Abstract
Reef-building corals depend for much of their energy on photosynthesis by symbiotic dinoflagellate algae (genus Symbiodinium) that live within their gastrodermal cells. However, the cellular mechanisms underpinning this ecologically critical symbiosis, including those governing the specificity of symbiont uptake by the host, remain poorly understood, in part because of the difficulties of working with corals in the laboratory. Here, we used the small symbiotic sea anemone Aiptasia as an experimentally tractable model system to analyze the specificity and timing of symbiosis onset in larval and adult animals under controlled laboratory conditions. Using four clonal, axenic Symbiodinium strains, we found no difference in uptake specificity between larvae (even when very young) and adults. Although both compatible and incompatible algal strains were found within the larval guts, only the former appeared to be internalized by gastrodermal cells, and they (but not incompatible algae) proliferated rapidly within the larvae in the absence of detectable exchange with other larvae. Older larvae showed reduced ingestion of both compatible and incompatible algae, and the addition of food failed to promote the uptake of an incompatible algal strain. Thus, Aiptasia adults and larvae appear to have similar mechanisms for discriminating between compatible and incompatible dinoflagellate types prior to phagocytosis by host gastrodermal cells. Whether a particular algal strain is compatible or incompatible appears to be stable during years of axenic culture in the absence of a host. These studies provide a foundation for future analyses of the mechanisms of symbiont-uptake specificity in this emerging model system.
KEY WORDS: Anemone, Cnidarian, Coral reefs, Dinoflagellate, Planulae larvae, Symbiosis
INTRODUCTION
Endosymbiosis is a driving factor in evolution that weaves together diverse lineages and is fundamental to the lives of many thousands of species (Wernegreen, 2012). Perhaps the best-known marine endosymbiosis is that of corals and other cnidarians with the dinoflagellate algae (genus Symbiodinium) that live within their gastrodermal cells. This symbiosis is critical for the survival and growth of coral reefs because algal photosynthesis provides most of the energy for the coral animals (Yellowlees et al., 2008). Coral reefs are deteriorating rapidly worldwide (Hoegh-Guldberg et al., 2007), in large part because the coral–algal symbiosis can break down under environmental stress (‘coral bleaching’). However, the cellular and molecular mechanisms that govern this symbiosis and its breakdown remain largely unknown (Davy et al., 2012).
Symbiodinium strains have been categorized into nine major lineages, known as clades A–I (Pochon and Gates, 2010), and the relationships between the many different algal strains and their hosts are governed by complex specificities. Many corals can associate with multiple Symbiodinium strains (van Oppen et al., 2001; Apprill and Gates, 2007; Silverstein et al., 2012), and many Symbiodinium strains can infect some cnidarian species but not others (Lajeunesse et al., 2004; Coffroth et al., 2010). Most species of reef-building corals produce larvae without symbionts (aposymbiotic) and must acquire them anew from the environment in each generation (Baird et al., 2009). In addition, bleached adult corals may need to re-acquire symbionts from the surrounding seawater to survive (Lewis and Coffroth, 2004; Coffroth et al., 2006). There is considerable evidence that the stress resistance of a particular coral is determined, at least in part, by the intrinsic stress resistance of the Symbiodinium type that it contains (Baker et al., 2004; Sampayo et al., 2008). Thus, understanding the specificity patterns and mechanisms of symbiosis establishment and maintenance is critical to both basic coral biology and efforts at coral conservation.
The specificity and dynamics of Symbiodinium uptake have been studied in larvae of the temperate anemone Anthopleura elegantissima (Schwarz et al., 2002) and of the corals Fungia (Schwarz et al., 1999; Weis et al., 2001; Rodriguez-Lanetty et al., 2004; Rodriguez-Lanetty et al., 2006) and Acropora (Harii et al., 2009; Bay et al., 2011). In all cases, larvae readily took up some Symbiodinium types but not others, and, in at least some cases, they could take up a wider variety of algae than were found in adult conspecifics from the same population (Bay et al., 2011). Similarly, comparisons of the naturally occurring symbiont populations in newly settled juvenile polyps and nearby conspecific adults have found a greater variety of algae in the juveniles than in the adults (Coffroth et al., 2001; Little et al., 2004; Gómez-Cabrera et al., 2008; Abrego et al., 2009). Taken together, these data have led to the prevailing hypothesis that juvenile hosts are typically more permissive than their adult counterparts.
Studies of symbiont specificity in corals face several major limitations that include (i) the intrinsic difficulties both of field studies and of studies of corals in the laboratory, (ii) the typical availability of larvae only during the once-yearly spawning season, and (iii) the difficulty or impossibility of keeping adult colonies alive while they are rendered fully aposymbiotic in preparation for subsequent re-infection experiments (Coffroth et al., 2010). In addition, the use of wild populations introduces an unavoidable element of genetic heterogeneity that is problematic for detailed studies of mechanisms.
The small sea anemone Aiptasia provides a way to avoid these limitations, as it is closely related to corals and houses similar symbionts, yet is far more tractable in the laboratory (Weis et al., 2008). Aiptasia also has a growing array of resources available, including large clonal populations of both symbiotic and aposymbiotic adults (Sunagawa et al., 2009; Lehnert et al., 2012; Xiang et al., 2013), transcriptomes for both aposymbiotic (Lehnert et al., 2012) and symbiotic (Lehnert et al., 2014) animals, metabolomic information (Burriesci et al., 2012), and a method for obtaining regular spawning in the laboratory throughout the year (S. F. Perez and J.R.P., unpublished resutls). Aiptasia has been used previously to study symbiosis specificity in aposymbiotic adult polyps (Schoenberg and Trench, 1980; Belda-Baillie et al., 2002); as with corals, the animals selectively took up certain Symbiodinium strains but not others.
In this study, we used aposymbiotic adult polyps from a clonal Aiptasia line, the naturally aposymbiotic larvae derived by mating this line (male) to any of several clonal female lines, and four clonal, axenic strains of Symbiodinium (Xiang et al., 2013) to perform a tightly controlled comparison of symbiont specificity and accumulation in larvae and adults. For the Symbiodinium strains tested, we could observe no differences in specificity between larvae and adults, and compatible strains accumulated rapidly in both larvae and adults even in the absence of further exposure. These studies provide a foundation for future mechanistic studies of these phenomena.
RESULTS
Symbiosis specificity in Aiptasia adults
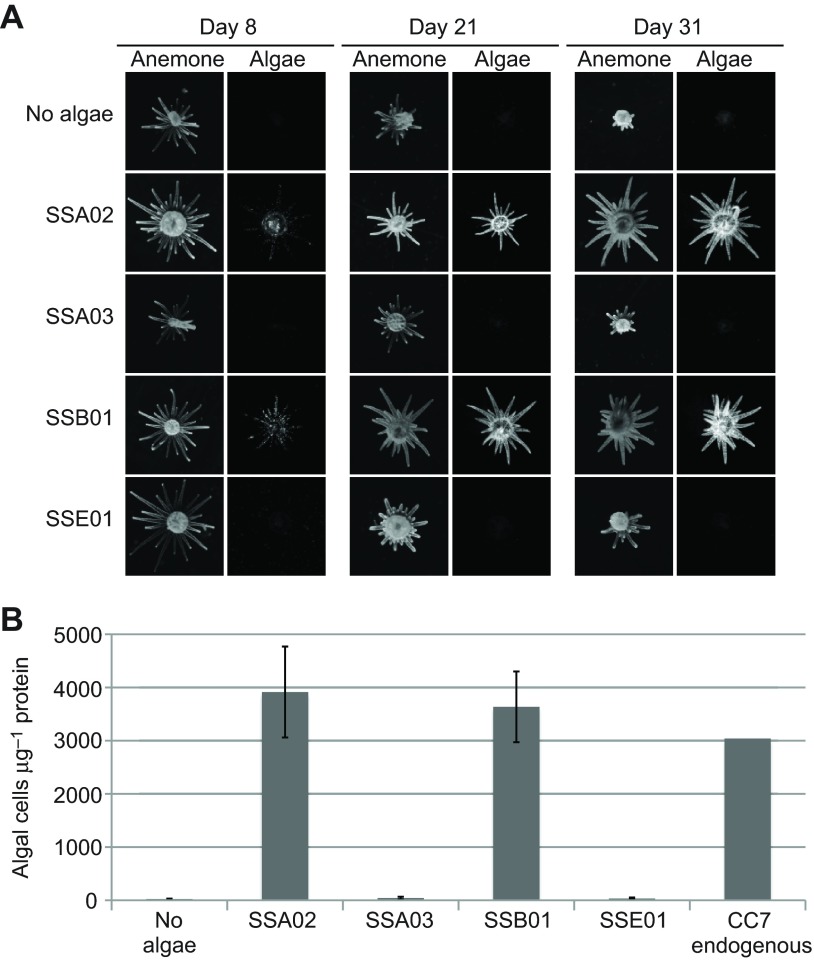
To examine the specificity of clonal CC7 Aiptasia adults for different Symbiodinium types, we exposed aposymbiotic animals to equal concentrations of four clonal, axenic algal strains (see Materials and methods). Strains SSA02 and SSB01 were taken up efficiently by the anemones, whereas strains SSA03 and SSE01 were not (Fig. 1A). Quantification of the algal populations of the anemones after 31 days of exposure showed that the compatible strains were present at concentrations similar to those of the endogenous clade A algae of stably infected CC7 adults that had been maintained for years under the same environmental conditions (Fig. 1B). The anemones were not fed during these experiments, and those containing compatible algae were larger and appeared healthier (stalks and tentacles more extended; more responsive to stimuli) than animals exposed only to incompatible algae (Fig. 1A); thus, it appears that the compatible algae begin contributing to the host's nutrition soon after uptake.
Fig. 1.
Symbiosis specificity in Aiptasia adults. In each of three experiments, aposymbiotic adults from clonal anemone line CC7 were incubated for 31 days in the absence of algae or in the presence of one of four clonal, axenic Symbiodinium strains, as indicated (see Materials and methods; 15 animals per treatment in each experiment). (A) At intervals, four randomly chosen anemones per treatment were photographed with the stereomicroscope using white light (Anemone) or a GFP filter set (to visualize the chlorophyll autofluorescence of any Symbiodinium present: Algae). Representative images are shown. (B) Quantification of Symbiodinium populations at day 31. In each experiment, all 15 animals for each treatment were pooled and homogenized. Algal number and total protein were then determined by Guava flow cytometry and the BCA assay (see Materials and methods). Means ± s.e.m. (N=3). Separately, two individual CC7 animals stably populated with their endogenous algae were homogenized and analyzed in the same way, yielding 3122 and 2959 algal cells μg−1 protein.
Symbiosis specificity in Aiptasia larvae
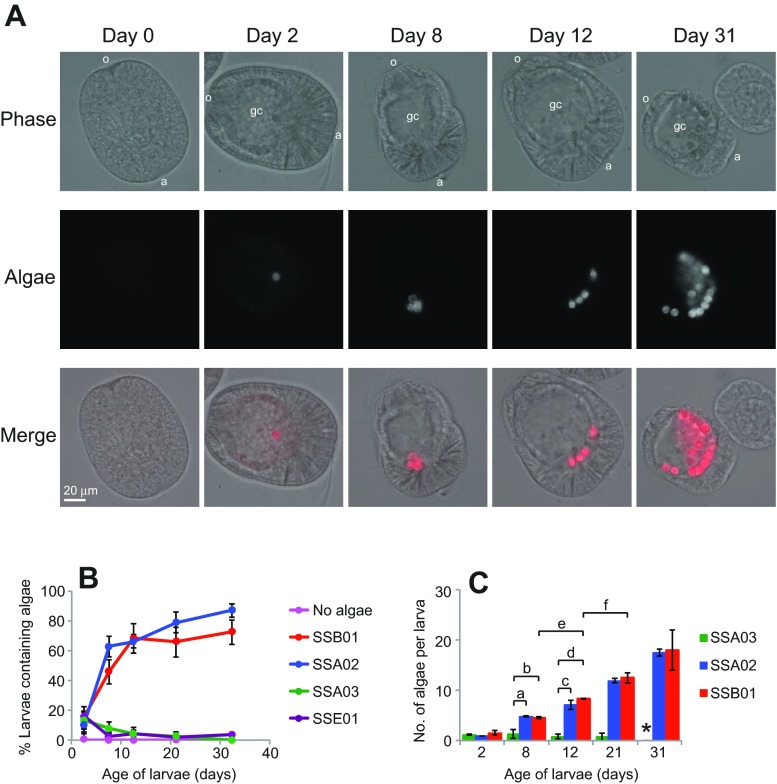
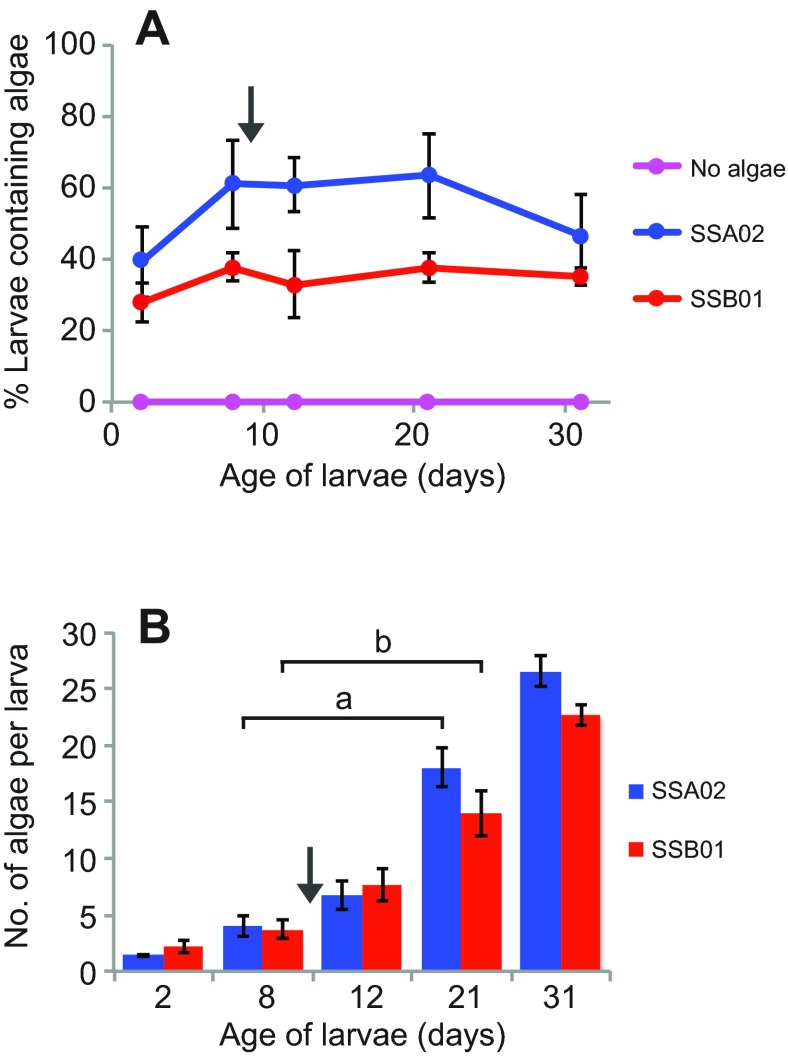
We next examined the specificity of the naturally aposymbiotic planula larvae (Fig. 2A, day 0) for the same Symbiodinium types; in the initial experiments, fresh algae were added with every water change (see Materials and methods). As in the adults, strains SSA02 and SSB01 were taken up readily: both the percentage of larvae containing algae and the number of algal cells per larva increased steadily over time (Fig. 2A–C). In contrast, strains SSA03 and SSE01 were never found in more than a small percentage of the larvae (Fig. 2B) or as more than one or two algal cells per larva (Fig. 2C). The data in Fig. 2 show that larvae discriminated strongly between compatible and incompatible algae by 8 days after fertilization, and even younger larvae also appeared capable of such discrimination. In experiments similar to but distinct from those of Fig. 2, we examined larvae at either 5 days or 4 days. At 5 days, 57 of 127 larvae (45%) exposed to the compatible strains appeared to contain algae, in contrast to only 13 of 84 larvae (15%) exposed to the incompatible strains. At 4 days, the corresponding numbers were 28 of 88 (32%) and 2 of 47 (4%). The differences were significant on both days with P<0.01 (binomial exact tests).
Fig. 2.
Symbiosis specificity in Aiptasia larvae. In each of three experiments, naturally aposymbiotic Aiptasia larvae (<1 day post-fertilization) were incubated for 31 days in the absence of algae or in the presence of one of four clonal, axenic Symbiodinium strains, as indicated (see Materials and methods). (A) Larvae exposed to strain SSA02 were sampled at intervals and imaged using an epifluorescence microscope to visualize overall structure (Phase) and endogenous chlorophyll autofluorescence (Algae; red in Merge). o, oral region; a, aboral region; gc, gastric cavity. Scale bar applies to all panels. (B,C) Quantification of Symbiodinium uptake as determined by epifluorescence microscopy as in A; for these counts, no attempt was made to distinguish algae in the gastric cavity from those within the gastrodermal cells. Means ± s.e.m. from the three experiments are shown. (B) Percentage of larvae that contained ≥1 algal cell. t-tests showed that the differences between compatible and incompatible strains at 8 days were significant (P-values of 0.0005, 0.005, 0.007 and 0.03 for the four comparisons). Neither the difference between the two compatible strains nor that between the two incompatible strains was significant. (C) Number of algal cells per larva in the larvae containing one or more algal cells; larvae containing no visible algal cells were not scored. Number of larvae scored: strain SSA03, 5–13 per time point; SSA02 and SSB01, 17–85 per strain per time point. *No larvae containing algae were observed. t-tests showed both that the number of algae per larva was significantly different for the compatible and incompatible algae from 8 days onward (P-values for the indicated comparisons are a, 0.01; b, 0.025; c, 0.0001; and d, 0.0001) and that the number of compatible algae per larva increased significantly over time (P-values for the indicated comparisons are e, 0.02 and f, 0.002).
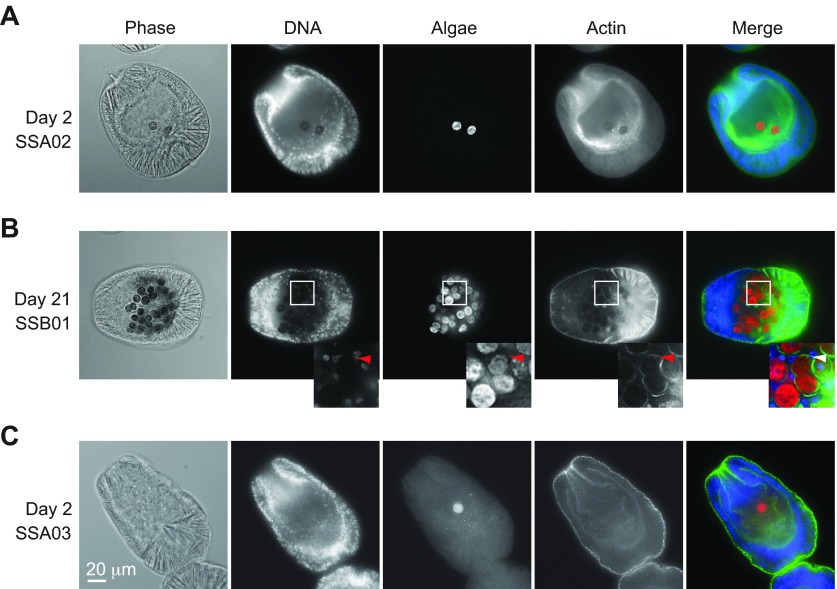
In unstained samples, compatible algal cells generally appeared to be either within the gastrodermal cells or apposed to the apical surfaces of these cells (Fig. 2A), whereas the incompatible algae generally appeared to be simply within the gut. To examine this question more closely, we stained larvae both with a DNA stain (to reveal the host cell nuclei) and with fluorescently tagged phalloidin (to stain actin and thus reveal the host cell cytoplasm). At both early times (Fig. 3A) and late (Fig. 3B), the compatible algae were almost always close to or within the gastrodermal cells: note the bright rims of phalloidin-stained host cytoplasm and/or plasma membrane surrounding the algal cells, as seen particularly clearly around the nucleus and the pair of algal cells – or dividing algal cell – in the region of the inset (Fig. 3B). In contrast, in 64 larvae observed that contained cells of incompatible algal strain SSA03 (37 stained with Hoechst plus phalloidin, 27 stained with Hoechst only), no examples were found of algal cells that appeared to be within the gastrodermal cells (Fig. 3C and data not shown).
Fig. 3.
Localization of algae in Aiptasia larvae. Phase-contrast (Phase) and deconvolved fluorescence images (see Materials and methods) of larvae exposed to compatible (A,B) or incompatible (C) algal types as in Fig. 2. DNA, Hoechst staining of nuclei (blue in Merge); Algae, chlorophyll autofluorescence of algal cells (red in Merge); Actin, staining of host cell cytoplasmic actin with AlexaFluor 488-conjugated phalloidin (green in Merge). (A,B) Accumulation of compatible algae within the gastrodermal cells of the host. Inset, a region in which a host nucleus (arrowheads) and two algal cells (or one dividing cell) can be seen surrounded by phalloidin-stained host cytoplasm. (C) Presence of incompatible algae within the gastric cavity but not the gastrodermal cells. Scale bar applies to all panels.
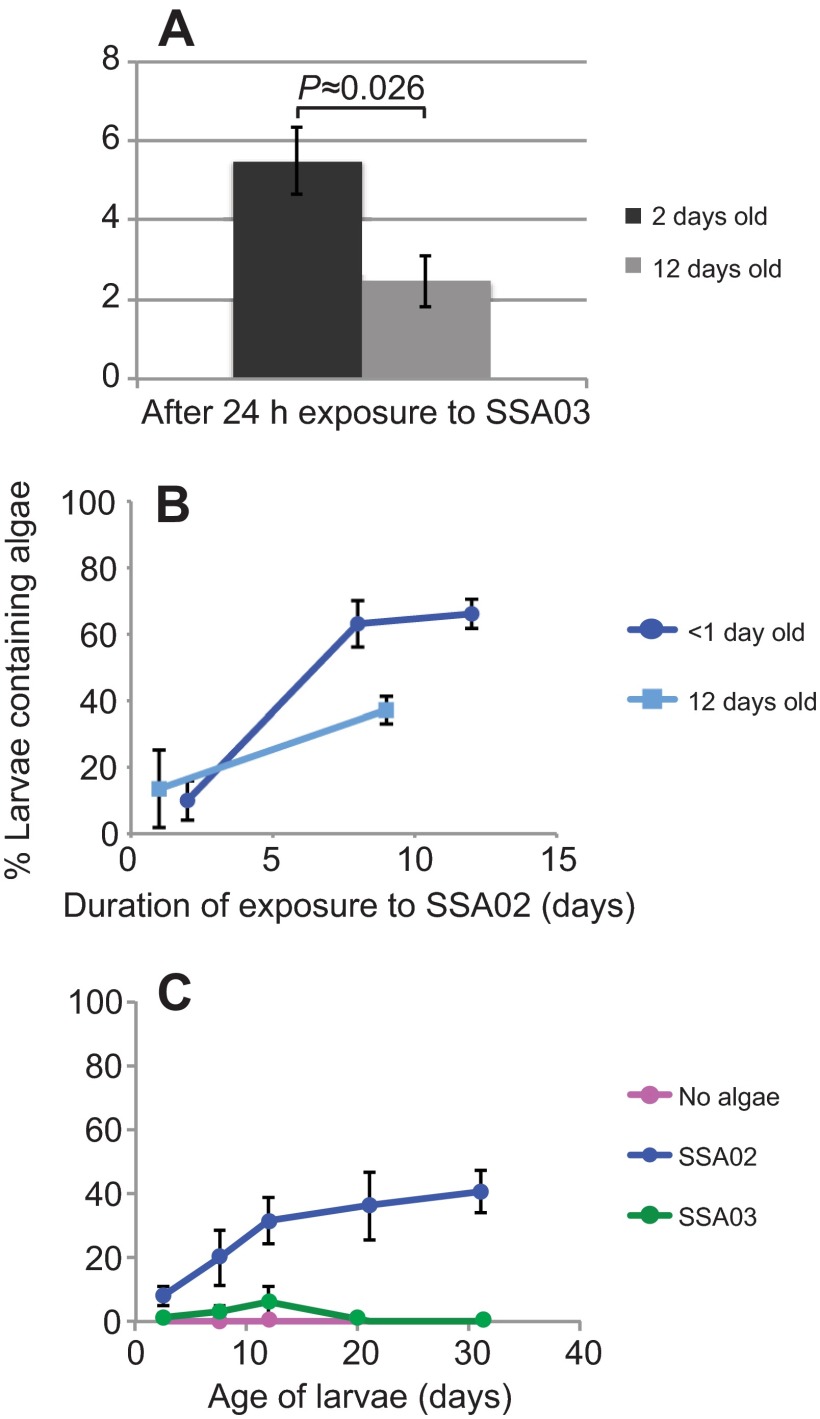
Of the young larvae exposed to the incompatible Symbiodinium strains, 10–15% contained one or two algal cells (Fig. 2B, 2 day time point), but larvae containing incompatible algae were rarely seen at later times despite the presence of algae in the environment throughout these experiments. These data initially suggested that older larvae might have an increased ability to discriminate between compatible and incompatible algae and to avoid ingesting the latter (or evict them more quickly). Indeed, when we allowed larvae to age in the absence of algae and then exposed them for 24 h to an incompatible algal type, fewer of them contained algae than when young larvae were given a similar exposure (Fig. 4A). However, in a similar experiment performed with a compatible algal strain, the older larvae also appeared to take up the algae more slowly (Fig. 4B), suggesting that the observations with the incompatible algae may reflect a general slowdown in algal ingestion in older larvae rather than an improved discrimination against incompatible algal types.
Fig. 4.
Reduced uptake of algae in older larvae or in the presence of food. Except as noted, all panels show the means ± s.e.m. from three replicate experiments. (A) Freshly collected larvae (<1 day old) were held in the absence of algae for 2 days (to allow their mouths to develop) or 12 days, exposed to an incompatible algal strain for 24 h, and scored by epifluorescence microscopy for the percentage that contained algal cells (as in Fig. 2). (B) Larvae that had been held in the absence of algae for 12 days were exposed for the indicated periods to a compatible algal strain and scored as in A for the percentage that contained algal cells; means ± s.d. are shown for two replicate experiments. For comparison, similar data for freshly collected larvae (re-plotted from Fig. 2B) are also shown. The younger larvae appeared to have taken up more algae by 8 days than the older larvae had by 9 days (P≈0.07). (C) Freshly collected larvae were exposed to compatible and incompatible algal types as in Fig. 2 but with food (homogenized brine shrimp) added together with each addition of algae (see Materials and methods). In contrast to the experiment of Fig. 2B, the apparent difference in uptake of the compatible and incompatible strains was not significant at 8 days (P≈0.1), and convincing differences did not appear until later (12 days, P≈0.04; 21 days, P≈0.03).
Food particles appear to enhance symbiont uptake in larvae of the corals Fungia scutaria and Acropora digitifera (e.g. Schwarz et al., 1999), presumably by stimulating a general feeding behavior. To test for a similar effect in Aiptasia larvae, and to ask whether feeding might promote the uptake (at least transiently) of otherwise incompatible algal types, we exposed larvae to compatible and incompatible Symbiodinium strains together with homogenized brine shrimp. Surprisingly, the presence of food appeared to reduce the uptake of both of the algal types tested (Fig. 4C, cf. Fig. 2B).
Algal proliferation in Aiptasia larvae
The results above suggested that compatible algae proliferated within larvae after they had been taken up, but this conclusion was uncertain because of the addition of fresh algae with each water change. Thus, we performed a wash-out experiment in which young larvae were exposed to compatible algal cells for 8 days and then washed and incubated further in the absence of added algae. After the wash-out, the percentage of larvae containing algae remained essentially constant (Fig. 5A), suggesting that there was little or no exchange of algae with the environment. However, the number of algae per infected larva increased steadily (Fig. 5B), showing that compatible algae indeed proliferate actively in the larvae. Similar proliferation in the absence of added algae is observed in re-infected adults (E.A.H., A.G. and J.R.P., unpublished results).
Fig. 5.
Proliferation of compatible algae in larvae. In each of three experiments, freshly collected larvae (<1 day old) were exposed to compatible algal strains for 8 days, at which time larvae were transferred to algae-free filter-sterilized artificial seawater (FASW, arrow indicates algae wash-out) and maintained without added algae for the duration of the experiment. At the indicated times, the percentage of larvae containing ≥1 algal cell (A) and the number of algal cells in the larvae containing ≥1 algal cell were scored as in Fig. 2. Means ± s.e.m. for the three experiments are shown; the number of larvae scored in B was 21–63 per strain per time point. Significant increases in algal number could be seen for both algal strains between 8 and 21 days (a, P≈0.01; b, P≈0.02).
DISCUSSION
We exposed aposymbiotic adult and larval Aiptasia to four clonal Symbiodinium strains representing three of the nine major clades; the algal strains had been grown in axenic culture for ≥1.5 years. The adult Aiptasia were from a clonal strain, and the larvae resulted from crosses between this strain and several maternal lines of unknown degrees of relatedness to the male line and to each other. Two of the Symbiodinium strains reproducibly infected both the adults and the larvae, whereas the other two did not. Thus, the compatibility of a particular Symbiodinium strain with a particular host is (or at least can be) a genetically determined characteristic that can survive many generations of growth apart from any host. Although the determinants of compatibility are not yet clear, the available evidence suggests that the interaction of Symbiodinium cell-surface glycans with host lectins may be important (Wood-Charlson et al., 2006; Davy et al., 2012), and the glycan profiles of several Symbiodinium strains indeed appear to be stable over multiple generations (Logan et al., 2010). It remains to be determined whether recent prior growth in a particular host can affect the subsequent compatibility of an algal strain with the same or other hosts. Importantly, the compatibility of a strain did not closely track its cladal identity, as the two compatible strains were from clades A and B, whereas the other clade A strain tested was incompatible with this host. This observation is not surprising given the substantial phylogenetic diversity within the major Symbiodinium clades (Lajeunesse et al., 2012).
The identical specificities of uptake observed for larvae (even very young larvae) and adults indicate that these life-cycle stages possess similar machineries for distinguishing among algal types; determining whether these machineries are identical or not will require testing a wider range of Symbiodinium strains and/or actually elucidating the molecular mechanisms involved in discrimination. Although our initial observations suggested that older larvae might be more discriminating than younger ones at the stage of initial algal ingestion (see below), further investigation suggested that the older larvae were simply less active in ingesting algae of any type. Our results contrast with several previous reports suggesting that coral larvae and juvenile polyps are less specific than adults in Symbiodinium uptake (Coffroth et al., 2001; Little et al., 2004; Rodriguez-Lanetty et al., 2004; Gómez-Cabrera et al., 2008; Abrego et al., 2009; Bay et al., 2011). This discrepancy may simply reflect differences in the behavior of the different host species or the limited variety of Symbiodinium strains that we have tested to date. However, it may also reflect the differences in experimental protocols. In the previous studies, the infecting algae either were those present naturally in the environment or were freshly isolated from a host animal. Thus, the results obtained may have reflected the presence of a variety of Symbiodinium types in the environment (perhaps including types with marginal compatibility with the hosts tested), environmental factors (such as the recent presence in a host) that conferred limited compatibility on an otherwise incompatible algal type, and/or other factors. Such complicating factors may, of course, play roles in governing Symbiodinium uptake by hosts in nature. However, our simplified protocol, using pairwise combinations of genetically homogeneous algae and hosts, should provide a more tractable system for elucidating the molecular mechanisms underlying the discrimination between compatible and incompatible algal strains.
In stable cnidarian–dinoflagellate symbioses, the algae reside within the gastrodermal cells of the host (Colley and Trench, 1983; Schwarz et al., 1999). Accordingly, we observed that cells of the two compatible algal strains were found within the gastrodermal cells of Aiptasia larvae. Moreover, these algae were observed to proliferate within the larvae after the removal of exogenous algae, although it remains unclear whether this proliferation involves division of algae within host cells accompanied or followed by division of those host cells, release of algal progeny into the gastric cavity followed by rapid uptake by other gastrodermal cells, or both. In contrast, incompatible algal cells that had been ingested were always found within the larval gastric cavity, but never within the gastrodermal cells. Although we cannot rule out the possibility that such cells are phagocytosed but then rapidly recognized as incompatible and evicted by exocytosis, these observations suggest that the ‘winnowing’ step (Nyholm and McFall-Ngai, 2004) of this symbiosis occurs at the level of phagocytosis, with only compatible cells actually being taken up by the host gastrodermal cells. We also found that the gastrodermal cells containing algae appeared to be concentrated in the aboral half of the gastric cavity (see Fig. 2A, Fig. 3A,B). These observations are similar to those made with coral larvae, where incompatible algae were scattered throughout the gastric cavity, but compatible algae were largely found in the larval equatorial plane (Rodriguez-Lanetty et al., 2006). Taken together, the data suggest that the gastrodermal cells in specific regions may be specialized for phagocytosis of Symbiodinium.
In a surprising contrast to several previous studies (Schwarz et al., 2002; Harii et al., 2009), we found that addition of food slowed, rather than accelerated, the acquisition of symbionts. Although the reasons for this discrepancy remain unclear, it seems likely that the presence of food (particularly at high concentrations) affects symbiosis establishment in complex ways, so that it is a complicating factor probably best avoided in future studies of the mechanisms underlying symbiosis establishment in cnidarians.
The model system described here should facilitate future studies of the specificity and dynamics of symbiont uptake and thus help to define the timing of host–symbiont signaling events, an important step in the molecular dissection of these signals. From an ecological perspective, it should also help to clarify the biological consequences of symbiosis establishment in larval stages, for example by comparing life-span and vitality parameters (such as protein content or growth rate) between larvae with and without compatible symbionts. The availability of two genetically distant but host-compatible Symbiodinium strains for the Aiptasia system should allow more precise evaluation than has previously been possible of the metabolic costs and benefits of harboring different symbiont types. Obtaining a deeper understanding of the parameters governing successful symbiosis establishment by cnidarian larvae has important implications for efforts to promote coral survival in the increasingly volatile global oceans.
MATERIALS AND METHODS
Organisms and culture conditions
Experiments with adult Aiptasia used animals of clonal line CC7 (Sunagawa et al., 2009) that were maintained in artificial sea water (ASW) as described previously (Xiang et al., 2013). Animals either contained their endogenous clade A Symbiodinium or had been rendered aposymbiotic as described previously (Xiang et al., 2013). Larvae were obtained using a spawning protocol to be described elsewhere (S. F. Perez and J.R.P., unpublished results); each small tub contained one large CC7 animal (male) and one large female (from one of several clonal lines). Spawning occurred during the night, and the aposymbiotic planula larvae (~100 μm in diameter) were collected in the morning, cleaned by rinsing with filter-sterilized ASW (FASW) on a cell strainer with a 40 μm mesh (BD Falcon, Franklin Lakes, NJ, USA), placed into sterile 6-well cell culture plates (BD Falcon) with 200–300 larvae in 5 ml FASW per well, and maintained at 27°C and 25 μmol photons m−2 s−1 light on a 12 h light:12 h dark cycle.
Clonal, axenic cultures of Symbiodinium strains SSA02, SSA03, SSB01 and SSE01 (in clades A, A, B and E, respectively) (see Xiang et al., 2013) were maintained in IMK medium (Ishikura et al., 2004) at 27°C and 25 μmol photons m−2 s−2 light on a 12 h light:12 h dark cycle, as described previously (Xiang et al., 2013).
Determination of algal cell number
For adjustment of inoculum size for infection experiments, we determined approximate algal number with a hemocytometer. For experiments with adults, we used a Guava flow cytometer (Millipore, Billerica, MA, USA) as described previously (Xiang et al., 2013). For experiments with larvae, we used direct microscopic counts (see below) of red-fluorescent cells. Protein concentrations of homogenates were determined with the BCA Assay (Thermo Fisher, Waltham, MA, USA) according to the manufacturer's protocol; absorbance readings of the reactions were taken on an Infinite Pro spectrophotometer (Tecan, Maennedorf, Switzerland).
Infection experiments
Prior to infection experiments, algae were gently rinsed by centrifugation three times at 1000 g in FASW and quantified (see above). Both adult and larval Aiptasia were exposed to algae at a final concentration of ~104 algal cells ml−1, and FASW was used as a negative control.
For infection of adults, small aposymbiotic polyps (1–2 mm in oral-disk diameter) were starved for 2 weeks, distributed into sterile 6-well cell culture plates (~15 anemones in 5 ml FASW per well), and held at 27°C and 25 μmol photons m−2 s−1 light on a 12 h light:12 h dark cycle. A few hours after transfer, rinsed algae (or FASW) were added and mixed with the animals by gentle agitation with a clean pipette. Water and algae were replaced every 7 days, with mixing as above, to maintain water quality and consistent algal exposure; no feeding was performed for the duration of the experiment. At intervals, six random anemones were removed from each well, rinsed gently with FASW, placed in FASW in a sterile Petri dish, photographed using the stereo-fluorescence microscope (see below), and returned to the culture plates. After photography on day 31, the animals from each well were pooled in 250 μl of 0.01% SDS (Sigma-Aldrich, St Louis, MO, USA) in distilled water, homogenized for 15 s with a hand-held homogenizer (PowerGen, Fisher Scientific, Waltham, MA, USA), and homogenized further by repeated passages through a sterile 25-gauge needle. Algal numbers and total protein were then determined as described above.
Except as noted, infection experiments with larvae were performed by adding rinsed algae (or FASW) to larvae in the 6-well dishes (see above) on the day of collection (day 0) and mixing by gentle agitation with a clean pipette. Water and algae were replaced every 7 days by transferring individual larvae into fresh wells using a plastic transfer pipette and the stereomicroscope (see below), with mixing as above, to maintain water quality and consistent algal exposure. For sampling, ≥30 random larvae were collected from each well and placed into 1 ml of 3.7% formaldehyde in FASW at 4°C.
In one experiment, we examined the effect of food on algal uptake by adding brine-shrimp homogenate (Lenhoff, 1983) to the wells with each addition of algae. Fresh Artemia nauplii were homogenized for 30 s with the hand-held homogenizer, and portions of the homogenate were added to each well. In another experiment, we tested the effect of larval age on algal uptake by holding larvae without algal exposure or feeding for various periods before adding algae. In a final experiment, we tested whether algal proliferation occurred in the larvae by adding compatible algae to larvae on day 0 as usual, transferring the larvae after 8 days of exposure into fresh wells containing FASW without algae, and continuing to sample at intervals as in the other experiments.
Except where noted, statistical tests were Student's two-tailed t-tests.
Microscopy
Adult anemones were observed and photographed using a model MZ16FA stereo-fluorescence microscope and model DFC500 camera (Leica, Wetzlar, Germany). The same camera settings were used for all images, and exposure times were identical for all images taken on a given day.
To observe and photograph larvae, fixed larvae (see above) were adhered onto glass slides that had been coated with poly-l-lysine (Sigma-Aldrich). The larvae were rinsed with Abdil (Tris-buffered saline, pH 7.4, containing 0.1% Triton-X100, 2% BSA and 0.1% sodium azide), and larval and algal nuclei were stained by incubation with Hoechst 33258 in Abdil (10 μg ml−1) for 10 min at room temperature. In some cases, larval actin was also stained by incubating slides with 33 nmol l−1 Alexa Fluor 488-phalloidin (Invitrogen, Carlsbad, CA, USA) in Abdil for 1 h at room temperature. Larvae were covered with mounting medium (20 mmol l−1 Tris, pH 8.8, 90% glycerol, 0.5% p-phenylenediamine) and sealed under a glass coverslip.
To determine the number of algae in larvae, samples were counted by eye using a Nikon Eclipse 80i microscope with a Nikon Plan Fluor 40× dry lens and Sedat Quad filter set (Chroma Technology, Bellows Falls, VT, USA) using the Texas Red channel. All larvae on each slide were scored, and algal cells were counted if they appeared to be anywhere inside the larva (i.e. either in the gut cavity or within the gastrodermal cells). When desired, images were also captured using a CoolSnapHQ charge-coupled device camera (Photometrics, Tucson, AZ, USA). To distinguish algae within the gut cavity from those within the gastrodermal cells, larvae stained with Hoechst and phalloidin were imaged using an Olympus Apo/340 40× oil-immersion lens on an Olympus IX70 microscope equipped with a Deltavision Core system (Applied Precision, Issaquah, WA, USA), a Sedat Quad filter set (Semrock, Rochester, NY, USA) and a CoolSnapHQ camera. The softWoRx 4.1.0 software package (Applied Precision) was used for microscope control and image deconvolution.
ACKNOWLEDGEMENTS
We thank Aaron Straight and Colin Fuller for support, discussions, and access to microscopes; Santiago Perez and Cawa Tran for providing Aiptasia larvae; Tingting Xiang and Arthur Grossman for help with Symbiodinium cultures; Julia Salzman for help with statistical tests; and other members of our laboratories for their support and helpful discussions.
FOOTNOTES
Competing interests
The authors declare no competing financial interests.
Funding
This study was supported by the Gordon and Betty Moore Foundation [grant no. 2629], a National Science Foundation EAGER award [award no. IOS-1138275], a National Science Foundation Graduate Research Fellowship to E.A.H., National Institutes of Health Training Grant 5 T32 HG000044, and a German Research Foundation (DFG) Emmy Noether Programme award to A.G. [grant no. GU 1128/3-1]. Deposited in PMC for release after 12 months.
References
- Abrego D., van Oppen M. J. H., Willis B. L. (2009). Highly infectious symbiont dominates initial uptake in coral juveniles. Mol. Ecol. 18, 3518-3531 [DOI] [PubMed] [Google Scholar]
- Apprill A. M., Gates R. D. (2007). Recognizing diversity in coral symbiotic dinoflagellate communities. Mol. Ecol. 16, 1127-1134 [DOI] [PubMed] [Google Scholar]
- Baird A. H., Guest J. R., Willis B. L. (2009). Systematic and biogeographical patterns in the reproductive biology of scleractinian corals. Annu. Rev. Ecol. Evol. Syst. 40, 551-571 [Google Scholar]
- Baker A. C., Starger C. J., McClanahan T. R., Glynn P. W. (2004). Coral reefs: corals' adaptive response to climate change. Nature 430, 741 [DOI] [PubMed] [Google Scholar]
- Bay L. K., Cumbo V. R., Abrego D., Kool J. T., Ainsworth T. D., Willis B. L. (2011). Infection dynamics vary between Symbiodinium types and cell surface treatments during establishment of endosymbiosis with coral larvae. Diversity 3, 356-374 [Google Scholar]
- Belda-Baillie C. A., Baillie B. K., Maruyama T. (2002). Specificity of a model cnidarian-dinoflagellate symbiosis. Biol. Bull. 202, 74-85 [DOI] [PubMed] [Google Scholar]
- Burriesci M. S., Raab T. K., Pringle J. R. (2012). Evidence that glucose is the major transferred metabolite in dinoflagellate–cnidarian symbiosis. J. Exp. Biol. 215, 3467-3477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffroth M. A., Santos S. R., Goulet T. L. (2001). Early ontogenetic expression of specificity in a cnidarian-algal symbiosis. Mar. Ecol. Prog. Ser. 222, 85-96 [Google Scholar]
- Coffroth M. A., Lewis C. F., Santos S. R., Weaver J. L. (2006). Environmental populations of symbiotic dinoflagellates in the genus Symbiodinium can initiate symbioses with reef cnidarians. Curr. Biol. 16, R985-R987 [DOI] [PubMed] [Google Scholar]
- Coffroth M. A., Poland D. M., Petrou E. L., Brazeau D. A., Holmberg J. C. (2010). Environmental symbiont acquisition may not be the solution to warming seas for reef-building corals. PLoS ONE 5, e13258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colley N. J., Trench R. K. (1983). Selectivity in phagocytosis and persistence of symbiotic algae in the scyphistoma stage of the jellyfish Cassiopeia xamachana. Proc. R. Soc. B 219, 61-82 [DOI] [PubMed] [Google Scholar]
- Davy S. K., Allemand D., Weis V. M. (2012). Cell biology of cnidarian-dinoflagellate symbiosis. Microbiol. Mol. Biol. Rev. 76, 229-261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Cabrera M. C., Ortiz J. C., Loh W. K. W., Ward S., Hoegh-Guldberg O. (2008). Acquisition of symbiotic dinoflagellates (Symbiodinium) by juveniles of the coral Acropora longicyathus. Coral Reefs 27, 219-226 [Google Scholar]
- Harii S., Yasuda N., Rodriguez-Lanetty M., Irie T., Hidaka M. (2009). Onset of symbiosis and distribution patterns of symbiotic dinoflagellates in the larvae of scleractinian corals. Mar. Biol. 156, 1203-1212 [Google Scholar]
- Hoegh-Guldberg O., Mumby P. J., Hooten A. J., Steneck R. S., Greenfield P., Gomez E., Harvell C. D., Sale P. F., Edwards A. J., Caldeira K., et al. (2007). Coral reefs under rapid climate change and ocean acidification. Science 318, 1737-1742 [DOI] [PubMed] [Google Scholar]
- Ishikura M., Hagiwara K., Takishita K., Haga M., Iwai K., Maruyama T. (2004). Isolation of new Symbiodinium strains from tridacnid giant clam (Tridacna crocea) and sea slug (Pteraeolidia ianthina) using culture medium containing giant clam tissue homogenate. Mar. Biotechnol. (NY) 6, 378-385 [DOI] [PubMed] [Google Scholar]
- Lajeunesse T. C., Thornhill D. J., Cox E. F., Stanton F. G., Fitt W. K., Schmidt G. W. (2004). High diversity and host specificity observed among symbiotic dinoflagellates in reef coral communities from Hawaii. Coral Reefs 23, 596-603 [Google Scholar]
- Lajeunesse T. C., Parkinson J. E., Reimer J. D. (2012). A genetics-based description of Symbiodinium minutum sp. nov. and S. psygmophilum sp. nov. (Dinophyceae), two dinoflagellates symbiotic with Cnidaria. J. Phycol. 48, 1380-1391 [DOI] [PubMed] [Google Scholar]
- Lehnert E. M., Burriesci M. S., Pringle J. R. (2012). Developing the anemone Aiptasia as a tractable model for cnidarian-dinoflagellate symbiosis: the transcriptome of aposymbiotic A. pallida. BMC Genomics 13, 271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnert E. M., Mouchka M. E., Burriesci M. S., Gallo N. D., Schwarz J. A., Pringle J. R. (2014). Extensive differences in gene expression between symbiotic and aposymbiotic cnidarians. G3 (Bethseda) 4, 277-295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenhoff H. M. (1983). Hydra: Research Methods. New York, NY: Plenum Press; [Google Scholar]
- Lewis C. L., Coffroth M. A. (2004). The acquisition of exogenous algal symbionts by an octocoral after bleaching. Science 304, 1490-1492 [DOI] [PubMed] [Google Scholar]
- Little A. F., van Oppen M. J. H., Willis B. L. (2004). Flexibility in algal endosymbioses shapes growth in reef corals. Science 304, 1492-1494 [DOI] [PubMed] [Google Scholar]
- Logan D. D. K., LaFlamme A. C., Weis V. M., Davy S. K. (2010). Flow-cytometric characterization of the cell-surface glycans of symbiotic dinoflagellates (Symbiodinium spp.). J. Phycol. 46, 525-533 [Google Scholar]
- Nyholm S. V., McFall-Ngai M. J. (2004). The winnowing: establishing the squid-vibrio symbiosis. Nat. Rev. Microbiol. 2, 632-642 [DOI] [PubMed] [Google Scholar]
- Pochon X., Gates R. D. (2010). A new Symbiodinium clade (Dinophyceae) from soritid foraminifera in Hawai'i. Mol. Phylogenet. Evol. 56, 492-497 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Lanetty M., Krupp D. A., Weis V. M. (2004). Distinct ITS types of Symbiodinium in clade C correlate with cnidarian/dinoflagellate specificity during onset of symbiosis. Mar. Ecol. Prog. Ser. 275, 97-102 [Google Scholar]
- Rodriguez-Lanetty M., Wood-Charlson E. M., Hollingsworth L. L., Krupp D. A., Weis V. M. (2006). Temporal and spatial infection dynamics indicate recognition events in the early hours of a dinoflagellate/coral symbiosis. Mar. Biol. 149, 713-719 [Google Scholar]
- Sampayo E. M., Ridgway T., Bongaerts P., Hoegh-Guldberg O. (2008). Bleaching susceptibility and mortality of corals are determined by fine-scale differences in symbiont type. Proc. Natl. Acad. Sci. USA 105, 10444-10449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenberg D. A., Trench R. K. (1980). Genetic variation in Symbiodinium (=Gymnodinium) microadriaticum Freudenthal, and specificity in its symbiosis with marine invertebrates. III. Specificity and infectivity of Symbiodinium microadriaticum. Proc. R. Soc. B 207, 445-460 [Google Scholar]
- Schwarz J. A., Krupp D. A., Weis V. M. (1999). Late larval development and onset of symbiosis in the scleractinian coral Fungia scutaria. Biol. Bull. 196, 70-79 [DOI] [PubMed] [Google Scholar]
- Schwarz J. A., Weis V. M., Potts D. C. (2002). Feeding behavior and acquisition of zooxanthellae by planula larvae of the sea anemone Anthopleura elegantissima. Mar. Biol. 140, 471-478 [Google Scholar]
- Silverstein R. N., Correa A. M. S., Baker A. C. (2012). Specificity is rarely absolute in coral-algal symbiosis: implications for coral response to climate change. Proc. Biol. Sci. 279, 2609-2618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunagawa S., Wilson E. C., Thaler M., Smith M. L., Caruso C., Pringle J. R., Weis V. M., Medina M., Schwarz J. A. (2009). Generation and analysis of transcriptomic resources for a model system on the rise: the sea anemone Aiptasia pallida and its dinoflagellate endosymbiont. BMC Genomics 10, 258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oppen M. J. H., Palstra F. P., Piquet A. M., Miller D. J. (2001). Patterns of coral-dinoflagellate associations in Acropora: significance of local availability and physiology of Symbiodinium strains and host-symbiont selectivity. Proc. Biol. Sci. 268, 1759-1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis V. M., Reynolds W. S., deBoer M. D., Krupp D. A. (2001). Host-symbiont specificity during onset of symbiosis between the dinoflagellates Symbiodinium spp. and planula larvae of the scleractinian coral Fungia scutaria. Coral Reefs 20, 301-308 [Google Scholar]
- Weis V. M., Davy S. K., Hoegh-Guldberg O., Rodriguez-Lanetty M., Pringle J. R. (2008). Cell biology in model systems as the key to understanding corals. Trends Ecol. Evol. 23, 369-376 [DOI] [PubMed] [Google Scholar]
- Wernegreen J. J. (2012). Endosymbiosis. Curr. Biol. 22, R555-R561 [DOI] [PubMed] [Google Scholar]
- Wood-Charlson E. M., Hollingsworth L. L., Krupp D. A., Weis V. M. (2006). Lectin/glycan interactions play a role in recognition in a coral/dinoflagellate symbiosis. Cell. Microbiol. 8, 1985-1993 [DOI] [PubMed] [Google Scholar]
- Xiang T., Hambleton E. A., DeNofrio J. C., Pringle J. R., Grossman A. R. (2013). Isolation of clonal, axenic strains of the symbiotic dinoflagellate Symbiodinium and their growth and host specificity. J. Phycol. 49, 447-458 [DOI] [PubMed] [Google Scholar]
- Yellowlees D., Rees T. A. V., Leggat W. (2008). Metabolic interactions between algal symbionts and invertebrate hosts. Plant Cell Environ. 31, 679-694 [DOI] [PubMed] [Google Scholar]