Abstract
Aim:
NYGGF4 is a novel gene that is abundantly expressed in the adipose tissue of obese patients. The purpose of this study was to investigate the effects of NYGGF4 on basal and insulin-stimulated glucose uptake in mature 3T3-L1 adipocytes and to understand the underlying mechanisms.
Methods:
3T3-L1 preadipocytes transfected with either an empty expression vector (pcDNA3.1Myc/His B) or an NYGGF4 expression vector were differentiated into mature adipocytes. Glucose uptake was determined by measuring 2-deoxy-D-[3H]glucose uptake into the adipocytes. Immunoblotting was performed to detect the translocation of insulin-sensitive glucose transporter 4 (GLUT4). Immunoblotting also was used to measure the phosphorylation and total protein contents of insulin signaling proteins such as the insulin receptor (IR), insulin receptor substrate (IRS)-1, Akt, ERK1/2, p38, and JNK.
Results:
NYGGF4 over-expression in 3T3-L1 adipocytes reduced insulin-stimulated glucose uptake and impaired insulin-stimulated GLUT4 translocation. It also diminished insulin-stimulated tyrosine phosphorylation of IRS-1 and serine phosphorylation of Akt without affecting the phosphorylation of IR, ERK1/2, p38, and JNK.
Conclusion:
NYGGF4 regulates the functions of IRS-1 and Akt, decreases GLUT4 translocation and reduces glucose uptake in response to insulin. These observations highlight the potential role of NYGGF4 in glucose homeostasis and possibly in the pathogenesis of obesity.
Keywords: obesity, glucose uptake, insulin receptor substrate-1, 3T3-L1 preadipocyte
Introduction
Obesity is a serious public health problem that is associated with increased risk of type 2 diabetes mellitus, hypertension, coronary heart disease, and certain cancers1, 2, 3. Obesity is increasing at an alarming rate. Recent data have shown that at a worldwide level, at least 1 in every 10 adults is obese. In some Western countries, the percentage is much higher (25% or more)4. Obesity is a multifactorial disease that arises from interactions between susceptibility genes and environmental factors5. Numerous epidemiological studies have recognized the contribution of genetic factors to an individual's susceptibility to obesity6.
In an earlier study7, we isolated and characterized NYGGF4, a novel gene whose expression was higher in adipose tissue from obese patients than in that from normal controls. We also found that ectopic expression of NYGGF4 could dramatically increase 3T3-L1 preadipocyte proliferation without affecting adipocyte differentiation7. Our findings suggest that NYGGF4 might be a new candidate gene for obesity. The aim of this study was to investigate the role of NYGGF4 in the pathogenesis of obesity and/or obesity-related metabolism disorders. We examined the effects of NYGGF4 on glucose transport in mature adipocytes and determined the molecular events underlying these effects. The results showed that NYGGF4 over-expression in 3T3-L1 adipocytes led to reduced insulin-stimulated glucose transport through attenuated phosphorylation of insulin receptor substrate (IRS)-1 and Akt. Thus, NYGGF4 might play an important role in the development of obesity-related insulin resistance.
Materials and methods
Antibodies
Primary polyclonal GLUT4 antibodies and horseradish peroxidase-conjugated secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-phospho-insulin receptor (IR) beta (Tyr1146), anti-IR beta, and anti-IRS-1 were purchased from Cell Signaling Technology (Danvers, MA, USA). The phospho-specific polyclonal antibody against IRS-1 (Tyr612) was from Biosource (Camarillo, CA, USA). Antibodies against Akt, ERK1/2, p38, and JNK and against the phosphorylated forms of these proteins were obtained from Kangchen (Shanghai, China).
Cell culture and differentiation
3T3-L1 preadipocytes that were stably transfected with either an empty expression vector (pcDNA3.1Myc/His B) or an NYGGF4 expression vector were established in our laboratory7. The transfected cells were maintained in Dulbecco's modified Eagle's medium (DMEM; Gibco Laboratories, Grand Island, NY, USA) supplemented with 10% calf serum (Gibco, Carlsbad, CA, USA) and 100 μg/mL neomycin (G418; Roche, Basel, Switzerland). Two days after complete confluence (day 0), the cells were cultured for 48 h in DMEM supplemented with 10% fetal bovine serum (FBS; Gibco, Carlsbad, CA, USA), 0.5 mmol/L 1-methyl-3-isobutylxanthine (Sigma, St Louis, MO, USA), 1 μmol/L dexamethasone (Sigma, St Louis, MO, USA), 10 μg/mL insulin (Sigma, St Louis, MO, USA), and 100 μg/mL G418. From d 2 to d 4, the medium was only supplemented with 100 nmol/L insulin. The cells were then transferred to DMEM containing 10% FBS and 100 μg/mL G418 for the remaining culture period. The cultures were replenished every 2 d. After induction for 10 d, more than 80% of the cells exhibited typical adipocyte morphology.
Glucose uptake
2-Deoxy-D-[3H]glucose (CIC, Beijing, China) uptake was assayed as described previously but with minor modifications8. The stably transfected cells were cultured in 6-well plates and induced into mature adipocytes. On d 10 of differentiation, the cells were serum-starved in DMEM containing 0.5% FBS for 3 h. The cells were then washed twice with phosphate-buffered saline (PBS) and incubated in KRP-HEPES buffer [30 mmol/L HEPES (pH 7.4), 10 mmol/L NaHCO3, 120 mmol/L NaCl, 4 mmol/L KH2PO4, 1 mmol/L MgSO4, and 1 mmol/L CaCl2] in the presence or absence of 100 nmol/L insulin for 30 min at 37 °C. Labeled 2-deoxy-D-[3H]glucose was added to a final concentration of 2 μCi/mL. After 10 min at 37 °C, the reaction was terminated by washing 3 times with ice-cold PBS supplemented with 10 mmol/L D-glucose. The cells were solubilized by adding 200 μL of 1 mol/L NaOH to each well, and aliquots of the cell lysate were transferred to scintillation vials for radioactivity counting; the remainder was used for the protein assay.
Immunoblotting
The transfected 3T3-L1 preadipocytes were induced to differentiate as described above. On day 10 of differentiation, the cells were serum-starved for 3 h and then incubated with or without 100 nmol/L insulin. Total proteins or phosphorylated proteins were extracted as described previously9. Plasma membrane (PM) proteins were extracted using the Eukaryotic Membrane Protein Extraction Reagent (Pierce, Rockford, IL, USA). Protein levels were quantified using the bicinchonic acid (BCA) protein assay kit (Pierce, Rockford, IL, USA) in accordance with the manufacturer's instructions. After sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), the proteins (20 μg/lane) were electrophoretically transferred onto a nitrocellulose membrane (Whatman, London, UK). After being blocked with Tris-buffered saline Tween-20 [TBST; 0.14 mol/L NaCl, 0.02 mol/L Tris base (pH 7.6), and 0.1% Tween] containing 3% bovine serum albumin (BSA) for 1 h at room temperature, the membrane was hybridized with primary antibodies at an appropriate dilution at 4 °C overnight. The membrane was then washed with TBST for 5 min. This step was repeated 5 times. After being washed, the membrane was incubated with horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature, washed with TBST, and developed with the enhanced chemiluminescence (ECL) kit (Amersham, Piscataway, NJ, USA).
Statistical analysis
All data are expressed as means± SEM. Statistical analysis was performed using one-way ANOVA with the SPSS 10.0 statistical software package (SPSS Inc, Chicago, IL, USA). The threshold of significance was defined as P<0.05.
Results
Effects of NYGGF4 on basal and insulin-stimulated glucose uptake in 3T3-L1 adipocytes
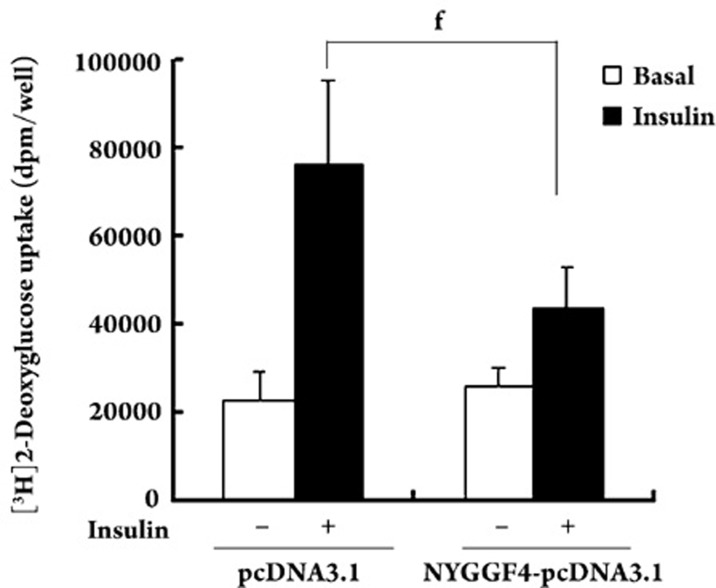
To determine whether NYGGF4 affects insulin sensitivity, we assessed glucose uptake in differentiated 3T3-L1 adipocytes. In NYGGF4-overexpressing cells, basal glucose uptake was similar to that observed in controls. However, in NYGGF4-overexpressing cells, insulin-stimulated glucose uptake was approximately 40% lower than that in controls (Figure 1).
Figure 1.
Effect of NYGGF4 on glucose uptake. 3T3-L1 preadipocytes transfected with NYGGF4 or the empty vector (pcDNA3.1Myc/His B) were induced to differentiate. After serum starvation for 3 h, the cells were incubated with (black columns) or without (white columns) 100 nmol/L insulin for 30 min, followed by measurement of the 2-deoxy-D-[3H]glucose uptake. Values represent the means±SD from three independent experiments. fP=0.001<0.01 vs insulin-stimulated control (cells transfected with the empty vector).
Effects of NYGGF4 on basal and insulin-stimulated GLUT4 translocation
In adipocytes, insulin-stimulated glucose uptake is dependent on translocation of the insulin-responsive glucose transporter GLUT4 from intracellular storage compartments to the PM10, 11. Therefore, using differentiated 3T3-L1 adipocytes, we examined the effects of NYGGF4 on GLUT4 translocation to the PM in response to insulin. The results demonstrated that NYGGF4 over-expression decreased insulin-stimulated GLUT4 translocation to the PM but did not alter either basal GLUT4 translocation or the total GLUT4 protein content (Figure 2).
Figure 2.
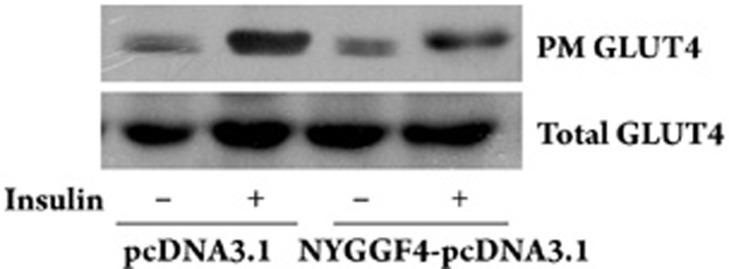
Effect of NYGGF4 on GLUT4 translocation. 3T3-L1 preadipocytes transfected with NYGGF4 or the empty vector were induced to differentiate. Membrane proteins and total proteins were extracted from the differentiated cells incubated with or without 100 nmol/L insulin for 30 min. Immunoblotting was performed using antibodies against GLUT4. The results are representative of those obtained from three independent experiments.
Effects of NYGGF4 on protein expression and insulin-stimulated phosphorylation of insulin signaling molecules
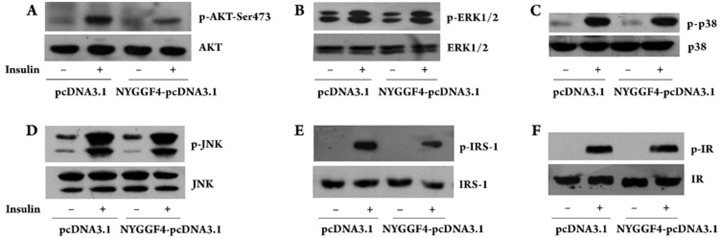
We next investigated the effect of NYGGF4 on the molecules involved in insulin signaling for glucose uptake. NYGGF4 over-expression resulted in significant inhibition of insulin-induced tyrosine phosphorylation of IRS-1 and serine phosphorylation of Akt, whereas NYGGF4 over-expression did not affect the tyrosine phosphorylation of IR. We also evaluated the phosphorylation of ERK1/2, p38, and JNK, which are downstream signaling molecules of the Ras/MAPK insulin signal pathway. We found that NYGGF4 over-expression had no effect on the insulin-induced phosphorylation of these molecules. As shown in Figure 3, there is no significant difference in the total protein contents of these signal molecules.
Figure 3.
Effect of NYGGF4 on insulin signaling transduction. 3T3-L1 preadipocytes transfected with NYGGF4 or the empty vector were induced to differentiate. After incubation with or without 100 nmol/L insulin for 30 min, the cell lysates were analyzed by SDS-PAGE, blotted onto a membrane, and then probed with antibodies against molecules involved in the insulin signal pathway. The results are representative of those obtained from three independent experiments. A–F represent the total protein concentration and phosphorylation level of AKT, ERK, p38, JNK, IRS-1, and IR, respectively.
Discussion
Obesity is a major public health problem that is strongly influenced by genetic factors that remain largely unknown12. In an earlier study, we identified NYGGF4 as a new obesity candidate gene. Our earlier study demonstrated that NYGGF4 was expressed at higher levels in obese individuals than in normal-weight controls. The purpose of this study was to further investigate the effects of NYGGF4 on insulin sensitivity in mature adipocytes.
In adipocytes, insulin acts on many steps of glucose metabolism. However, one of its most important effects is the ability to increase the rate of cellular glucose transport13. In this study, we found that NYGGF4 over-expression could significantly decrease insulin-stimulated glucose transport in mature adipocytes and had no effect on basal glucose uptake. Insulin-stimulated glucose uptake in adipose tissue is mediated by the translocation of insulin-sensitive GLUT4 from intracellular vesicles to the PM. We studied GLUT4 translocation to determine the mechanism by which NYGGF4 decreases insulin-stimulated glucose uptake. The results indicated that NYGGF4 affected insulin-stimulated glucose uptake by decreasing GLUT4 translocation to the PM.
To further investigate the mechanism by which NYGGF4 decreases insulin-stimulated GLUT4 translocation and glucose uptake, we examined the protein contents and phosphorylation levels of molecules involved in insulin signaling for glucose transport. In the presence of insulin14, 15, the insulin receptor (IR) autophosphorylates and catalyzes several intracellular substrates, including IRS proteins and Shc (in the adipose tissue). These substrates are involved in the activation of two main signaling pathways: (1) the phosphatidylinositol 3-kinase (PI3K)-protein kinase B/Akt (PKB) pathway, which is responsible for most of the metabolic actions of insulin, and (2) the Ras-mitogen-activated protein kinase (MAPK) pathway, which regulates the expression of some genes and also participates in glucose transport16, 17. In this study, we examined the protein contents and phosphorylation levels of Akt and three different MAPKs (ERK, p38, and JNK). We found that NYGGF4 attenuated the phosphorylation level of Akt and had no effect on the phosphorylation levels of ERK, p38, and JNK. Therefore, we deduced that NYGGF4 reduced insulin-stimulated glucose uptake through attenuated Akt phosphorylation. Subsequently, we examined the protein content and phosphorylation level of IR and IRS-1, the upstream molecules of Akt that play a central role in the metabolic effects of insulin on adipocytes18. Intriguingly, NYGGF4 decreased insulin-stimulated tyrosine phosphorylation of IRS-1 without changing the tyrosine phosphorylation of IR. Based on these results, we speculated that the downregulated phosphorylation level of Akt resulting from attenuated tyrosine phosphorylation of IRS-1 might impair insulin-stimulated GLUT4 translocation and glucose uptake in NYGGF4-overexpressing adipocytes.
A domain-predicting program indicated that the NYGGF4 protein contained a phosphotyrosine binding (PTB) domain. The PTB domain usually binds to phosphorylated tyrosine residues and plays an important role in signal transduction by growth factor receptors19. In the insulin signaling pathway, the PTB domain of IRS-1 binds to the NPXpY motif in the β subunit of the insulin receptor, leading to tyrosine phosphorylation. Therefore, we hypothesized that NYGGF4 might impair tyrosine phosphorylation in IRS-1 by competitively inhibiting the binding of IRS-1 to the insulin receptor via its PTB domain.
In conclusion, the results of this study demonstrate that NYGGF4 over-expression inhibits insulin-stimulated glucose transport in mature adipocytes by attenuating the phosphorylation levels of IRS-1 and Akt. Although additional studies are necessary to elucidate the mechanism by which NYGGF4 regulates IRS-1 tyrosine phosphorylation, our results provide evidence that NYGGF4, a new obesity candidate gene, plays an important role in the development of obesity. Furthermore, this gene may be a potential target for the treatment of obesity and obesity-related insulin resistance.
Author contribution
Professor Xi-rong GUO and Rong-hua CHEN designed research; Chun-mei ZHANG and Xiao-hui CHEN performed research; Bin WANG, Feng LIU, Xia CHI, Mei-ling TONG and Yu-hui NI contributed new analytical tools and reagents; chun-mei ZHANG analyzed data; chun-mei ZHANG wrote the paper.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No 30571978), Health Bureau of Jiangsu Province (No RC2002061).
References
- Kopelman PG. Obesity as a medical problem. Nature. 2000;404:635–43. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001;104:531–43. doi: 10.1016/s0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- Visscher TL, Seidell JC. The public health impact of obesity. Annu Rev Public Health. 2001;22:355–75. doi: 10.1146/annurev.publhealth.22.1.355. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- Samaras K, Kelly PJ, Chiano MN, Arden N, Spector TD, Campbell LV. Genes versus environment. The relationship between dietary fat and total and central abdominal fat. Diabetes Care. 1998;21:2069–76. doi: 10.2337/diacare.21.12.2069. [DOI] [PubMed] [Google Scholar]
- Rankinen T, Zuberi A, Chagnon YC, Weisnagel SJ, Argyropoulos G, Walts B, et al. The human obesity gene map: the 2005 update. Obesity (Silver Spring) 2006;14:529–644. doi: 10.1038/oby.2006.71. [DOI] [PubMed] [Google Scholar]
- Wang B, Zhang M, Ni YH, Liu F, Fan HQ, Fei L, et al. Identification and characterization of NYGGF4, a novel gene containing a phosphotyrosine-binding (PTB) domain that stimulates 3T3-L1 preadipocytes proliferation. Gene. 2006;379:132–40. doi: 10.1016/j.gene.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Ceddia RB, Somwar R, Maida A, Fanq X, Bikopouls G, Sweeney G. Globular adiponectin increases GLUT4 translocation and glucose uptake but reduces glycogen synthesis in rat skeletal muscle cells. Diabetoloqia. 2005;48:132–9. doi: 10.1007/s00125-004-1609-y. [DOI] [PubMed] [Google Scholar]
- Andreozzi F, Laratta E, Sciacqua A, Perticone F, Sesti G. Angiotensin II impairs the insulin signaling pathway promoting production of nitric oxide by inducing phosphorylation of insulin receptor substrate-1 on Ser312 and Ser616 in human umbilical vein endothelial cells. Circ Res. 2004;94:1211–8. doi: 10.1161/01.RES.0000126501.34994.96. [DOI] [PubMed] [Google Scholar]
- Bryant NJ, Govers R, James DE. Regulated transport of the glucose transporter GLUT4. Nat Rev Mol Cell Biol. 2002;3:267–77. doi: 10.1038/nrm782. [DOI] [PubMed] [Google Scholar]
- K anzaki M. Insulin receptor signals regulating GLUT4 translocation and actin dynamics. Endocr J. 2006;53:267–93. doi: 10.1507/endocrj.kr-65. [DOI] [PubMed] [Google Scholar]
- Liu YJ, Liu XG, Wang L, Dina C, Yan H, Liu JF, et al. Genome-wide association scans identified CTNNBL1 as a novel gene for obesity. Hum Mol Genet. 2008;17:1803–13. doi: 10.1093/hmg/ddn072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducluzeau PH, Fletcher LM, Vidal H, Laville M, Tavaré JM. Molecular mechanisms of insulin-stimulated glucose uptake in adipocytes. Diabetes Metab. 2002;28:85–92. [PubMed] [Google Scholar]
- Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7:85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- Samuelsson AM, Bollano E, Mobini R, Larsson BM, Omerovic E, Fu M. H yperinsulinemia: effect on cardiac mass/function, angiotensin II receptor expression, and insulin signaling pathways. Am J Physiol Heart Circ Physiol. 2006;291:H787–96. doi: 10.1152/ajpheart.00974.2005. [DOI] [PubMed] [Google Scholar]
- Fujishiro M, Gotoh Y, Katagiri H, Sakoda H, Ogihara T, Anai M, et al. Three mitogen-activated protein kinases inhibit insulin signaling by different mechanisms in 3T3-L1 adipocytes. Mol Endocrinol. 2003;17:487–97. doi: 10.1210/me.2002-0131. [DOI] [PubMed] [Google Scholar]
- González-Yanes C, Serrano A, Bermúdez-Silva FJ, Hernández-Dominguez M, Páez-Ochoa MA, Rodríguez de Fonseca F, et al. Oleylethanolamide impairs glucose tolerance and inhibits insulin-stimulated glucose uptake in rat adipocytes through p38 and JNK MAPK pathways. Am J Physiol Endocrinol Metab. 2005;289:E923–9. doi: 10.1152/ajpendo.00555.2004. [DOI] [PubMed] [Google Scholar]
- Giovannone B, Scaldaferri ML, Federici M, Porzio O, Lauro D, Fusco A, et al. Insulin receptor substrate (IRS) transduction system: distinct and overlapping signaling protein. Diabetes Metab Res Rev. 2000;16:434–4. doi: 10.1002/1520-7560(2000)9999:9999<::aid-dmrr159>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Margolis B, Borg JP, Straight S, Meyer D. The function of PTB domain proteins. Kidney Int. 1999;56:1230–7. doi: 10.1046/j.1523-1755.1999.00700.x. [DOI] [PubMed] [Google Scholar]