Abstract
The purpose of this study was to examine upper and lower limb vasoconstrictor responses to changes in transmural pressure in humans. Brachial and femoral blood mean blood velocity (MBV) and vessel diameter (Doppler ultrasound) were measured in 20 supine healthy subjects (10 men and 10 women; 27 ± 1 yr; mean ± SE) during four levels of limb suction at −25, −50, −75, and −100 mmHg, respectively. Limb suction led to an initial rise in MBV followed by a rapid fall in flow velocity to a level below MBV baseline, indicating a vasoconstriction effect. Femoral compared with brachial vessels exhibited a greater fall in flow velocity at all levels of suction (−89 ± 17 vs. −10 ± 2, −142 ± 11 vs. −14 ± 2, −156 ± 22 vs. −13 ± 2, and −162 ± 29 vs. −12 ± 2 ml/min for −25, −50, −75, and −100 mmHg, respectively; interaction effect, P < 0.05). Even at low tank suction levels (i.e., −10 and −20 mmHg), significant brachial flow velocity vasoconstriction from baseline values was demonstrated, reflecting downstream resistance vessel changes (n = 14). Brachial and femoral diameters did not change during changes in negative tank pressure. During suction, changes in limb volumes were significantly greater in the forearm (1.4 ± 0.5%, 2.4 ± 0.8%, 3.5 ± 1.0%, and 4.3 ± 1.1%) compared with the calf (0.9 ± 0.5%, 1.4 ± 0.7%, 2.0 ± 0.8%, and 2.8 ± 1.1%) at all levels of negative tank pressures (−25, −50, −75, and −100 mmHg, respectively). Simultaneous measurements of both upper limbs and both lower limbs suggested that the majority of the reduction in flow was due to myogenic influences except when −100 mmHg of suction was applied to the lower limb. The greater vasoconstriction responses in the leg compared with the arm with suction appear to be influenced by both myogenic and sympathetic mechanisms.
Keywords: vasoconstriction, local circulatory control, myogenic reflex, autoregulation
during postural stress, blood pressure is maintained. This occurs in large part because peripheral vascular resistance rises, thereby maintaining orthostatic tolerance. Causes of this constriction include a rise in sympathetic tone and engagement of myogenic autoregulatory vasoconstriction (8, 14, 21, 49). It has been suggested that ∼55% of cutaneous vasoconstriction with upright posture is due to engagement of the myogenic reflex (37); whether this same degree of vasoconstriction in skeletal muscle arteries with postural change can be attributed to myogenic influences is not known. Briefly, the myogenic response is the inherent ability of blood vessel to respond to changes in transmural pressure. Blood vessels, particularly arteries and arterioles, exhibit a strong myogenic response, resulting in smooth muscle contraction as transmural pressure rises and relaxation as transmural pressure falls (25, 27). In vitro animal studies demonstrate that as intraluminal pressure is increased beyond 40 cmH20, blood vessels incrementally vasoconstrict (9, 29). Animal studies have demonstrated that the myogenic response can occur independently of the sympathetic nervous system (SNS) (15, 26, 28). Yet, the SNS may modulate the myogenic response (39).
A number of human studies have examined and compared upper and lower extremity neurovascular adjustments to changes in posture and/or orthostatic stress (12, 13, 23, 44, 47, 48). Heterogeneous responses to pharmacological vasodilators and vasoconstrictors have been observed, suggesting that the vascular response of the arm and leg may be different (2, 48). At least four studies have shown that muscle sympathetic nerve activity (MSNA), an index of central sympathetic outflow, increases similarly in the arm and leg during orthostatic stress (23, 52–54). Our laboratory has previously shown that despite similar MSNA responses in the arm and leg, flow fell more in the lower limbs than in the upper limbs during tilt testing (23). This finding suggested that local mechanisms such as the myogenic response might be engaged to a greater degree in the legs compared with the arms during orthostatic stress. We chose to further evaluate limb myogenic differences using limb-pressurized tanks to minimize the involvement of the SNS. The negative tank pressure approach (31, 32) is advantageous in that transmural pressures can be easily adjusted and transmitted through the limb tissue (3, 33). Two components of flow responses have been demonstrated with negative tank pressure application: dynamic and sustained (8). The dynamic phase involves the rapid decrease in mean blood velocity (MBV), which occurs 2–4 s after the initial transient increase in MBV with the abrupt application of negative pressure. The sustained phase involves continuous vasoconstriction below baseline levels 30 to 60 s after the abrupt application of negative pressure.
The purpose of this study was to examine the flow responses in the arm and leg of the same subject under different levels of negative tank pressure (−25, −50, −75, and −100 mmHg) and to differentiate between myogenic and sympathetic influences. Based on animal studies, our hypothesis was that increasing transmural pressure would be associated with increases in limb vasoconstriction, and greater vasoconstriction would be observed in the lower compared with the upper limbs. We also hypothesized that lower levels of suction would be associated with myogenic regulation, whereas higher levels of suction would be influenced to a greater degree by sympathetic activation.
METHODS
Study Subjects
Thirty-four healthy nonobese subjects with a mean (± SE) age of 27 ± 1 yr participated in the study (Table 1). Subjects were nonsmokers, normotensive, and not on any medications. All subjects were free of symptoms and/or history of cardiac, vascular, pulmonary, metabolic, diabetes, or neurological disease. The women were tested 18 ± 2 days (range 2–31 days) into their menstrual cycle, and 4 of 18 were on oral contraceptives. All subjects were recreationally active, but none were involved in a regular exercise program. The Institutional Review Board of the Milton S. Hershey Medical Center approved the experimental protocol. Each person had the purposes and risks of the protocol explained to her or him before written consent was obtained.
Table 1.
Demographic and anthropometric data
| Value | |
|---|---|
| Age, yr | 27±1 |
| Sex, n (men/women) | 16/18 |
| Height, cm | 173±2 |
| Weight, kg | 74±3 |
| BMI, kg/m2 | 24.6±0.5 |
Values are means ± SE. BMI, body mass index.
Experimental Measurements
Measurement of heart rate and blood pressure.
A standard electrocardiogram was used to monitor heart rate (HR). Systolic and diastolic blood pressures were continuously measured using the volume-clamp method (Finapres, Ohmeda, Madison, WI) with mean arterial pressure (MAP) calculated from the Finapres waveform. Before testing, Finapres pressure was confirmed by an automated sphygmomanometer (Dinamap, Critikon, Tampa, FL). HR and MAP were recorded continuously and collected online at 200 Hz using a PowerLab system (AD Instruments, Castle Hill, Australia).
Measurement of limb volumes and circumferences.
Anthropometric measurements taken included forearm and leg volume by water displacement technique, and lower forearm and calf circumferences. To estimate limb volume, we used a water displacement technique in which the limb (arm or leg) is placed into a cylinder of water and the amount of displaced water is measured [limb volume = (πd2/4)·(h), where h = change in water level with/without the limb, and d =diameter of the test vessel (16)]. Changes in limb volume (i.e., limb circumference) from baseline (%) during tank suction were measured with use of a mercury-in-Silastic strain gauge (Hokanson, Belleave, WA) placed around the largest circumference of the lower forearm and calf (31, 32).
Measurement of mean blood velocity and diameter.
Brachial and femoral MBV was measured on a beat-by-beat basis using a 4-MHz pulsed wave Doppler ultrasound (USN) probe (500M Multigon, Yonkers, NY). The flat Doppler probe was securely taped into a fixed position to the skin over the common brachial artery approximately 8–10 cm proximal to the antecubital fossa and over the common femoral artery, approximately 2–3 cm above its bifurcation into the superficial and deep femoral branches. Since the arteries are nearly parallel to the skin surface, the insonation angle with the artery was ∼45°. The gate for USN was also set to insonate the total width of the artery's diameter. Maximal Doppler frequency shift based on the strength of the velocity spectra was obtained with slight manual adjustments of the Doppler probe avoiding any pressure on the skin, which could change the angle of insonation. MBV was measured continuously and collected online as noted above. The coefficients of variability (CV) for MBV brachial measurements at rest and during suction were 8.9 ± 3.1% and 10.0 ± 4.5% and for femoral MBV measurements at rest and during suction were 7.7 ± 1.5% and 8.7 ± 1.8%, respectively. Brachial and femoral diameters were measured using Doppler Ultrasound (12–5 MHz; Advanced Technology Laboratories, model HDI 5000CV, Bothell, WA). The CV for brachial and femoral diameters measurements at rest were 2.0 ± 0.2% and 1.2 ± 0.1% and during suction were 1.1 ± 0.1% and 1.9 ± 0.2%, respectively. Blood flow was calculated from diameters and MBV using the following equation: flow (ml/min) = MBV × π × [diameter (cm)/2]2 × 60. Conductance (ml·min−1·mmHg−1) was calculated as flow/MAP. In addition, shear rate was calculated from diameter and MBV using the following equation: shear rate (s−1) = MBV/diameter (43).
Experimental Design
Experimental testing.
Subjects were instructed to abstain from products containing caffeine and alcohol, as well as to abstain from any exercise 24 h before testing. All studies were performed in the morning after a 2-h fast in a quiet, dimly lit, and temperature-controlled room (21–24°C). Subjects were positioned supine and instrumented with ECG electrodes. The subject's nondominant forearm and the ipsilateral leg were inserted into the limb tanks. On a finger of the dominant forearm, a Finapres blood pressure device was attached. Both arms were positioned at heart level. The arm and leg were sealed above the elbow and at midthigh level, respectively, in air-tight pressure tanks using neoprene cuffs to create a snug nonconstricting seal. After a 20-min rest period, baseline measurements were taken.
Experiment 1: vasoconstriction responses to tank suction.
Increases in transmural pressure were elicited with the application of suction (i.e., negative pressure) to a single arm or leg via a pressurized limb tank (n = 20). This method reflects a sustained increase in transmural pressure from ambient transmural pressure. We chose to use different levels of tank suction to replicate the changes in transmural pressure due to increases in intravascular pressure when standing up. Twenty subjects were exposed to four separate levels of negative pressure (−25, −50, −75, and −100 mmHg) with each limb (arm and leg) tested separately. A primer trial was performed to ensure that the appropriate pressure was present within the limb tank. After 1 min at baseline, the tank pressure was abruptly changed to negative pressure (−25 mmHg) within 0.2–0.4 s for 1 min and then abruptly released for 1 min. Two trials were done for each pressure, and the responses were averaged. A brief rest period (2–3 min) was included between each trial to ensure that blood flow had returned to baseline. This procedure was repeated using −50, −75, and −100 mmHg with the sequence of pressure application remaining constant. During the application of suction, desired tank pressures remained stable (±0.2 mmHg). Diameters and MBV of each limb were measured during separate arm and leg trials.
Experiment 2: potential SNS and local influences on vasoconstriction.
We measured opposing limb blood flow in the contralateral limb during changes in negative limb tank pressure (−25, −50, −75, and −100 mmHg) in 20 subjects using the experiment protocol above to examine for any systemic sympathetic effects evoked by limb suction. In addition, we also measured changes in limb volume as an estimate of change in venous distension. The arm and leg limb volumes of the limbs within the tank were measured during changes in negative tank pressure with strain gauges placed at the widest part of the forearm and calf.
Experiment 3: vasoconstriction at low forearm tank pressures.
Last, in a separate group of men and women (n = 14), we chose to examine blood flow responses in just the forearm using a series of lower levels of negative pressures (−10, −20, −30, −40, and −50 mmHg). We reasoned that myogenic vasoconstriction would be apparent at all levels of suction. After 1 min at baseline, forearm tank pressure was abruptly changed to negative pressure (−10 mmHg) within 0.2–0.4 s for 1 min and then abruptly released for 1 min. Two trials were done for each pressure, and the responses were averaged. A brief rest period (2–3 min) was included between each trial to ensure that blood flow had returned to baseline values. This procedure was repeated using −20, −30, −40, and −50 mmHg with the sequence of pressure application remaining constant.
Data Analysis
The following variables were measured on a beat-by-beat basis: HR, MAP, and MBV. Intraluminal diameters were analyzed at the end of diastole at the following time periods: baseline, and 5–10 s and 50–60 s after change in tank pressure (5). Trials for each pressure time period were averaged for each individual. If there were no significant differences before all negative tank pressures, resting hemodynamic variables were averaged. To normalize the data for any differences in baseline, the absolute change in MBV from baseline was calculated (MBVx − MBVbaseline). Within-comparisons between limbs and pressures for HR, MAP, MBV, flow, diameter, and conductance were evaluated using ANOVA for repeated measures. Greenhouse Geisser adjustments for degrees of freedom were used when sphericity assumptions were violated. Post hoc testing was performed using Tukey and pairwise testing where appropriate. A mixed-effect model was used to estimate the correlation coefficient between any two continuous variables having repeated observations per subject (46). To determine whether the correlation coefficient from the mixed-effects model was significantly different from zero, a 95% bootstrapped confidence interval was obtained using 2,500 bootstrapped samples (11). Statistical analyses were performed using SPSS (version 13 for Mac OSX, SPSS, Chicago, IL). Data are presented as means ± SE, and level of significance used was P < 0.05.
RESULTS
Resting femoral MBV, diameter, flow, and conductance were greater than resting flow parameters from the forearm (see Table 2). Limb volume was greater in the leg compared with forearm (6,860 ± 310 vs. 1,341 ± 77 ml; P < 0.05). Resting flow and conductance relationships were still observed when resting variables were normalized by limb volume and resting diameters.
Table 2.
Absolute hemodynamic and flow responses to changes in suction
| Arm |
Leg |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Under Sustained Negative Pressure (mmHg) |
Baseline | Under Sustained Negative Pressure (mmHg) |
|||||||||||||||
| −25 | −50 | −75 | −100 | −25 | −50 | −75 | −100 | |||||||||||
| HR, beats/min | 62±2 | 61±2 | 61±2 | 61±2 | 63±2 | 62±2 | 61±2 | 61±2 | 61±2 | 62±2 | ||||||||
| MAP, mmHg | 84±2 | 85±2 | 85±2 | 86±1 | 90±2* | 83±2 | 82±2 | 82±2 | 85±2* | 86±2* | ||||||||
| Limb volume, % | 1.41±0.11 | 2.35±0.17 | 3.55±0.24 | 4.40±0.23 | 0.87±0.12 | 1.42±0.15 | 1.99±0.18 | 2.82±0.24 | ||||||||||
| MBV, cm/s | 5.67±0.38 | 4.40±0.34* | 3.77±0.26* | 3.57±0.24* | 4.16±0.65* | 11.65±0.70† | 8.87±0.94* | 7.32±0.59* | 7.42±0.76* | 7.56±0.85* | ||||||||
| Diameter, cm | 0.38±0.02 | 0.38±0.02 | 0.38±0.01 | 0.38±0.02 | 0.39±0.01 | 0.87±0.03† | 0.86±0.03 | 0.88±0.04 | 0.89±0.03 | 0.87±0.04 | ||||||||
| Flow, ml/min | 38±3 | 30±2* | 26±2* | 24±2* | 27±3* | 412±31† | 309±33* | 262±27* | 270±28* | 257±26* | ||||||||
| Conductance, ml·min−1·mmHg−1 | 0.46±0.04 | 0.35±0.03* | 0.30±0.03* | 0.28±0.02* | 0.31±0.04* | 4.99±0.37† | 3.79±0.39* | 3.21±0.35* | 3.19±0.34* | 2.99±0.31* | ||||||||
Values are means ± SE. Resting baseline values represent the average of all resting baselines before the change in suction levels. HR, heart rate; MAP, mean arterial pressure; MBV, mean blood velocity.
Significantly different from baseline.
Significantly different from arm baseline.
Experiment 1: Vasoconstriction Responses to Tank Suction
With the abrupt application of all negative tank pressures, MBV rose quickly (∼2–3 s) and within 20–30 s fell below baseline values in both the brachial and femoral arteries. Figure 1 provides an example of the hemodynamic and flow responses to changes in tank suction in one subject.
Fig. 1.
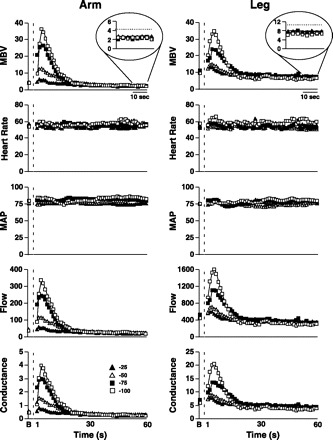
Flow and hemodynamic responses in the brachial (left panels) and femoral artery (right panels) to changes in suction (−25, −50, −75, and −100 mmHg) in 1 subject. Dashed line, change to suction. Negative pressures used: ▴, −25 mmHg; ▵, −50 mmHg; ▪, −75 mmHg; □, −100 mmHg. MBV, mean blood velocity (cm/s); MAP, mean arterial pressure (mmHg). Heart rate is in beats/min; flow is in ml/min; conductance is in ml·min−1·mmHg−1.
Dynamic Responses to Changes in Limb Tank Pressures of −25, −50, −75, and −100 mmHg
At the higher tank pressures (−50, −75 and −100 mmHg), the initial transient rise in MBV was greater in the arms compared with the legs (Fig. 2A). When the peak MBV data were expressed as peak shear rate, similar relationships were shown (arm: 72 ± 8, 215 ± 15, 469 ± 31, and 711 ± 53 s−1; Leg: 26 ± 4, 76 ± 8, 152 ± 12, and 281 ± 27 s−1; P < 0.05). There was no significant change in brachial or femoral diameters at peak MBV compared with baseline diameters (Fig. 2B). The timing of this initial peak MBV was delayed in the leg compared with the arm (3.1 ± 0.2 vs. 2.1 ± 0.1 s; P < 0.05, Fig. 2C). Immediately after the initial rise in MBV, MBV fell at a dynamic rate that was greater in the arms compared with legs (−1.91 ± 0.15 vs. −1.25 ± 0.08 cm·s−1·s−1; P < 0.05, Fig. 2D). Each increase in tank pressure led to a greater initial rise in MBV and dynamic rate of MBV reduction in both limbs (main effect, P < 0.05). This dynamic rate of MBV reduction in both limbs was dependent on the initial rise in peak MBV.
Fig. 2.

Absolute peak MBV and diameter changes (Δ), timing of peak MBV, and dynamic response to abrupt changes in suction (−25, −50, −75, and −100 mmHg) were examined. Arms compared with legs had a higher peak MBV at the higher tank pressures (−50, −75, and −100 mmHg; A). There were no changes in limb diameters at peak velocity across all limb tank pressures (B). Legs had a delayed response to all applications of tank pressure compared with arms (C). Arms had a greater dynamic response after the peak velocity (D). Baseline, baseline levels at ambient pressure where Δ = 0; • and solid line, arm; ○ and dashed line, leg; *Significant difference between arm and leg at a specific negative tank pressure. **Significant main effect difference between arm and leg.
Sustained Responses to Changes in Limb Tank Pressures of −25, −50, −75, and −100 mmHg
Sixty seconds after the application of limb suction in the arm and leg, the reduction of MBV below baseline levels was greater for the higher tank pressures (−50, −75, and −100 mmHg) compared with the lower tank pressure (−25 mmHg) (P < 0.05). The level of vasoconstriction appeared to plateau after −50 mmHg of tank suction. Legs exhibited a significantly greater reduction in MBV compared with arm at all tank pressures (−3.86 ± 0.37 vs. −1.70 ± 0.37 cm/s; P < 0.05, Fig. 3A); however, there was no change in vessel diameters in either limb with any of the sustained tank pressure changes (Fig. 3B). When the data were examined in terms of flow and conductance, legs exhibited a greater vasoconstriction effect at every level of suction compared with the arms (Fig. 3, C and D). When data were corrected for differences in limb volume, a greater lower limb constrictor effect was still noted (Fig. 4). When data were normalized to resting baseline flow representing 100%, the percentage of flow reduction with suction was not significantly different between limbs. There were no significant differences in HR during negative tank pressures to either limb; however, MAP was significantly increased at the highest tank pressures in both limbs (Table 2). Overall, MAP had a greater increase with exposure to suction in the arm compared with the leg (2.4 ± 0.6 vs. 1.0 ± 0.4 mmHg; main effect, P < 0.05). Last, there was no significant correlation between the reductions of flow from baseline values during the different levels of tank suction and the initial rise in peak MBV or between the vasoconstrictor response and peak shear rate.
Fig. 3.
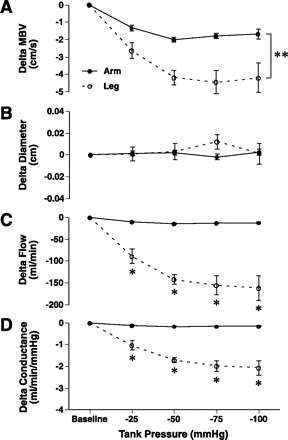
Absolute MBV, diameter, flow, and conductance changes (Δ) under sustained increases in tank suction (−25, −50, −75, and −100 mmHg) in the brachial and femoral arteries. Legs compared with arms had greater velocity reductions in response to changes in suction (A) with no change in diameters in either limb (B). Leg flow and conductance changes were greater at every tank suction level compared with the arm (C and D). Baseline, baseline levels at ambient pressure where Δ = 0; • and solid line, arm; ○ and dashed line, leg; *Significant difference between arm and leg at a specific negative tank pressure. **Significant main effect difference between arm and leg.
Fig. 4.
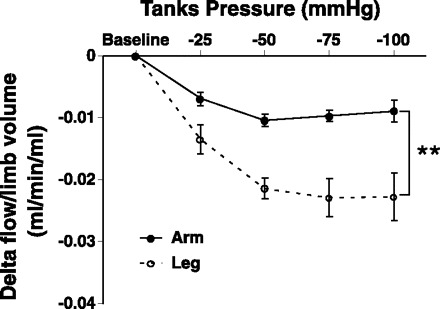
Absolute flow changes (Δ) normalized by limb volumes under sustained increases in tank suction (−25, −50, −75, and −100 mmHg) in the brachial and femoral arteries. Leg flow and conductance when normalized by limb volume was lower at every tank suction level compared with the arm (A and B). Baseline, baseline levels at ambient pressure where Δ = 0; • and solid line, arm; ○ and dashed line, leg. **Significant main effect difference between arm and leg.
Experiment 2: Potential SNS and Venous Influences on Vasoconstriction
We measured MBV in the contralateral limb during changes in negative limb tank pressure (−25, −50, −75, and −100 mmHg) to examine for any systemic sympathetic effects evoked by limb suction. We reasoned that this opposite nontank limb flow subtracted from the flow responses measured from the limb enclosed in the limb tank would be representative of a myogenic component to the vasoconstriction response. Complete opposing limb data for both limb tanks was obtained in 17 subjects (Fig. 5, A and B). In the arm, the majority of the vasoconstriction with forearm tanks pressure appeared to be influenced by a myogenic mechanism (∼84% average; Fig. 5C). In the leg, the myogenic response accounted for ∼64% at the three lower tank pressures (−25, −50, and −75 mmHg) and ∼40% at the highest leg tank pressure (−100 mmHg; Fig. 5D). Changes in limb volumes within the limb tanks were significantly greater in the forearm (1.4 ± 0.5%, 2.4 ± 0.8%, 3.5 ± 1.0%, and 4.3 ± 1.1%) compared with calf (0.9 ± 0.5%, 1.4 ± 0.7%, 2.0 ± 0.8%, and 2.8 ± 1.1%) at all levels of negative tank pressures (−25, −50, −75, and −100 mmHg, respectively). Last, there was no significant correlation between the changes in limb volume and degree of MBV vasoconstriction during the different levels of tank suction in the arm (r= −0.21) or leg (r= −0.14).
Fig. 5.
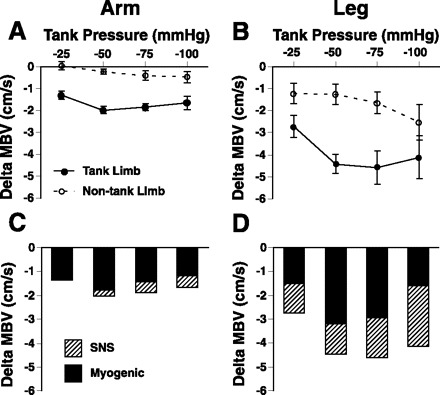
Sympathetic nervous system (SNS) and myogenic influences on vasoconstriction during changes in limb tank pressures (n = 17). There was little change in contralateral flow in the arm during changes in tank suction (A), whereas opposing leg limb blood exhibited vasoconstrictor responses (B). Myogenic contribution to the vasoconstriction was calculated from limb MBV within the tank minus limb MBV in opposing limb for the arm (C) and leg (D). The myogenic response compared with SNS influence appeared to have a greater contribution to vasoconstriction in the arm with changes in tank suction. Although the myogenic response still had a strong influence in leg vasoconstriction, sympathetic activation also appears to contribute to vasoconstriction in the lower limbs, especially at the highest tank pressure (−100 mmHg). Baseline, baseline levels at ambient pressure where Δ = 0; • and solid line, tank limb; ○ and dashed line, nontank limb; solid bars, myogenic influence; hatched bars, SNS influence. **Significant main effect difference between arm and leg.
Experiment 3: Vasoconstriction at Low Tank Pressure
In a separate study with eight women and six men of similar age (27.9 ± 2.6 yr) and body mass index (23.6 ± 0.6 kg/m2), blood flow responses to changes in arm tank pressures (−10, −20, −30, −40, and −50 mmHg) were also examined for 1 min. Blood velocity dropped to below baseline values within 20–30 s for all negative tank pressures (P < 0.05). Forearm vasoconstriction at the higher negative pressures (−30, and −40 mmHg) was significantly greater than at the lowest negative pressures (−10 mmHg; Fig. 6).
Fig. 6.
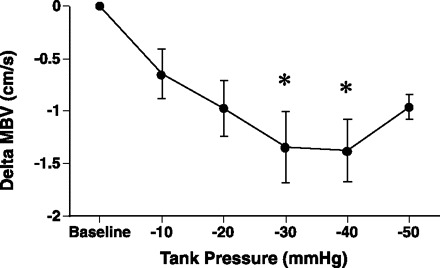
In a separate group of subjects (n = 14), forearm MBV responses were examined 60 s after application of −10, −20, −30, −40, and −50 mmHg tank pressure. At 60 s under negative pressure, MBV was below baseline levels for all levels of suction (P < 0.05). A greater vasoconstriction response (ΔMBV) was observed with higher negative pressures (−30 and −40 mmHg) compared with lowest negative pressure (−10 mmHg). Baseline, baseline levels at ambient pressure where Δ = 0. *Significantly different from −10 mmHg.
DISCUSSION
In these studies, we examined upper and lower limb constrictor responses during a series of negative tank pressures in healthy individuals. The main findings of this study were: 1) legs compared with the arms exhibited greater vasoconstriction at all levels of suction; 2) vasoconstrictor responses occurred at all negative pressures (−10 through −100 mmHg) with the degree of vasoconstriction plateauing with tank pressures greater than −50 mmHg; 3) limb tank pressures greater than −25 mmHg appear to also elicit systemic sympathetic engagement in the leg and arm; 4) the contribution of sympathetic activation to the constrictor response was much smaller for the arm than the leg; and 5) greater vasoconstrictor responses in the leg are due to both greater sympathetic and myogenic influences. The physiological significance of these findings is that increases in transmural pressure with standing invariably evoke a larger lower limb increase in vascular tone that is due to both sympathetic and myogenic factors.
Animal studies suggest there are differences in the regulation of vascular tone in the upper and lower limbs of swines (30). Several human studies have examined regional variations in arterial dilator and constrictor responses (35, 36, 38). Reactive hyperemic studies show greater peak flow responses and vasodilator capacity in the forearm compared with the calf (35, 41). Yet the calf compared with forearm has been shown to have greater vasoconstriction response to pharmacological stimuli (12, 24, 38). A potential mechanism for this is felt to be due to greater α-adrenergic responsiveness in the calf relative to the forearm (38); however, α1- and α2-adrenergic responsiveness may be different between limbs (10, 51). Thus the majority of the pharmacological and physiological studies of the arm and leg have shown that the arm has a greater vasodilator response than the legs but that the legs compared with the arms have a greater vasoconstrictor response (42).
Classic studies examining human forearm (17) and calf (4) blood flow regulation have demonstrated that on the release of negative pressure (−50 to −200 mmHg) a vasoconstriction effect occurred. It was suggested that the forearm had a stronger vasoconstrictor response with lower pressures (−50 mmHg), whereas calf vessels responded more regularly and strongly to all other higher negative pressures. A limitation to these studies was that blood flow was measured after the release of negative pressure. In our study, we measured limb velocity and diameters during the application of suction to replicate the effects of standing. When an individual stands, intraluminal pressure increases in the dependent limbs, leading to an increase in transmural pressure (45). Since conduit diameters did not change with the application of suction in either limb, we reasoned that changes in vessel diameter were occurring in the downstream smaller resistance vessels of the arm and leg within the tank. Thus the conduit velocity and conductance data were more representative of the lower resistance vessels located within the enclosed tank responding to the changes in transmural pressure. In our study, although limb differences were not present when baseline flows were normalized to 100%, absolute changes in flow demonstrated that there was greater vasoconstriction in the leg compared with arm during all levels of suction. These limb vasoconstrictor differences were still apparent when the absolute changes were normalized by limb volume. Thus we feel that the absolute changes in flow were more representative of the vasoconstrictor effect in the limbs with increasing levels of suction. Interestingly, the vasoconstriction response appeared to plateau beyond 50 mmHg of negative pressure. This ceiling effect may have been due to endothelial influences from the initial rise in flow that opposed vasoconstriction, leading to a plateau effect.
When an individual moves from a supine to standing position, there is an increase in the lower limb's transmural pressure (45) due to an increase in intraluminal pressure. Additionally, vascular resistance has been shown to be greater in the lower compared with upper limbs during orthostatic stress (23). Yet similar increases in MSNA have been shown in the arm and leg with tilt (23), static exercise (55), and lower-body negative pressure (44). Previous animal studies have demonstrated that the myogenic response can occur independently of SNS but that the SNS may modulate the myogenic response (39, 40). In cutaneous tissues, it has recently been suggested that vascular sensory fibers are involved in mediating the myogenic response (37). Prior studies using lower-body negative pressure tanks enclosing the pelvis and both or one leg have demonstrated engagement of sympathetic activity (7, 22, 34). Thus we questioned whether our limb tank model also evoked sympathetic activation, contributing to the vasoconstriction responses observed. We speculated that the higher levels of suction would be associated with an increase in opposing limb vasoconstriction. In our control limb, we saw mild to moderate levels of vasoconstriction, which we felt was due to a potential baroreflex response based on the minor blood pressure fluctuations with activation of the limb tanks. Using measurements of limb flow in the limbs contralateral to those exposed to suction, we found that suction of the leg evoked a greater sympathetic engagement than the arm. Previously it has been suggested that the myogenic response contributes ∼55% of cutaneous vasoconstriction with upright posture (37). In our study, forearm vasoconstriction appeared to be due primarily to myogenic influences (∼84% average). In the leg, the myogenic influence was responsible for more than 50% of the constriction at all levels of suction except at −100 mmHg. Thus the contribution of the vasoconstriction response due to nonadrenergic mechanisms appeared to be greater in the arm than leg.
Several prior studies have suggested that the cutaneous, subcutaneous, and muscle vasoconstriction effect observed with the limb dependent below heart level is due to a local venoarteriolar axon reflex in which increases in venous pressure leads to arterial constriction (1, 6, 18–20, 37, 50). In cutaneous tissue, the dominant part of the axon reflex's vasoconstriction effects is suggested to occur when vascular transmural pressure increased from 20 to 40 mmHg (18, 50). Similar cutaneous nonadrenergic vasoconstrictor responses (∼50%) to venous congestion (56) and leg in a dependent position (37) have been reported in both the arm and leg. To further examine for the possible influence of the axon reflex, we measured limb volume changes as an indirect measure of change in venous volume during tank suction. We observed a greater increase in arm compared with leg volume with all negative pressures. Thus, if our changes in blood flow were due to purely an axon reflex mechanism, we speculated that the greater limb volumes in the arm would have evoked a greater vasoconstrictor effect in the forearm. We did not observe this phenomenon. We also did not observe any association between the magnitude of change in limb volume and degree of vasoconstriction during changes in limb suction. Last, in our study, we also examined forearm vascular responses to a range of lower negative suction (−10, −20, −30, −40, and −50 mmHg). We reasoned that myogenic vasoconstriction would occur at thresholds lower than predicted for the venoarterial axon reflex (18, 50). Even though there appeared to be a plateau of the forearm responses beyond −50 mmHg of suction, we demonstrated forearm vasoconstriction effects below baseline values with tank suction as little as −10 and −20 mmHg. Thus we feel that the above evidence concurs that it is unlikely that the axon reflex made a major contribution to our findings.
Several signaling pathways have been suggested to be involved in the myogenic response. Animal studies suggest that, as vascular smooth muscle depolarizes, calcium enters the cell via voltage-gated calcium channels increasing intracellular calcium, leading to smooth muscle vasoconstriction (8, 21). Studies in rat mesenteric vessels also suggest that increases in intraluminal pressure may also lead to the generation of arachidonate metabolites activating vanilloid receptors (TRPV1) on C fiber nerve endings. Activation of these receptors is suggested to lead to depolarization of nerves and the release of vasoactive sensory neuropeptides, which bind to tachykinin NK1 receptors, leading to vasoconstriction (49). Whether these signaling pathways are different in the arm and leg vasculature is unknown. Another potential cause for the greater leg vasoconstriction may be due to the fact that α1-adrenergic responsiveness is higher in the calf relative to the forearm (38). In other studies, the arm exhibited greater α1- compared with α2-adrenergic responsiveness to infused adrenergic agonists (10), whereas the leg exhibited similar levels of α1 - and α2-adrenergic responsiveness, respectively (51). Thus the exact cellular mechanism responsible for the enhanced vasoconstriction response in the legs compared with arms will require further study.
Limitations to the Study
There are several limitations to be addressed. First, previous studies have demonstrated that the tank pressures are transmitted through the tissue (33). Due to differences in muscle mass and limb circumference, it is possible that the timing of pressure transmission throughout the tissue may have influenced the initial dynamic rate of MBV change with the abrupt application of suction. However, we think it is unlikely that differences in transmission rate altered our conclusion regarding the relative size of the sustained myogenic response in the arm and leg. Second, it may be argued that the initial increase in velocity could have affected the magnitude of the constrictor responses. To examine this possibility, we examined MBV vasoconstrictor responses in the arm and leg at 25 mmHg of suction. At this pressure, the initial increases in flow (peak MBV) were similar, but sustained vasoconstriction was greater in the legs compared with the arms. At higher levels of suction, peak MBV in the arms was greater than that in the legs. Thus this, coupled with the smaller diameters in the forearm, yields greater shear rates for the brachial than the femoral arteries. This response could lead to the greater release of nitric oxide in the forearm, and this could act to oppose forearm myogenic influences. Thus endothelial modulation via changes in shear stress may influence the degree of vasoconstriction with changes in negative tank suction.
Third, for technical reasons, we used the same order for tank suction (i.e., −25, −50, −75, and −100 mmHg) in both limbs when measuring velocity and diameters separately. To minimize for any potential order effects, trials were separated by rest periods to ensure that flow measurements returned to baseline levels before starting the next trial. Fourth, elevating the limbs may have minimized the venoarterial reflex, but it would have also altered the transmural pressure across the blood vessel. Thus we chose to keep the limb tank at heart level to avoid changes in hydrostatic pressure. Due to the increases in limb volume, we cannot exclude the role of the local venoarterial reflex especially at the high suction levels; however, we did not observe an association between increases in limb volume and MBV vasoconstriction. Fifth, since these subjects were part of a larger study, we were unable to control for the phase of the menstrual cycle in our female subjects. Last, since we did not measure skin blood flow with changes in tank pressure, we cannot evaluate skin blood flow and muscle flow responses.
Conclusion
Myogenic reactivity in combination with the SNS leads to vasoconstriction in the limbs, which helps regulate blood pressure with posture changes. Responses to lower levels of tank pressures especially in the arm are likely to be more representative of local myogenic vasoconstriction effects, whereas higher levels of tank pressure appear to elicit a combination of sympathetic and myogenic influences.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants R01-HL-070222 (L. I. Sinoway) and P01-HL-077670 (L. I. Sinoway) and National Institutes of Health/National Center for Research Resources Grants M01-RR-010732 and C06-RR-016499.
Acknowledgments
We thank the support staff of the Heart and Vascular Institute Research and General Clinical Research Center, Amy Fogelman, and the other staff of Dr. Chester Ray's research group for recruitment and technical support, Kristen Gray for graphical support, and Jennifer Stoner for excellent secretarial skills. Last, we thank the volunteers who participated in the study.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Andersen EB, Boesen F, Henriksen O, Sonne M. Blood flow in skeletal muscle of tetraplegic man during postural changes. Clin Sci (Lond) 70: 321–325, 1986 [DOI] [PubMed] [Google Scholar]
- 2.Brede M, Wiesmann F, Jahns R, Hadamek K, Arnolt C, Neubauer S, Lohse MJ, Hein L. Feedback inhibition of catecholamine release by two different alpha2-adrenoceptor subtypes prevents progression of heart failure. Circulation 106: 2491–2496, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Coles DR, Greenfield ADM. The reactions of the blood vessels of the hand during increases in transmural pressure. J Physiol 131: 277–289, 1956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coles DR, Kidd BSL, Patterson GC. The reactions of the blood vessels of the human calf to increases in transmural pressure. J Physiol 134: 665–674, 1956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 39: 257–265, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Crandall CG, Shibasaki M, Yen TC. Evidence that the human cutaneous venoarteriolar response is not mediated by adrenergic mechanisms. J Physiol 538: 599–605, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui J, Wilson TE, Crandall CG. Muscle sympathetic nerve activity during lower body negative pressure is accentuated in heat-stressed humans. J Appl Physiol 96: 2103–2108, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev 79: 387–423, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Davis MJ, Sikes PJ. Myogenic responses of isolated arterioles: test for a rate-sensitive mechanism. Am J Physiol Heart Circ Physiol 259: H1890–H1900, 1990 [DOI] [PubMed] [Google Scholar]
- 10.Dinenno FA, Dietz NM, Joyner MJ. Aging and forearm postjunctional alpha-adrenergic vasoconstriction in healthy men. Circulation 106: 1349–1354, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Efron B, Tibshirani RJ. An Introduction to the Bootstrap. Boca Raton, FL: Chapman and Hall/CRC, 1993
- 12.Essandoh LK, Duprez DA, Shepherd JT. Postural cardiovascular reflexes: comparison of responses of forearm and calf resistance vessels. J Appl Physiol 63: 1801–1805, 1987 [DOI] [PubMed] [Google Scholar]
- 13.Essandoh LK, Houston DS, Vanhoutte PM, Shepherd JT. Differential effects of lower body negative pressure on forearm and calf blood flow. J Appl Physiol 61: 994–998, 1986 [DOI] [PubMed] [Google Scholar]
- 14.Folkow B. Hypertensive structural changes in systemic precapillary resistance vessels: how important are they for in vivo haemodynamics? J Hypertens 13: 1546–1559, 1995 [PubMed] [Google Scholar]
- 15.Folkow B. Intravascular pressure as a factor regulating the tone of small vessels. Acta Physiol Scand 17: 289–310, 1949 [DOI] [PubMed] [Google Scholar]
- 16.Going Densitometry SB. Chapter 1. In: Human Body Composition, edited by Roche AF, Heymsfield SB, Lohman TG. Champaign, IL: Human Kinetics, 1996, p. 3–23.
- 17.Greenfield ADM, Patterson GC. Reactions of the blood vessels of the human forearm to increases in transmural pressure. J Physiol 125: 508–524, 1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henriksen O. Local sympathetic reflex mechanism in regulation of blood flow in human subcutaneous adipose tissue. Acta Physiol Scand 101: 6–43, 1977 [PubMed] [Google Scholar]
- 19.Henriksen O. Sympathetic reflex control of blood flow in human peripheral tissues. Acta Physiol Scand 603: 33–39, 1991 [PubMed] [Google Scholar]
- 20.Henrikson O, Nielsen SL, Paaske WP. Autoregulation of blood flow in human adipose tissue. Acta Physiol Scand 89: 531–537, 1973 [DOI] [PubMed] [Google Scholar]
- 21.Hill MA, Zou H, Potocnik SJ, Meininger GA, Davis MJ. Arteriolar smooth muscle mechanotransduction: Ca2+ signaling pathways underlying myogenic reactivity. J Appl Physiol 91: 973–983, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Ichinose M, Saito M, Fujii N, Kondo N, Nishiyasu T. Modulation of the control of muscle sympathetic nerve activity during severe orthostatic stress. J Physiol 576: 947–958, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imadojemu VI, Lott MEJ, Gleeson K, Hogeman CS, Ray CA, Sinoway LI. Contribution of perfusion pressure to vascular resistance response during head-up tilt. Am J Physiol Heart Circ Physiol 281: H371–H375, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Jacob G, Costa F, Shannon J, Robertson D, Biaggioni I. Dissociation between neural and vascular responses to sympathetic stimulation : contribution of local adrenergic receptor function. Hypertension 35: 76–81, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Johnson PC. Autoregulation of blood flow. Circ Res 59: 483–495, 1986 [DOI] [PubMed] [Google Scholar]
- 26.Johnson PC. Myogenic nature of increase in intestinal vascular resistance with venous pressure elevation. Circ Res 7: 992–999, 1959 [DOI] [PubMed] [Google Scholar]
- 27.Johnson PC. The myogenic response. In: Handbook of Physiology. The Cardiovascular System. Vascular Smooth Muscle. Bethesda, MD: Am. Physiol Soc., sect. 2, vol. II, 1980, p. 409–442.
- 28.Jones RD, Berne RM. Local regulation of blood flow in skeletal muscle. Circ Res XIV and XV: I30–I38, 1964 [PubMed] [Google Scholar]
- 29.Kuo L, Davis MJ, Chilian WM. Myogenic activity in isolated subepicardial and subendocardial coronary arterioles. Am J Physiol Heart Circ Physiol 255: H1558–H1562, 1988 [DOI] [PubMed] [Google Scholar]
- 30.Laughlin MH, McAllister RM, Jasperse JL, Hitchcock LS, Bonagura JD. Acetylcholine is a vasodilator of porcine skeletal muscle arteries. Comp Biochem Physiol A Mol Integr Physiol 120: 345–354, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Lott ME, Herr MD, Sinoway LI. Effects of age on brachial artery myogenic responses in humans. Am J Physiol Regul Integr Comp Physiol 287: R586–R591, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Lott MEJ, Herr MD, Sinoway LI. Effects of transmural pressure on brachial artery mean blood flow velocity dynamics in humans. J Appl Physiol 93: 2137–2146, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Lundvall J, Länne T. Transmission of externally applied negative pressure to the underlying tissue. A study on the upper arm of man. Acta Physiol Scand 136: 403–409, 1989 [DOI] [PubMed] [Google Scholar]
- 34.Monahan KD, Ray CA. Gender affects calf venous compliance at rest and during baroreceptor unloading in humans. Am J Physiol Heart Circ Physiol 286: H895–H901, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Newcomer SC, Leuenberger UA, Hogeman CS, Handly BD, Proctor DN. Different vasodilator responses of human arms and legs. J Physiol 556: 1001–1011, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Newcomer SC, Leuenberger UA, Hogeman CS, Proctor DN. Heterogeneous vasodilator responses of human limbs: influence of age and habitual endurance training. Am J Physiol Heart Circ Physiol 289: H308–H315, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Okazaki K, Fu Q, Martini ER, Shook R, Conner C, Zhang R, Crandall CG, Levine BD. Vasoconstriction during venous congestion: effects of venoarteriolar response, myogenic reflexes, and hemodynamics of changing perfusion pressure. Am J Physiol Regul Integr Comp Physiol 289: R1354–R1359, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Pawelczyk JA, Levine BD. Heterogeneous responses of human limbs to infused adrenergic agonists: a gravitational effect? J Appl Physiol 92: 2105–2113, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Ping P, Johnson PC. Mechanism of enhanced myogenic response in arterioles during sympathetic nerve stimulation. Am J Physiol Heart Circ Physiol 263: H1185–H1189, 1992 [DOI] [PubMed] [Google Scholar]
- 40.Ping P, Johnson PC. Role of myogenic response in enhancing autoregulation of flow during sympathetic nerve stimulation. Am J Physiol Heart Circ Physiol 263: H1177–H1184, 1992 [DOI] [PubMed] [Google Scholar]
- 41.Proctor DN, Le KU, Ridout SJ. Age and regional specificity of peak limb vascular conductance in men. J Appl Physiol 98: 193–202, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Proctor DN, Newcomer SC. Is there a difference in vascular reactivity of the arms and legs? Med Sci Sports Exerc 38: 1819–1828, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Pyke KE, Dwyer EM, Tschakovsky ME. Impact of controlling shear rate on flow-mediated dilation responses in the brachial artery of humans. J Appl Physiol 97: 499–508, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Rea RF, Wallin BG. Sympathetic nerve activity in arm and leg muscles during lower body negative pressure in humans. J Appl Physiol 66: 2778–2781, 1989 [DOI] [PubMed] [Google Scholar]
- 45.Rowell LB. Passive effects of gravity. In: Human Cardiovascular Control. New York: Oxford Univ. Press, 1993, chapt. 1, p. 3–35.
- 46.Roy A. Estimating correlation coefficient between two variables with repeated observations using mixed effects model. Biom J 48: 286–301, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Schrage WG, Woodman CR, Laughlin MH. Hindlimb unweighting alters endothelium-dependent vasodilation and ecNOS expression in soleus arterioles. J Appl Physiol 89: 1483–1490, 2000 [DOI] [PubMed] [Google Scholar]
- 48.Schulz R, Schmidt D, Blum A, Lopes-Ribeiro X, Lucke C, Mayer K, Olschewski H, Seeger W, Grimminger F. Decreased plasma levels of nitric oxide derivatives in obstructive sleep apnoea: response to CPAP therapy. Thorax 55: 1046–1051, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scotland RS, Chauhan S, Davis C, De Felipe C, Hunt S, Kabir J, Kotsonis P, Oh U, Ahluwalia A. Vanilloid receptor TRPV1, sensory C-fibers, and vascular autoregulation: a novel mechanism involved in myogenic constriction. Circ Res 95: 1027–1034, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Skagen K, Henriksen O. Changes in subcutaneous blood flow during locally applied negative pressure to the skin. Acta Physiol Scand 117: 411–414, 1983 [DOI] [PubMed] [Google Scholar]
- 51.Smith EG, Voyles WF, Kirby BS, Markwald RR, Dinenno FA. Ageing and leg postjunctional alpha-adrenergic vasoconstrictor responsiveness in healthy men. J Physiol 582: 63–71, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsunoda S, Shindo K, Shiozawa Z, Mano T. Comparison between muscle sympathetic nerve activity and calf vascular resistance with head-up tilting in humans. J Auton Nerv Syst 33: 277–282, 1991 [DOI] [PubMed] [Google Scholar]
- 53.Victor RG, Leimbach WN Jr. Effects of lower body negative pressure on sympathetic discharge to leg muscles in humans. J Appl Physiol 63: 2558–2562, 1987 [DOI] [PubMed] [Google Scholar]
- 54.Vissing SF, Scherrer U, Victor RG. Relation between sympathetic outflow and vascular resistance in the calf during perturbations in central venous pressure. Evidence for cardiopulmonary afferent regulation of calf vascular resistance in humans. Circ Res 65: 1710–1717, 1989 [DOI] [PubMed] [Google Scholar]
- 55.Wallin BG, Victor RG, Mark AL. Sympathetic outflow to resting muscles during static handgrip and postcontraction muscle ischemia. Am J Physiol Heart Circ Physiol 256: H105–H110, 1989 [DOI] [PubMed] [Google Scholar]
- 56.Wilson TE, Shibasaki M, Cui J, Levine BD, Crandall CG. Effects of 14 days of head-down tilt bed rest on cutaneous vasoconstrictor responses in humans. J Appl Physiol 94: 2113–2118, 2003 [DOI] [PubMed] [Google Scholar]


