease in case of screening and early treatment versus no screening.
Table 2.
The results of time trade off in Shiraz University of Medical Sciences, 2010
| Disease | Screening status | Number of interviewed nurses | Mean score of utility (SD) | Maximum | Minimum |
|---|---|---|---|---|---|
| Phenylketonuria | Unscreened | 36 | 0.397 (0.2348) | 1 | 0 |
| Screened | 36 | 0.849 (0.2068) | 1 | 0.3 | |
| Hypothyroidism | Unscreened | 36 | 0.469 (0.2847) | 1 | 0 |
| Screened | 36 | 0.899 (0.1697) | 1 | 0.3 | |
| Galactosemia | Unscreened | 36 | 0.475 (0.2771) | 1 | 0 |
| Screened | 36 | 0.896 (0.1514) | 1 | 0.4 | |
| Favism | Unscreened | 36 | 0.793 (0.2715) | 1 | 0 |
| Screened | 36 | 0.975 (0.0485) | 1 | 0.8 |
SD: Standard Deviation
The results obtained from calculation of ICER for the four mentioned diseases are presented in Table 3. As the table depicts, a negative value was obtained for the amount of increase in costs, which means that the individuals’ costs of screening and early treatment of the disease have been less than when they were not screened. Also, the data regarding the effectiveness of screening show the increased quality-adjusted life years for the screened patients.
Table 3.
ICER of neonatal screening in Shiraz University of Medical Sciences, 2010
| Type of disease | Incremental cost (US$) | Incremental QALYs | ICER |
|---|---|---|---|
| Phenylketonuria | - 33.714 | 0.001 | Dominant |
| Congenital hypothyroidism | - 755.43 | 0.0055 | Dominant |
| Galactosemia | - 42.174 | 0.002 | Dominant |
| Favism | -345.53 | 0.88540 | Dominant |
ICER: Incremental Cost Effectiveness Ratio; QALY: Quality-Adjusted Life Years
Discussion
Early diagnosis and treatment of hereditary metabolic disorders are of great importance in preventing or delaying the onset of the disease. Moreover, screening at birth reduces mortality, diseases, and the social burden accompanied by irreversible effects of the diseases among the population.
Fars province neonatal screening program was started in 2004 and all the infants born in the province are examined for phenylketonuria, congenital hypothyroidism, galactosemia, and favism.
The results of economic evaluation (cost-utility analysis) obtained from comparison of executing and not executing the neonatal screening program and treating the patients with these diseases from social perspective showed that performing the neonatal screening program was far more cost-effective. In this section of the study, the results related to each disease are going to be discussed.
Phenylketonuria
In the present study, the total cost of each phenylketonuria screening test was 23.422 Rials ($2,28). In case screening was not performed, the 1-year cost of treating such patients was 94.599.684 Rials ($9.223). The mean utility of the patients who were screened and treated was measured as 0.849 through time trade off method, while the mean utility of the unscreened patients was 0.397.
ICER measured for phenylketonuria showed that in case screening was executed, 34.727.047,73 Rials ($3.386) were saved per patient. As the results show, the annual cost of taking care of a phenylketonuria patient was far more than the cost of caring for a screened individual. Quality of life among screened patients was two times more than the unscreened ones. Therefore, executing phenylketonuria screening program is highly efficient and cost-effective.
Aaron's et al study in USA in 2005 revealed the cost of each phenylketonuria screening test as $3,43 which is quite close to the result of the present study. On the other hand, the treatment cost of the affected patients was equal to $1.042.110 and its difference with the findings of the current study might be due to the calculation of indirect medical costs, such as transportation expenditures, in that study as well as the difference in medical services tariffs in USA[10]. In Aaron's study, utility of the patients suffering from severe mental retardation was obtained as 0.3909 which is consistent with the measure computed in our study (0.397). Also, in line with the present study, estimation of ICER in Aaron's study showed that executing the phenylketonuria screening was dominant. Overall, Carrol and Downs showed that, except for galactosemia and congenital adrenal hyperplasia, screening of all their study diseases including phenylketonuria had the required cost-effectiveness[10].
Another study was conducted from social perspective in Australia in 2001. In that study, Geelhoed et al showed phenylketonuria screening to be quite cost-effective[11]. Lord et al also conducted a study in 2000. They revealed that 143.500 pounds were saved for each detected case of phenylketonuria and this shows the cost-effectiveness of phenylketonuria screening program[12]. Furthermore, the results of a study performed by Lauren et al in Canada in 2005 showed that ICER of using the new technology for screening phenylketonuria was equal to 5.114.492 CAD per each life year gained[13].
In the current study, one-way sensitivity analysis for phenylketonuria was performed by adding and subtracting 20% of the study variables and the results were most affected by discount rate, while least influenced by life expectancy. Overall, the study findings were verified by sensitivity analysis of most of the study variables.
The results of two-way sensitivity analysis (worst-best analysis) which was performed by simultaneous change of two variables of cost and utility showed that screening was accompanied by 35.597.101 Rials ($3.471) saving per patient in the worst scenario; i.e., minimum utility and maximum costs, and 74.757.441 Rials ($7.289) saving per patient in the best scenario; i.e., maximum QALYs (+20%) and minimum costs (–20%). Of course, it should be noted that the cost of treating phenylketonuria patients and providing them with special foods can be barriers to complete treatment of the disorder.
Congenital hypothyroidism
In this study, the annual cost of treating the patients suffering from congenital hypothyroidism was 77.416.450 Rials ($7.548). Nevertheless, the mean utility of the patients who were screened and treated was measured as 0.899 through the time trade off method, while the mean utility of the unscreened infected patients was 0.469.
ICER measured for congenital hypothyroidism showed that executing the screening had resulted in 134.126.566,08 Rials ($1.3078) saving per patient. The results obtained from assessing the congenital hypothyroidism screening program showed that the costs of not performing the screening program were far more than the costs of its execution. On the other hand, utility of the unscreened patients was quite less than the utility of the screened and treated ones. Thus, performing the congenital hypothyroidism is cost-effective.
In the study by Carrol and Downs, the cost of each screening test was $4,59 which is highly different from the results of the present study. In addition, the cost of treating a patient with congenital hypothyroidism was $1.110.042, which is also more than the findings of this study. The difference between the two studies might be due to the estimation of non-medical costs in Aaron's research, while only considering the direct medical costs in the present one. In that study, the utility of the patients with congenital hypothyroidism was 0.3909 and close to the measure obtained in the current study (0.469). Overall, Carrol and Downs showed execution of congenital hypothyroidism to be quite cost-effective[10]. Similar results were also obtained in the study conducted by Geelhoed et al in Australia[11].
Furthermore, Yarahmadi et al conducted a study in 2011 and compared the IQ of the screened patients and the normal individuals. The study results revealed no considerable difference between the IQs of the two study groups. Thus, the program revealed to be cost-effective, led to saving in consuming the resources, and prevented mental retardation[14].
In the present study, one-way sensitivity analysis was performed by adding and subtracting 20% of the study variables and confirmed the study findings. The results of two-way sensitivity analysis of the two variables of cost and utility also revealed 129.258.720,26 Rials ($12.603) saving per patient in the best scenario; ie, maximum utility and minimum costs.
According to the study findings, congenital hypothyroidism screening is not only economically beneficial, but it also preserves the patients’ IQ and prevents mental retardation as well as growth complications. Yarahmadi et al also showed that the average IQ of the patients who were treated at birth was 15.7 higher compared to the patients diagnosed 30 days after birth[14].
Galactosemia
According to the results of time trade off method, the mean utility of the screened and treated patients and the unscreened ones was 0.896 and 0.475, respectively. Moreover, ICER measured for galactosemia showed that in case screening was performed, 201.443.240,99 Rials ($19.641) would be saved per patient. As the results depict, executing the galactosemia screening program is quite cost-effective and the saving per patient resulting from this program is even more than the two previously mentioned diseases. On the other hand, Carrol and Downs showed galactosemia screening program not to be cost-effective which might be due to the calculation of non-medical costs in that study[10].
In 2011, Junior et al conducted a study in Brazil and found that health improvement in the screened galactosemia patients was 1.33 folds more than its costs and, as a result, executing the program was cost-effective.
Similar to phenylketonuria, high costs of treatment and special foods may prevent the achievement of complete treatment of galactosemia patients.
Favism
Due to the families’ information and preventing the children from dangerous cases, this disease has no costs from the second year on. Of course, one-year cost of the unscreened patients was measured as 2.991.840 Rials ($292). In addition, the mean utility of the screened and treated patients was obtained as 0.975 through time trade off method, while it was measured as 0.793 for the unscreened patients. Moreover, ICER measured for favism showed that screening was accompanied by 11.161.717,20 Rials ($1.088) saving per patient.
Up to now, only a limited number of studies have been conducted on the cost-effectiveness of favism screening program. For instance, Khneisser et al (2004) performed a study in Lebanon and showed that the treatment cost of each patient with favism was equal to 1450 Lebanese Lira. On the other hand, the cost of performing the screening test was revealed to be 3 Lira. Furthermore, hospitalization of the screened newborns due to anemia was 3 times less than that of the unscreened ones[16]. Cohan et al also showed that favism screening was highly effective in reducing the newborns’ rate of hospitalization due to favism[17].
The findings of the present study were also confirmed by the results of the sensitivity analysis of evaluation of favism screening.
Overall, the study results showed that 126.127.576 Rials ($12.298) have been spent for executing the neonatal screening program and treating the infected cases in Fars province in 2010 and, consequently, patient utility has had a two-fold increase. Therefore, performing the program is both acceptable and cost-effective from social perspective. Moreover, considering the savings resulting from executing the screening program for the 4 diseases and increase in the patients’ quality of life, one can conclude that appropriate and cost-effective design of the screening program can open the way for performing the program for other inherited metabolic diseases, as well. In fact, the sample used in the present study can be utilized for examination of other diseases and this will lead to the higher efficiency of the program.
Neonatal screening program is one of the health interventions which is beneficial for the patients, has long-term savings for the society, and is cost-effective even with pessimistic assumptions[11]. In fact, screening leads the patients to a longer and healthier life and, in other words, improves their life quality as well as quantity.
Conclusion
The current study was conducted with the assumption that the diagnosed patients would completely receive the treatment and observe the special food diets in case of phenylketonuria and galactosemia; of course, these diets may not be followed during the adolescence. Furthermore, the results obtained from this study were only related to the patients; however, the patients’ families, particularly their parents, also benefit from these results and this increases the effectiveness of executing the program.
Acknowledgements
This research was performed by Mr. Samad Shirvani, in partial fulfillment of the requirements for certification as a MSc in Health Care Management at Shiraz University of Medical Sciences in Shiraz, Iran.
The present article was adopted from the proposal number 90-5887 approved by Shiraz University of Medical Sciences. The authors would like to thank the research deputy of Shiraz University of Medical Sciences for financially supporting the present work and Vice-chancellor for Health of Shiraz University of Medical Sciences and Dr. M. Moghadami for friendly cooperation.
Conflict of Interest
None
References
- 1.Smith LK, Budd JL, Field DJ, et al. Socioeconomic inequalities in outcome of pregnancy and neonatal mortality associated with congenital anomalies: population based study. BMJ. 2011;343:d4306. doi: 10.1136/bmj.d4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burgard P, Rupp K, Lindner M, et al. Newborn screening programmes in Europe; arguments and efforts regarding harmonization. Part 2. From screening laboratory results to treatment, follow-up and quality assurance. J Inherit Metab Dis. 2012;35(4):613–25. doi: 10.1007/s10545-012-9484-z. [DOI] [PubMed] [Google Scholar]
- 3.Delavari A, Yar Ahmadi Sh, Birjandi R, et al. Cost-Benefit Analysis of the Neonatal Screening Program Implementation for Congenital Hypothyroidism in I. R. Iran. Int J Endocrinol Metab. 2006;4:84–87. [Google Scholar]
- 4.Downing M, Pollitt R. Newborn bloodspot screening in the UK--past, present and future. Ann Clin Biochem. 2008;45(Pt 1):11–7. doi: 10.1258/acb.2007.007127. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization (WHO) Control of Genetic Diseases. http://apps.who.int/gb/archive/pdf_files/EB116/B116_3-en.pdf. Assess date: Jul 26, 2013.
- 6.Azizi F, Oladi B, Nafarabadi M, et al. Screening for congenital hypothyroidism in Tehran. Effect of iodine deficiency on transient elevation of neonatal TSH. J Shaheed Beheshti School Med. 1994;18(1):34–8. [Google Scholar]
- 7.Spahis JK, Bowers NR. Navigating the maze of newborn screening. MCN Am J Matern Child Nurs. 2006;31(3):190–6. doi: 10.1097/00005721-200605000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Vice-Chancellor of Health, Shiraz University of Medical Sciences. Neonatal Screening. http://fhc.sums.ac.ir/files/gh-vagir/screening.pdf. Access date: Jul 26, 2013.
- 9.Foxrushby J, Cairns J, editors. London: Brunel University, London School of Hygiene and Tropical Medicine; 2005. Economic Evaluation; pp. 210–5. [Google Scholar]
- 10.Carroll AE, Downs SM. Comprehensive cost-utility analysis of newborn screening strategies. Pediatrics. 2006;117(5 pt 2):s95–s287. doi: 10.1542/peds.2005-2633H. [DOI] [PubMed] [Google Scholar]
- 11.Geelhoed EA, Lewis B, Hounsome D, et al. Economic evaluation of neonatal screening for phenylketonuria and congenital hypothyroidism. J Pediatr Child Health. 2005;41(11):575–9. doi: 10.1111/j.1440-1754.2005.00725.x. [DOI] [PubMed] [Google Scholar]
- 12.Lord J, Thomason MJ, Littlejohns P, et al. Secondary analysis of economic data: a review of cost-benefit studies of neonatal screening for phenylketonuria. J Epidemiol Community Health. 1999;53(3):179–86. doi: 10.1136/jech.53.3.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cipriano LE, Rupar CA, Zaric GS. The cost-effectiveness of expanding newborn screening for up to 21 inherited metabolic disorders using tandem mass spectrometry: results from a decision-analytic model. Value Health. 2007;10(2):83–97. doi: 10.1111/j.1524-4733.2006.00156.x. [DOI] [PubMed] [Google Scholar]
- 14.Yarahmadi Sh, Tabibi SJ, Alimohammadzade Kh, et al. Cost-benefit and effectiveness of newborn screening of congenital hypothyroidism. Int J Endocrinol Metab. 2010;8(1):1–6. [In Persian] [Google Scholar]
- 15.Camelo Junior JS, Fernandes MI, Jorge SM, et al. Newborn screening for galactosemia a health economic evaluation. Cad Saude Publica. 2011;27(4):667–76. doi: 10.1590/s0102-311x2011000400006. [DOI] [PubMed] [Google Scholar]
- 16.Khneisser I, Adib SM, Loiselet J, et al. Cost-benefit analysis of G6PD screening in Lebanese newborn males. J Med Liban. 2007;55(3):129–32. [PubMed] [Google Scholar]
- 17.Cohan N, Karimi M, Khalili AH, et al. The efficacy of a neonatal screening programme in decreasing the hospitalization rate of patients with G6PD deficiency in southern Iran. J Med Screen. 2010;17(2):66–7. doi: 10.1258/jms.2010.009105. [DOI] [PubMed] [Google Scholar]