Abstract
Hepatitis B virus (HBV) or hepatitis C virus (HCV) infections are a major threat worldwide. Combination therapy of interferon-α and ribavirin is currently the treatment of choice for HCV-infected patients. However, this regimen is only effective in approximately 50% of patients and provokes severe side-effects. Numerous natural alternatives for treating HCV have been suggested. Deoxynojirimycin and its derivatives are iminosugars which exert anti-HCV activity by inhibiting α-glucosidases. A non-immunosuppressive derivate of cyclosporine A, NIM811, exerts anti-HCV activity by binding to cyclophilin. Other natural products with promising anti-HCV activity are 2-arylbenzofuran derivatives, Mellein, and pseudoguaianolides. For HBV treatment, several drugs are available, specifically targeting the virus polymerase (lamivudine, entecavir, telbivudine, and adefovir dipivoxil). The efficacy of these drugs is hampered by the development of resistance due to point mutations in the HBV polymerase. Due to drug resistance and adverse side-effects, the search for novel drugs is mandatory. Wogonin, ellagic acid, artemisinin and artesunate, chrysophanol 8-O-β-D-glucoside, saikosaponin C, and protostane triterpenes are active against HBV. Natural products need to be investigated in more detail to explore their potential as novel adjuncts to established HBV or HCV therapy.
Keywords: antiviral agent, chemotherapy, drug resistance, hepatitis B, hepatitis C, natural product, pharmacognosy
Introduction
The major cause of liver cancer and cirrhosis in the Western world is hepatitis C virus (HCV) infections. Worldwide, approximately 170 million people are affected. The infection is often clinically silent (at least until cirrhosis), and therefore, frequently diagnosed only by chance. Current anti-HCV therapies are only effective in approximately half of patients and have strong side-effects.
The HCV belongs to the Flaviviridae family and contains a genome three times as large as the hepatitis B virus (HBV) genome (9.6 kb). It is made up of linear, single-stranded RNA. One single open reading frame (ORF) codes 10 different structural and non-structural proteins (NS). The resulting polyprotein precursor has to be cleaved by several viral and host enzymes, which all provide potential targets for antiviral therapy1. The low fidelity of the RNA-dependent RNA polymerase (RdRp) is the main reason for a heterogeneous virus population and the emergence of resistance to antiviral drugs2.
Approximately two billion people worldwide have been infected with HBV, and more than 350 million people are estimated to be chronic carriers of HBV3. There are 500 000–1.2 million deaths per year caused by HBV infections, which lead to cirrhosis and hepatocellular carcinoma in many cases after years and decades of infection4. Young children in particular are likely to become persistently infected. As current therapies are not able to eradicate the virus completely, prolonged treatment is necessary, giving rise to resistance mutations5.
The HBV genome consists of partly double-stranded DNA (3.2 kb in size). The host cell contains covalently-closed circular DNA, relatively resistant to antiviral therapies, making complete eradication difficult. Replication occurs via reverse transcription of an RNA intermediate in the host cell. Since the reverse transcriptase lacks a proof-reading function, the mutation rate of HBV is quite high. Nevertheless, the overlapping arrangement of the 4 ORF limits the viability of mutants, and therefore, has up to a 1000-fold lower mutation rate compared to HIV6.
With the current treatment options and their efficacy in mind, new agents for HBV and HBC treatment are urgently needed7, 8.
Current treatment
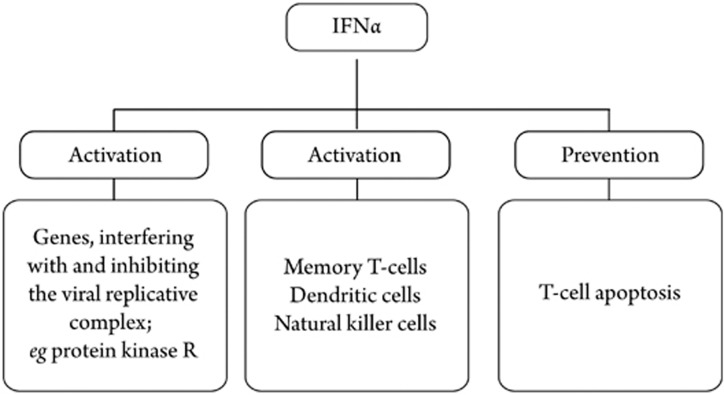
Combination therapy of interferon-α (IFN-α) and ribavirin formulations is currently recommended for HCV-infected patients. Since the immune system of chronic carriers is not able to eradicate the virus completely, IFN-α is believed to strengthen the host's innate antiviral immune response. The activation of Janus-activated and tyrosine kinases result in a signal cascade, which finally causes transcription of various genes encoding for proteins that interfere with the virus replicative complex.
Furthermore, IFN-α activates the proliferation of memory T cells, the maturation of dendritic and natural killer cells, and prevents T-cell apoptosis (Figure 1)5. The nucleoside analog ribavirin has broad antiviral activity. However, its mechanism of action is not well understood. The proposed modes of action are immune modulatory effects, direct inhibition of RdRp, and the depletion of intracellular guanosine triphosphate that is essential for viral RNA synthesis and for causing lethal viral mutagenesis9. The combination of IFN-α and ribavirin is only effective in approximately 50% of patients. Individuals infected with genotypes 2 and 3 achieve sustained viral response (SVR) after treatment in approximately 90% of cases, but those infected with genotype 1 (mostly in Europe and the USA) achieve SVR in only 33%–42% of cases10. Nearly all treated patients suffer from side-effects. Frequently-observed side-effects of high-dose IFN treatment are flu-like symptoms, thrombocytopenia, leukopenia, and anemia. In as much as 19% of patients, severe adverse effects, like impotence, thyroid disorders, or intestinal bleeding, lead to early discontinuation11. The most common side-effects of ribavirin are hemolysis and anemia, which are teratogenic effects5. Taken into account this data, new, more effective, and less toxic agents are essential for successful HCV treatment.
Figure 1.
Effects of IFN-α on innate immune response.
In contrast to HCV, for HBV treatment, there are several agents that specifically target the virus polymerase. Lamivudine, a cytosine analog, was the first polymerase inhibitor approved for hepatitis B treatment. Predominantly, combination therapy of IFN-α and lamivudine is the treatment of choice for HBV infections. The recently approved drugs entecavir (guanine analog) and telbivudine (L-deoxythymidine) offer new possibilities in treatment and combination therapies, but the best strategy for fighting HBV is still unknown. Adefovir dipivoxil is another competitive inhibitor of the HBV polymerase, structurally similar to dATP and the potential to be nephrotoxic. However, in contrast to IFN-α and ribavirin, those nucleoside analogs have fewer side-effects due to the specific viral target. Though, exactly this is the reason for resistance susceptibility6, 12.
Resistance problems
Since combination therapy of IFN-α and ribavirin against HCV does not target the virus directly, or in case of ribavirin, the mechanism of action is not well understood, conclusions about resistance mechanisms are complex and difficult to make. The effectiveness of this therapy depends on the virus genotype. Patients infected with genotype 2 or 3 have a higher treatment success rate than those infected with genotype 1. Different regions have been suggested to antagonize the effect of IFN-α. An interaction of NS5A, the 9th NS of HCV, with the double-stranded RNA protein kinase, for example, is supposed to block the signaling pathway in cell culture13. Of particular interest are resistance studies from recent approaches of anti-HCV agents currently in the production pipeline. These drugs target specific viral proteins, especially NS3-4A serine protease and NS5B polymerase. Different peptidomimetic inhibitors, nucleoside analogs, and non-nucleoside analogs are at various stages of development and show high potency of anti-HCV activity. Nevertheless, in vitro studies have revealed resistance emergence or each of these agents. In most cases, 1 single point mutation is sufficient to achieve tolerance against the drug, or worse, cross mutations against another one14. These observations indicate that drug resistance is likely to remain a problem, and solely targeting viral proteins will not be enough to prevent resistance emergence.
Since lamivudine has been available for the longest period time among all approved anti-HBV agents, resistance emergence has been extensively studied. Clinical trials have shown that the rate of mutations increases with prolonged lamivudine use (Table 1). After 1 year of treatment, 24% of patients become resistant, and after 3 years, 69% achieve resistance15. The tyrosine-methionine-aspartate-aspartate (YMDD) locus within the catalytic C domain of the HBV reverse transcriptase plays a major role in resistance development. In most resistance patterns, the methionine within this locus is replaced by an isoleucine or a valine, and occasionally a serine. The substitution of methionine by isoleucine is sufficient to achieve resistance. Other patterns include 1 or 2 more point mutations. Those amino acid substitutions at the YMDD motif cause steric hindrance, and therefore, prevent the incorporation of lamivudine into the viral DNA. Another mechanism causing resistance concerns diminished catalytic efficiency. Geometric changes can result in a suboptimal nucleophilic attack, thus influencing catalysis negatively16. Interestingly, lamivudine-resistant patients show a low HBV–DNA level at the beginning. In vitro studies have revealed that rtM204V/I lowers the replication fitness of the virus. However, drug withdrawal leads to the re-emergence of the wild type after some time. Further lamivudine administration increases the risk of additional mutations, which are able to restore viral replication fitness6.
Table 1. Common resistance mutations in domains of the HBV polymerase.
| Lamivudine | Adefovir dipivoxil | Entecavir | Telbivudine | |
|---|---|---|---|---|
| Domain A | L80V/I | |||
| Domain B | V173L/L180M | A181V | T184G | |
| Domain C | M204V/I/S | S202I | M204I | |
| Domain D | N236T |
Adefovir dipivoxil resistance has been less frequently observed (Table 1). In a study with treatment–naïve patients, resistant mutations were detected in only 6%. A substitution of asparagine for threonine within the D domain of the polymerase (rtN236T) is the most important point mutation, leading to increased sensitivity for adefovir. As this mutation occurs in a different region than mutations causing lamivudine resistance, those mutants remain sensitive to lamivudine treatment as well as to telbivudine and entecavir6.
Experience with entecavir and telbivudine resistance is limited due to their recent approval. However, resistance to entecavir has been reported in patients with prior lamivudine resistance, indicating an increased risk for resistance emergence in patients with no response to lamivudine (Table 1). Higher mutation rates in patients receiving lamivudine and telbivudine at the same time suggest similar mutation patterns of both agents and antagonistic effects in combination therapy, respectively6.
Natural products
In the following section, selected natural products with anti-HBV and -HCV activities are discussed. The main focus is on products that exhibit new or different mechanisms than those known by approved drugs. Other selection criteria include knowledge about clinical trials, in vivo data, lack of cytotoxicity, and general mechanisms of action.
Iminosugars
One way to reduce the development of resistance might be the targeting of host cell factors. During the maturation of several viruses, envelope proteins are first hyperglycosylated and have to be processed. Endoplasmic reticulum (ER) α-glycosidases trim those N-linked glycosylations, and are therefore essential for correct viral maturation. The inhibition of those enzymes has been shown to reduce the production of various ER-budding viruses, especially flaviviruses17. Potent inhibitors of ER α-glucosidases are derivatives of deoxynojirimicin (DNJ) iminosugars. Those iminosugars occur naturally, for example in Bombyx mori L (silkworm)18. The antiviral effect of this inhibition is supposed to be the result of 2 actions. First, due to incorrect glycosylation, envelope proteins are not able to interact with chaperones, which are essential for proper folding. Therefore, misfolding of the proteins induces the inhibition of enveloped virus maturation18. Furthermore, it is suggested that treatment with iminosugars reduces the infectivity of virus particles. The infectivity reduction is supposed to be caused by incorporated misfolded envelope proteins in secreted particles19. Derivatives of iminosugars provide antiviral activity against both HBV and HCV infections. 1-DNJ, a compound derived from Morus alba L, suppresses the secretion of HBV particles in a dose-dependent manner18. Of greater interest is the effect of the potency of some iminosugars on anti-HCV activity. Compounds with long alkyl side chains, like NN-DNJ (N-nonyl deoxynojirimycin), have particularly strong effects. Those derivatives are potent inhibitors of the HCV p7 ion channel. Thus, compounds with both a long alkyl side chain, which seems to be necessary for inhibitory effects on p7, and a DNJ head group, may combine 2 mechanisms, which could be an advantage for susceptibility to resistance20. Currently, there is 1 compound in clinical development that targets α-glycosidase I. Celgosivir, a prodrug of the naturally-occurring castanospermine (derived from Castanospermum australe), has strong in vitro inhibitory effects21. Since castanospermine itself exhibits strong antiviral activity, but was found to also have inhibitory effects on intestinal sucrases leading to diarrhea, the apparently non-toxic 6-O-butanoyl derivative celgosivir was chosen for further development and is currently undergoing phase II of clinical trials22.
Cyclophilin as an anti-HCV target
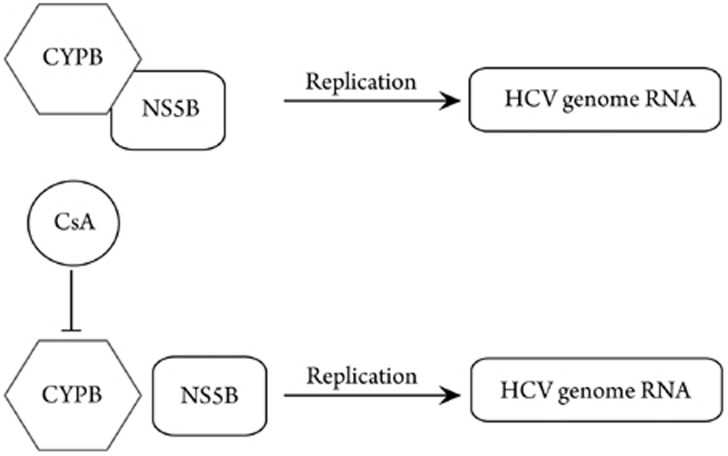
Cell culture-based screening for anti-HCV compounds identified cyclosporin A (CsA) as a potential agent. CsA, produced by the fungus Tolypocladium inflatum Gams, is known for its immunosuppressive effects and organ transplant application23. The responsible target for CsA anti-HCV activity was cyclophilin PB (CyPB; Figure 2)24. CyPB is a cellular peptidyl prolyl cis-trans isomerase. It is mainly localized at the cytoplasmic site of the ER membrane, exactly like the NS5B polymerase of HCV. Furthermore, both components form complexes with HCV–RNA. Specific knockdown experiments showed that CyPB stimulated the RNA binding activity performed by NS5B, and a lack of CyPB led to reduced replication, respectively25. Since CsA disrupts the complex of CyPB and NS5B, as a consequence, HCV genome replication is less efficient. Due to its immunosuppressive activities, CsA is not suitable as an anti-HCV drug. However, NIM811, a derivative with only 1 amino acid substitution, completely lacks the immunosuppressive effect and even provides approximately 2-fold stronger binding affinity to CyPB. Clinical trials are now underway. The fact that the CsA treatment of patients with HCV recurrence after liver transplantation, who did not respond to HCV standard therapy, resulted in a decrease of HCV–RNA below the detection level in 5 of 8 patients, gives hope for positive results of cyclosporins24.
Figure 2.
Influence of CsA and CyPB on HCV genome replication. If CyPB is inhibited by CsA, it is no longer able to form a complex with NS5B. Consequently, HCV genome replication is reduced.
Natural products with anti-HCV activity
The possibility of infectious HCV particle production in tissue culture has been shown only very recently26. Screenings of natural products with potential anti-HCV activity are not as frequent as anti-HBV screenings. Therefore, as mentioned earlier, many synthetic and designed drugs are currently under development, mostly targeting NS3-4A protease and viral NS5B polymerase8. New host targets as cyclophilins and α-glucosidases have already been discussed. Nevertheless, there are also some other natural products with promising anti-HCV activity.
2-Arylbenzofuran derivatives
Derived from Mori cortex radicis, 2-arylbenzofuran derivatives have shown anti-HCV activity in an HCV replicon system27. Two compounds are of special interest, as a NS3 helicase assay revealed potent inhibitory activities (IC50=42.9 and 27.0 μmol/L, respectively). The helicase of the viral NS3 unwinds RNA×RNA and RNA ×DNA duplexes, and therefore, is essential for viral replication. Thus, targeting this enzyme is ideal, and the reported derivatives could serve as models for the future development of potent helicase inhibitors27.
While the carboxyterminal group of NS3 provides helicase function, its amino terminal group exhibits protease activity. Mellein, a compound isolated from the fungus Aspergillus ochraceus, exhibits anti-HCV protease activity with an IC50 value of 35 μmol/L28.
Testing the effects of various pseudoguaianolides from Parthenium hispitum in an HCV subgenomic replicon system revealed anti-HCV activities of many compounds. Three of these led to more than 90% inhibition of the reporter replicon at a 2 μmol/L concentration. Another 3 exhibited at least 50% inhibition. Moreover, all of these compounds were shown to have no cytotoxicity at the tested concentrations, demanding further investigations regarding their high potential29.
Natural products with anti-HBV activity
Wogonin is a monoflavonoid isolated from Scutellaria radix. This herb has been used for thousands of years in Asia for inflammatory diseases and also for hepatitis. The anti-HBV activity of wogonin was already reported in 2000, demonstrating its ability to suppress hepatitis B surface antigen (HBsAg) secretion in cell culture30. Recently, the suppression of both HBsAg and hepatitis B e-antigen (HBeAg) secretion was shown with an IC50 of 4 μg/mL31. Moreover, the HBV–DNA level was reduced in a dose-dependent manner. Interestingly, these observations have been confirmed in vivo with duck hepatitis B virus (DHBV)-infected ducks. Plasma HBsAg and the DHBV–DNA level were significantly reduced in ducks treated with wogonin, and an additional histopathological evaluation of their liver showed considerable improvement. Furthermore, immunohistological staining of human HBV-transgenic mouse livers confirmed the potential of wogonin in HBsAg reduction. Therefore, it is currently under early development as anti-HBV drug31.
Another flavonoid molecule, ellagic acid, isolated from Phyllanthus urinaria exhibits a rather peculiar anti-HBV function. Ellagic acid has been found to effectively block HBeAg secretion in cell culture with an IC50 of 0.07 μg/mL, but does not have any effects on HBsAg secretion, HBV replication, or polymerase activity32. Since intracellular HBeAg amounts remain constant during treatment with ellagic acid, it has been suggested that it exhibits its function by blocking HBeAg secretion. HBeAg is believed to contribute to immune tolerance of the host33, therefore, inhibiting its secretion could be a good way to weaken this tolerance. Recent investigations with HBeAg-producing transgenic mice showed tolerance to HBeAg. There was no production of antibodies to the antigen, the levels of cytokines were minimal, and cytotoxic T-lymphocyte (CTL) responses decreased. However, feeding mice with ellagic acid inhibited this immune tolerance and suggested that ellagic acid was an agent that could overcome this essential mechanism for chronic HBV infection34.
Artemisinin
Derived from Artemisia annua and mainly known for its antimalaria activity, artemisinin was also found out to have anti-HBV activity35. Its semisynthetic derivative, artesunate, even showed better effects. Artesunate inhibits HBsAg secretion with an IC50 of 2.3 μmol/L and reduces the HBV–DNA level with an IC50 of 0.5 μmol/L. Compared to lamivudine, those values are not as good (IC50=0.2 μmol/L and 0.3 μmol/L, respectively), but by combining both agents, a synergic effect could be observed. Concerning the susceptibility of lamivudine to drug resistance, this combination might be an effective strategy to minimize resistance emergence against lamivudine35. Another point for further investigations of artemisinin and artesunate regarding its anti-HBV activities is the lack of serious side-effects. Because of their antimalaria properties, there have been evaluations in large populations without any hint of serious adverse effects36.
An analysis of various anthraquinones from Rheum palmatum L ethanol extracts revealed chrysophanol 8-O-β-D-glucoside to have potent anti-HBV activity37. Simultaneously, no toxicity, even at high concentrations, could be observed. A HBV–DNA polymerase assay found chrysophanol 8-O-β-D-glucoside to be a potential inhibitor of this viral enzyme37.
Testing the effects of different saikosaponins present in Bupleurum species, saikosaponin C was found to have inhibitory effects against HBsAg secretion (IC50=11 μg/mL) and HBV–DNA (IC50=13.4 μg/mL). The inhibition of HBeAg secretion was even more effective, and the reduction of the HBV–DNA level was more potent than that obtained with lamivudine. Significant cytotoxicity of saikosaponin C could not be observed38.
Protostane triterpenes from Alisma orientalis have also been reported for their anti-HBV activity. Compound 7 in particular showed promising effects, inhibiting HBsAg and HBeAg with an IC50 of 7.7 μmol/L and 5.1 μmol/L, respectively, whereas cytotoxicity was only observed at a much higher concentration [50% cytotoxicity concentration (CC50)=142.7 μmol/L]39.
Among the 10 different alkaloids isolated from Corydalis saxicola, a traditional herb used as folk medicine to treat hepatitis in China, dihydrochelerythrine was shown to exhibit extraordinary effects against HBsAg and HBeAg secretions. With an IC50 value of less than 0.5 μmol/L and a SI>3.5, these results prompted further investigations of the mechanism, which are now underway40.
Discussion
Only a few drugs are available for HBV, and in particular, HCV treatment, so there is undoubtedly an urgent need for new agents. A lot of natural products have been reported for their anti-HBV effects. Further investigations concerning the underlying mechanisms will be the main task in near future. New mechanisms of action could provide additional treatment options and would be very desirable for more and better possibilities of effective combination therapy.
The lack of any virus-specific treatment for HCV therapy, poor response rates, and serious side-effects demand the development of new drugs targeting viral proteins. On the one hand, agents currently in development that target NS5B polymerase and NS3-4A protease show potent inhibitory activities. On the other hand, all those drugs show a high susceptibility for resistance emergence. To get this problem under control, other potential viral targets have to be explored. Therefore, iminosugars inhibiting the p7 ion channel or 2-arylbenzofuran derivatives that have been suggested to exhibit inhibitory activities against NS3 helicase could mark new possibilities. In the future, combinations of drugs with various targets and different mechanisms of action, including host targets, are most likely to result in high response rates, and of course, much lower, if any, drug resistance. As many natural products show promising and potent effects, they should be included in further investigations and developments in order to get away from the current, aggressive standard therapy.
References
- Qureshi SA. Hepatitis C virus — biology, host evasion strategies, and promising new therapies on the horizon. Med Res Rev. 2007;27:353–73. doi: 10.1002/med.20063. [DOI] [PubMed] [Google Scholar]
- De Francesco R, Migliaccio G. Challenges and successes in developing new therapies for hepatitis C. Nature. 2005;436:953–60. doi: 10.1038/nature04080. [DOI] [PubMed] [Google Scholar]
- Safioleas M, Lygidakis NJ, Manti C. Hepatitis B today. Hepatogastroen-terology. 2007;54:545–8. [PubMed] [Google Scholar]
- Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Vir Hepat. 2004;11:97–107. doi: 10.1046/j.1365-2893.2003.00487.x. [DOI] [PubMed] [Google Scholar]
- Arbuthnot P, Longshaw V, Naidoo T, Weinberg MS. Opportunities for treating chronic hepatitis B and C virus infection using RNA interference. J Viral Hepat. 2007;14:447–59. doi: 10.1111/j.1365-2893.2006.00818.x. [DOI] [PubMed] [Google Scholar]
- Fung SK, Lok AS. Management of hepatitis B patients with antiviral resistance. Antiviral Ther. 2004;9:1013–26. [PubMed] [Google Scholar]
- Waters L, Nelson M. New therapeutic options for hepatitis C. Curr Opin Infect Dis. 2006;19:615–22. doi: 10.1097/QCO.0b013e328010a869. [DOI] [PubMed] [Google Scholar]
- Hoofnagle JH, Seeff LB. Peginterferon and ribavirin for chronic hepatitis C. N Engl J Med. 2006;355:2444–51. doi: 10.1056/NEJMct061675. [DOI] [PubMed] [Google Scholar]
- Lutchman G, Danehower S, Song BC, Liang TJ, Hoofnagle JH, Thomson M, et al. Mutation rate of hepatitis C virus NS5B in patients undergoing treatment with ribavirin monotherapy. Gastroenterology. 2007;132:1757–66. doi: 10.1053/j.gastro.2007.03.035. [DOI] [PubMed] [Google Scholar]
- Parfieniuk A, Jaroszewicz J, Flisiak R. Specifically targeted antiviral therapy for hepatitis C virus. World J Gastroenterol. 2007;13:5673–81. doi: 10.3748/wjg.v13.i43.5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartal ED, Alpat SN, Ozgunes I, Usluer G. Adverse effects of high-dose interferon-alpha-2a treatment for chronic hepatitis B. Adv Ther. 2007;24:963–71. doi: 10.1007/BF02877700. [DOI] [PubMed] [Google Scholar]
- Zoulim F. Current data on the treatment of chronic hepatitis B. Presse Med. 2008;37:287–93. doi: 10.1016/j.lpm.2007.06.021. [DOI] [PubMed] [Google Scholar]
- Veillon P, Payan C, Le Guillou-Guillemette H, Gaudy C, Lunel F. Quasispecies evolution in NS5A region of hepatitis C virus genotype 1b during interferon or combined interferon-ribavirin therapy. World J Gastroenterol. 2007;13:1195–203. doi: 10.3748/wjg.v13.i8.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Francesco R, Migliaccio G. Challenges and successes in developing new therapies for hepatitis C. Nature. 2005;436:953–60. doi: 10.1038/nature04080. [DOI] [PubMed] [Google Scholar]
- Bartholomeusz A, Tehan BG, Chalmers DK. Comparisons of the HBV and HIV polymerase, and antiviral resistance mutations. Antiviral Ther. 2004;9:149–60. [PubMed] [Google Scholar]
- Locarnini S. Hepatitis B viral resistance: mechanisms and diagnosis. J Hepatol. 2003;39:124–32. doi: 10.1016/s0168-8278(03)00318-0. [DOI] [PubMed] [Google Scholar]
- Wu SF, Lee CJ, Liao CL, Dwek RA, Zitzmann N, Lin YL. Antiviral effects of an iminosugar derivative on flavivirus infections. J Virol. 2002;76:3596–604. doi: 10.1128/JVI.76.8.3596-3604.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob JR, Mansfield K, You JE, Tennant BC, Kim YH. Natural iminosugar derivatives of 1-deoxynojirimycin inhibit glycosylation of hepatitis viral envelope proteins. J Microbiol. 2007;45:431–40. [PubMed] [Google Scholar]
- Chapel C, Garcia C, Bartosch B, Roingeard P, Zitzmann N, Cosset FL, et al. Reduction of the infectivity of hepatitis C virus pseudoparticles by incorporation of misfolded glycoproteins induced by glucosidase inhibitors. J Gen Virol. 2007;88:1133–43. doi: 10.1099/vir.0.82465-0. [DOI] [PubMed] [Google Scholar]
- Steinmann E, Whitfield T, Kallis S, Dwek RA, Zitzmann N, Pietschmann T, et al. Antiviral effects of amantadine and iminosugar derivatives against hepatitis C virus. Hepatology. 2007;46:330–8. doi: 10.1002/hep.21686. [DOI] [PubMed] [Google Scholar]
- Dugourd D, Fenn J, Siu R, Coulson R, Clement JJ.Characterization of celgosivir, a clinical stage compound for the treatment of HCV infections[Cited 8 Mar 2008.] Available from URL: http://www.migenix.com/annuals/ICAR_2005_2.pdf
- Sorbera LA, Castañer J, García-Capdevila L. Celgosivir. Drugs Fut. 2005;30:545. [Google Scholar]
- Doutre MS. Cyclosporin. Ann Dermatol Venereol. 2002;129:392–404. [PubMed] [Google Scholar]
- Watashi K, Shimotohno K. Chemical genetics approach to hepatitis C virus replication: cyclophilin as a target for anti-hepatitis C virus strategy. Rev Med Virol. 2007;17:245–52. doi: 10.1002/rmv.534. [DOI] [PubMed] [Google Scholar]
- Watashi K, Ishii N, Hijikata M, Inoue D, Murata T, Miyanari Y, et al. Cyclophilin B is a functional regulator of hepatitis C virus RNA polymerase. Mol Cell. 2005;19:111–22. doi: 10.1016/j.molcel.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Zeisel MB, Baumert TF. Production of infectious hepatitis C virus in tissue culture: a breakthrough for basic and applied research. J Hepatol. 2006;44:436–9. doi: 10.1016/j.jhep.2005.11.031. [DOI] [PubMed] [Google Scholar]
- Lee HY, Yum JH, Rho YK, Oh SJ, Choi HS, Chang HB, et al. Inhibition of HCV replicon cell growth by 2-arylbenzofuran derivatives isolated from Mori Cortex Radicis. Planta Med. 2007;73:1481–5. doi: 10.1055/s-2007-990249. [DOI] [PubMed] [Google Scholar]
- Dai J, Carté BK, Sidebottom PJ, Sek Yew AL, Ng S, Huang Y, et al. Circumdatin G, a new alkaloid from the fungus Aspergillus ochraceus. J Nat Prod. 2001;64:125–6. doi: 10.1021/np000381u. [DOI] [PubMed] [Google Scholar]
- Hu JF, Patel R, Li B, Garo E, Hough GW, Goering MG, et al. Anti-HCV bioactivity of pseudoguaianolides from Parthenium hispitum. J Nat Prod. 2007;70:604–7. doi: 10.1021/np060567e. [DOI] [PubMed] [Google Scholar]
- Huang RL, Chen CC, Huang HL, Chang CG, Chen CF, Chang C, et al. Anti-hepatitis B virus effects of wogonin isolated from Scutellaria baicalensis. Planta Med. 2000;66:694–8. doi: 10.1055/s-2000-9775. [DOI] [PubMed] [Google Scholar]
- Guo Q, Zhao L, You Q, Yang Y, Gu H, Song G, et al. Anti-hepatitis B virus activity of wogonin in vitro and in vivo. Antiviral Res. 2007;74:16–24. doi: 10.1016/j.antiviral.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Shin MS, Kang EH, Lee YI. A flavonoid from medicinal plants blocks hepatitis B virus-e antigen secretion in HBV-infected hepatocytes. Antiviral Res. 2005;67:163–8. doi: 10.1016/j.antiviral.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Milich DR, Jones JE, Hughes JL, Price J, Raney AK, McLachlan A. Is a function of the secreted hepatitis B e antigen to induce immunologic tolerance in utero. Proc Natl Acad Sci USA. 1990;87:6599–603. doi: 10.1073/pnas.87.17.6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang EH, Kown TY, Oh GT, Park WF, Park SI, Park SK, et al. The flavonoid ellagic acid from a medicinal herb inhibits host immune tolerance induced by the hepatitis B virus-e antigen. Antiviral Res. 2006;72:100–6. doi: 10.1016/j.antiviral.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Romero MR, Efferth T, Serrano MA, Gastaño B, Macias RI, Briz O, et al. Effect of artemisinin/artesunate as inhibitors of hepatitis B virus production in an “in vitro” replicative system. Antiviral Res. 2005;68:75–83. doi: 10.1016/j.antiviral.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Price R, van Vugt M, Phaipun L. Adverse effects in patients with acute falciparum malaria treated with artemisinin derivatives. Am J Trop Med Hyg. 1999;60:547–55. doi: 10.4269/ajtmh.1999.60.547. [DOI] [PubMed] [Google Scholar]
- Li Z, Li LJ, Sun Y, Li J. Identification of natural compounds with anti-hepatitis B virus activity from Rheum palmatum L. ethanol extract. Chemotherapy. 2007;53:320–6. doi: 10.1159/000107690. [DOI] [PubMed] [Google Scholar]
- Chiang LC, Ng LT, Liu LT, Shieh DE, Lin CC. Cytotoxicity and anti-hepatitis B virus activities of saikosaponins from Bupleurum species. Planta Med. 2003;69:705–9. doi: 10.1055/s-2003-42797. [DOI] [PubMed] [Google Scholar]
- Jiang ZY, Zhang XM, Zhang FX, Liu N, Zhao F, Zhou J, et al. A new triterpene and anti-hepatitis B virus active compounds from Alisma orientalis. Planta Med. 2006;72:951–4. doi: 10.1055/s-2006-947178. [DOI] [PubMed] [Google Scholar]
- Wu YR, Ma YB, Zhao YX, Yao SY, Zhou J, Zhou Y, et al. Two new quaternary alkaloids and anti-hepatitis B virus active constituents from Corydalis saxicola. Planta Med. 2007;73:787–91. doi: 10.1055/s-2007-981549. [DOI] [PubMed] [Google Scholar]