Abstract
Introduction
The aim of the present study was to identify the relationships between the uptake of radiotracers – namely pentavalent dimercaptosuccinic acid [(V)DMSA] and sestamibi (MIBI) – and the following parameters in primary breast cancer: steroid receptor concentrations (i.e. estrogen receptor [ER] and progesterone receptor [PR]), Ki-67 expression, tumor size, tumor grade, age, and levels of expression of p53 and c-erbB-2. In addition, by multivariate regression analysis, we further isolated those factors with independent associations with (V)DMSA and/or MIBI uptake in primary breast cancer.
Methods
Thirty-four patients with histologically confirmed breast carcinoma underwent preoperative scintimammography with technetium-99m (99mTc)-(V)DMSA and/or 99mTc-MIBI in consecutive sessions 10 and 60 min after administration of 925–1110 MBq of each radiotracer. The tumor-to-background ratio was calculated and correlated with the presence of ER, PR, Ki-67, tumor size, tumor grade, p53, and c-erbB-2. ER, PR, p53, and c-erbB-2 were determined immunohistochemically. The analysis included tumor-to-background ratio of (V)DMSA and MIBI uptake as dependent and all of the other parameters as independent variables.
Results
Correlation was positive between Ki-67 and (V)DMSA (r = 0.37 at 10 min, P = 0.038; r = 0.42 at 60 min, P = 0.018) and inverse between PR and (V)DMSA uptake (r = -0.46 at 10 min, P = 0.010; r = -0.51 at 60 min, P = 0.003). Multivariate regression analysis demonstrated a positive correlation between Ki-67 and (V)DMSA at 60 min (P = 0.045). Ki-67 was not significantly correlated with MIBI uptake, whereas tumor size was positively correlated with MIBI uptake at 60 min both in univariate (r = 0.45, P = 0.027) and multivariate analysis (P = 0.024). Negative correlations were observed between (V)DMSA uptake and ER, as well as between ER/PR and MIBI uptake, but these were not significant.
Conclusion
Ki-67 appears to represent the major independent factor affecting (V)DMSA uptake in breast cancer. Tumor size was the only independent parameter influencing MIBI uptake in breast cancer. (V)DMSA appears to have an advantage over MIBI in that it can be used to visualize tumors with intense proliferative activity, and thus it can identify those tumors that are more aggressive.
Keywords: breast cancer, Ki-67, 99mTc-MIBI, 99mTc-(V)DMSA, scintimammography
Introduction
Several factors have been reported to be able to predict the biological behavior and clinical outcome of breast cancer, including proliferation index, tumor suppressor gene p53, over-expression of oncoprotein c-erbB-2, tumor size, age, steroid receptors, and grade of malignancy. Of these, proliferation rate is the most important parameter in predicting tumor aggressiveness and prognosis. Many attempts have recently been made to define more accurately a specific agent with which to image the proliferative activity of breast lesions using [18F]-fluoro-2-deoxy-D-glucose positron emission tomography, [18F]3'-deoxy-3'-fluorothymidine positron emission tomography, and deoxyuridine, with ambiguous results [1-3].
Pentavalent dimercaptosuccinic acid [(V)DMSA] and hexakis-2-methoxyisobutylisonitrile (sestamibi [MIBI]) appear to have different mechanisms of accumulation in tumor, which have not yet been completely clarified. Technetium-99m (99mTc)-MIBI has been widely used for the past 5 years and has contributed significantly to the diagnosis of breast cancer, especially in circumstances that hinder mammographic diagnosis, such as dense breasts, scars, and implants. 99mTc-MIBI is concentrated in cancer cells by an energy requiring transport mechanism and transmembrane electronegative potential, in addition to nonspecific mechanisms, and it is stored within the mitochondria. 99mTc-(V)DMSA is a tumor seeking agent [4,5] that is known for its ability to detect medullary thyroid carcinoma [6], soft tissue tumors [7,8], lung cancer [9], metastatic disease [10], and brain tumors [11,12]. Its mechanism of accumulation has been thought to be related to the structural similarity between (V)DMSA core and PO4-3, which is avidly taken up by some cancer cells [13,14]. In a poorly differentiated breast cancer model, Palmedo and coworkers [15] reported that uptake of (V)DMSA was more than twofold greater than that of MIBI. Horiuchi and colleagues [16] reported that 99mTc-(V)DMSA uptake by tumors was related to glucose mediated acidosis.
We have conducted many comparative studies with (V)DMSA and MIBI in an attempt to identify their characteristics in detecting primary breast cancer and lymph node involvement, in defining tumor grade, and in visualizing preinvasive lesions such as ductal carcinoma in situ (DCIS)/lobular carcinoma in situ and epithelial hyperplasia, as well as their relationship with prognostic factors [17-21]. Apart from what was considered until now to be its main mechanism of concentration in breast cancer, (V)DMSA was found by simple [22] and multivariate regression analysis [23] to be related to proliferative activity in invasive and preinvasive breast lesions, which is directly related to tumor grade.
The aim of the present study was to determine the possible independent correlation between proliferative activity and other important prognostic factors, and (V)DMSA and MIBI uptake in breast cancer.
Materials and method
Patients
The series consisted of 34 patients (age 61.6 ± 12.49 years [mean ± standard deviation], range 38–85 years) with histologically proven breast cancer (infiltrating ductal 13, infiltrating lobular 9, tubular 1, mixed ductal and lobular 3, and infiltrating ductal plus in situ ductal 8). Twenty one of these patients underwent both 99mTc-(V)DMSA and 99mTc-MIBI scintigraphy in a double phase study, with an interval of 48 hours between the two tests. Ten patients underwent only 99mTc-(V)DMSA scintigraphy and three patients underwent only 99mTc-MIBI scintigraphy. Thus, a total of 31 patients underwent 99mTc-(V)DMSA and 24 underwent 99mTc-MIBI scintigraphy. Lateral prone and anterior supine images were acquired at approximately 15 and 60 min after administration of 925–1110 MBq of each radiotracer. Acquisitions were obtained using a special positioning pad (PBI-2 Scintimammography Pad Set®; Pinestar Technology Inc., Greenville, PA, and Somerset, NJ, USA).
Inclusion criteria for entry into the study were as follows: female sex, age greater than 21 years, not pregnant, suspicious lesion of the breast detected by palpation or mammography, recommendation for excisional biopsy after mammography, and informed consent. Exclusion criteria were recurrent disease, previous mastectomy, medically unstable patient with severe arrhythmia, heart failure or recent surgery, fine needle aspiration within 1 week before scintimammography, core biopsy during the previous 4 weeks, and previous chemotherapy.
Scintimammography was performed using a single-head γ camera (Sophycamera DS7; Sopha Medical Vision International, Buc Cedex, France) equipped with a high-resolution parallel hole collimator connected to a dedicated computer (Sophy NxT; Sopha Medical Vision International). The matrix was 256 × 256 pixels and the photo-peak was focused at 140 KeV with a symmetric 10% window. Scintimammography findings were characterized by two experienced nuclear medicine physicians as positive, suspicious, or negative. Disagreement was resolved by consensus or by obtaining a third opinion. The presence of focal increased activity, as compared with surrounding tissue, was classified as a positive study.
99mTc-(V)DMSA was prepared using a commercially available kit (DMS(V)/Demoscan®; distributed by the National Center of Physical Sciences, Institute of Radioisotopes and Radiodiagnostics 'Democritos', Athens, Greece), containing 1.36 mg dimercaptosuccinic acid, 0.105 mg SnCl2-2 H2O, 0.175 mg ascorbic acid, 12.5 mg inositole, 10 mg dextrose, 16.52 mg and 200 μl 7% NaHCO3, which was reconstituted with 2–3 ml 99mTc-pertechnetate solution (approximately 925–1110 MBq). Sestamibi kit (Cardiolite®), obtained from Bristol Myers Squibb GmbH (Regensburg, Germany), was labeled with 99mTc within the Department of Nuclear Medicine.
Diagnosis was made by histopathology of the specimens obtained surgically. Scintigraphic results and mammograms were compared with histologic findings.
Image analysis
Accumulation of radiopharmaceutical in breast tumors was evaluated in early and delayed images visually and by calculating tumor-to-background ratio using regions of interest of standardized shape and size over the site of the greatest activity and the surrounding normal breast tissue. The same procedure was also performed with regions of maximum and minimum activity in the contralateral normal breast.
Immunostaining
An immunohistochemical method (avidin–biotin–peroxidase complex; ABC-HRP) was performed on paraffin embedded sections from breast cancer patients for demonstration of estrogen receptor (ER), progesterone receptor (PR), p53, c-erbB-2, and Ki-67 protein expression. For ER and PR receptor proteins the 1D5 and 1A6 monoclonal antibodies (DAKO, Glostrup, Denmark) were used at dilutions of 1 : 400 and 1 : 150, respectively. For p53 protein determination the specimens were incubated with 1 : 15 diluted monoclonal antibody (Clone BPS3.12.1; Oncogene Science, Cambridge, MA, USA). CB11 monoclonal antibody (Biogenex, San Ramon, CA, USA) was used for c-erbB-2 detection at a dilution of 1 : 150. A polyclonal antibody for Ki-67 (DAKO) was used at a dilution of 1 : 350. To enhance antigen retrieval, sections for ER, PR, and Ki-67 were microwave treated in 0.01 M citrate buffer (pH 6.0) at 750 W for 10 min.
A semiquantitative estimation based on the staining intensity and the percentage of positive cells was performed. Staining for ER and PR was evaluated using the H score system, and a score greater than 50 was considered positive for both antigens (0 = negative [0–50], 1 = mild reactivity [51–100], 2 = moderate reactivity [101–200], 3 = strong reactivity [201–300]). Staining for p53, c-erbB-2, and Ki-67 was scored on a scale from 0 to 3 in half steps (0 = negative [0–10% positive cells], 1 = 11–30% positive cells, 2 = 31–50% positive cells, 3 = > 51% positive cells).
Data analysis
Patients were classified into four subgroups: groups 1 and 2 for early and late (V)DMSA acquisitions, respectively; and groups 3 and 4 for early and late MIBI acquisitions, respectively. To investigate which factors play important roles in the uptake of these radiotracers, we used both univariate and multivariate analysis. In univariate analysis we used the method of Spearman rank correlation to test whether there was a significant correlation between the uptake ratio (tumor-to-background ratio) and the variables of interest. Each analysis was performed within the four subgroups. In multivariate analysis, we used the method of stepwise multivariate linear regression for each subgroup. For all tests, P < 0.05 was considered statistically significant.
Results
Tumor-to-background ratios for (V)DMSA and MIBI ranged from 1.13 to 2.80 (1.69 ± 0.49 [mean ± standard deviation]) and from 1.13 to 4.78 (1.96 ± 0.92), respectively. Descriptive statistics for all dependent parameters are listed in Table 1. Information concerning tumor grade, tumor size, and age of patients is presented in Table 2.
Table 1.
Values for parameters studied in the tumors
| Minimum | Maximum | Mean | Standard deviation | |
| Age (years) | 38 | 85 | 61.58 | 12.49 |
| Tumor Size (cm) | 1 | 6 | 2.82 | 1.34 |
| Estrogen receptor | 0 | 250 | 134 | 84.53 |
| Progesterone receptor | 0 | 240 | 75.52 | 69.85 |
| p53 | 0 | 100 | 10.42 | 13.95 |
| Ki-67 | 0 | 210 | 62.58 | 63.49 |
| c-erbB-2 | 0 | 100 | 11.54 | 23.46 |
Table 2.
Distribution of studied material according to grade and size of tumor and patient age
| Frequency | Percentage | |
| Grade | ||
| I | 4 | 11.7% |
| II | 20 | 58.8% |
| III | 10 | 29.4% |
| Size | ||
| T1 | 5 | 14.7% |
| T2 | 27 | 79.4% |
| T3 | 2 | 5.8% |
| Age (years) | ||
| < 40 | 4 | 11.7% |
| 41–50 | 6 | 17.6% |
| 51–60 | 6 | 17.6% |
| 61–70 | 12 | 35.2% |
| ≥ 71 | 6 | 17.6% |
Proliferative index (Ki-67)
Linear regression univariate analysis revealed a significant positive correlation between Ki-67 and (V)DMSA uptake in early and late phases (r = 0.37 at 10 min, P = 0.038; r = 0.42 at 60 min, P = 0.018; Fig. 1). Simple regression analysis could not identify any correlation of Ki-67 with MIBI uptake.
Figure 1.
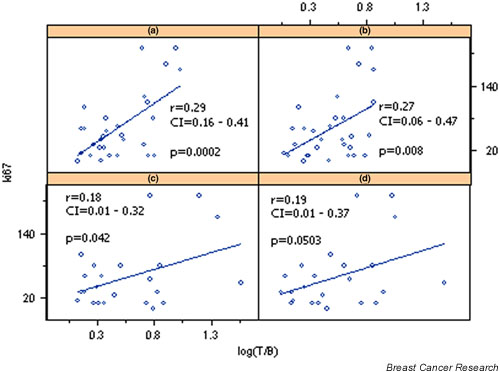
Correlation of Ki-67 with log transformed tumor-to-background ratios (T/B) of pentavalent dimercaptosuccinic acid [(V)DMSA] uptake at (a) 10 min and (b) 60 min, and sestamibi (MIBI) uptake at (c) 10 min and (d) 60 min. CI, confidence interval.
Multivariate analysis demonstrated a positive correlation of Ki-67 with (V)DMSA (P = 0.045) but not with MIBI (Table 3). If c-erbB-2 and p53 are excluded from the multivariate analysis, then the statistical significance of the correlation becomes greater (P = 0.023).
Table 3.
Significant regression coefficients with standard errors and P values of the full model for 99mTc-(V)DMSA and 99mTc-MIBI uptake
| Standard error | P | |
| (V)DMSA at 60 min: Ki-67 | 0.002 | 0.045 |
| MIBI at 60 min: tumor size | 0.141 | 0.024 |
MIBI, sestamibi; 99mTc, technitium-99m; (V)DMSA, pentavalent dimercaptosuccinic acid.
Estrogen and progesterone receptors
Using simple univariate analysis, inverse (negative) correlations between PRs and (V)DMSA uptake were found at 10 and 60 min (r = -0.46 at 10 min, P = 0.010; r = -0.51 at 60 min, P = 0.003; Table 4, Figs 2 and 3). MIBI uptake was inversely but not significantly correlated with ER/PR receptors, despite its tendency to be related to lower ER/PR values.
Table 4.
Correlation coefficients and their significance for the four groups
| ER | PR | Ki-67 | Tumor size | Age | p53 | c-erbB-2 | |
| (V)DMSA 10 min | |||||||
| r | -0.24 | -0.46 | 0.37 | 0.32 | 0.30 | -0.06 | -0.09 |
| P | 0.187 | 0.010 | 0.038 | 0.081 | 0.093 | 0.745 | 0.640 |
| (V)DMSA 60 min | |||||||
| r | -0.26 | -0.51 | 0.42 | 0.25 | 0.22 | 0.23 | 0.09 |
| P | 0.157 | 0.003 | 0.018 | 0.178 | 0.226 | 0.206 | 0.617 |
| MIBI 10 min | |||||||
| r | -0.02 | -0.25 | 0.23 | 0.29 | 0.19 | 0.33 | -0.11 |
| P | 0.917 | 0.250 | 0.279 | 0.166 | 0.372 | 0.121 | 0.626 |
| MIBI 60 min | |||||||
| r | -0.01 | -0.27 | 0.35 | 0.45 | 0.27 | 0.22 | -0.04 |
| P | 0.953 | 0.211 | 0.097 | 0.027 | 0.202 | 0.305 | 0.848 |
ER, estrogen receptor; MIBI, sestamibi; PR, progesterone receptor; (V)DMSA, pentavalent dimercaptosuccinic acid.
Figure 2.
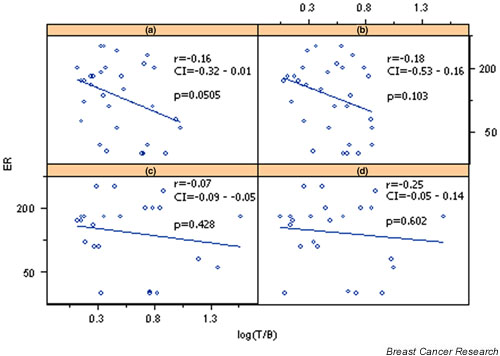
Correlation of estrogen receptor (ER) with log transformed tumor-to-background ratios (T/B) of pentavalent dimercaptosuccinic acid [(V)DMSA] uptake at (a) 10 min and (b) 60 min, and sestamibi (MIBI) uptake at (c) 10 min and (d) 60 minutes. CI, confidence interval.
Figure 3.
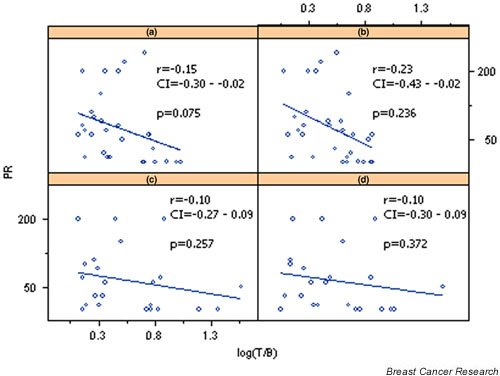
Correlation of progesterone receptor (PR) with log transformed tumor-to-background ratios (T/B) of pentavalent dimercaptosuccinic acid [(V)DMSA] uptake at (a) 10 min and (b) 60 min, and sestamibi (MIBI) uptake at (c) 10 min and (d) 60 min. CI, confidence interval.
Multivariate analysis did not reveal any significant correlation of uptake of either radiotracer with ER/PR receptor status.
Tumor size
Tumor size was positively correlated in univariate and multivariate analyses with MIBI uptake at 60 min (r = 0.45, P = 0.027 for univariate analysis; and P = 0.024 for multivariate analysis; Tables 3 and 4, Fig. 4). (V)DMSA was not found to be correlated with tumor size.
Figure 4.
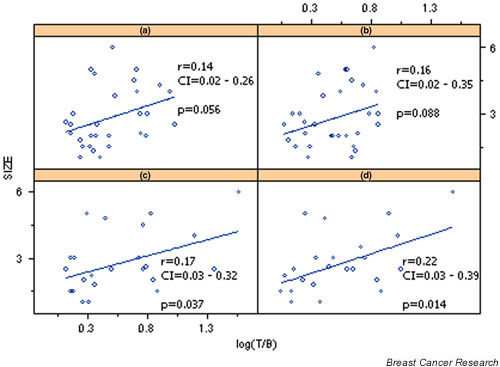
Correlation of tumor size (TS) with log transformed tumor-to-background ratios (T/B) of pentavalent dimercaptosuccinic acid [(V)DMSA] uptake at (a) 10 min and (b) 60 min, and sestamibi (MIBI) uptake at (c) 10 min and (d) 60 min. CI, confidence interval.
Grade of malignancy, age, and expressions of p53 and c-erbB-2
None of these parameters was significantly correlated with tumor (V)DMSA or MIBI uptake.
Discussion
The prognosis of breast cancer depends not only on the extent of the disease but also on the biological behavior of the tumor, which is based on physical, histologic, and immunohistochemical characteristics. Steroid receptor status, loss of differentiation, increasing proliferative activity, inactivation of tumor suppressor genes, over-expression of oncogenes, tumor size, and age are related to tumor progression and may be used to predict prognosis. Although the exact mechanism of tumor accumulation and the kinetics of the tracers MIBI and (V)DMSA have not yet been fully clarified, their possible correlation with some of these parameters should be of great importance in preoperative evaluation and prognostication in this disease.
Scintimammography with 99mTc-MIBI and 99mTc-(V)DMSA is a useful technique that is complementary to mammography in the assessment of suspected breast lesions. It has been reported to have high sensitivity (88.4%), specificity (93.3%), positive predictive value (95%), negative predictive value [82% for MIBI, 80% for (V)DMSA], and accuracy [90% for MIBI, 89% for (V)DMSA] for lesions greater than 1 cm in size; however, these values are less for lesions sized smaller than 1 cm, which might be considered a limitation of the procedure [20,24,25].
Arrest of tumor cell proliferation is the ultimate objective of anticancer therapy. Breast cancer aggressiveness appears to be directly related to the percentage of Ki-67 positive cancer cells [26]. The immunohistochemical expression of Ki-67 appears to be associated with the grade of differentiation, angiogenic invasion, lymph node metastases, and the absence of ER and PR [26]. In our group of patients there was a positive correlation in univariate analysis between 99mTc-(V)DMSA tumor uptake and Ki-67 expression in both early and late phases, which was more prominent in the late phase. This relationship was found in multivariate regression analysis to be independent of the presence of other prognostic factors, which indicates the existence of a separate primary pathway for the mechanism of accumulation of (V)DMSA in tumor tissue. This is considered to reflect its uptake in structures that participate in mitotic activity of cancerous and precancerous cell populations. The inverse correlation found between PR and (V)DMSA uptake strengthened our previous finding, because Ki-67 positivity is inversely correlated with the presence of PR receptors.
99mTc-MIBI exhibited no correlation with Ki-67 in univariate and multivariate analyses. This indicates that this parameter could not represent an important regulating factor in MIBI accumulation in tumor tissue.
Cwikla and coworkers [27], in a group of 79 patients with breast cancer, also found a negative correlation of PR and a borderline negative correlation of ER with MIBI uptake. However, they did not find a significant correlation between Ki-67 expression and MIBI uptake (P = 0.09). Cutrone and coworkers [28] reported a moderate positive correlation between MIBI uptake and cellular proliferation (P < 0.05) in a group of 42 patients with breast cancer. Ohira and coworkers [29] found that MIBI uptake did not reflect tumor growth rate in three animal models of breast cancer. However, it must be borne in mind that all of those investigators used only simple univariate analysis.
For (V)DMSA, preliminary reports from our center [20,30-32] indicate that preinvasive breast lesions such as DCIS/lobular carcinoma in situ and epithelial hyperplasia, and especially those with moderate to intense Ki-67 expression (unpublished data), can be identified with high sensitivity by (V)DMSA scintimammography. Mitotic activity and cellular proliferation are the hallmarks not only of invasive cancers but also of in situ or premalignant lesions such as atypical epithelial hyperplasia and tumors with unpredictable biologic behaviour such as phyllodes tumors. These lesions have been identified more frequently and more intensely with (V)DMSA than with MIBI [20].
Tumor size was positively correlated with MIBI uptake at 60 min (r = 0.45, P = 0.027), but not with (V)DMSA uptake, in univariate analysis. Multivariate analysis demonstrated independent correlation only with MIBI uptake at 60 min (P = 0.024). This finding indicates that MIBI concentration reflects more accurately the number of metabolically active cancer cells but does not represent tumor growth or cell proliferation. This independent correlation was found only at 60 min and shows that tumor vascularity was not involved in this process. The nondependency of (V)DMSA uptake with tumor size could be explained as a result of the heterogeneity of Ki-67 expression in different parts of the tumor. Cwikla and coworkers [27] also found a positive correlation between MIBI uptake and size of the tumor in a group of 79 patients with breast cancer (P = 0.01).
Tumor grading using Bloom–Richardson's criteria could in theory be considered another variable that is closely related to proliferative activity and to tumor uptake of the radiotracers (V)DMSA and MIBI. However, this was not demonstrated in univariate or multivariate analysis for either tracer.
Cwikla and coworkers [27] reported a positive correlation between MIBI uptake and malignant grade only for infiltrating ductal carcinomas (P = 0.03). Recently, in 45 breast cancer patients, we found that the difference in (V)DMSA and MIBI uptake between lower and higher tumor grades was not statistically different [21]. However, by calculating the retention index ([delayed uptake – early uptake] × 100/early uptake), we found that lower grade tumors had lower retention indices than did higher grade ones.
In our group of patients the majority of cancers were of grade II or III. The lack of grade I cancers and the possible individual subjective over- or underestimation of close consecutive grades (II and III) could offer an alternative explanation for these observations.
The relationship between p53 expression and other prognostic parameters is not yet clear. Contradictory findings concerning tumor size and lymph node status have been reported, but a strong relationship has been reported with in situ carcinomas and their histologic types. Furthermore, it has been demonstrated that p53 overexpression reflects tumor aggressiveness and decreased disease free interval following therapy. Our findings indicated no p53 dependency of (V)DMSA or MIBI uptake in breast tumor in simple and multivariate analyses.
C-erbB-2 over-expression has also been suggested to be a prognostic factor in breast cancer. C-erbB-2 is found in almost 60% of cases of high grade comedo-type DCIS [33,34], in 10–40% of infiltrating ductal carcinomas, and in only a few cases of infiltrating lobular carcinomas. C-erbB-2 expression has a close positive relationship with degree of differentiation and number of infiltrated lymph nodes, and an inverse correlation with the presence of hormonal receptors. In contrast, in our series no significant correlation was found in univariate and multivariate analyses between (V)DMSA or MIBI uptake and c-erbB-2 expression.
Conclusion
In summary, the results of the present study suggest that proliferative activity is a major independent factor affecting (V)DMSA uptake in breast cancer. Tumor size was the only independent parameter influencing MIBI uptake in breast cancer. The negative correlation found between PR and (V)DMSA uptake can be interpreted as indirect and dependent on the negative correlation between Ki-67 and PR. The clinical significance of such an in vivo indicator of cell proliferation activity could be useful for predicting the prognosis and clinical behavior of an infiltrating breast cancer, and in the detection of early preinvasive lesions.
Competing interests
None declared.
Abbreviations
DCIS = ductal carcinoma in situ; ER = estrogen receptor; MIBI = sestamibi; 99mTc = technetium-99m; (V)DMSA = pentavalent dimercaptosuccinic acid; PR = progesterone receptor.
References
- Rasey JS, Grierson JR, Wiens LW, Kolb PD, Schwartz JL. Validation of FLT uptake as a measure of thymidine kinase-1 activity in A549 carcinoma cells. J Nucl Med. 2002;43:1210–1217. [PubMed] [Google Scholar]
- Toyohara J, Hayashi A, Sato M, Tanaka H, Haraguchi K, Yoshimura Y, Yonekura Y, Fujubayashi Y. Rationale of 5-125I-iodo-4'-thio-2'-deoxyuridine as a potential iodinated proliferation marker. J Nucl Med. 2002;43:1218–1226. [PubMed] [Google Scholar]
- Buck A, Schirrmeister H, Kühn Th, Shen Ch, Kalker Th, Kotzerke J, Dankerl A, Glatting G, Reske S, Mattfeldt T. FDG uptake in breast cancer: correlation with biological and clinical prognostic parameters. Eur J Nucl Med. 2002;29:1317–1323. doi: 10.1007/s00259-002-0880-8. [DOI] [PubMed] [Google Scholar]
- Westera G, Gadze A, Horst W. A convenient method for the preparation of 99mTc(V) dimercaptosuccinic acid (99mTc(V)-DMSA) Int J Appl Radiat Isot. 1985;36:311–312. doi: 10.1016/0020-708X(85)90091-2. [DOI] [PubMed] [Google Scholar]
- Blower PJ, Singh J, Clarke SEM. The chemical identity of pentavalent 99mTc-dimercaptosuccinic acid. J Nucl Med. 1991;32:845–849. [PubMed] [Google Scholar]
- Ohta H, Yamamoto K, Endo K, Mori T, Hamanaka D, Shimazu A, Ikekubo K, Makimoto K, Iida Y, Konishi J. A new imaging agent for medullary carcinoma of the thyroid. J Nucl Med. 1984;25:323–325. [PubMed] [Google Scholar]
- Ohta H, Endo K, Fujita T, Nakajima T, Sakahara H, Torizuka K, Shimizu Y, Hata N, Masuda H, Horiuchi K. Imaging of soft tissue tumors with 99mTc(V)-dimercaptosuccinic acid, a new tumor seeking agent. Clin Nucl Med. 1984;9:564–573. doi: 10.1097/00003072-198410000-00007. [DOI] [PubMed] [Google Scholar]
- Ohta H, Endo K, Fujita T, Konishi J, Torizuka K, Horiuchi K, Yokoyama A. Clinical evaluation of tumor imaging using 99mTc(V)-dimercaptosuccinic acid, a new tumor seeking agent. Nucl Med Commun. 1988;9:105–116. [PubMed] [Google Scholar]
- Hirano T, Otake H, Yoshida I, Endo K. Primary lung cancer SPECT imaging with pentavalent 99mTc-DMSA. J Nucl Med. 1995;36:202–207. [PubMed] [Google Scholar]
- Lamki L, Shearer R. Technetium-99m-DMSA uptake by metastatic carcinoma of the prostate. J Nucl Med. 1985;25:733–734. [PubMed] [Google Scholar]
- Hirano T, Otake H, Shibasaki T, Tamura M, Endo K. Differentiating histologic malignancy of primary brain tumors: pentavalent Technetium-99m-DMSA. J Nucl Med. 1997;38:20–26. [PubMed] [Google Scholar]
- Hirano T, Otake H, Kazama K, Wakabayashi K, Zama A, Shibasaki T, Tamura M, Endo K. Technetium-99m-(V)-DMSA and Thallium-201 in brain tumor imaging: correlation with histology and malignant grade. J Nucl Med. 1997;38:1741–1749. [PubMed] [Google Scholar]
- Yokoyama A, Saji H. Tumor diagnosis using radioactive metal ions and their complexes. In: Siegel H, editor. In Metal Ions in Biological Systems. Vol. 10. New York: Marcel Dekker; 1980. pp. 313–340. [Google Scholar]
- Wulfrank D, Schelstraete K, Small F, Charles F. Analogy between tumor uptake of technetium-99m-(V) dimercaptosuccinic acid (DMSA) and Technetium-99m-MDP. Clin Nucl Med. 1989;14:488–593. doi: 10.1097/00003072-198908000-00007. [DOI] [PubMed] [Google Scholar]
- Palmedo H, Hensel J, Bender H, Wagner U, Bangard M, Bierrsack 18F-FDG, 99mTc-(V)DMSA and 99mTc-MIBI in an animal breast cancer-model: comparison of tumor-uptake and correlation with scintigraphic and PET-detection [abstract] Eur J Nucl Med. 2000;27:1130. doi: 10.1007/s002590050017. [DOI] [Google Scholar]
- Horiuchi K, Saji H, Yokoyama A. Tc(V)-DMS tumor localization mechanism: a pH-sensitive Tc(V)-DMS-enhanced target/non-target ratio by glucose-mediated acidosis. Nucl Med Biol. 1998;25:549–555. doi: 10.1016/S0969-8051(98)00012-2. [DOI] [PubMed] [Google Scholar]
- Papantoniou V, Karganzis P, Stipsanelli A, Arka A, Keramopoulos A, Kostamis P. Technetium-99m (V) dimercaptosuccinic acid [Tc-99m (V) DMSA] in the detection of primary breast cancer. Preliminary results [abstract] Eur J Nucl Med. 1996;23:1216. [Google Scholar]
- Papantoniou V, Stipsanelli A, Arka A, Keramopoulos A, Kostamis P, Michalas S. Comparison of Technetium-99m-[V]-DMSA with Technetium-99m-MDP in diagnosis of breast lesions [abstract] Eur J Nucl Med. 1998;25:1040. [Google Scholar]
- Papantoniou V, Stipsanelli A, Galeros C, Feida E, Kostamis P, Keramopoulos A, Michalas S. Similarity between 99mTc-V-DMSA and 99mTc sestamibi in the detection of primary breast cancer. Preliminary results [abstract] Eur J Nucl Med. 1998;25:1040. [Google Scholar]
- Papantoniou V, Stipsaneli A, Christodoulidou J, Louvrou A, Lazaris D, Sotiropoulou M, Papadaki E, Valotassiou V, Pampouras G, Keramopoulos A, Michalas S, Zerva C. 99mTc-(V)DMSA scintimammography in the assessment of breast lesions. Comparative study with 99mTc MIBI. Eur J Nucl Med. 2001;28:923–928. doi: 10.1007/s002590100545. [DOI] [PubMed] [Google Scholar]
- Papantoniou V, Christodoulidou J, Papadaki E, Valotassiou V, Souvatzoglou M, Louvrou A, Feida H, Sotiropoulou M, Pampouras G, Michalas S, Zerva C. Uptake and washout of 99mTc-V-dimercaptosuccinic acid and 99m-Tc-sestamibi in the assessment of histological type and grade in breast cancer. Nucl Med Com. 2002;23:461–467. doi: 10.1097/00006231-200205000-00006. [DOI] [PubMed] [Google Scholar]
- Papantoniou V, Stipsaneli A, Arka A, Louvrou A, Lazaris D, Nakopoulou L, Palikarona P, Keramopoulos A, Michalas S. Immunohistologic assessment of 99mTc-(V)DMSA and 99mTc-MIBI uptake in breast cancer [abstract] Eur J Nucl Med. 1999;26:977. [Google Scholar]
- Papantoniou V, Nakopoulou L, Christodoulidou J, Papadaki E, Souvatzoglou M, Stipsaneli A, Lazaris D, Sotiropoulou M, Keramopoulos A, Michalas S, Zerva C. Correlation and multivariate regression analysis between 99mTc (V)DMSA and 99mTc MIBI uptake and steroid receptors, proliferation index, tumor size, age, malignant grade, p53, and c-erbB-2 in primary breast cancer [abstract] Eur J Nucl Med. 2001;28:1120. doi: 10.1007/s002590100545. [DOI] [Google Scholar]
- Palmedo H, Schomburg A, Grunwald F, Mallmann P, Krebs D, Biersack H. Technetium-99m-MIBI scintimammography for suspicious breast lesions. J Nucl Med. 1996;37:626–639. [PubMed] [Google Scholar]
- Scopinaro F, Schillaci O, Ussof W, Nordling K, Capoferro R, De Vincentis G, Danieli R, Ierardi M, Picardi V, Tavolaro R, Colella AC. A three-center study on the diagnostic accuracy of 99mTc-MIBI scintimammography. Anticancer Res. 1997;17:1631–1634. [PubMed] [Google Scholar]
- Tavassoli FN. Pathology of the Breast. Norwalk: Appleton & Lange; 1992. [Google Scholar]
- Cwikla JB, Buscombe JR, Kolasinska AD, Rarbhaoo SP, Thakrar DS, Hilson AJW. Correlation between uptake of 99mTc Ses-taMIBI and prognostic factors of breast cancer. Anticancer Res. 1999;19:2299–2304. [PubMed] [Google Scholar]
- Cutrone JA, Yospur LS, Khalkhali I, Tolmos J, Devito A, Diggles L, Vargas MP, Shitabata P, French S. Immunohistologic assessment of technetium-99m-MIBI uptake in benign and malignant breast lesions. J Nucl Med. 1998;39:449–453. [PubMed] [Google Scholar]
- Ohira H, Kubota K, Ohuchi N, Harada Y, Fukuda H, Satomi S. Comparison of intratumoral distribution of 99mTc-MIBI and deoxyglucose in mouse breast cancer models. J Nucl Med. 2000;41:1561–1568. [PubMed] [Google Scholar]
- Papantoniou V, Sotiropoulou M, Stipsaneli A, Louvrou A, Feda H, Christodoulidou J, Pampouras G, Zerva C, Keramopoulos A, Michalas S. Scintimammographic findings of in situ ductal breast carcinoma in a double-phase study with 99mTc (V) DMSA and 99mTc MIBI. Value of 99mTc (V) DMSA. Clin Nucl Med. 2000;25:434–439. doi: 10.1097/00003072-200006000-00009. [DOI] [PubMed] [Google Scholar]
- Papantoniou V, Stipsanelli A, Christodoulidou J, Papadaki E, Valotassiou V, Louvrou A, Bebi M, Sotiropoulou M, Pampouras G, Keramopoulos A. Scintimammographic detection of ductal in situ carcinomas. Comparative study with 99mTc(V)DMSA and 99mTc-MIBI [abstract] Eur J Nucl Med. 2000;27:1129. [Google Scholar]
- Papantoniou V, Sotiropoulou M, Stipsanelli A, Souvatzoglou M, Valotassiou V, Koutsikos I, Louvrou A, Ambela C, Lazaris D, Arka A, Kounadi E, Melissinou M, Zerva C, Michalas S. 99mTc (V)DMSA breast uptake in usual type hyperplasia (HUT) and apocrine metaplasia (AM) in ralation with cell proliferation index (Ki-67) and the presence of estrogen receptors (ER). Comparative study with 99mTc-MIBI [abstract] Eur J Nucl Med. 2003;Suppl 2:S290. [Google Scholar]
- Dawkins HJ, Robbins PD, Smith KL, Sarna M, Harvey JM, Sterrett GF, Papadimitriou JM. What's new in breast cancer: molecular perspectives of cancer development and the role of the oncogene c-erbB-2 in prognosis and disease. Pathol Res Pract. 1993;189:1233–1252. doi: 10.1016/S0344-0338(11)80853-8. [DOI] [PubMed] [Google Scholar]
- De Potter CR, Schellhom AM. The neu-protein and breast cancer. Virchows Archiv. 1995;426:107–115. doi: 10.1007/BF00192631. [DOI] [PubMed] [Google Scholar]


