Abstract
Organisms are continuously exposed to reactive chemicals capable of causing oxidative stress and cellular damage. Antioxidant enzymes, such as superoxide dismutases (SODs) and catalases, are present in both prokaryotes and eukaryotes and provide an important means of neutralizing such oxidants. Studies in cnidarians have previously documented the occurrence of antioxidant enzymes (transcript expression, protein expression and/or enzymatic activity), but most of these studies have not been conducted in species with sequenced genomes or included phylogenetic analyses, making it difficult to compare results across species due to uncertainties in the relationships between genes. Through searches of the genome of the sea anemone Nematostella vectensis Stephenson, one catalase gene and six SOD family members were identified, including three copper/zinc-containing SODs (CuZnSODs), two manganese-containing SODs (MnSODs) and one copper chaperone of SOD (CCS). In 24 h acute toxicity tests, juvenile N. vectensis showed enhanced sensitivity to combinations of ultraviolet radiation (UV) and polycyclic aromatic hydrocarbons (PAHs, specifically pyrene, benzo[a]pyrene and fluoranthene) relative to either stressor alone. Adult N. vectensis exhibited little or no mortality following UV, benzo[a]pyrene or crude oil exposure but exhibited changes in gene expression. Antioxidant enzyme transcripts were both upregulated and downregulated following UV and/or chemical exposure. Expression patterns were most strongly affected by UV exposure but varied between experiments, suggesting that responses vary according to the intensity and duration of exposure. These experiments provide a basis for comparison with other cnidarian taxa and for further studies of the oxidative stress response in N. vectensis.
KEY WORDS: Cnidarian, Phototoxicity, Polycyclic aromatic hydrocarbon, Superoxide dismutase
INTRODUCTION
Reactive oxygen species (ROS), such as superoxide radical, hydroxyl radical and hydrogen peroxide, can damage cellular DNA, lipids and proteins (reviewed by Lesser, 2006). Organisms are exposed to ROS from several sources, including endogenously produced cellular metabolites, environmental contaminants and photochemical processes. When ROS accumulate and overwhelm the defensive capacity of the cell, oxidative stress results and leads to cellular damage. Cells are able to repair some oxidative damage and to neutralize ROS through the actions of both antioxidant enzymes [e.g. superoxide dismutases (SODs), catalases, peroxidases] and non-enzymatic antioxidants (e.g. glutathione and ascorbic acid). In addition, heat shock proteins (HSPs) help to prevent and repair damage to cellular proteins, and their expression can be induced by a broad range of stressors (Feder and Hofmann, 1999) including ROS exposure (Kim et al., 2011; Landis et al., 2004).
SODs and catalases are evolutionarily ancient classes of antioxidant enzymes that are present in both prokaryotes and eukaryotes (reviewed by Chelikani et al., 2004; Landis and Tower, 2005). Animals typically have a single catalase gene (Zámocký et al., 2012) and multiple SOD genes that are specific in their subcellular location and function. SODs catalyze both superoxide oxidation to molecular oxygen and reduction to hydrogen peroxide. SODs are divided into multiple families, classified in part by the metallic ion present at the active site. In animals, copper/zinc-containing SODs (CuZnSODs) may be cytosolic or extracellular, manganese/iron-containing SODs (MnFeSODs, usually MnSODs in animals) are mitochondrial, and copper chaperones of superoxide dismutase (CCS) lack enzymatic activity but facilitate transfer of copper to the CuZnSODs (reviewed by Landis and Tower, 2005; Zelko et al., 2002). Catalase in turn converts hydrogen peroxide to molecular oxygen and water.
Antioxidant enzymes, particularly SODs and catalase, have been identified in several cnidarians through catalytic assays, antibody-based detection of proteins and/or cloning of the corresponding genes. In addition, many cnidarian species in which oxidative stress has been studied (e.g. reef-building corals) contain dinoflagellate symbionts, which also contain SOD and catalase enzymes (Richier et al., 2003; Tytler and Trench, 1986). Several SODs have been identified in the symbiotic sea anemone Anemonia viridis based on their enzymatic activity, specific enzyme inhibition and reactivity against an antibody targeted to human SODs (Richier et al., 2003). In A. viridis, host protein expression and catalase activity primarily occur in ectodermal tissues (Merle et al., 2007). Catalase-like activity has also been identified within microperoxisomes (Hand, 1976) and regenerating foot cells (Hoffmeister and Schaller, 1985) of Hydra spp. At the sequence level, searches of genomic and expressed sequence tag (EST) databases of the sea anemones Nematostella vectensis (Goldstone, 2008; Reitzel et al., 2008b) and Aiptasia pallida (Sunagawa et al., 2009), the coral Acropora digitifera (Shinzato et al., 2012) and the hydrozoan Hydra magnapapillata (Shinzato et al., 2012) have demonstrated the presence of multiple genes encoding antioxidant enzymes, including CuZnSODs, MnSODs, catalase, thioredoxins and glutathione peroxidases. Two CuZnSODs have been cloned from A. viridis (Plantivaux et al., 2004), and an extracellular CuZnSOD and a mitochondrial MnSOD have been identified in the freshwater hydrozoan Hydra vulgaris (Dash et al., 2007). Catalase has been cloned from A. viridis (Merle et al., 2007) and H. vulgaris (Dash and Phillips, 2012).
List of symbols and abbreviations
- B[a]P
benzo[a]pyrene
- CCS
copper chaperone of SOD
- EST
expressed sequence tag
- HSP
heat-shock protein
- JGI
Joint Genome Institute
- LC50
lethal concentration for 50% of organisms
- PAH
polycyclic aromatic hydrocarbon
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- UV
ultraviolet
The activity and transcript expression of SOD and catalase genes can be broadly induced by conditions producing oxidative stress. Exposure of Hydra to elevated temperatures or to a variety of metals induced expression of catalase and one or more SODs within 6 h (Dash et al., 2007; Dash and Phillips, 2012). SOD and catalase activity and/or expression can be induced in corals by elevated temperatures and ultraviolet (UV) radiation, and these levels have been used as stress biomarkers in corals (Barshis et al., 2013; Császár et al., 2009; Downs et al., 2000; Souter et al., 2011). Production of ROS under these conditions can cause or contribute to coral bleaching (Downs et al., 2002; Lesser, 1997; Lesser, 2006). A wide variety of other genes, in addition to SOD and catalase, may be induced in response to oxidative stress. Among these, HSPs, particularly HSP70 homologs, are robustly induced by a variety of stressors (Coles and Brown, 2003).
Like metals and physical stressors, many chemical contaminants can cause oxidative stress, either directly or through metabolic processes. Petroleum-derived pollutants including polycyclic aromatic hydrocarbons (PAHs) are widespread, persistent and particularly well studied (Wolska et al., 2012). Acute toxicity of PAHs occurs primarily through narcosis at high concentrations, but chronic PAH exposure can lead to genotoxicity, carcinogenesis and a variety of sublethal effects. As in other animals, exposure of corals or anemones to PAHs can lead to upregulation of the mixed-function oxygenase system and antioxidant enzymes (Downs et al., 2006; Gomez-Gutierrez and Guerra-Rivas, 2010; Ramos and Garcia, 2007; Rougee et al., 2006), although metabolism and elimination of PAHs by corals is relatively slow (Kennedy et al., 1992). Crude oil contains a diverse mixture of compounds including PAHs and other aromatic hydrocarbons [reviewed by the National Research Council (NRC, 1985)]. Exposure of corals to water-accommodated fractions of crude oil results in decreased survival (Shafir et al., 2007), decreased reproductive output (Rinkevich and Loya, 1979), and changes in protein composition, including increased CuZnSOD concentration (Rougee et al., 2006).
Organisms inhabiting shallow coastal environments are often exposed to combinations of stressors, which are likely to interact. One mechanism for interaction is the activation of some PAHs and structurally related compounds by UV radiation, which can lead to enhanced production of ROS and greater toxicity than either stressor alone (reviewed by Arfsten et al., 1996; Fu et al., 2012). Phototoxicity of PAHs or oil has been observed in a variety of marine invertebrates, particularly in transparent larvae (Bellas et al., 2008; Lyons et al., 2002; Pelletier et al., 1997; Saco-Alvarez et al., 2008); however, very little research has been conducted on the phototoxic effects of PAHs on cnidarians. Brief reports have indicated that polyps of the anemone Anthopleura aureoradioata are resistant (Ahrens and Hickey, 2002) and that larvae of the coral Fungia scutaria are sensitive (Peachy and Crosby, 1996) to phototoxicity, but these studies were relatively small in scale and few experimental details were provided.
Because of the availability of a sequenced genome, its relatively quick development, and ease of breeding and rearing in the laboratory, N. vectensis Stephenson has become widely used as a model for evolutionary and developmental studies (Darling et al., 2005; Technau and Steele, 2011). Nematostella vectensis inhabits surficial sediments within the high marsh (Hand and Uhlinger, 1994), has a native distribution along the Atlantic coast of the USA and Canada (Reitzel et al., 2008a), and has been suggested as an ecotoxicological model (Ambrosone and Tortiglione, 2013; Harter and Matthews, 2005). In this study, we provide a phylogenetic analysis of SOD and catalase diversity in N. vectensis and experimentally characterize their induction by PAHs, crude oil and UV radiation, both individually and in combination.
RESULTS
Diversity of SOD and catalase genes in N. vectensis
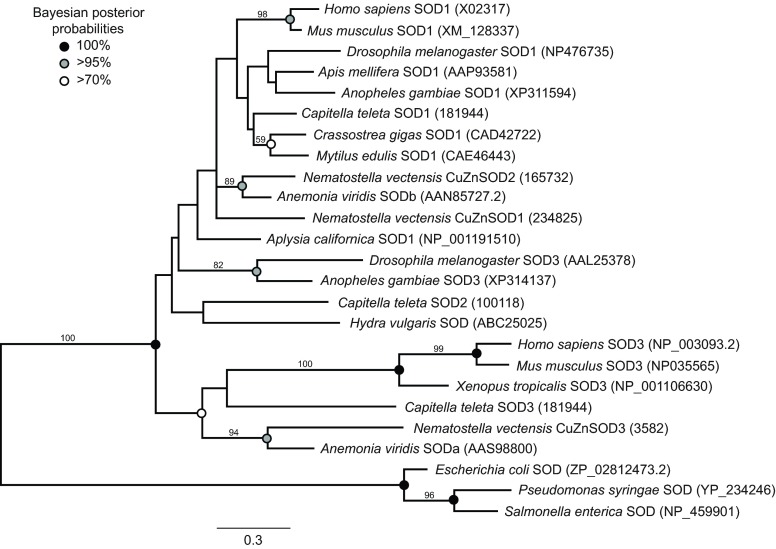
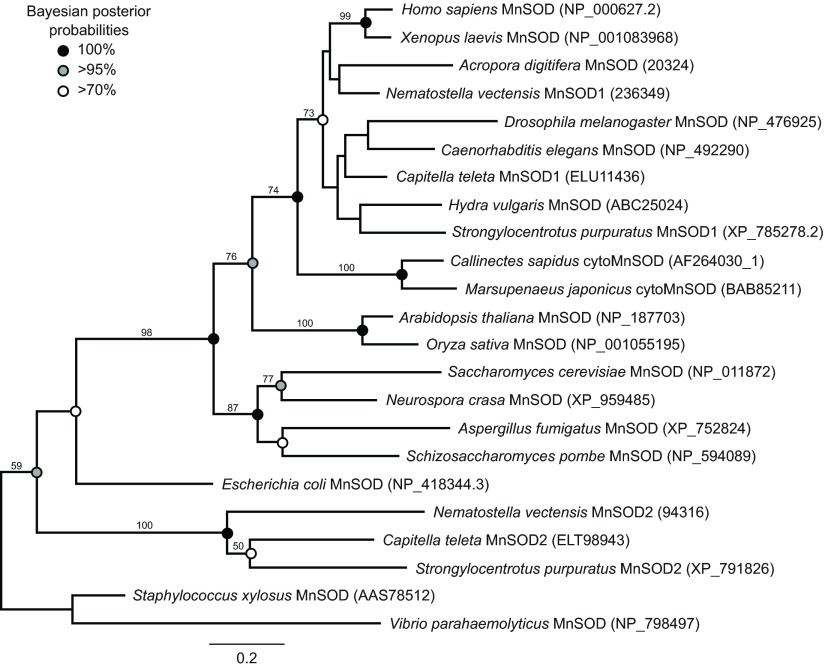
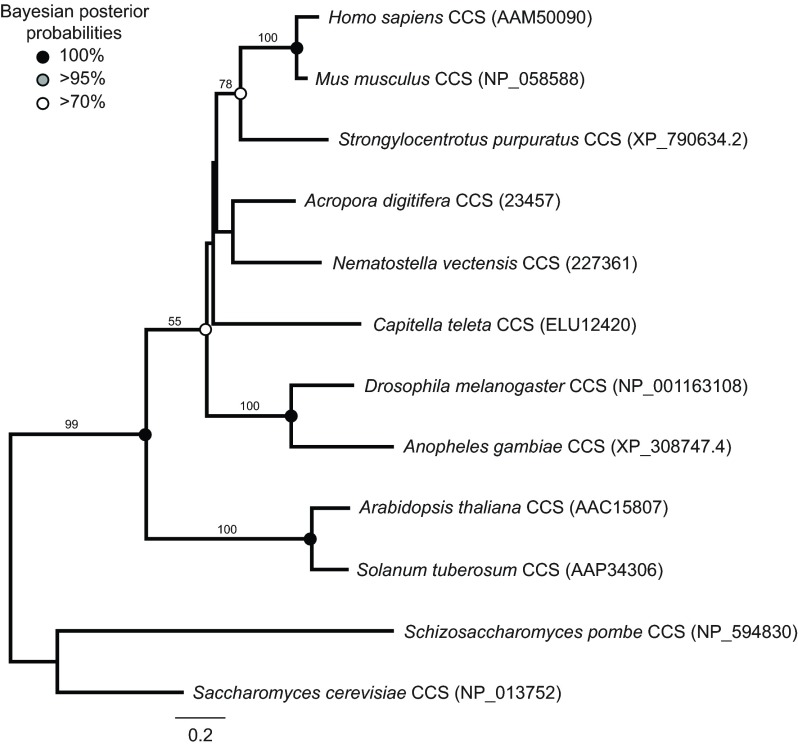
From the N. vectensis genome, we recovered seven predicted genes belonging to the SOD superfamily, six of which were supported by ESTs. Three of the sequences corresponded to members of the CuZnSOD family (Fig. 1). NvCuZnSOD1 is not recognizable as an ortholog of any previously reported cnidarian SOD. NvCuZnSOD2 is most closely related to SODb from the anemone A. viridis (Plantivaux et al., 2004). NvCuZnSOD3 is positioned within a well-supported clade that includes SODa from A. viridis as well as SOD3 from human and Xenopus. Two N. vectensis genes belong to the MnFeSOD family (Fig. 2). NvMnSOD1 is orthologous to a predicted SOD from the coral A. digitifera, and falls into a clade that includes MnFeSODs from protostomes, deuterostomes and the hydrozoan H. vulgaris. NvMnSOD2 groups with strong support in a clade that includes genes from the annelid Capitella teleta and the sea urchin Strongylocentrotus purpuratus. NvCCS belongs to a family corresponding to copper chaperones of SODs (Fig. 3). We were consistently unable to amplify the seventh predicted gene (JGI 231554). The predicted gene had no introns, was on a short genomic scaffold and was not supported by ESTs. We consider this sequence most likely to have resulted from contamination during the sequencing of the reference genome.
Fig. 1.
Maximum likelihood tree of copper/zinc-containing superoxide dismutases (CuZnSODs) derived from a 145 amino acid alignment of sequences from selected species. Accession numbers for each sequence are in parentheses. Sequences for Nematostella vectensis and Capitella teleta are from respective genomic databases at the Joint Genome Institute. All other sequences are from NCBI. The tree was rooted with sequences from prokaryotes. Values above nodes indicate percentage of 1000 bootstraps. Bootstrap values below 40 were removed. Circles indicate posterior probabilities from Bayesian analysis for shared clades between analyses.
Fig. 2.
Maximum likelihood tree of manganese/iron-containing SODs (MnFeSODs) derived from a 195 amino acid alignment of sequences from selected species. Accession numbers for each sequence are in parentheses. Sequences for N. vectensis and C. teleta are from respective genomic databases at the Joint Genome Institute. The sequences for Acropora digitifera are from the genomic database at the Okinawa Institute of Science and Technology. All other sequences are from NCBI. The tree was rooted with sequences from prokaryotes. Values above nodes indicate percentage of 1000 bootstraps. Bootstrap values below 40 were removed. Circles indicate posterior probabilities from Bayesian analysis for shared clades between analyses.
Fig. 3.
Maximum likelihood tree of copper chaperones of superoxide dismutase (CCS) derived from a 208 amino acid alignment of sequences from selected species. Accession numbers for each sequence are in parentheses. Sequences for N. vectensis and C. teleta are from respective genomic databases at the Joint Genome Institute. The CCS sequence for A. digitifera is from the genomic database at the Okinawa Institute of Science and Technology. All other sequences are from NCBI. The tree was rooted with sequences from fungi. Values above nodes indicate percentage of 1000 bootstraps. Bootstrap values below 40 were removed. Circles indicate posterior probabilities from Bayesian analysis for shared clades between analyses.
Using BLASTp searches with full-length catalase protein sequences from human (NP_001743.1) and A. viridis (AAZ50618.1), we identified two N. vectensis partial proteins (JGI 103289 and 103340) in the reference protein dataset. The N. vectensis proteins were reciprocal BLAST matches to catalase from various animals. The two predicted proteins were on the same genomic scaffold (scaffold 68) and were adjacent to each other, separated by almost 30 kb of sequence that contained large sections of poor assembly (i.e. long stretches of N). To assemble a more complete catalase gene and protein sequence, we queried the N. vectensis EST database, where we identified numerous sequences mapping to this genomic location (e.g. CAGN26665, CAIC6716, CAGF11616). We assembled all matching ESTs in silico to produce a transcript with a clear stop codon but ambiguous start codon. To identify the putative start codon, we aligned the predicted open reading frame from the assembled ESTs (536 amino acids) to the full-length catalase sequence from the anemone A. viridis. In this alignment, position 27 in the assembled N. vectensis catalase matches the start site from A. viridis (supplementary material Fig. S1). If this is the correct start site, the N. vectensis catalase is 510 amino acids long, and has high similarity (e=0.0, identity=82%, positives=89%) to catalase from A. viridis. When this assembled transcript was mapped to the reference genome, the transcript encompassed both of the predicted proteins, indicating that the two predicted proteins comprise a single catalase gene.
Effects of UV radiation on acute toxicity of PAHs
In experiments with juvenile N. vectensis, exposure to UV radiation dramatically enhanced the acute toxicity of benzo[a]pyrene (B[a]P), pyrene and fluoranthene (Table 1). Low and moderate UV resulted in no mortality without PAHs [diluted seawater and solvent (DMSO) controls]. Similarly, PAH concentrations up to 500 μg l−1 resulted in >90% survival when animals were shielded from UV. In contrast, complete mortality was observed for all three chemicals at 50 μg l−1 under medium UV and at 500 μg l−1 under both medium and low UV. At 50 μg l−1 under low UV, we observed survival rates of 3% for pyrene, 87% for B[a]P, and 100% for fluoranthene.
Table 1.
Percentage survival of Nematostella vectensis juveniles following a 24 h exposure to UV radiation and/or PAHs
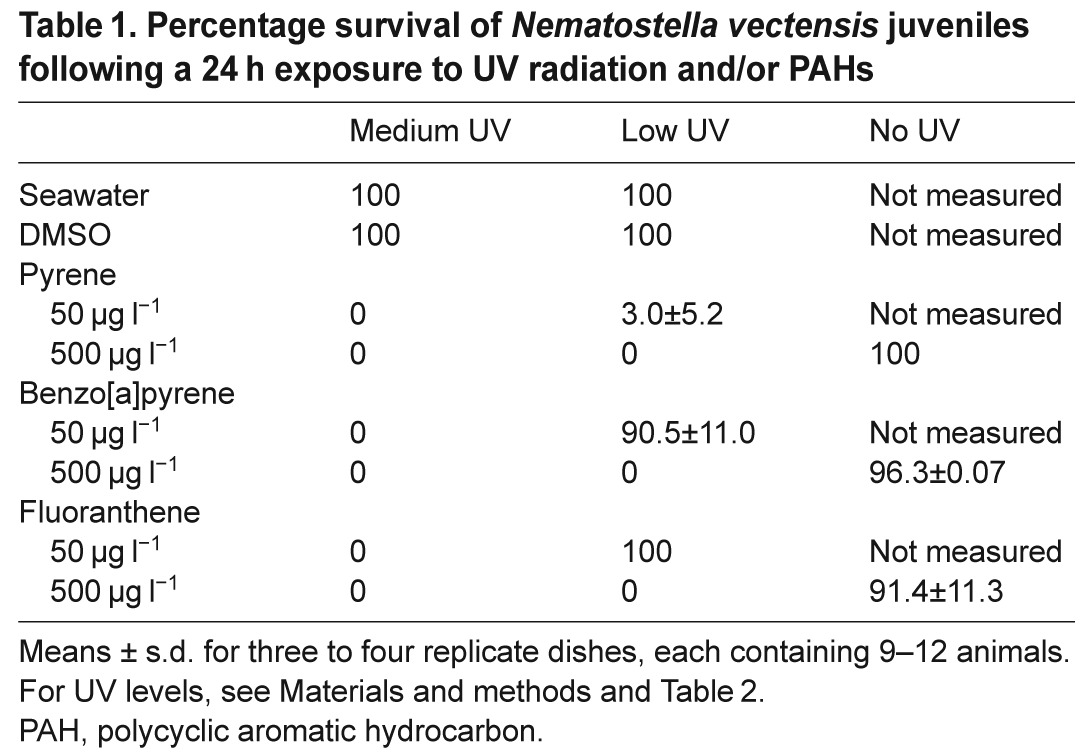
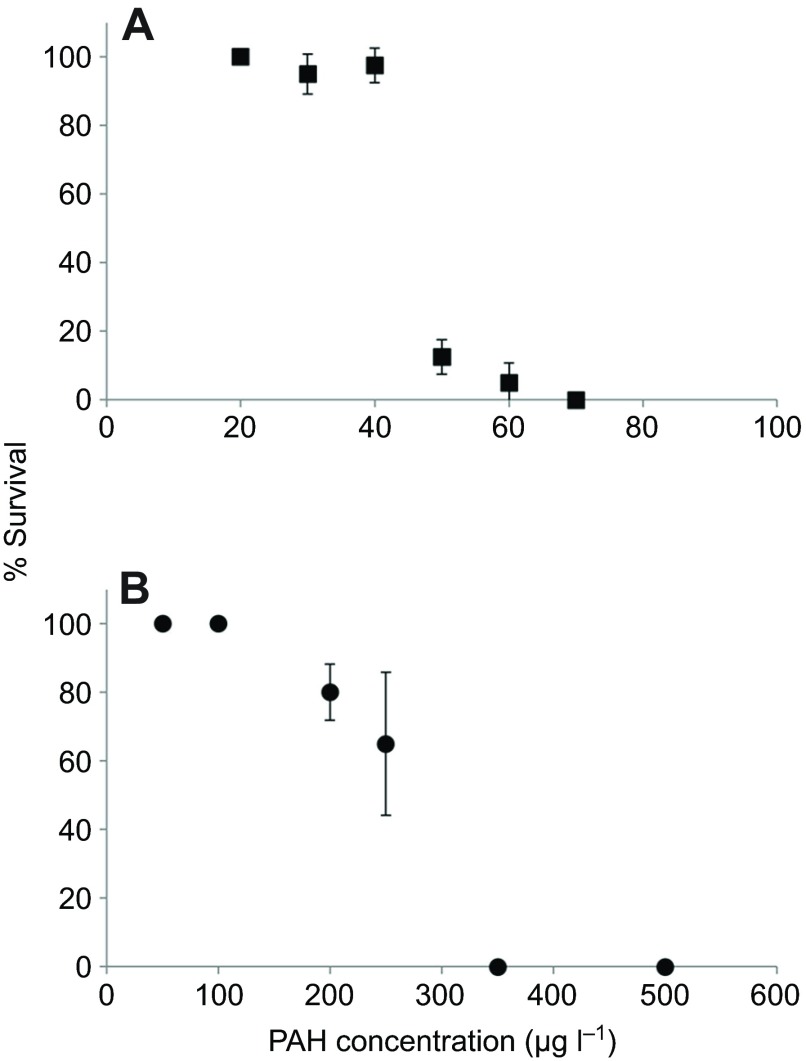
In a subsequent experiment with low UV and a narrower range of chemical concentrations, we observed partial mortality with pyrene beginning with 30 μg l−1, an LC50 (lethal concentration for 50% of organisms) of 49 μg l−1, and complete mortality with 70 μg l−1 (Fig. 4A). Under the same UV conditions, with B[a]P exposure we observed partial mortality beginning with 200 μg l−1, an LC50 of 271 μg l−1, and complete mortality with 350 μg l−1 (Fig. 4B). As before, mortality was only observed in animals co-exposed to UV and either PAH.
Fig. 4.
Percentage survival of N. vectensis juveniles following a 24 h exposure to low UV and/or polycyclic aromatic hydrocarbons (PAHs). The PAHs used were (A) pyrene and (B) benzo[a]pyrene (B[a]P). Symbols represent the mean ± s.d. for three to four replicate dishes, each containing 9–12 animals. No mortality was observed in control animals: seawater and UV, DMSO and UV, 70 μg l−1 pyrene without UV, and 500 μg l−1 B[a]P without UV (not shown).
In the two experiments designed to test the effects of PAH and UV on gene expression in adult N. vectensis, mortality was only observed on one occasion. Following 96 h of exposure to the high level of UV and 500 μg l−1 B[a]P, the animals in one dish were dead and had begun to disintegrate (leaving two replicates within the treatment for analysis of gene expression).
Effects of UV and chemical treatment on gene expression
Two experiments were conducted in which adult N. vectensis were exposed to UV radiation and/or chemicals. Based on their known roles in neutralization of ROS and/or repair of cellular damage, we predicted that catalase, HSP70 and one or more SOD genes would be induced by UV and/or chemical exposure. In the first experiment, gene expression was measured following 96 h of exposure to high UV or darkness and varying concentrations of B[a]P (Fig. 5). Broadly, CuZnSOD3, CCS, MnSOD1, catalase and HSP70 were either induced by UV exposure or were induced by UV within some chemical treatments. Both CuZnSOD2 and CuZnSOD3 were induced by UV in animals that were not exposed to either B[a]P or DMSO. Expression of CuZnSOD2 decreased following B[a]P exposure, but significant differences were only observed between animals exposed to 100 or 500 μg l−1 B[a]P relative to some groups exposed to control or lower concentrations (e.g. 500 μg l−1 treatment different from all controls and most B[a]P treatments up to 1 μg l−1). For CuZnSOD3, expression generally increased following UV exposure, but high expression in UV-exposed DMSO-treated animals resulted in a significant interaction. Other pairwise comparisons were not statistically significant. CCS expression increased in response to UV exposure and was also significantly affected by chemical treatment; however, no chemical treatments were significantly different from one another in pairwise comparisons. MnSOD1 expression increased following UV exposure in some treatments with no or relatively low B[a]P exposure (water, DMSO, 10 μg l−1 B[a]P). MnSOD2 expression decreased following 10, 100 or 500 μg l−1 B[a]P exposure relative to the chemical-free control. Catalase was induced by UV exposure. Hsp70 was very strongly induced by UV exposure, and showed a further trend toward increased expression with chemical exposure in UV-treated animals (Fig. 5G, note log scale).
Fig. 5.
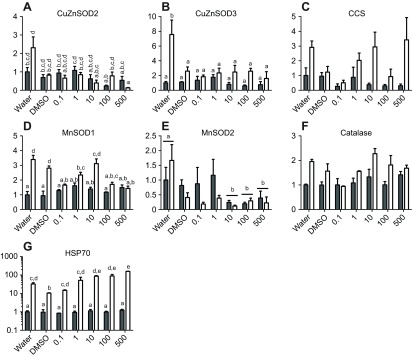
Transcript expression of putative stress-response genes following a 96 h exposure to high UV and/or varying concentrations of B[a]P. Open bars indicate UV-exposed animals and filled bars indicate animals not exposed to UV. Data were analyzed using two-way ANOVA with significance at P<0.05. Pairwise comparisons for significant effects were conducted using Tukey's test. Each bar represents the mean ± s.e.m. of three replicates, except the 500 μg l−1 B[a]P and UV treatment, which had two replicates because of mortality in the third. Some data were transformed to achieve a normal distribution of residuals (no transform: D,F; log10: A,E,G; square root: B,C). For consistency of presentation, untransformed data are shown, except in G, where data are shown on a log scale because of the ~100-fold range in values. Significant UV effects were found in C and F. Significant chemical effects were found in C and E. In post hoc tests, significantly different chemical groups are indicated by bars with lowercase letters in C; no groups were significantly different in pairwise comparisons in E. When there was a significant interaction, lowercase letters are used to indicate statistically different groups. Unlabeled groups are not statistically different from any other group.
In the second experiment, gene expression was measured following 24 h of exposure to low UV or darkness, and diluted seawater, 250 μg l−1 B[a]P or a water-accommodated fraction prepared from 20 mg l−1 crude oil in diluted seawater (Fig. 6). Expression in animals exposed to DMSO (vehicle control) was not different from that of unexposed animals (not shown). CuZnSOD2 and MnSOD2 expression were not affected by these treatments. CuZnSOD3 was induced by B[a]P in the presence of low UV radiation. CCS was downregulated by UV and by crude oil. MnSOD1 expression was highest following exposure to UV and B[a]P but lowest following exposure to UV and crude oil. In contrast to the previous experiment, expression of both catalase and HSP70 decreased with UV exposure.
Fig. 6.
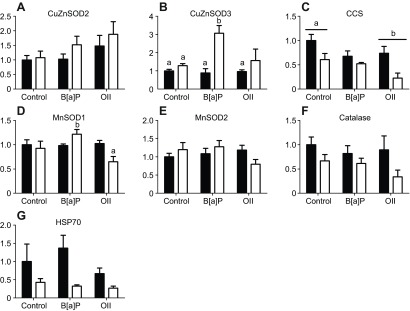
Transcript expression of putative stress-response genes following a 24 h exposure to low UV and/or either B[a]P or crude oil. B[a]P was used at 250 μg l−1; crude oil was at 20 mg l−1 (water-accommodated fraction). Open bars indicate UV-exposed animals and filled bars indicate animals not exposed to UV. Data were analyzed using two-way ANOVA with significance at P<0.05. Pairwise comparisons for significant effects were conducted using Tukey's test. Each bar represents the mean ± s.e.m. of three to four replicates. Some data were transformed to achieve a normal distribution of residuals (no transform: D; log10: E,F; square root: A,B,C,G). For consistency of presentation, untransformed data are shown. Significant UV effects were found in C, F and G; significant chemical effects (in C) are indicated by bars and lowercase letters over significantly different treatments. When there was a significant interaction (in D), lowercase letters are used to indicate statistically different groups. Unlabeled groups are not statistically different from any other group.
DISCUSSION
Antioxidant enzymes and stress-inducible chaperones are deeply conserved within the animal kingdom and more broadly in both prokaryotes and eukaryotes. This represents the first comprehensive study of SOD diversity in a cnidarian. Phylogenetic analysis of the N. vectensis SOD genes provides a glimpse into the diversification of SODs early in the evolution of animals and within the cnidarian lineage. Two CuZnSODs have previously been identified in the anemone A. viridis (Plantivaux et al., 2004), one in the hydrozoan H. vulgaris (Dash et al., 2007) and eight from an initial search of the genome of the coral A. digitifera (Shinzato et al., 2012). We have identified three CuZnSODs in N. vectensis, two of which are orthologs of the genes from A. viridis. NvCuZnSOD3 is orthologous to SODa from A. viridis. Based on the presence of a signal peptide at the N-terminus, SODa is predicted to be extracellular, like the mammalian SOD3 genes (Plantivaux et al., 2004). In N. vectensis the gene prediction of NvCuZnSOD3 appears to be incomplete at the 5′ end, so it is not currently known whether a signal peptide is present. NvCuZnSOD2 is orthologous to SODb from A. viridis, which is predicted to be cytosolic. We identified one CCS gene, which is an ortholog of the CCS from A. digitifera.
Most animals have a single MnSOD gene, and this study represents the first documentation of two MnSOD genes in a cnidarian. We identified two MnSOD genes in N. vectensis, one of which (NvMnSOD1) is most closely related to the predicted MnSOD from A. digitifera and which falls into a clade with previously described MnSODs from H. vulgaris and many protostomes and deuterostomes. The second, NvMnSOD2, falls into a distantly related clade that also contains MnSODs from the annelid C. teleta and the urchin S. purpuratus. The presence of a second clade of MnSODs among such distantly related taxa implies that the origin of this gene is ancient, although it is not clear whether it resulted from gene duplication or a gene transfer event. None of the genes within this clade have been functionally characterized, and specific physiological roles of the two MnSODs from N. vectensis are not yet clear.
In this study, UV and chemical exposures were used to identify whether and how transcription of antioxidant enzymes can be induced by environmentally relevant stressors; however, these laboratory exposures differed from natural exposures in several ways. On a cloudless July day at latitude 42°N (the collection site at Sippewisset Marsh, MA, USA), calculated noon UV-A and UV-B levels are 54 and 1.6 W m−2, respectively (calculated using the FastRT program, available at http://nadir.nilu.no/~olaeng/fastrt/fastrt.html. Thus, the levels tested in our experiments (0.37–2.6 W m−2 UV-A, 0.15–1.1 W m−2 UV-B) were lower than peak noon exposure; however, animals would not be naturally exposed to continuous levels of UV radiation for 24–96 h. PAH concentrations in contaminated seawater or porewater are generally lower than (<1 μg l−1) the highest exposures used in this study (Liu et al., 2013; Lu et al., 2011; Maruya et al., 1996); however, PAH concentrations in sediments can be several orders of magnitude higher (>1 μg g−1) (Liu et al., 2013; Lu et al., 2011; Maruya et al., 1996; White et al., 2005). While the PAH levels used in our study are high relative to environmental concentrations, the exposures were short relative to the chronic exposures associated with continued partitioning of PAHs between the sediment and seawater, and co-exposure to chemical dispersants can enhance partitioning of PAHs into porewater (Zuijdgeest and Huettel, 2012). Field studies and longer term mesocosm experiments would be necessary to fully explore responses to environmentally realistic contaminant exposures.
While the effects of UV, PAH and crude oil have been previously studied in corals (Downs et al., 2006; Kuffner, 2001; Lesser et al., 1990; Ramos and Garcia, 2007), relatively few studies have been conducted in other cnidarians including N. vectensis. A previous study demonstrated that UV exposure from a Stratalinker selectively induces apoptosis in developing gametes within N. vectensis polyps (Pankow and Bamberger, 2007). While these authors provided limited details regarding the UV exposure conditions, a conventional Stratalinker produces UV-C radiation (254 nmol l−1) over short intervals (25–50 s for recommended uses in DNA cross-linking, although the exposure duration can be adjusted); these conditions are very different from the extended exposures to UV-A and UV-B that were conducted within our experiments.
In coastal environments, animals are commonly co-exposed to UV radiation and contaminants including PAHs, both of which can lead to the production of ROS. UV exposure can alter the reactivity of some chemicals, including the three PAHs tested in this study, resulting in enhanced toxicity (Diamond, 2003). As would be predicted by a phototoxicity mechanism, survival of N. vectensis juveniles decreased dramatically upon co-exposure to UV and PAHs. In contrast, short-term survival of adults was not substantially affected by any of the treatment conditions. The differential sensitivity may be due to thicker or more pigmented tissue in adults, maturation of defense mechanisms, and/or the presence of greater energetic reserves to fuel defensive or cellular repair processes. Nematostella vectensis contains a suite of fluorescent proteins (Ikmi and Gibson, 2010) as well as enzymes needed to synthesize mycosporine-like amino acids (Starcevic et al., 2008). These compounds are photoprotective in other cnidarians (Salih et al., 2000; Shick and Dunlap, 2002), but their physiological role in N. vectensis has not been determined, and it is yet unknown how the photoprotective capacity varies during development. In natural environments, burrowing into sediments could reduce UV exposure. The behavior of N. vectensis has not been extensively documented, but individuals have been observed with their tentacles extended on the surface of the sediment (Crowell, 1946; Rudy and Rudy, 1983) (A.M.T., unpublished observation) as well as with their entire body extended across the sediment (Rudy and Rudy, 1983). In addition, even short exposures may result in photoactivation of accumulated contaminants and lead to enhanced toxicity to benthic invertebrates (Arfsten et al., 1996; Boese et al., 1998).
CuZnSODs appear to be an important part of the cnidarian oxidative stress response. CuZnSOD activity can be induced in corals by thermal, chemical and UV stress, generally to a greater extent than MnSOD activity (Brown et al., 2002; Downs et al., 2000), and accounts for about two-thirds of the total SOD activity in the anemone A. viridis (Richier et al., 2003). Of the two CuZnSOD transcripts quantified in N. vectensis, NvCuZnSOD3 expression was upregulated in response to high UV and in response to the combination of low UV and B[a]P. NvCuZnSOD2 expression showed a statistically complex set of interactions in the high UV experiment and no significant effects in the low UV experiment. Under a prolonged exposure (96 h) to high UV, both NvCuZnSOD2 and NvCuZnSOD3 were most highly expressed in animals exposed to UV without exposure to B[a]P or DMSO. One interpretation is that prolonged exposure to DMSO and/or B[a]P prevented the animals from mounting a response to UV; however, the error bar was relatively large.
The activity of some CuZnSODs (mammalian and insect cytosolic CuZnSODs, traditionally called ‘SOD1’) is regulated post-translationally through interactions with CCS (Banci et al., 2012; Kirby et al., 2008; Wong et al., 2000). In N. vectensis, CCS was induced by a 96 h exposure to high UV and downregulated following a 24 h exposure to low UV. CCS expression was significantly reduced in response to the crude oil water-accommodated fraction. If the cnidarian CCS serves a similar function to that described in bilaterians, a decrease in CCS expression may result in a decreased capacity for cytosolic CuZnSOD function.
Changes in NvMnSOD expression were primarily seen following 96 h exposure to B[a]P and/or high UV. This resulted in induction of NvMnSOD1 expression by UV when animals were not exposed to B[a]P. As was observed for NvCuZnSOD2 and NvCuZnSOD3, co-exposure to B[a]P appears to prevent the induction of NvMnSOD1 by high UV. In contrast, NvMnSOD2 transcript expression was downregulated following a 96 h exposure to B[a]P. The reason for the downregulation of MnSODs is not known. Because MnSODs are localized to the mitochondria, one possibility is that some experimental conditions resulted in a reduction in metabolic rate and endogenous production of ROS through oxidative respiration.
Catalase and HSP70 were both induced by a prolonged exposure to high UV and were downregulated by a shorter exposure to low UV. HSP70 was further induced by the combination of high UV and B[a]P. Within the experimental conditions tested, it is not possible to separate the effects of intensity and duration of UV exposure, although both are likely to be important. Future studies in which the intensity and duration of UV exposure are varied independently and in combination will provide further insight into the dynamics of the UV response. While HSP70 can be induced by acute thermal stress within a few hours, responses to milder changes can be slow to develop or not observed (Coles and Brown, 2003; Richier et al., 2008). Similarly, some cnidarian stress responses to chemicals require prolonged periods of exposure to produce measureable effects at the molecular and organismal level. For example, in the coral Montastrea faveolata, catalase and SOD activity were induced by B[a]P (100 μg l−1) after 72 h, but not after 24 h (Ramos and Garcia, 2007). Porites astreoides coral larvae exposed to 10 μg l−1 B[a]P appeared normal during the first 24 h, but experienced a decreased survivorship after 48 h (Farina et al., 2008).
We did not observe effects of DMSO on survival or gene expression relative to animals not exposed to any chemicals (except for the previously noted difference in NvCuZnSOD3 expression in the high UV experiment); however, the experimental design did not account for the possibility that the anti- or pro-oxidant activity of DMSO modulates the effects of PAH toxicity or phototoxicity. DMSO is frequently used as a vehicle in studies requiring aqueous exposures to highly lipophilic chemicals because it exhibits relatively low toxicity, mixes readily into aqueous media and increases cell permeability, enabling delivery of the test chemical. While relatively non-toxic, DMSO can alter processes including cellular differentiation, transcription and methylation (Iwatani et al., 2006; Preisler and Giladi, 1975). Of particular relevance to the present study, DMSO can act as an antioxidant by scavenging hydroxyl radicals, but can also act as a pro-oxidant through interactions with protein thiol groups (Sanmartín-Suárez et al., 2011). UV-A-induced oxidative DNA damage largely occurs through production of singlet oxygen, which is not scavenged by DMSO (Wei et al., 1997). Superoxide, singlet oxygen and hydroxyl radical can all be produced upon exposure to PAHs including pyrene, B[a]P and B[a]P metabolites (Bryla and Weyand, 1991; Sun et al., 2008; Tobit et al., 2011; Woo et al., 2008; Xia et al., 2013), so scavenging of hydroxyl by DMSO could reduce the toxicity or phototoxicity of PAHs. Future experiments utilizing a range of solvents could address this issue but will need to account for potential differences in solvent toxicity, delivery of test compounds and pharmacokinetic effects (Sanderson et al., 1998).
Overall, the effects of B[a]P and crude oil on SOD, catalase and HSP70 expression were mild. As discussed above, this may be due in part to the relatively short nature of the exposures or, in the case of B[a]P, to antioxidant effects of DMSO. Phototoxicity from the combination of UV and PAHs was clearly observed in mortality experiments with juveniles; however, adult gene expression patterns consistent with phototoxicity were observed only in a few cases (HSP70 in the high UV experiment, CuZnSOD3 and MnSOD1 in the low UV experiment). Responses to such stressors are dynamic, and the qPCR approach provided only a snapshot of the transcriptional response, which at best indirectly indicates corresponding protein or activity levels. Another important consideration is that the genes measured in this study comprise only a portion of the antioxidant enzymes and do not include non-enzymatic antioxidants or other stress-responsive genes within N. vectensis. Informative directions for future research include measurement of the activity of enzymatic antioxidants as well as assessment of non-enzymatic antioxidants (e.g. measurement of glutathione concentration and redox state). In addition, transcriptome-wide measurements of changes in gene expression may reveal more sensitive biomarkers as well as identify novel mechanisms deployed in response to exposure to ROS.
MATERIALS AND METHODS
Gene identification and sequence analysis
Members of the SOD and catalase gene families were identified within the N. vectensis genome (v1.0, http://genome.jgi-psf.org/Nemve1/Nemve1.home.html) through BLASTp searches of protein models using the Joint Genome Institute (JGI) browser. Gene predictions were supported by ESTs available within the JGI database and through RT-PCR followed by cloning and sequencing.
We collected a set of published SOD sequences from cnidarians and other representative bilaterians. Amino acid sequences for all taxa were aligned using Muscle v3.8 (Edgar, 2004), and alignments were manually trimmed. Within the SOD superfamily, separate alignments were constructed for members of the CuZnSOD, MnFeSOD and CCS families. Maximum likelihood analyses were conducted with RAxML v7.0.4, (Stamatakis, 2006) and Bayesian analyses with MrBayes v3.1.2 (Ronquist and Huelsenbeck, 2003) using the WAG + G model. In the maximum likelihood analysis, support for individual nodes was assessed through 1000 bootstrap replicates. Bayesian analysis of each family was determined with 5 million generation runs of five chains, where trees were sampled every 500 generations. The first 25% of generations were discarded as burn-in, well after each analysis had reached stationarity, and a consensus tree and node probabilities were determined from the remaining generations. Trees were visualized and annotated with FigTree v1.1.2 (http://tree.bio.ed.ac.uk/software/figtree/).
Animal culture
Adult N. vectensis were reared in glass dishes containing filtered natural seawater that was diluted to 20 ppt (referred to as ‘diluted seawater’ throughout this paper). These animals were maintained at room temperature on an 8 h:16 h light:dark cycle with lighting provided by overhead fluorescent lights. Animals were fed freshly hatched brine shrimp nauplii four times per week, and the water was changed weekly. To induce spawning, animals were placed in the dark at 18°C for 2.5 days. At the end of this period, animals were returned to the bench top, the water was changed, and over the course of the day fertilized bundles of eggs were removed into fresh glass dishes that contained diluted seawater.
Chemical exposures
B[a]P, pyrene and fluoranthene were obtained from Sigma-Aldrich (St Louis, MO, USA). Chemical solutions were gently evaporated under liquid nitrogen, reconstituted in DMSO, and serially diluted in DMSO as needed. Exposures were conducted in glass dishes, 30 mm diameter×12 mm depth (Electron Microscopy Sciences, Hatfield, PA, USA), containing 3 ml diluted seawater. Test chemicals were added in a volume of 15 μl DMSO per dish (final concentration of 0.5% DMSO in the assay).
To test the effects of crude oil on N. vectensis gene expression, a water-accommodated fraction was prepared. Macondo sweet crude oil was added into an amber-colored glass bottle containing diluted seawater to give a nominal concentration of 20 ppm oil (typically 2 μl into 100 ml). The bottle was capped tightly and stirred overnight with a Teflon-coated magnetic stir bar such that a vortex was formed that reached one-third of the way toward the bottom of the bottle.
UV radiation
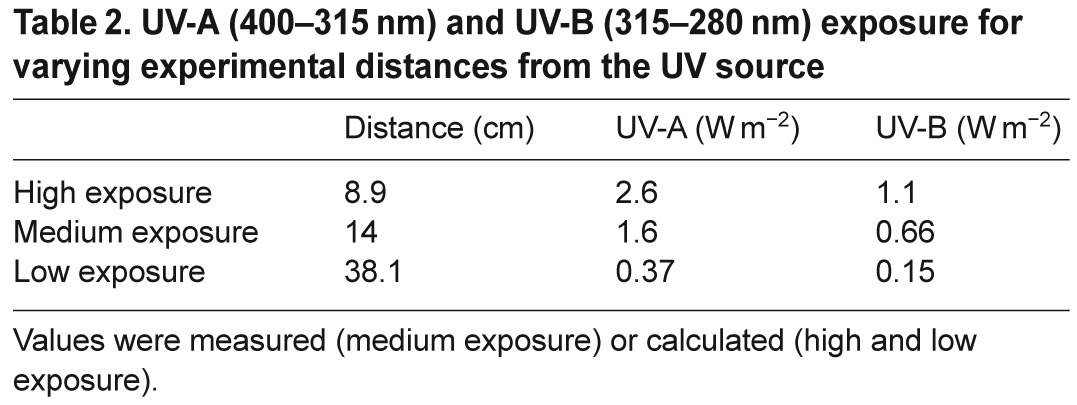
UV radiation was provided by a UV-B-enriched bulb (Zilla Desert Series fluorescent T5 bulb, purchased from a local pet store). The spectral quality and intensity of the emitted radiation were measured 14 cm from the bulb using an OL 756 UV-vis spectroradiometer (Optronic Laboratories, Orlando, FL, USA). Most of the UV radiation emitted ranged from 300 to 350 nmol l−1, and no UV-C radiation was detected over the range scanned (250–280 nmol l−1, supplementary material Fig. S2). The distance of the dishes from the bulb varied among experiments to give three UV levels (high, medium and low), as summarized in Table 2. Because of physical constraints within the incubator, it was not possible to measure UV levels at each experimental position using the spectroradiometer. Instead, the light sensor on a HOBO pendant temperature/light data logger (Onset, Bourne, MA, USA) was used to measure the relative light intensity at each position. These values were used to calculate the UV intensity within the high and low UV treatments. High, medium and low represent relative levels within our experimental regime. Because little is known regarding the UV sensitivity of N. vectensis, we do not imply that the levels necessarily span a range from high to low physiological impact.
Table 2.
UV-A (400–315 nm) and UV-B (315–280 nm) exposure for varying experimental distances from the UV source
Acute toxicity tests
In acute toxicity tests, 10 juvenile N. vectensis (10–21 days old) were added to each dish, and the dishes were covered either with UV-transparent plastic wrap or UV-opaque glass lids. Animals were maintained for 24 h at 25°C in an incubator continuously lit by a UV-B-enriched bulb. After 24 h, dishes were removed from the incubator, and the number of surviving anemones was assessed visually under a dissecting microscope. Each treatment was replicated in three dishes.
Two experiments were conducted: the first tested toxicity of a broad range of B[a]P, pyrene and fluoranthene concentrations under medium and low UV levels, and the second tested a narrower range of B[a]P and pyrene concentrations under low UV.
Sublethal toxicity tests
Two experiments were conducted to characterize effects of UV radiation and/or chemical exposure on transcript expression of antioxidant enzymes and HSP70. Three or four adult N. vectensis were added to each glass dish, with chemical and UV exposures similar to those described for acute toxicity tests. Each treatment was replicated in three or four dishes. Within a dish, animals were pooled and stored in RNAlater (Invitrogen Life Technologies, Grand Island, NY, USA) at −20°C until analysis.
In the first experiment, to test effects of prolonged exposure to UV radiation and B[a]P, animals were exposed to either continuous darkness or high UV (2.6 W m−2 UV-A, 1.1 W m−2 UV-B) and a range of B[a]P concentrations (0.1–500 μg l−1) for 96 h. Chemical solutions were partially renewed every 12 h by exchanging 2 ml of the test solution.
In the second experiment, to test effects of a shorter exposure to B[a]P and lower UV levels, animals were maintained for 24 h in continuous darkness or with continuous exposure to UV radiation (low UV: 0.37 W m−2 UV-A, 0.15 W m−2 UV-B). In combination with the UV treatments, animals were exposed either to 250 μg l−1 B[a]P or to 20 ppm crude oil.
Measurement of gene expression
Total RNA was extracted from the pooled animals within each glass dish using an Aurum Fatty and Fibrous Tissue Kit (Bio-Rad, Hercules, CA, USA), according to the manufacturer's protocol, including DNase treatment. RNA quantity and purity were assessed using a NanoDrop 1000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA). RNA quality was assessed using denaturing agarose gels. cDNA was synthesized from 1 μg of total RNA in a 20 μl reaction using an iScript cDNA synthesis kit (Bio-Rad). Expression of genes of interest was measured using a MyCycler Real-Time PCR detection system and a 20 μl reaction mixture consisting of 10 μl of SsoFast EvaGreen Supermix (Bio-Rad), 500 nmol l−1 gene-specific primers and 0.8 μl of cDNA (0.25 μl in the 18S assay). Expression was calculated by comparing the threshold cycle of amplification against a standard curve constructed from a serially diluted plasmid standard containing the amplicon of interest. PCR conditions were as follows: 95°C for 2 min followed by 40 cycles of 95°C for 5 s and 60°C for 10 s. After 40 cycles, the products were subjected to melt curve analysis to ensure that only a single specific product was amplified. Most genes (five SOD family members: catalase, HSP70, HSC71, EF1 and 18S) produced narrow single peaks. Amplification of one of the SOD genes (CuZnSOD1) resulted in melt curves with a strong shoulder, inconsistent amplification and low apparent expression; expression values for this gene were not considered further. All primer sequences are given in supplementary material Table S1.
We have previously identified five predicted N. vectensis genes within the HSP70 family (A.M.R. and A.M.T., unpublished data). Among these, one (JGI 234533, referred to here as HSP70) is most strongly induced by exposure to either cadmium or extreme temperatures, and a second (JGI 195315, referred to here as HSC71) exhibits relatively constant expression throughout a normal 24 h period (Reitzel et al., 2010) and following exposure to cadmium or extreme temperatures (A.M.R. and A.M.T., unpublished data).
Three genes were tested as potential normalizer genes in this study: elongation factor 1a (EF1), heat shock cognate protein 71 (HSC71) and the 18S ribosomal protein (18S). Expression of EF1 and HSC71 was found to vary with treatment, generally resulting in decreased expression in UV-exposed samples (data not shown). In contrast, two-way ANOVA showed that 18S expression did not vary by treatment in either experiment (P>0.05), so SOD, catalase and HSP70 expression was normalized to that of 18S.
Each treatment had three to four biological replicates, except for one treatment within the 96 h high UV experiment (animals exposed to UV and 500 μg l−1 B[a]P), in which all the animals from one dish died, leaving two replicates. Within each experiment, fold-change was calculated by dividing expression for each sample by the mean expression for the control (no UV or chemical exposure). Normality was evaluated by the Kolmogorov–Smirnov test, and equality of variance was evaluated through visual inspection of residuals. Data were either left untransformed or transformed by the square root or base-10 logarithm to satisfy these assumptions. Expression of each gene was analyzed using a two-way analysis of variance with UV exposure and chemical treatments as fixed factors. Transformation, evaluation of residuals and ANOVA were conducted using SYSTAT 13 (Systat Software Inc., Chicago, IL, USA).
Supplementary Material
ACKNOWLEDGEMENTS
We thank Lars Behrendt for assistance with initial animal exposures and Jed Goldstone for measuring spectral properties of the UV bulbs.
FOOTNOTES
Competing interests
The authors declare no competing financial interests.
Funding
This work was supported by the National Science Foundation [award nos MCB1057354 to A.M.T. and MCB1057152 to M.J.J.]. A.M.R. was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD) [award no. F32HD062178] from the National Institute of Health and incentive funding from the University of North Carolina at Charlotte. C.K.K. was supported by a fellowship in the Hong Kong Joint Universities Summer Teaching Laboratory (JUSTL) Program, which is funded by the Croucher Foundation and the Government of the Hong Kong SAR. Deposited in PMC for release after 12 months.
Supplementary material
Supplementary material available online at http://jeb.biologists.org/lookup/suppl/doi:10.1242/jeb.093690/-/DC1
References
- Ahrens M. J., Hickey C. W. (2002). UV-photoactivation of polycyclic aromatic hydrocarbons and the sensitivity of sediment-dwelling estuarine organisms. In Transcript of the National Institute of Water and Atmospheric Research Workshop on UV-Radiation and its Effects: An Update. New Zealand: Antarctic Center, Christchurch; [Google Scholar]
- Ambrosone A., Tortiglione C. (2013). Methodological approaches for nanotoxicology using cnidarian models. Toxicol. Mech. Methods 23, 207-216 [DOI] [PubMed] [Google Scholar]
- Arfsten D. P., Schaeffer D. J., Mulveny D. C. (1996). The effects of near ultraviolet radiation on the toxic effects of polycyclic aromatic hydrocarbons in animals and plants: a review. Ecotoxicol. Environ. Saf. 33, 1-24 [DOI] [PubMed] [Google Scholar]
- Banci L., Bertini I., Cantini F., Kozyreva T., Massagni C., Palumaa P., Rubino J. T., Zovo K. (2012). Human superoxide dismutase 1 (hSOD1) maturation through interaction with human copper chaperone for SOD1 (hCCS). Proc. Natl. Acad. Sci. USA 109, 13555-13560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barshis D. J., Ladner J. T., Oliver T. A., Seneca F. O., Traylor-Knowles N., Palumbi S. R. (2013). Genomic basis for coral resilience to climate change. Proc. Natl. Acad. Sci. USA 110, 1387-1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellas J., Saco-Alvarez L., Nieto O., Beiras R. (2008). Ecotoxicological evaluation of polycyclic aromatic hydrocarbons using marine invertebrate embryo-larval bioassays. Mar. Pollut. Bull. 57, 493-502 [DOI] [PubMed] [Google Scholar]
- Boese B. L., Lamberson J. O., Swartz R. C., Ozretich R., Cole F. (1998). Photoinduced toxicity of PAHs and alkylated PAHs to a marine infaunal amphipod (Rhepoxynius abronius). Arch. Environ. Chem. 34, 235-240 [DOI] [PubMed] [Google Scholar]
- Brown B. E., Downs C. A., Dunne R. P., Gibb S. W. (2002). Exploring the basis of thermotolerance in the reef coral Goniastrea aspera. Mar. Ecol. Prog. Ser. 242, 119-129 [Google Scholar]
- Bryla P., Weyand E. H. (1991). Role of activated oxygen species in benzo[a]pyrene:DNA adduct formation in vitro. Free Radic. Biol. Med. 11, 17-24 [DOI] [PubMed] [Google Scholar]
- Chelikani P., Fita I., Loewen P. C. (2004). Diversity of structures and properties among catalases. Cell. Mol. Life Sci. 61, 192-208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles S. L., Brown B. E. (2003). Coral bleaching – capacity for acclimatization and adaptation. Adv. Mar. Biol. 46, 183-223 [DOI] [PubMed] [Google Scholar]
- Crowell S. (1946). A new sea anemone from Woods Hole, Massachusetts. J. Wash. Acad. Sci. 36, 57-60 [Google Scholar]
- Császár N. B. M., Seneca F. O., van Oppen M. J. H. (2009). Variation in antioxidant gene expressionin the scleractinian coral Acropora millepora under laboratory thermal stress. Mar. Ecol. Prog. Ser. 392, 93-102 [Google Scholar]
- Darling J. A., Reitzel A. R., Burton P. M., Mazza M. E., Ryan J. F., Sullivan J. C., Finnerty J. R. (2005). Rising starlet: the starlet sea anemone, Nematostella vectensis. Bioessays 27, 211-221 [DOI] [PubMed] [Google Scholar]
- Dash B., Phillips T. D. (2012). Molecular characterization of a catalase from Hydra vulgaris. Gene 501, 144-152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash B., Metz R., Huebner H. J., Porter W., Phillips T. D. (2007). Molecular characterization of two superoxide dismutases from Hydra vulgaris. Gene 387, 93-108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond S. A. (2003). Phototoxicity inaquatic environments. In UV Effects in Aquatic Organisms and Environments (ed. Helbling W. E., Zagarese H.), pp. 219-250 Vienna, Austria: European Society for Photobiology; [Google Scholar]
- Downs C. A., Mueller E., Phillips S., Fauth J. E., Woodley C. M. (2000). A molecular biomarker system for assessing the health of coral (Montastraea faveolata) during heat stress. Mar. Biotechnol. 2, 533-544 [DOI] [PubMed] [Google Scholar]
- Downs C. A., Fauth J. E., Halas J. C., Dustan P., Bemiss J., Woodley C. M. (2002). Oxidative stress and seasonal coral bleaching. Free Radic. Biol. Med. 33, 533-543 [DOI] [PubMed] [Google Scholar]
- Downs C. A., Richmond R. H., Mendiola W. J., Rougée L., Ostrander G. K. (2006). Cellular physiological effects of the MV Kyowa violet fuel-oil spill on the hard coral, Porites lobata. Environ. Toxicol. Chem. 25, 3171-3180 [DOI] [PubMed] [Google Scholar]
- Edgar R. C. (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792-1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina O., Ramos R., Bastidas C., García E. (2008). Biochemical responses of cnidarian larvae to mercury and benzo(a)pyrene exposure. Bull. Environ. Contam. Toxicol. 81, 553-557 [DOI] [PubMed] [Google Scholar]
- Feder M. E., Hofmann G. E. (1999). Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu. Rev. Physiol. 61, 243-282 [DOI] [PubMed] [Google Scholar]
- Fu P. P., Xia Q., Sun X., Yu H. (2012). Phototoxicity and environmental transformation of polycyclic aromatic hydrocarbons (PAHs)-light-induced reactive oxygen species, lipid peroxidation, and DNA damage. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 30, 1-41 [DOI] [PubMed] [Google Scholar]
- Goldstone J. V. (2008). Environmental sensing and response genes in cnidaria: the chemical defensome in the sea anemone Nematostella vectensis. Cell Biol. Toxicol. 24, 483-502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Gutiérrez C. M., Guerra-Rivas G. (2010). Uptake and biochemical response of B[a]P in the sea anemone Anthopleura elegantissima. J. Environ. Sci. Health A Tox. Hazard Subst. Environ. Eng. 45, 42-48 [DOI] [PubMed] [Google Scholar]
- Hand A. R. (1976). Ultrastructural localization of catalase and l-alpha-hydroxy acid oxidase in microperoxisomes of Hydra. J. Histochem. Cytochem. 24, 915-925 [DOI] [PubMed] [Google Scholar]
- Hand C., Uhlinger K. (1994). The unique, widely distributed sea anemone, Nematostella vectensis Stephenson; a review, new facts, and questions. Estuaries 17, 501-508 [Google Scholar]
- Harter V. L., Matthews R. A. (2005). Acute and chronic toxicity test methods for Nematostella vectensis Stephenson. Bull. Environ. Contam. Toxicol. 74, 830-836 [DOI] [PubMed] [Google Scholar]
- Hoffmeister S., Schaller H. C. (1985). A new biochemical marker for foot-specific cell differentiation in hydra. Roux's Arch. Develop. Biol. 194, 453-461 [Google Scholar]
- Ikmi A., Gibson M. C. (2010). Identification and in vivo characterization of NvFP-7R, a developmentally regulated red fluorescent protein of Nematostella vectensis. PLoS ONE 5, e11807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatani M., Ikegami K., Kremenska Y., Hattori N., Tanaka S., Yagi S., Shiota K. (2006). Dimethyl sulfoxide has an impact on epigenetic profile in mouse embryoid body. Stem Cells 24, 2549-2556 [DOI] [PubMed] [Google Scholar]
- Kennedy C. J., Gassman N. J., Walsh P. J. (1992). The fate of benzo[a]pyrene in the scleractinian corals Favia fragum and Montastrea annularis. Mar. Biol. 113, 313-318 [Google Scholar]
- Kim R. O., Rhee J. S., Won E. J., Lee K. W., Kang C. M., Lee Y. M., Lee J. S. (2011). Ultraviolet B retards growth, induces oxidative stress, and modulates DNA repair-related gene and heat shock protein gene expression in the monogonont rotifer, Brachionus sp. Aquat. Toxicol. 101, 529-539 [DOI] [PubMed] [Google Scholar]
- Kirby K., Jensen L. T., Binnington J., Hilliker A. J., Ulloa J., Culotta V. C., Phillips J. P. (2008). Instability of superoxide dismutase 1 of Drosophila in mutants deficient for its cognate copper chaperone. J. Biol. Chem. 283, 35393-35401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffner I. B. (2001). Effects of ultraviolet (UV) radiation on larval settlement of the reef coral Pocillopora damicornis. Mar. Ecol. Prog. Ser. 217, 251-261 [Google Scholar]
- Landis G. N., Tower J. (2005). Superoxide dismutase evolution and life span regulation. Mech. Ageing Dev. 126, 365-379 [DOI] [PubMed] [Google Scholar]
- Landis G. N., Abdueva D., Skvortsov D., Yang J., Rabin B. E., Carrick J., Tavaré S., Tower J. (2004). Similar gene expression patterns characterize aging and oxidative stress in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 101, 7663-7668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesser M. P. (1997). Oxidative stress causes coral bleaching during exposure to elevated temperatures. Coral Reefs 16, 187-192 [Google Scholar]
- Lesser M. P. (2006). Oxidative stress in marine environments: biochemistry and physiological ecology. Annu. Rev. Physiol. 68, 253-278 [DOI] [PubMed] [Google Scholar]
- Lesser M. P., Stochaj W. R., Tapley D. W., Shick J. M. (1990). Bleaching in coral reef anthozoans: effects of irradiance, ultraviolet radiation, and temperature on the activities of protective enzymes against active oxygen. Coral Reefs 8, 225-232 [Google Scholar]
- Liu X., Jia H., Wang L., Qi H., Ma W., Hong W., Guo J., Yang M., Sun Y., Li Y. F. (2013). Characterization of polycyclic aromatic hydrocarbons in concurrently monitored surface seawater and sediment along Dalian coast after oil spill. Ecotoxicol. Environ. Saf. 90, 151-156 [DOI] [PubMed] [Google Scholar]
- Lu X., Skwarski A., Drake B., Reible D. D. (2011). Predicting bioavailability of PAHs and PCBs with porewater concentrations measured by solid-phase microextraction fibers. Environ. Toxicol. Chem. 30, 1109-1116 [DOI] [PubMed] [Google Scholar]
- Lyons B. P., Pascoe C. K., McFadzen I. R. (2002). Phototoxicity of pyrene and benzo[a]pyrene to embryo-larval stages of the Pacific oyster Crassostrea gigas. Mar. Environ. Res. 54, 627-631 [DOI] [PubMed] [Google Scholar]
- Maruya K. A., Risebrough R. W., Horne A. J. (1996). Partitioning of polynuclear aromatic hydrocarbons between sediments from San Francisco Bay and their porewaters. Environ. Sci. Technol. 30, 2942-2947 [Google Scholar]
- Merle P.-L., Sabourault C., Richier S., Allemand D., Furla P. (2007). Catalase characterization and implication in bleaching of a symbiotic sea anemone. Free Radic. Biol. Med. 42, 236-246 [DOI] [PubMed] [Google Scholar]
- NRC (1985). Oil in the Sea: Inputs, Fates and Effects: Report of the National Research Council. Washington, DC: National Academy Press; [Google Scholar]
- Pankow S., Bamberger C. (2007). The p53 tumor suppressor-like protein nvp63 mediates selective germ cell death in the sea anemone Nematostella vectensis. PLoS ONE 2, e782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peachey R. L., Crosby D. G. (1996). Phototoxicity in tropical reef animals. Mar. Environ. Res. 42, 359-362 [Google Scholar]
- Pelletier M. C., Burgess R. M., Ho K. T., Kuhn A., McKinney R. A., Ryba S. A. (1997). Phototoxicity of individual polycyclic aromatic hydrocarbons and petroleum to marine invertebrate larvae and juveniles. Environ. Toxicol. Chem. 16, 2190-2199 [Google Scholar]
- Plantivaux A., Furla P., Zoccola D., Garello G., Forcioli D., Richier S., Merle P.-L., Tambutté E., Tambutté S., Allemand D. (2004). Molecular characterization of two CuZn-superoxide dismutases in a sea anemone. Free Radic. Biol. Med. 37, 1170-1181 [DOI] [PubMed] [Google Scholar]
- Preisler H. D., Giladi M. (1975). Differentiation of erythroleukemic cells in vitro: irreversible induction by dimethyl sulfoxide (DMSO). J. Cell. Physiol. 85, 537-545 [DOI] [PubMed] [Google Scholar]
- Ramos R., Garcia E. (2007). Induction of mixed-function oxygenase system and antioxidant enzymes in the coral Montastrea faveolata on acute exposure to benzo(a)pyrene. Comp. Biochem. Physiol. 144C, 348-355 [DOI] [PubMed] [Google Scholar]
- Reitzel A. M., Darling J. A., Sullivan J. C., Finnerty J. R. (2008a). Global population genetic structure of the starlet anemone Nematostella vectensis: multiple introductions and implications for conservation policy. Biol. Invasions 10, 1197-1213 [Google Scholar]
- Reitzel A. M., Sullivan J. C., Traylor-Knowles N., Finnerty J. R. (2008b). Genomic survey of candidate stress-response genes in the estuarine anemone Nematostella vectensis. Biol. Bull. 214, 233-254 [DOI] [PubMed] [Google Scholar]
- Reitzel A. M., Behrendt L., Tarrant A. M. (2010). Light entrained rhythmic gene expression in the sea anemone Nematostella vectensis: the evolution of the animal circadian clock. PLoS ONE 5, e12805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richier S., Merle P.-L., Furla P., Pigozzi D., Sola F., Allemand D. (2003). Characterization of superoxide dismutases in anoxia- and hyperoxia-tolerant symbiotic cnidarians. Biochim. Biophys. Acta 1621, 84-91 [DOI] [PubMed] [Google Scholar]
- Richier S., Rodriguez-Lanetty M., Schnitzler C. E., Weis V. M. (2008). Response of the symbiotic cnidarian Anthopleura elegantissima transcriptome to temperature and UV increase. Comp. Biochem. Physiol. 3D, 283-289 [DOI] [PubMed] [Google Scholar]
- Rinkevich B., Loya Y. (1979). Laboratory experiments on the effects of crude oil on the Red Sea coral Stylophora pistillata. Mar. Pollut. Bull. 10, 328-330 [Google Scholar]
- Ronquist F., Huelsenbeck J. P. (2003). MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19, 1572-1574 [DOI] [PubMed] [Google Scholar]
- Rougée L., Downs C. A., Richmond R. H., Ostrander G. K. (2006). Alteration of normal cellular profiles in the Scleractinian coral (Pocillopora damicornis) following laboratory exposure to fuel oil. Environ. Toxicol. Chem. 25, 3181-3187 [DOI] [PubMed] [Google Scholar]
- Rudy P., Jr, Rudy L. H. (1983). Oregon Estuarine Invertebrates. Charleston, OR: Oregon Institute of Marine Biology; [Google Scholar]
- Saco-Álvarez L., Bellas J., Nieto O., Bayona J. M., Albaigés J., Beiras R. (2008). Toxicity and phototoxicity of water-accommodated fraction obtained from Prestige fuel oil and Marine fuel oil evaluated by marine bioassays. Sci. Total Environ. 394, 275-282 [DOI] [PubMed] [Google Scholar]
- Salih A., Larkum A., Cox G., Kühl M., Hoegh-Guldberg O. (2000). Fluorescent pigments in corals are photoprotective. Nature 408, 850-853 [DOI] [PubMed] [Google Scholar]
- Sanderson J. T., Kennedy S. W., Giesy J. P. (1998). In vitro induction of ethoxyresorufin-o-deethylase and porphyrins by halogenated aromatic hydrocarbons in avian primary hepatocytes. Environ. Toxicol. Chem. 17, 2006-2018 [Google Scholar]
- Sanmartín-Suárez C., Soto-Otero R., Sánchez-Sellero I., Méndez-Álvarez E. (2011). Antioxidant properties of dimethyl sulfoxide and its viability as a solvent in the evaluation of neuroprotective antioxidants. J. Pharmacol. Toxicol. Methods 63, 209-215 [DOI] [PubMed] [Google Scholar]
- Shafir S., Van Rijn J., Rinkevich B. (2007). Short and long term toxicity of crude oil and oil dispersants to two representative coral species. Environ. Sci. Technol. 41, 5571-5574 [DOI] [PubMed] [Google Scholar]
- Shick J. M., Dunlap W. C. (2002). Mycosporine-like amino acids and related Gadusols: biosynthesis, acumulation, and UV-protective functions in aquatic organisms. Annu. Rev. Physiol. 64, 223-262 [DOI] [PubMed] [Google Scholar]
- Shinzato C., Hamada M., Shoguchi E., Kawashima T., Satoh N. (2012). The repertoire of chemical defense genes in the coral Acropora digitifera genome. Zoolog. Sci. 29, 510-517 [DOI] [PubMed] [Google Scholar]
- Souter P., Bay L. K., Andreakis N., Császár N., Seneca F. O., van Oppen M. J. H. (2011). A multilocus, temperature stress-related gene expression profile assay in Acropora millepora, a dominant reef-building coral. Mol. Ecol. Resour. 11, 328-334 [DOI] [PubMed] [Google Scholar]
- Stamatakis A. (2006). RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22, 2688-2690 [DOI] [PubMed] [Google Scholar]
- Starcevic A., Akthar S., Dunlap W. C., Shick J. M., Hranueli D., Cullum J., Long P. F. (2008). Enzymes of the shikimic acid pathway encoded in the genome of a basal metazoan, Nematostella vectensis, have microbial origins. Proc. Natl. Acad. Sci. USA 105, 2533-2537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Yin Y., Zhang J., Yu H., Wang X., Wu J., Xue Y. (2008). Hydroxyl radical generation and oxidative stress in Carassius auratus liver, exposed to pyrene. Ecotoxicol. Environ. Saf. 71, 446-453 [DOI] [PubMed] [Google Scholar]
- Sunagawa S., Wilson E. C., Thaler M., Smith M. L., Caruso C., Pringle J. R., Weis V. M., Medina M., Schwarz J. A. (2009). Generation and analysis of transcriptomic resources for a model system on the rise: the sea anemone Aiptasia pallida and its dinoflagellate endosymbiont. BMC Genomics 10, 258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Technau U., Steele R. E. (2011). Evolutionary crossroads in developmental biology: Cnidaria. Development 138, 1447-1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobit V., Verma O. P., Ramteke P. W., Ray R. S. (2011). Phototoxic assessment of polycyclic aromatic hydrocarbons by using NIH-3T3 and L-929 cell lines. J. AIDS Clinic. Res. 2, 123 [Google Scholar]
- Tytler E. M., Trench R. K. (1986). Activities of enzymes in b-carboxylation reactions and catalase in cell-free preparations from the symbiotic dinoflagellates Sybiodinium spp. from a coral, a clam, a zoanthid and two sea anemones. Proc. R. Soc. B 228, 483-492 [Google Scholar]
- Wei H., Cai Q., Rahn R., Zhang X. (1997). Singlet oxygen involvement in ultraviolet (254 nm) radiation-induced formation of 8-hydroxy-deoxyguanosine in DNA. Free Radic. Biol. Med. 23, 148-154 [DOI] [PubMed] [Google Scholar]
- White H. K., Xu L., Lima A. L. C., Eglinton T. I., Reddy C. M. (2005). Abundance, composition, and vertical transport of PAHs in marsh sediments. Environ. Sci. Technol. 39, 8273-8280 [DOI] [PubMed] [Google Scholar]
- Wolska L., Mechlinska A., Rogowska J., Namiesnik J. (2012). Sources and fate of PAHs and PCBs in the marine environment. Crit. Rev. Environ. Sci. Technol. 42, 1172-1189 [Google Scholar]
- Wong P. C., Waggoner D., Subramaniam J. R., Tessarollo L., Bartnikas T. B., Culotta V. C., Price D. L., Rothstein J., Gitlin J. D. (2000). Copper chaperone for superoxide dismutase is essential to activate mammalian Cu/Zn superoxide dismutase. Proc. Natl. Acad. Sci. USA 97, 2886-2891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo H. D., Kim B. M., Kim Y. J., Lee Y. J., Kang S. J., Cho Y. H., Choi J. Y., Chung H. W. (2008). Quercetin prevents necrotic cell death induced by co-exposure to benzo(a)pyrene and UVA radiation. Toxicol. In Vitro 22, 1840-1845 [DOI] [PubMed] [Google Scholar]
- Xia Q., Chiang H. M., Yin J. J., Chen S., Cai L., Yu H., Fu P. (2013). UVA photoirradiation of benzo[a]pyrene metabolites: induction of cytotoxicity, reactive oxygen species, and lipid peroxidation. Toxicol. Ind. Health doi:10.1177/0748233713484648 [DOI] [PubMed] [Google Scholar]
- Zámocký M., Gasselhuber B., Furtmüller P. G., Obinger C. (2012). Molecular evolution of hydrogen peroxide degrading enzymes. Arch. Biochem. Biophys. 525, 131-144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelko I. N., Mariani T. J., Folz R. J. (2002). Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic. Biol. Med. 33, 337-349 [DOI] [PubMed] [Google Scholar]
- Zuijdgeest A., Huettel M. (2012). Dispersants as used in response to the MC252-spill lead to higher mobility of polycyclic aromatic hydrocarbons in oil-contaminated Gulf of Mexico sand. PLoS ONE 7, e50549 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.