Abstract
Rationale
There is tight coupling between Akt activation and suppression of cell death. Full Akt activation requires mTOR complex 2 (mTORC2), but the regulation of mTORC2 is unclear.
Objective
To gain new insights into mechanisms of mTORC2/Akt signaling.
Methods and Results
The role of mTORC2 in cardioprotection was examined. In perfused mouse hearts, ischemic preconditioning (IPC) increased mTORC2 activity, leading to phosphorylation of Akt on Ser473. The protective effect of IPC was lost by pretreatment with dual mTORC inhibitors but not with rapamycin, a mTORC1 inhibitor, which indicates the fundamental role of mTORC2 activation in cardioprotection. Next, the regulation and downstream targets of mTORC2/Akt signaling were explored. We have found that IPC and other Akt activators (insulin and opioids) result in phosphorylation of ribosomal protein S6 (Rps6) at Ser235/236 in mouse hearts and neonatal rat ventricular myocytes. Rps6 interacts with components of mTORC2, and siRNA-mediated knockdown of Rps6 attenuates insulin-induced mTORC2 activation and Akt-Ser473 phosphorylation. On the other hand, Rps6 overexpression enhanced Akt-Ser473 phosphorylation, indicating that Rps6 activation amplifies mTORC2/Akt signaling. Disruption of the Rps6/mTORC2 pathway by knockdown of Rps6 or rictor abrogated insulin-induced cytoprotection against oxidative stress. Although rapamycin blocks Rps6-dependent mTORC2 activation, mTORC2 is still activated by an alternative signaling pathway, demonstrating the redundancy in cardioprotective signaling.
Conclusion
Activation of mTORC2 plays a pivotal role in cardioprotection, and Rps6 is a convergence point of cardioprotective signaling, providing positive feedback regulation of mTORC2/Akt signaling.
Keywords: mTOR, Akt, preconditioning, rapamycin, insulin, signaling pathways, ischemic reperfusion injury
INTRODUCTION
Reperfusion therapy has improved the prognosis of patients with acute myocardial infarction, but is insufficient in 25% patients, who have poor prognosis.1 Therefore, there is a need for adjunctive therapy in addition to reperfusion to improve clinical outcome. Several signaling pathways induced by cardioprotective interventions, such as ischemic preconditioning (IPC), ischemic postconditioning, and their mimetics, have been demonstrated. However, how these signaling pathways confer protection from cell death and how these pathways are amplified are not well understood.2
Phosphatidylinositol-3-kinase (PI3K)/Akt signaling is a major branch in cytoprotective signaling. Interventions that activate the PI3K/Akt pathway such as IPC, insulin, erythropoietin, δ-opioid receptor agonists, and ischemic postconditioning protect the heart from ischemia/reperfusion injury.2,3,4,5 The protective effect of IPC is abolished by PI3K inhibitors3 and in PI3Kγ knockout mice or mice with a catalytically inactive mutant PI3Kγ.6,7 Full activation of Akt requires phosphorylation at Thr308 by PDK1 and Ser473 by mTOR complex 2 (mTORC2), respectively. Using PDK1 hypomorphic mutant mice with reduced expression of PDK1, a previous study showed that PDK1 was essential for IPC in perfused mouse hearts.8 However, there are no available data concerning the role of mTORC2 on IPC-induced protection. A recent thorough study by Miyamoto et al. demonstrates that enhancement of Akt phosphorylation at Ser473, by PHLPP-1 deletion, suppresses cell death and ischemia/reperfusion injury, which suggests that mTORC2 is involved in cardioprotection.9
There is evidence of tight coupling between activation of PI3K/Akt signaling and suppression of mitochondrial permeability transition pore (mPTP) opening, which triggers cell death. First, multiple pro-survival signaling pathways, including Akt, converge on GSK-3β and inhibition of GSK-3β suppresses opening of the mPTP after reperfusion and ATP hydrolysis during ischemia.2,10 Second, Akt/endothelial nitric oxide synthase (eNOS)/Nitric oxide (NO) signaling induces S-nitrosylation (SNO) of proteins, which is proposed to reduce ischemia/reperfusion injury and increase the threshold for mPTP opening.11,12 Finally, treatment with leukemia inhibitory factor suppresses mPTP opening through Akt-mediated binding of hexokinase II with mitochondria, which is implicated in the protective mechanism of IPC.13,14
In the present study, we characterized the regulation of mTORC2 by cardioprotective interventions and obtained new insights into the mechanisms by which Akt signaling suppresses cell death. We demonstrate that mTORC2 plays a crucial role in cardioprotection. Furthermore, ribosomal protein S6 (Rps6) is identified as a downstream target of the PI3K/Akt/mTOR signaling cascade. Interestingly, Rps6 regulates pro-survival ribosomal mTORC2 signaling, which serves as a positive feedback loop in Akt activation. Akt signaling can have both beneficial and detrimental effects, cardioprotection with acute activation, hypertrophy with more chronic activation. Better understanding of the role of this signaling in cardioprotection will provide opportunities for development of novel cardioprotective interventions, with fewer detrimental effects.
METHODS
Detailed Methods are in the Online Supplement at http://circres.ahajournals.org.
Animals
This study was conducted in accordance with The Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication No. 85-23, revised 1996) and approved by the Institutional Laboratory Animal Care and Use Committee. Male C57BL/6 mice (11 to 15 weeks) were obtained from The Jackson Laboratory (Bar Harbor, ME).
Cell culture
Neonatal rat ventricular myocytes (NRVM) were isolated as described previously.15 HEK293 cells, human embryonic kidney cells, were obtained from ATCC.
Perfusion protocols
Hearts were perfused as previously reported,11 and IPC was 4 cycles of 5 min ischemia and 5 min reperfusion. Ischemia/reperfusion injury was induced by 20 min global ischemia, with 120 min reperfusion for infarct measurement.11
Immunoblotting and Immunoprecipitation
Samples for electrophoresis were total tissue homogenates or mitochondrial fractions prepared by differential centrifugation as previously reported.16,17
mTORC2 activity
We used a method reported by Huang with slight modification.18
RESULTS
IPC activates mTORC2
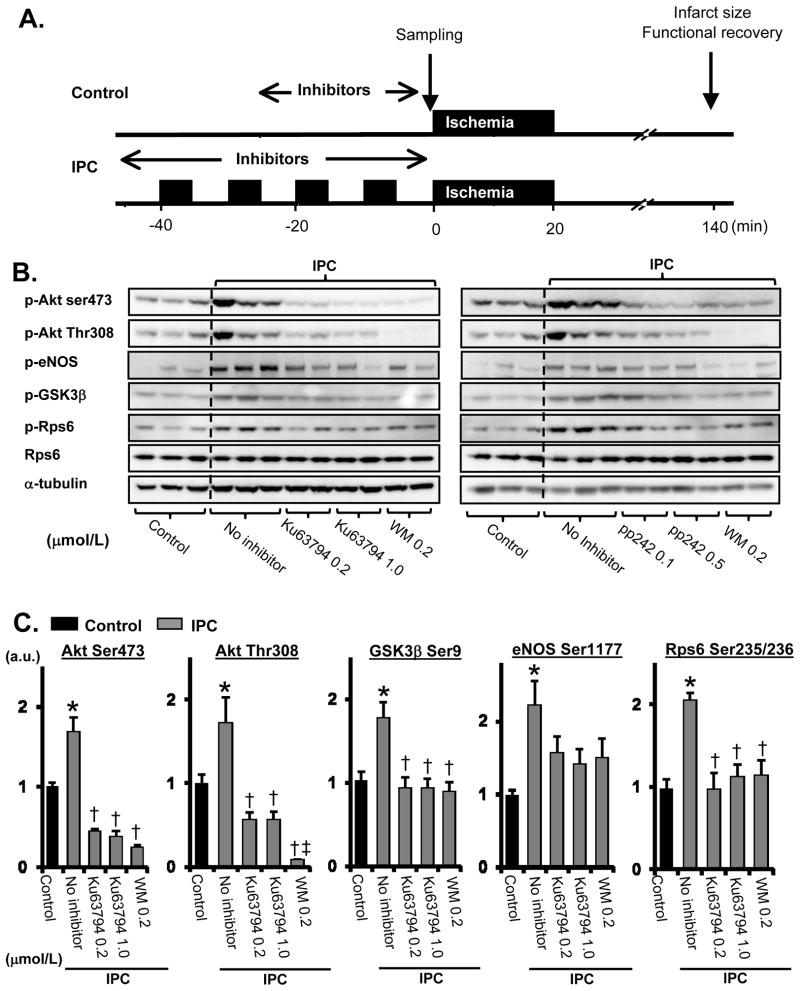
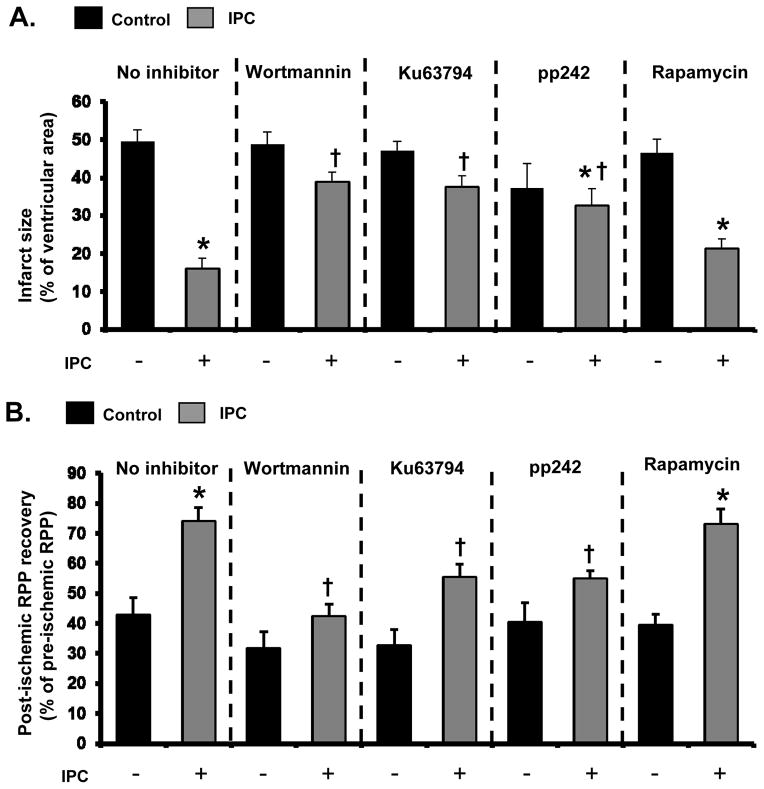
We studied the role of mTOR in IPC-induced phosphorylation of proteins involved in cardioprotection, using the protocols in Figure 1A. The effect of different inhibitors was assessed on several key signaling molecules. IPC significantly increased phosphorylation of Akt-Ser473, Akt-Thr308, GSK3β, eNOS, p70S6K, and Rps6 in mouse heart (Figure 1B–C, Online Figure II), and the ATP competitive mTOR inhibitors Ku63794 and pp242, inhibited the phosphorylation of all of these proteins. Wortmannin, a PI3K inhibitor, also blocked the increase in phosphorylation of these proteins, and inhibition of Akt-Thr308 phosphorylation was greater than that observed with mTOR inhibitors (Figure 1B–C). To further explore the role of mTORC2 on Akt-Ser473 phosphorylation, we measured mTORC2 activity. We immunoprecipitated mTORC2 using an antibody against rictor and recombinant Akt was used as substrate. IPC increased mTORC2 activity by 1.8 fold (Figure 1D). When preconditioning was performed in the presence of wortmannin or Ku63794, mTORC2 activity was markedly reduced, as indicated by less phosphorylation of recombinant Akt on Ser473. A recent study showed that IKKε can direct phosphorylate Akt on Ser473 in a PI3K-dependent manner. 19 IPC enhanced the ability of immunoprecipitated IKKε to phosphorylate Akt-Ser473 (Online Figure III). However, Ku63794 did not prevent IKKε activation by IPC (Online Figure III) but blocked phosphorylation of Akt-Ser473 by IPC, indicating the importance of mTORC2 in IPC. Thus, since mTORC2 is responsible for Ser473 phosphorylation and Rps6 is a downstream target of the Akt/mTORC1/p70S6K pathway, our results suggest that both mTORC1 and mTORC2 are involved in IPC-induced phosphorylation of keys molecules involved in cardioprotection.
Figure 1. IPC induces mTORC2 activation in perfused mouse heart.
A, Experimental protocol. The following inhibitors were perfused: Wortmannin 200 nmol/L, Ku63794 1 μmol/L, pp242 0.5 μmol/L, Rapamycin 1 nmol/L. B, Effects of mTOR inhibitors on IPC-induced Akt signaling. Two structurally different mTOR inhibitors (Ku63794 or pp242) and wortmannin (WM) were infused from 5 min before the IPC protocol until the end of IPC. Samples were taken at the end of IPC. C, Summarized data of effect of Ku63794 on IPC-induced Akt signaling. Levels of phosphorylated kinases were normalized to α-tubulin levels. Black bar = Control, Gray bar = IPC. N = 4~5 in each group. D, Effects of Ku63794 and wortmannin on IPC-induced mTORC2 activation. Representative immunoblots and summarized data are shown. mTORC2 was immunoprecipitated using the antibody against rictor. The level of recombinant Akt-Ser473 phosphorylation was normalized to the level of total recombinant Akt. a.u. = arbitrary unit. N = 3 in each group. *P<0.05 vs. Control. †P<0.05 vs. IPC, no inhibitor. ‡P<0.05 vs. IPC+Ku63794 1.0.
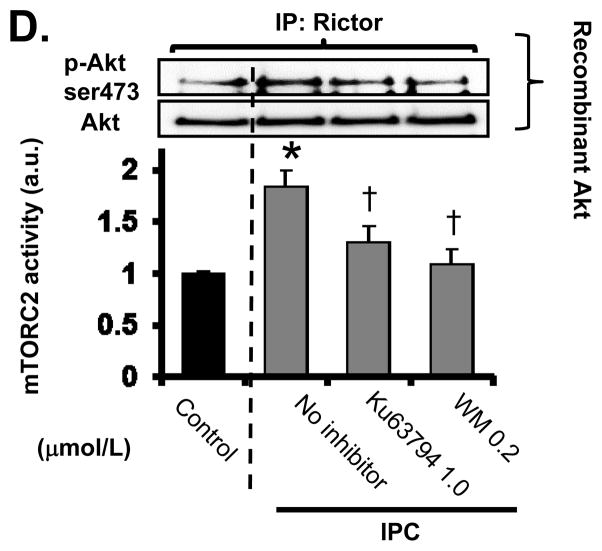
Cardioprotection afforded by IPC is mediated by PI3K/mTORC2 activation
We next evaluated the effect of PI3K/mTORC2 inhibition on IPC-mediated cardioprotection. As shown in Figure 2, 4 cycles of IPC limited infarct size from 49.6±3.1% to 15.9±2.8% and improved rate-pressure product (RPP) recovery from 42.7±6.0% to 74.1±4.6%. This protective effect of IPC on infarct size and RPP recovery was blocked by wortmannin (infarct size: 38.8±2.7%, RPP recovery: 42.3±4.2%) or the mTOR inhibitors Ku63794 (infarct size: 37.5±3.1%, RPP recovery: 55.4±4.3%) and pp242 (infarct size 32.5±4.6% and RPP recovery 55.0±2.5%). In contrast to the effect of dual mTOR inhibitors, infusion of rapamycin, a mTORC1 inhibitor, did not modify the effect of IPC on infarct size (21.3±2.7%) and RPP recovery (73.1±5.0%). These findings suggest that mTORC2 activation is involved in the mechanism by which acute IPC reduces ischemia/reperfusion injury, and mTORC1 activation is not required.
Figure 2. Cardioprotection afforded by IPC is mediated by mTORC2.
A, Effect of inhibitors on infarct size. Infarct size is expressed as a percentage of ventricular area. B, Post-ischemic rate pressure product (RPP) recovery. RPP recovery after reperfusion is shown as a percentage of pre-ischemic RPP after stabilization. Black bar = Control, Gray bar = IPC. N = 5~7 in each group. *P<0.05 vs. Control. †P<0.05 vs. IPC, No inhibitor.
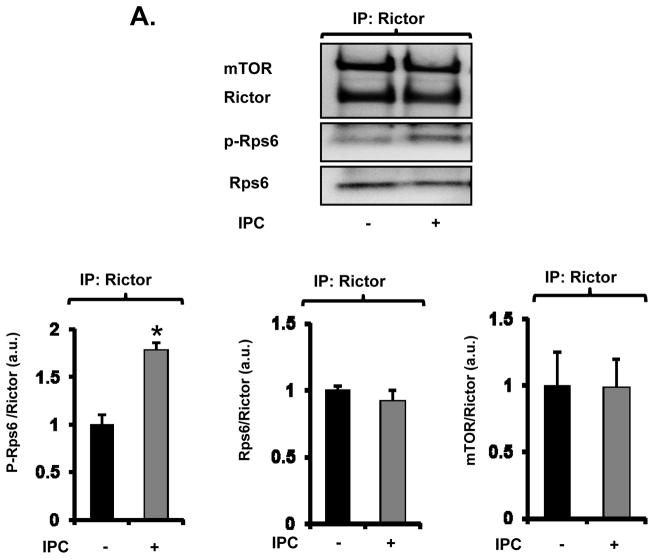
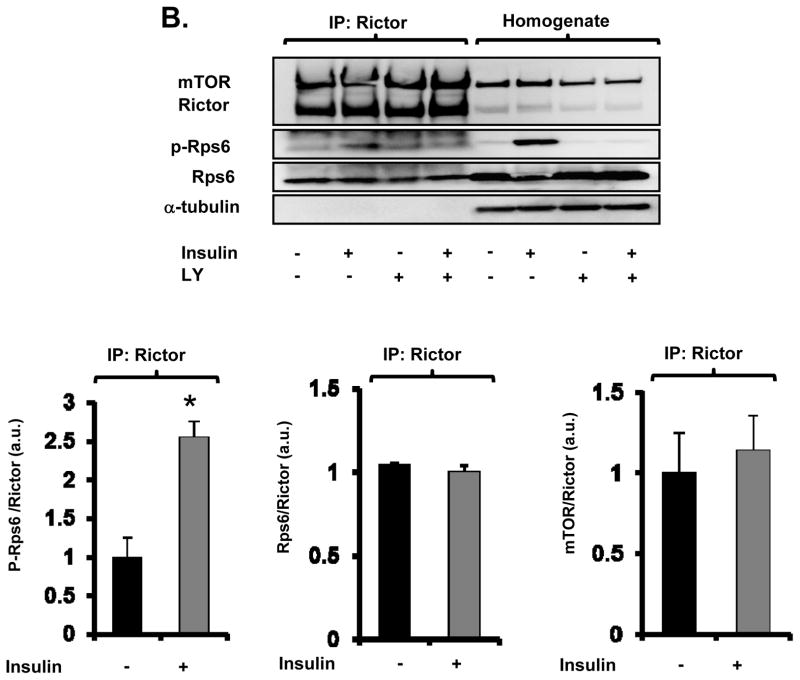
Figure 4. Rps6 is associated with mTORC2.
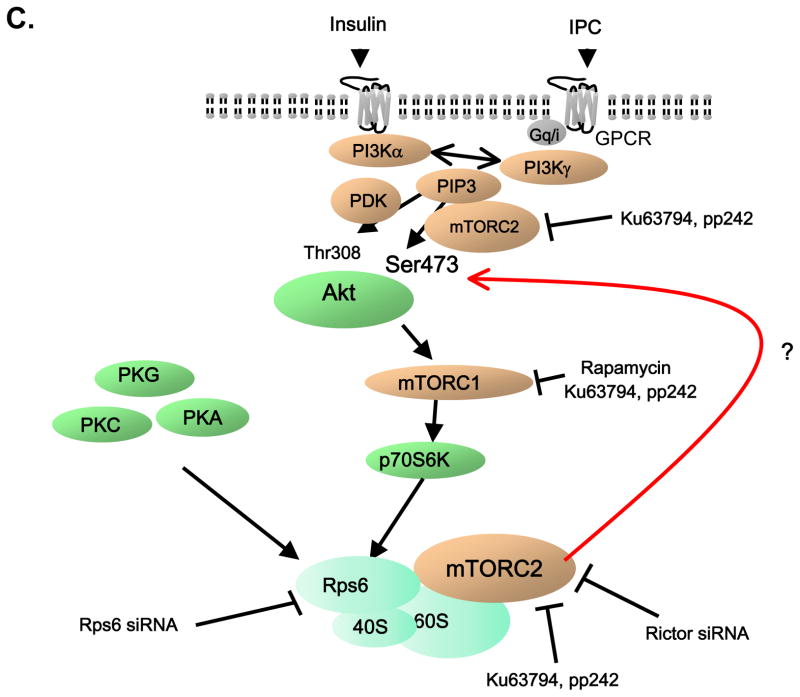
A and B, Effect of IPC (A) or insulin (B) on interaction of mTORC2 with Rps6 in mouse hearts (A) or HEK293 cells (B), respectively. mTORC2 was immunoprecipitated with anti-rictor antibody from mouse heart treated with or without IPC. Representative immunoblots and summarized data are presented. IP = Immunoprecipitation. a.u. = arbitrary unit. * P<0.05 vs. Control. N = 3 in each group. C, Schema of protective signaling by IPC and insulin, highlighting the stimulatory effect of Rps6 phosphorylation on mTORC2 (Red line).
Cardioprotective signals mediated by the PI3K/Akt pathway converge on Rps6
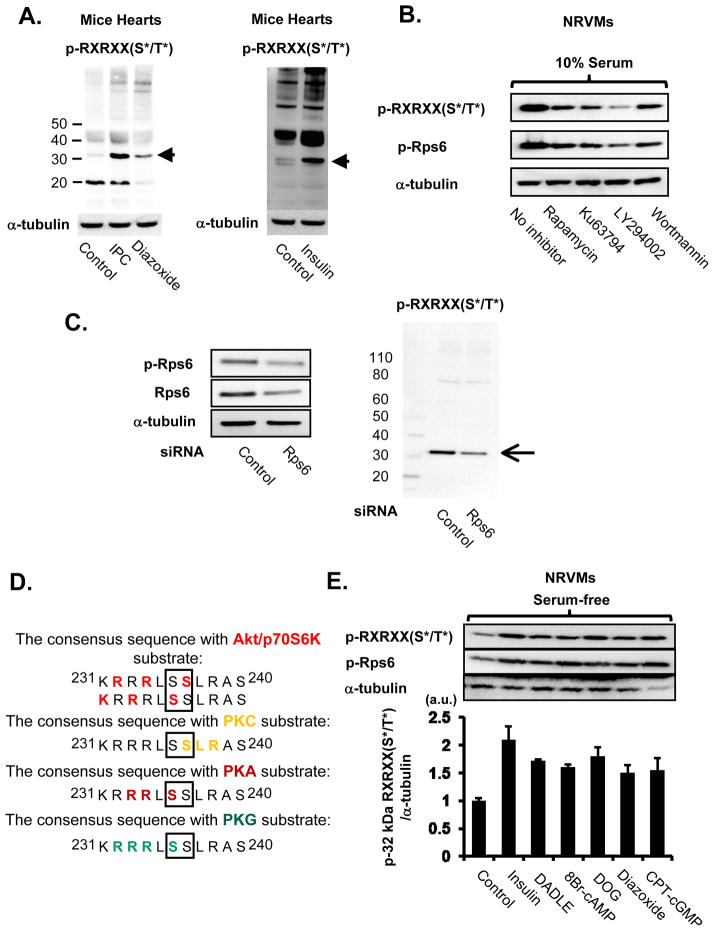
Akt activation is important in cardioprotective signaling, and we sought to identify new possible downstream targets in the PI3K/Akt/mTOR signaling cascade. We initially screened for downstream proteins that were modulated by cardioprotective interventions, using an antibody that detects phospho-Ser/Thr on the conserved motif RXRXX(S*/T*), where X is any amino acid, R is Arg (or Lys), and S*/T* denotes phosphorylatable serine/threonine residues.20,21 Several kinases can phosphorylate this motif (Figure 3D), including Akt, PKC, PKA, PKG, and the 70kDa protein S6 kinase (p70S6K). First, the effects of IPC and insulin, PI3K/Akt activators, were examined. Among the proteins that showed increased phosphorylation by IPC or insulin was a 32 kDa protein (Figure 3A). Surprisingly, 100 μmol/L of diazoxide, another well-known IPC mimetic, also increased phosphorylation of this 32 kDa protein (Figure 3A). In NRVM, wortmannin and LY294004, PI3K inhibitors, reduced phosphorylation of this 32kDa protein (Figure 3B). Because the 70kDa protein S6 kinase (p70S6K) favors substrates that have the conserved motif RXRXX(S*/T*) and previous studies identified a 30–32 kDa protein that is phosphorylated by receptor agonists that activate PI3K in cell culture as Rps6 20,21, we examined the effect of mTOR inhibitors on phosphorylation of this 32kDa protein. Phosphorylation was reduced by rapamycin, a mTORC1 inhibitor, and Ku63794, a ATP-competitive dual mTOR inhibitor (Figure 3B), indicating that phosphorylation of this 32 kDa protein is regulated by the mTORC1/p70S6K pathway (Figure 3B).
Figure 3. Cardioprotective signals phosphorylate Rps6.
A, Effect of IPC, diazoxide, and insulin on protein phosphorylation. Diazoxide (100 μmol/L) and insulin (1 mU/ml) were perfused for 20 min. B, Effect of protein kinase inhibitors on a 32 kDa protein phosphorylation. The following drugs were applied to 10% serum-stimulated NRVM for 30 min: Rapamycin 10 nmol/L, Ku63794 1 μmol/L, LY294004 20 μmol/L, Wortmannin 200 nmol/L. C, Effect of siRNA-mediated knockdown of Rps6 on expression level of a 32 kDa protein in NRVM. Representative immunoblots for p-Rps6 Ser235/236, Rps6, α-tubulin, and p-Akt substrate are presented. D, Amino acid sequence of mouse Rps6. The sequence around Ser235 and Ser236 is potentially a site for phosphorylation by Akt, p70S6K, PKC, PKA, and PKG. E, Effect of cytoprotective signals on Rps6 phosphorylation. The following drugs were applied to serum-depleted NRVM: Insulin 200 nmol/L, DADLE 300 nmol/L, 8Br-cAMP (PKA activator) 500 μmol/L, DOG (PKC activator) 10 μmol/L, Diazoxide 100 μmol/L, 8-CPT-cGMP (PKG activator) 500 μmol/L. Summarized data are shown in the lower panel (N = 3).
To corroborate our hypothesis that this 32kDa protein is Rps6, we performed western blots with an antibody that detects phosphorylation of Rps6 on Ser235 or Ser236, and found that the bands for the 32kDa phosphoprotein identified by the phospho-RXRXX(S*/T*) antibody and by the p-Rps6 antibody migrated in exactly the same location on the gel and the intensity changed in parallel (Figure 3B, Online Figure IV). We transfected HA-tagged Rps6S235A/S236A, with Ser replaced by Ala at 235 and 236 on RpS6, and its control (Rps6WT) in HEK293 cells. Rps6WT but not Rps6S235A/S236A was clearly detected by immunoblotting with the phospho-RXRXX(S*/T*) antibody (Online Figure IV). Furthermore, we used siRNA against Rps6 and observed a decrease in the 32 kDa phosphorylation band, confirming that Rps6 is the main 32kDa phosphoprotein that increases with cardioprotective interventions (Figure 3C). Stimulation with insulin, DADLE, 8Br-cAMP, DOG, diazoxide, and 8-CPT-cGMP all increased phosphorylation of Rps6 at Ser235 or Ser236 (Figure 3E, Online Figure V). Previous studies showed that Akt phosphorylation is reduced during ischemia and increased after reperfusion.22. We found that in perfused mouse hearts, the pattern of Rps6 phosphorylation during ischemia/reperfusion was also similar to Akt phosphorylation and GSK3β phosphorylation, a direct Akt substrate (Online Figure VI). IPC alone significantly increased phosphorylation of Rps6, and a significant difference between control and IPC hearts was maintained during ischemia and after reperfusion (Online Figure VI). Taken together, the data indicate that Rps6 phosphorylation is a convergence point of multiple cardioprotective signals.
Finally, we examined whether Akt can directly phosphorylate Rps6. In vitro analysis showed that active Akt could not phosphorylate Rps6 immunoprecipitated from Rps6-overexpressed HEK293 cells (Online Figure VII). Thus, phosphorylation of Rps6 by IPC and insulin is primarily regulated by the Akt/mTORC1/p70S6K pathway, but further analysis is needed to assess whether Akt can directly phosphorylate Rps6 in vivo.
Rps6 is associated with mTORC2 and regulates pro-survival mTORC2/Akt signaling
The primary role of Rps6 is protein translation, but the involvement of protein translation in the protective mechanisms of classical/early IPC is controversial.2 A recent study demonstrated that insulin induces the association of ribosomes with mTORC2 and enhances mTORC2 activity in a protein translation-independent mechanism.23 However, how insulin activates the mTORC2-ribosome complex remains unclear. We sought to determine if Rps6 phosphorylation induced by protective signaling is a key step to mTORC2 activation.
First, the association of Rps6 with mTORC2 was examined. mTOR exists in two different complexes. mTOR interacts with raptor to form mTORC1 and with rictor to form mTORC2. To immunoprecipitate mTORC2, anti-rictor antibody was used. In mouse hearts, phospho-Rps6 was co-immunoprecipitated with mTORC2 under basal conditions and the amount of phospho-Rps6 bound to mTORC2 was increased by IPC (Figure 4A). IPC did not modulate the levels of mTOR and Rps6 in the rictor immunoprecipitates (Figure 4A). Insulin treatment in HEK293 cells also increased the levels of phospho-Rps6 but not Rps6 bound to mTORC2, which was blocked by pretreatment with the PI3K inhibitor LY294004 (Figure 4B). To examine whether Rps6 bound to mTORC2 in the ribosomal protein complex is phosphorylated, immunoprecipitation using the antibody against Rpl26, a protein in the 60S ribosomal subunit, was performed.23 Insulin increased phosphorylation of Rps6 in the homogenate and the amount of p-Rps6 in the ribosomal complex, though mTORC2 levels in the ribosomal complex were not modulated by insulin (Online Figure VIII). These findings suggest that Akt signaling increases the amount of phospho-Rps6 that is bound to mTORC2 in the ribosomal complex.
Next, we examined the role of Rps6 in mTORC2 signaling. mTORC2 is the kinase that phosphorylates Akt on Ser473. We hypothesized that activation of PI3K/Akt signaling induces Rps6 phosphorylation, which in turn leads to phosphorylation of Ser473 through mTORC2 activation. In this way, Rps6 serves as a positive feedback loop in Akt signaling (Figure 4C). We investigated the importance of Rps6 phosphorylation in signaling and in cytoprotection.
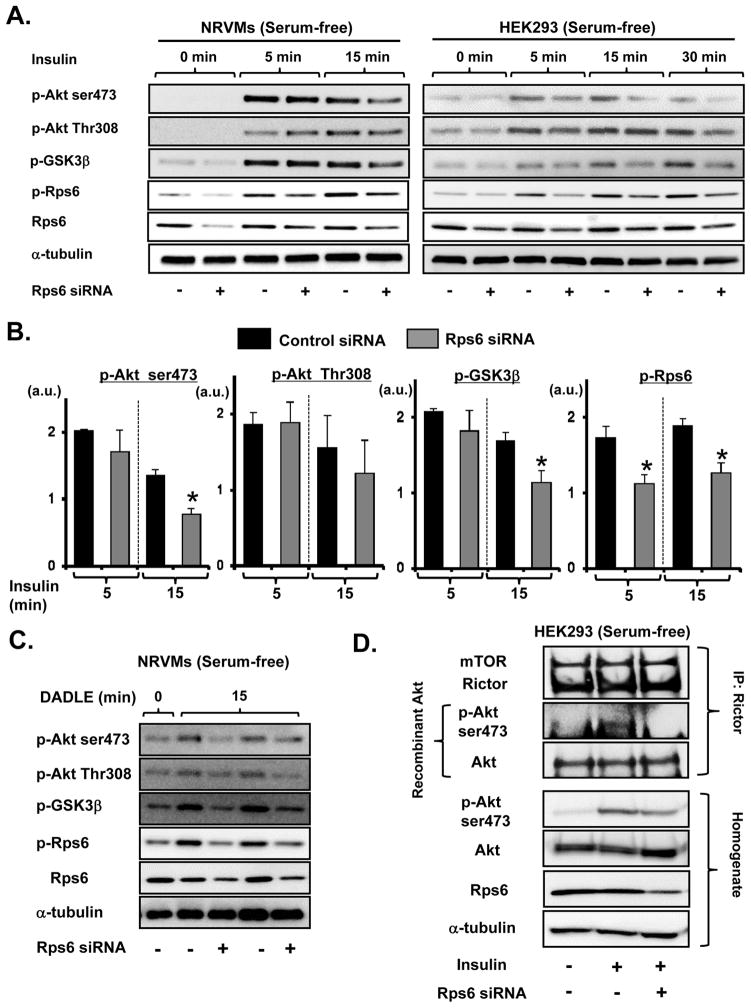
To study the role of Rps6 phosphorylation in these signaling pathways, the level of Rps6 phosphorylation was reduced by siRNA-mediated knockdown of Rps6 and by mTOR inhibitors. Insulin induced significant phosphorylation of Akt, GSK3β, and Rps6 in serum-free NRVM and HEK293 cells. The level of Akt-Ser473 phosphorylation was increased at 5 min after insulin addition and then declined (Figure 5A–B). On the other hand, the level of Rps6 phosphorylation was gradually augmented and sustained. Knockdown of Rsp6 in NRVM significantly reduced phosphorylation of Akt-Ser473 and GSK3β, but not Akt-Thr308 phosphorylation, after 15 min of insulin stimulation compared to control siRNA (Figure 5B). A similar trend in the effect of Rps6 knockdown on Akt/GSK3β signaling was observed in HEK293 cells (Figure 5A). Next, we examined the effect of pp242, a mTOR inhibitor, on phosphorylation of Akt and Rps6. Unlike the effect of Rps6 knockdown, phosphorylation of Akt-Ser473 was attenuated by pp242 even at 5 min after insulin addition (Online Figure IX). Thus, Rps6 is critically involved in the sustained mTORC2-mediated Akt-Ser473 phosphorylation and downstream effects of Akt signaling, but an Rps6-independent mechanism also contributes to phosphorylation of Akt-Ser473 by insulin.
Figure 5. Rps6 regulates mTORC2/Akt signaling.
A, Representative immunoblots showing the effect of siRNA-mediated knockdown of Rps6 on insulin-induced Akt/GSK3β signaling in NRVM and HEK293 cells. Insulin (200 nmol/L) treatment was performed at 24 h (HEK293 cells) or 40 h (NRVM) after siRNA transfection. B, Summarized data from insulin-induced Akt/GSK3β signaling in NRVM. Levels of phosphorylated kinases were normalized to α-tubulin levels. Black bar = Control siRNA transfection, Gray bar = Rps6 siRNA transfection. N = 4 in each group. *P<0.05 vs. Control. C, Effect of siRNA-mediated knockdown of Rps6 on DADLE-induced Akt/GSK3β signaling in NRVM. DADLE (300 nmol/L) treatment was performed at 40 h after siRNA transfection. Representative blots are shown. D, Effect of siRNA-mediated knockdown of Rps6 on insulin-induced mTORC2 activation in HEK293 cells. Insulin stimulation was performed at 24 h after siRNA transfection. Rictor immunoprecipitates from whole cell homogenates were prepared for in vitro kinase assay. Three independent experiments showed similar results.
While PI3Kα is involved in Akt activation by insulin, PI3Kγ mediates Akt phosphorylation through G-protein-coupled receptors (GPCRs) such as the δ-opioid receptor. To assess whether Rps6 is involved in the signaling pathway induced by [D-Ala2,D-Leu5]-Enkephalin (DADLE), a δ-opioid receptor agonist, we examined the effect of Rps6 knockdown on DADLE signaling. Rps6 knockdown attenuated phosphorylation of Akt-ser473 and GSK3β following 15 min of DADLE treatment (Figure 5C). Rsp6 down-regulation also decreased insulin-induced mTORC2 activity, assessed by an in vitro kinase assay of Akt phosphorylation in HEK293 cells (Figure 5D) and NRVM (data not shown). Conversely, overexpression of HA-Rps6 increased phosphorylation of Akt-ser473 under non-stimulated conditions, resulting in enhancement of endogenous Rps6 phosphorylation (Online Figure X). These findings demonstrate that Rps6 regulates mTORC2 activity induced by PI3K activation and mediates positive feedback signaling in mTORC2/Akt activation.
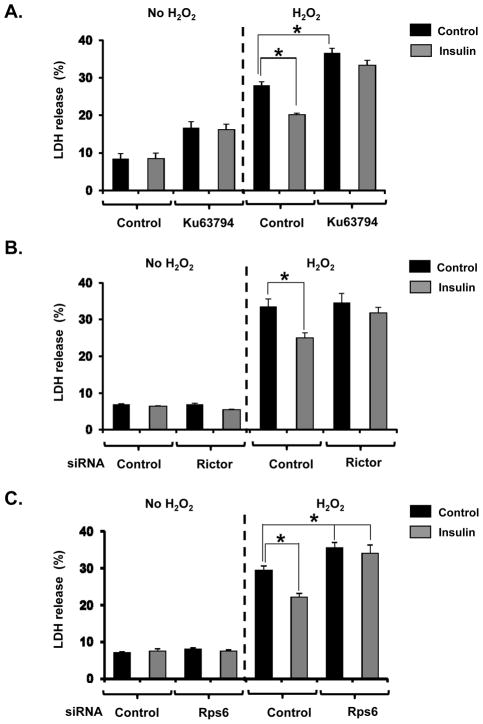
Finally, the role of mTORC2 and Rps6 in cytoprotection afforded by insulin was examined in NRVM. Incubation with 100 μmol/L H2O2 for 18 h induced cell death in ~30% of NRVM. Pretreatment with insulin significantly suppressed cell death by 70% compared with no pretreatment controls (Figure 6). mTOR inhibition by Ku63794 blocked cytoprotection induced by insulin, indicating that this experimental model simulated IPC-induced cardioprotection in perfused hearts. Consistent with our hypothesis, knockdown of rictor (Online Figure XI) or Rps6 abolished the protection afforded by insulin. Thus, mTORC2 and Rps6 are critical for the protective effect induced by insulin.
Figure 6. Rps6 and mTORC2 mediated insulin-induced cytoprotection.
A, Effect of Ku63794, a mTOR inhibitor, on insulin-induced cytoprotection in NRVM. Summarized data are shown. To induce cell death, cells were incubated with 100 μmol/L H2O2 for 16 h. One hour before addition of H2O2, cells were pretreated with insulin or vehicle. Half an hour before addition of insulin or vehicle, cells were incubated with Ku63794 or DMSO. LDH activity is expressed as the percentage of total LDH activity. Black bar = Control, Gray bar = Insulin. N = 7 in each group. *P<0.05 vs. Control cells treated with H2O2. B and C, Effects of siRNA-mediated knockdown of rictor (B) or Rps6 (C) on insulin-induced protection against oxidative stress in NRVM. Summarized data is shown. Experiments were performed at 48 h after siRNA transfection in NRVM. N = 8 in each group. *P<0.05 vs. Control siRNA-transfected cells treated with H2O2.
Because it is possible that Rps6 knockdown itself could inhibit overall protein synthesis and induce cell injury, the effect of Rps6 knockdown on confounding factors in these experimental results was examined. Protein levels of mTOR, rictor, Akt, GSK3β, Rpl26 and PHLPP-1, a phosphatase that dephosphorylates p-Akt at ser473, were comparable between NRVM transfected with control siRNA and Rps6 siRNA (Online Figure XII). Although deficiency of ribosomal proteins including Rps6 can induce p53-dependent cell cycle arrest and apoptosis, 24 p53 was not induced by Rps6 knockdown in our experimental protocol (Online Figure XIII). Thus, our protocol, which reduced Rps6 by ~50%, had no detectable effect on protein levels or cell injury.
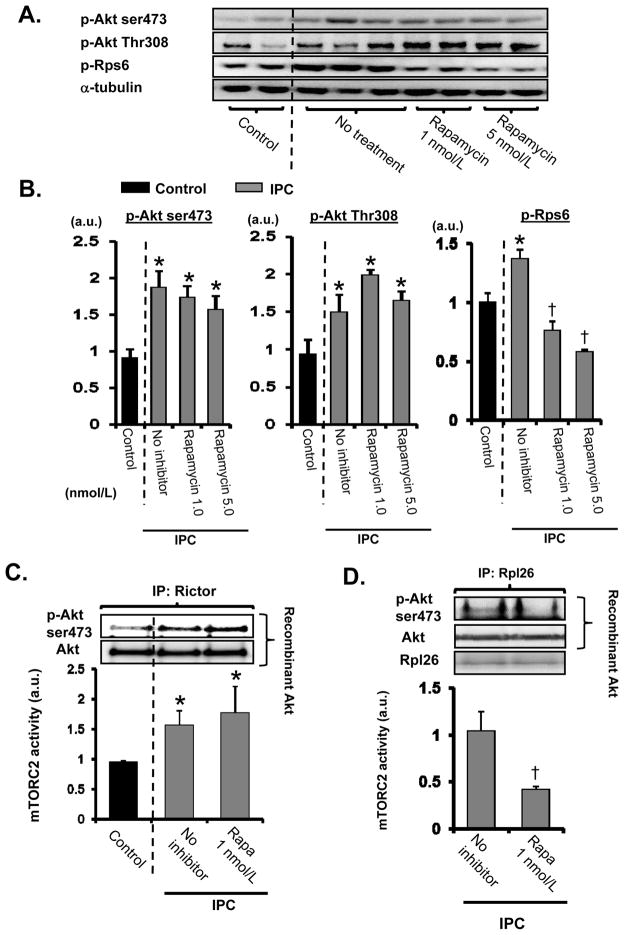
Reduction of Rps6 phosphorylation by rapamycin reduced ribosomal mTORC2 activity, but did not eliminate the IPC-induced overall increase in mTORC2 activity
Rapamycin reduces Rps6 phosphorylation through mTORC1 inhibition, which should reduce mTORC2 activation according to our Rps6 knockdown data. Phosphorylation of Rps6 by IPC was significantly reduced by rapamycin (Figure 7A–B). Nevertheless, rapamycin did not affect the IPC-induced increase in Akt phosphorylation at Thr308 or Ser473 (Figure 7A–B). In addition, rapamycin treatment failed to reduce the increase in mTORC2 activity by IPC (Figure 7C). It is well known that the PI3K/Akt pathway is up-regulated by mTORC1 inhibition due to rapamycin treatment through disruption of negative feedback control of PI3K/Akt signaling.25 We hypothesize that rapamycin reduces the activity of mTORC2 bound to ribosomes, but enhances ribosome independent-mTORC2 activation (Online Figure XIV). To evaluate ribosomal mTORC2 activity, we immunoprecipitated mTORC2 using an antibody against Rpl26 (Online Figure VIII). In contrast to total mTORC2 activity, measured using mTORC2 immunoprecipitated with rictor antibody, IPC-induced ribosomal mTORC2 activity was reduced by rapamycin (Figure 7D). These data suggest that enhancement of mTORC2 activity by IPC is maintained by activation of mTORC2 through a ribosome-independent mechanism, despite a decrease in ribosomal mTORC2 activation through reduction of Rps6 phosphorylation (Online Figure XIV).
Figure 7. Effects of rapamycin on IPC-induced PI3K/Akt signaling and mTORC2 activation.
A and B, Rapamycin was infused from 5 min before the start of IPC until the end of IPC. Ventricular samples were prepared at the end of the protocol. Representative immunoblots (A) and summarized data (B) are presented. Levels of phosphorylated proteins were normalized to α-tubulin levels. IPC = ischemic preconditioning. a.u. = arbitrary unit. N = 4~5 in each group. C, The effect of rapamycin on IPC-induced mTORC2 activation. mTORC2 was immunoprecipitated using antibody against rictor. N = 3 in each group. D, The effect of rapamycin on IPC-induced ribosomal mTORC2 activation. mTORC2 was immunoprecipitated using antibody against Rpl26. a.u. = arbitrary unit. N = 3 in each group. *P<0.05 vs. control. †P<0.05 vs. IPC no inhibitor.
DISCUSSION
This is the first study to examine specifically the role of mTORC2 in cardioprotection. The novel findings obtained from this study are as follows: (1) mTORC2 activation plays a pivotal role in the cardioprotective mechanisms of IPC including phosphorylation of GSK3β and eNOS. (2) Rps6 was identified as a downstream target of PI3K/Akt pathway and multiple cytoprotective signals phosphorylated Rps6 at Ser235/236. (3) Direct reduction of Rps6 phosphorylation by Rps6 knockdown attenuated insulin- and opioid-induced mTORC2 activation and Akt-Ser473 phosphorylation. (4) Pharmacological and siRNA-mediated disruption of the Rps6/mTORC2 pathway eliminated the protective effect of insulin-induced pharmacologic preconditioning. (5) Up-regulation of Rps6 increased phosphorylation of Akt-ser473. (6) Reduction of Rps6 phosphorylation by rapamycin also decreased ribosomal mTORC2 activity, but IPC-induced mTORC2 activation and Akt-Ser473 phosphorylation persisted, through stimulation of nonribososmal mTORC2 activity. These data show the essential role of mTORC2 activation in cytoprotection induced by PI3K/Akt signaling, and the importance of Rps6 phosphorylation in mTORC2 activation.
The pivotal role of mTORC2 in cardioprotective signaling
Cardioprotection requires activation of Akt. Full activation of Akt requires phosphorylation at Thr308 and Ser473.26 The literature suggests that only PDK1 phosphorylates Akt at Thr308 in the activation loop, while mTORC2 is the primary Akt-Ser473 kinase, though this is not the sole mechanism of Akt phosphorylation at Ser473.19,26,27 Using PDK1 hypomorphic mutant mice with reduced expression of PDK1, a previous study showed that PDK1 was essential for IPC in perfused mouse hearts.8 However, there are no available data concerning the role of mTORC2 on IPC-induced protection. In our report, the infarct size limitation afforded by IPC was blocked by wortmannin, a PI3K inhibitor, and ATP competitive dual mTOR inhibitors, which inhibit both mTORC1 and mTORC2 activity, but not rapamycin, a mTORC1 inhibitor. IPC-induced mTORC2 activation and Akt-Ser473 phosphorylation were blocked by wortmannin and dual mTOR inhibitors but not rapamycin. Although mTOR inhibition can attenuate IPC-induced Akt-Thr308 phosphorylation,28 the decrease in Akt-Thr308 phosphorylation by mTOR inhibitors was significantly less than by wortmannin, indicating that mTOR inhibitors did not completely inhibit PI3K or PDK1 activity. Finally, constitutive phosphorylation of Akt-Ser473 by knockout of PHLPP-1, an Akt-Ser473 specific phosphatase, protected myocardium from ischemia/reperfusion injury in mice.9 Thus, mTORC2 activation plays a crucial role in IPC-induced cardioprotection. Although we focused on the role of mTORC2 in the setting of acute ischemia/reperfusion injury, a very recent study showed that mTORC2 activation reduced apoptosis in remote areas after myocardial infarction induced by permanent coronary artery occlusion and suppressed post-infarct ventricular remodeling.29 Taken together with our present data, mTORC2 is an attractive target in the therapy of myocardial infarction. Interestingly, mTORC2 may regulate additional kinases other than Akt. It is well known that mTORC2 phosphorylates PKCα, and a recent study showed that mTORC2 phosphorylated PKCε at Ser729.30 Because PKCε has a pivotal role in IPC-induced cardioprotection, it is worth considering that IPC-induced mTORC2 activation may also regulate PKCε activity. Further analysis is needed to demonstrate the role of mTORC2 in phosphorylation of PKCε and other protective kinases in the heart.
The involvement of Rps6 in cardioprotective signaling
Rps6 is a component of the small 40S ribosomal subunit. The fundamental role of ribosomes is protein translation, but some protein translation-independent functions have been reported.31 A recent study by Zinzalla et al. demonstrated that ribosomes are involved in insulin-induced mTORC2 activation in cancer cell lines.23 In that study, knockdown of three different ribosomal proteins decreased levels of other ribosomal proteins, indicating impaired ribosomal assembly. Under those conditions, H2O2-induced apoptosis was higher and insulin-induced mTORC2 activation was reduced. Further analysis suggested that increased association of mTORC2 with ribosomes by insulin is the mechanism responsible for insulin-induced mTORC2 activation. In our report, IPC and insulin did not affect the level of association of mTORC2 with ribosomes, but enhanced phosphorylation of Rps6 in the ribosome/mTORC2 complex (Figure 4). Rps6 knockdown inhibited mTORC2 activation by insulin and DADLE (Figure 5), and insulin-induced cytoprotection was lost by Rps6 knockdown (Figure 6). Thus, Rps6 is a primary mediator in the cytoprotection that occurs when the PI3K/mTORC2 pathway is activated by pharmacologic agents such as insulin, in cardiomyocytes and mouse hearts.
The involvement of protein translation in the mechanism of IPC is controversial. We have found that cardioprotective interventions modulate subcellular protein levels by post-translational modification and regulation of mitochondrial import and degradation,32,33 thus in a protein translation-independent manner An earlier study by Downey’s group showed that pharmacological inhibition of protein translation did not affect IPC-induced infarct size limitation.34 Furthermore, Zinzalla et al. reported that protein translation inhibitors did not affect phosphorylation of Akt-Ser473 by insulin. 23 Thus, it appears that cardioprotection can be achieved independent of changes in protein translation.
Our data and the literature suggest that there is a complex connection between PI3K signaling and mTORC2 activation.35,36 A previous study using an in vitro kinase assay showed that immunoprecipitated mTORC2 was activated by PIP3, which can explain prompt phosphorylation of Akt-Ser473 by insulin or by IPC.36 In the present study, the level of Akt-Ser473 phosphorylation was increased at 5 min after insulin addition and then declined. On the other hand, Rps6 phosphorylation was maintained at a high level after insulin treatment (Figure 5A–B). Furthermore, significant differences in Akt-Ser473 phosphorylation between control siRNA- and Rps6 siRNA-transfected cells were observed at 15 min but not 5 min after insulin addition. In contrast, phosphorylation of Akt-Ser473 was attenuated by pp242 even at 5 min after insulin addition (Online Figure IX). These findings suggested that Rps6 phosphorylation contributed more to mTORC2 activation at 15 min after insulin addition than at earlier times, and that early activation of mTORC2 was induced by a different mechanism, presumably through direct activation by PIP3. Thus, there is Rps6-dependent and Rps6–independent mTORC2 activation, and Rps6–dependent mTORC2 activation serves as a positive feedback loop in Akt signaling (Online Figure XIV and XV). Similarly, with IPC, rapamycin blocks Rps6 phosphorylation but does not prevent Akt-Ser473 phosphorylation, which can occur by the Rps6-independent process. Since there is only a 5-minute gap between IPC and the sustained period of ischemia, the ability to sustain mTORC2 activation would be less important in our protocol of IPC than in some models of pharmacologic preconditioning. Multiple negative feedback mechanisms in PI3K/Akt/mTOR signaling have been proposed and demonstrated, but there are little data regarding positive feedback mechanisms, including the Rps6-dependent pathway, prior to our study.
Our data suggest that Rps6 phosphorylation is important for mTORC2 activation through the mTORC1/Rps6-dependent pathway, particularly for sustained activation. Ruvinsky et al. generated a knock-in mouse (rps6P−/−) encoding a mutant Rps6 harboring Ala substitutions at all five C-terminal phosphorylation sites (Ser235, Ser236, Ser241, Ser244, Ser247).37 In that study, comparable phosphorylation of Akt-Ser473 at 5 min after intraperitoneal injection of insulin was observed in rps6P−/− compared with wild type mice. Although this early response to insulin in rps6P−/− was similar to that in Rps6 siRNA-transfected NRVM (Figure 5A–B), the time course of Akt phosphorylation by insulin stimulation in rps6P−/− was not examined. Furthermore, there is the possibility that other ribosomal proteins might also mediate this signaling, particularly in a chronic model of impaired Rps6 signaling. In addition to the study by Zinzalla et al., previous studies reported that Rps3 knockdown increased H2O2-induced cell death in HEK293 cells, and Rps3 was identified as an Akt substrate.38 Interestingly, our study showed that IPC induced SNO of ribosomal proteins including Rps6 in perfused mouse hearts.39 The role of other ribosomal proteins and SNO of ribosomal proteins in regulation of mTORC2 warrants further investigation. Nevertheless, Rps6 is an important transducer of mTORC2 activation because our data show that Rps6 knockdown resulted in less phosphorylation of Akt-Ser473 at 15 minutes after insulin treatment, and Rps6 knockdown eliminated the cytoprotection induced by pharmacologic preconditioning.
The effect of rapamycin on ischemia/reperfusion injury and cardioprotection
Previous studies have shown that infarct size limitation afforded by IPC, ischemic postconditioning, and their mimetics can be blocked by rapamycin in rat regional ischemia models and a mouse perfused heart model, though there are conflicting results.2,4,40,41,42,43 Because p70S6K can directly phosphorylate GSK3β, rapamycin-induced reduction of GSK3β phosphorylation was proposed as a primary mechanism of the inhibitory effect of rapamycin on cardioprotection. In contrast, pretreatment with rapamycin alone reduced infarct size in mouse global ischemia models and protected from hypoxia/reoxygenation-induced cardiomyocyte death.43,44 In addition, our previous study showed that the infarct size-limiting effect of IPC was not necessarily correlated with the level of p70S6K phosphorylation.7 The effect of rapamycin on signaling downstream of PI3K is highly complex. Rapamycin reduces mTORC1 activity, which decreases phosphorylation of p70S6K and Rps6. Because a decrease in Rps6 phosphorylation by Rps6 knockdown reduced mTORC2 activity (Figure 5), it is possible that rapamycin could attenuate IPC-induced Akt phosphorylation and mTORC2 activation. However, it is well known that the PI3K/Akt pathway is up-regulated by mTORC1 inhibition due to rapamycin treatment or raptor knockout through disruption of negative feedback control of PI3K/Akt signaling (Online Figure XIV).25,45 In our report, rapamycin did not affect the IPC-induced increase in Akt phosphorylation and overall mTORC2 activity, though reduction of Rps6 phosphorylation by rapamycin decreased ribosomal mTORC2 activity (Figure 7). In addition, infarct size limitation afforded by multiple cycles of IPC was not blocked by rapamycin (Figure 2). A plausible explanation is that IPC activates pro-survival mTORC2 by two mechanisms, through a Rps6/ribosome-independent mechanism, and through Rps6-dependent ribosomal mTORC2 activation, and only the latter is reduced by rapamycin (Online Figure XIV). It will be important to analyze the time-dependent changes mediated by rapamycin in signaling pathways responsible for cardioprotection in future studies.
Conclusions
In summary, IPC activates multiple classes of receptors, leading to activation of redundant pro-survival signaling pathways. In particular, repetitive IPC augments activation of redundant signaling pathways so that blockade of a single signaling pathway in IPC does not abrogate protection because of compensation by other signaling pathways. Activation of multiple signaling pathways strengthens the protective effect. We find that mTORC2 plays a key role in cardioprotection through phosphorylation of Akt-Ser473, which results in full Akt activation. mTORC2 can be activated by a ribosomal Rps6-dependent pathway and by a nonribosomal Rps6-independent pathway. The ribosomal Rps6-dependent pathway confers sustained Akt activation which could be more useful for cardioprotection. Because multiple cardioprotective signaling pathways converge on mTORC2 and phosphorylation of Rps6, Rps6/mTORC2 signaling may be an attractive target for development of novel cardioprotective interventions.
Supplementary Material
Novelty and Significance.
What Is Known?
PI3K/Akt activation initiates multiple pathways of cytoprotective signaling induced by agonists of G-protein coupled receptors and ischemic preconditioning.
Full Akt activation requires dual phosphorylation, and mTOR complex 2 (mTORC2) has been shown to phosphorylate Akt on Ser473.
There is tight coupling between Akt activation and suppression of cell death.
What New Information Does This Article Contribute?
mTORC2 activation plays a pivotal role in cardioprotection.
Rps6 was identified as a downstream target of PI3K/Akt/p70S6K signaling and multiple cytoprotective signals increase phosphorylation of Rps6 on Ser235/236.
Phosphorylation of Rps6 by the PI3K/Akt/p70S6K pathway increases mTORC2 activity, leading to enhanced and longer-lived phosphorylation of Akt on Ser473.
The Rps6/mTORC2 signaling module is responsible for insulin-induced cytoprotection against oxidative stress.
Multiple cardioprotective signaling pathways have been identified, but the mechanisms by which these signaling pathways are amplified and how they confer protection from cell death are not well understood. We show that activation of mTORC2 plays a pivotal role in cardioprotection afforded by IPC and other cardioprotective interventions. We propose dual pathways of mTORC2 activation that are involved in cardioprotection. Activation of the PI3K/Akt pathway, a well-known cardioprotective signal, leads to Rps6 phosphorylation-mediated mTORC2 activation. Thus, Akt activation begets further Akt activation through Rps6/mTORC2 signaling. The reduction of Rps6 phosphorylation by rapamycin also blocks Rps6-dependent mTORC2 activation, but mTORC2 is still activated by an alternative signaling pathway, demonstrating the redundancy in cardioprotective signaling. However, ribosomal Rps6-dependent mTORC2 activation results in greater and more persistent Akt activation. Better understanding of the role of the dual pathways of mTORC2 activation in cardioprotection will likely provide opportunities for development of novel cardioprotective interventions.
Acknowledgments
SOURCES OF FUNDING
This study was supported by NIH grants 5R01HL039752 (to C.S.), Banyu Life Science Foundation International (to T.Y.), and the National Heart, Lung, and Blood Institute/NIH intramural program (to A.A. and E.M.), and AHA0830395N (to M.F.).
We thank Dr. Phillipe Roux for kindly providing Rps6 plasmids.
Nonstandard Abbreviations and Acronyms
- 8Br-cAMP
8-Bromoadenosine 3′,5′-cyclic monophosphate
- 8-CPT-cGMP
8-(4-Chlirophenylthio)-guanosine 3′,5′-cyclic monophosphorothioate
- DADLE
[D-Ala2, D-Leu5]-Enkephalin
- eNOS
Endothelial nitric synthase
- IPC
Ischemic preconditioning
- mTOR
Mammalian target of rapamycin
- mTORC2
mTOR complex 2
- mPTP
Mitochondrial permeability transition pore
- NRVM
Neonatal rat ventricular myocytes
- PI3K
Phosphatidylinositol-3-kinase
- Rpl26
Ribosomal protein L26
- Rps6
Ribosomal protein S6
- SNO
S-nitrosylation
Footnotes
DISCLOSURES
None.
References
- 1.Miura T, Miki T. Limitation of myocardial infarct size in the clinical setting: current status and challenges in translating animal experiments into clinical therapy. Basic Res Cardiol. 2008;103:501–513. doi: 10.1007/s00395-008-0743-y. [DOI] [PubMed] [Google Scholar]
- 2.Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev. 2008;88:581–609. doi: 10.1152/physrev.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tong H, Chen W, Steenbergen C, Murphy E. Ischemic preconditioning activates phosphatidylinositol-3-kinase upstream of protein kinase C. Circ Res. 2000;87:309–315. doi: 10.1161/01.res.87.4.309. [DOI] [PubMed] [Google Scholar]
- 4.Gross ER, Hsu AK, Gross GJ. Opioid-induced cardioprotection occurs via glycogen synthase kinase beta inhibition during reperfusion in intact rat hearts. Circ Res. 2004;94:960–966. doi: 10.1161/01.RES.0000122392.33172.09. [DOI] [PubMed] [Google Scholar]
- 5.Tsang A, Hausenloy DJ, Mocanu MM, Yellon DM. Postconditioning: a form of “modified reperfusion” protects the myocardium by activating the phosphatidylinositol 3-kinase-Akt pathway. Circ Res. 2004;95:230–232. doi: 10.1161/01.RES.0000138303.76488.fe. [DOI] [PubMed] [Google Scholar]
- 6.Ban K, Cooper AJ, Samuel S, Bhatti A, Patel M, Izumo S, Penninger JM, Backx PH, Oudit GY, Tsushima RG. Phosphatidylinositol 3-kinase gamma is a critical mediator of myocardial ischemic and adenosine-mediated preconditioning. Circ Res. 2008;103:643–653. doi: 10.1161/CIRCRESAHA.108.175018. [DOI] [PubMed] [Google Scholar]
- 7.Tong H, Rockman HA, Koch WJ, Steenbergen C, Murphy E. G protein-coupled receptor internalization signaling is required for cardioprotection in ischemic preconditioning. Circ Res. 2004;94:1133–1141. doi: 10.1161/01.RES.0000126048.32383.6B. [DOI] [PubMed] [Google Scholar]
- 8.Budas GR, Sukhodub A, Alessi DR, Jovanović A. 3′Phosphoinositide-dependent kinase-1 is essential for ischemic preconditioning of the myocardium. FASEB J. 2006;20:E1924–E1934. doi: 10.1096/fj.06-6252fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyamoto S, Purcell NH, Smith JM, Gao T, Whittaker R, Huang K, Castillo R, Glembotski CC, Sussman MA, Newton AC, Brown JH. PHLPP-1 negatively regulates Akt activity and survival in the heart. Circ Res. 2010;107:476–484. doi: 10.1161/CIRCRESAHA.109.215020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Das S, Wong R, Rajapakse N, Murphy E, Steenbergen C. Glycogen synthase kinase 3 inhibition slows mitochondrial adenine nucleotide transport and regulates voltage-dependent anion channel phosphorylation. Circ Res. 2008;103:983–991. doi: 10.1161/CIRCRESAHA.108.178970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun J, Morgan M, Shen RF, Steenbergen C, Murphy E. Preconditioning results in S-nitrosylation of proteins involved in regulation of mitochondrial energetics and calcium transport. Circ Res. 2007;101:1155–1163. doi: 10.1161/CIRCRESAHA.107.155879. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen TT, Stevens MV, Kohr M, Steenbergen C, Sack MN, Murphy E. Cysteine 203 of cyclophilin D is critical for cyclophilin D activation of the mitochondrial permeability transition pore. J Biol Chem. 2011;286:40184–40192. doi: 10.1074/jbc.M111.243469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyamoto S, Murphy AN, Brown JH. Akt mediates mitochondrial protection in cardiomyocytes through phosphorylation of mitochondrial hexokinase-II. Cell Death Differ. 2008;15:521–529. doi: 10.1038/sj.cdd.4402285. [DOI] [PubMed] [Google Scholar]
- 14.Smeele KM, Southworth R, Wu R, Xie C, Nederlof R, Warley A, Nelson JK, van Horssen P, van den Wijngaard JP, Heikkinen S, Laakso M, Koeman A, Siebes M, Eerbeek O, Akar FG, Ardehali H, Hollmann MW, Zuurbier CJ. Disruption of hexokinase II-mitochondrial binding blocks ischemic preconditioning and causes rapid cardiac necrosis. Circ Res. 2011;108:1165–1169. doi: 10.1161/CIRCRESAHA.111.244962. [DOI] [PubMed] [Google Scholar]
- 15.Ferlito M, Fulton WB, Zauher MA, Marbán E, Steenbergen C, Lowenstein CJ. VAMP-1, VAMP-2, and syntaxin-4 regulate ANP release from cardiac myocytes. J Mol Cell Cardiol. 2010;49:791–800. doi: 10.1016/j.yjmcc.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun J, Kohr MJ, Nguyen T, Aponte AM, Connelly PS, Esfahani SG, Gucek M, Daniels MP, Steenbergen C, Murphy E. Disruption of caveolae blocks ischemic preconditioning-mediated S-nitrosylation of mitochondrial proteins. Antioxid Redox Signal. 2012;16:45–56. doi: 10.1089/ars.2010.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yano T, Miki T, Tanno M, Kuno A, Itoh T, Takada A, Sato T, Kouzu H, Shimamoto K, Miura T. Hypertensive hypertrophied myocardium is vulnerable to infarction and refractory to erythropoietin-induced protection. Hypertension. 2011;57:110–115. doi: 10.1161/HYPERTENSIONAHA.110.158469. [DOI] [PubMed] [Google Scholar]
- 18.Huang J. An in vitro assay for the kinase activity of mTOR complex 2. Methods Mol Biol. 2012;821:75–86. doi: 10.1007/978-1-61779-430-8_6. [DOI] [PubMed] [Google Scholar]
- 19.Xie X, Zhang D, Zhao B, Lu MK, You M, Condorelli G, Wang CY, Guan KL. IkappaB kinase epsilon and TANK-binding kinase 1 activate AKT by direct phosphorylation. Proc Natl Acad Sci U S A. 2011;108:6474–6479. doi: 10.1073/pnas.1016132108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang H, Zha X, Tan Y, Hornbeck PV, Mastrangelo AJ, Alessi DR, Polakiewicz RD, Comb MJ. Phosphoprotein analysis using antibodies broadly reactive against phosphorylated motifs. J Biol Chem. 2002;277:39379–39387. doi: 10.1074/jbc.M206399200. [DOI] [PubMed] [Google Scholar]
- 21.Kane S, Sano H, Liu SC, Asara JM, Lane WS, Garner CC, Lienhard GE. A method to identify serine kinase substrates. Akt phosphorylates a novel adipocyte protein with a Rab GTPase-activating protein (GAP) domain. J Biol Chem. 2002;277:22115–22118. doi: 10.1074/jbc.C200198200. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi H, Miura T, Ishida H, Miki T, Tanno M, Yano T, Sato T, Hotta H, Shimamoto K. Limitation of infarct size by erythropoietin is associated with translocation of Akt to the mitochondria after reperfusion. Clin Exp Pharmacol Physiol. 2008;35:812–819. doi: 10.1111/j.1440-1681.2008.04925.x. [DOI] [PubMed] [Google Scholar]
- 23.Zinzalla V, Stracka D, Oppliger W, Hall MN. Activation of mTORC2 by association with the ribosome. Cell. 2011;144:757–768. doi: 10.1016/j.cell.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 24.Panić L, Tamarut S, Sticker-Jantscheff M, Barkić M, Solter D, Uzelac M, Grabusić K, Volarević S. Ribosomal protein S6 gene haploinsufficiency is associated with activation of a p53-dependent checkpoint during gastrulation. Mol Cell Biol. 2006;26:8880–8891. doi: 10.1128/MCB.00751-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carew JS, Kelly KR, Nawrocki ST. Mechanisms of mTOR inhibitor resistance in cancer therapy. Target Oncol. 2011;6:17–27. doi: 10.1007/s11523-011-0167-8. [DOI] [PubMed] [Google Scholar]
- 26.Liao Y, Hung MC. Physiological regulation of Akt activity and stability. Am J Transl Res. 2010;2:19–42. [PMC free article] [PubMed] [Google Scholar]
- 27.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 28.Feldman ME, Apsel B, Uotila A, Loewith R, Knight ZA, Ruggero D, Shokat KM. Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol. 2009;7:e38. doi: 10.1371/journal.pbio.1000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Völkers M, Konstandin MH, Doroudgar S, Toko H, Quijada P, Din S, Joyo A, Ornelas L, Samse K, Thuerauf DJ, Gude N, Glembotski CC, Sussman MA. Mechanistic target of rapamycin complex 2 protects the heart from ischemic damage. Circulation. 2013;128:2132–2144. doi: 10.1161/CIRCULATIONAHA.113.003638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cameron AJ, Linch MD, Saurin AT, Escribano C, Parker PJ. mTORC2 targets AGC kinases through Sin1-dependent recruitment. Biochem J. 2011;439:287–297. doi: 10.1042/BJ20110678. [DOI] [PubMed] [Google Scholar]
- 31.Warner JR, McIntosh KB. How common are extraribosomal functions of ribosomal proteins? Mol Cell. 2009;34:3–11. doi: 10.1016/j.molcel.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong R, Aponte AM, Steenbergen C, Murphy E. Cardioprotection leads to novel changes in the mitochondrial proteome. Am J Physiol Heart Circ Physiol. 2010;298:H75–91. doi: 10.1152/ajpheart.00515.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen T, Wong R, Wang G, Gucek M, Steenbergen C, Murphy E. Acute inhibition of GSK causes mitochondrial remodeling. Am J Physiol Heart Circ Physiol. 2012;302:H2439–2445. doi: 10.1152/ajpheart.00033.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thornton J, Striplin S, Liu GS, Swafford A, Stanley AW, Van Winkle DM, Downey JM. Inhibition of protein synthesis does not block myocardial protection afforded by preconditioning. Am J Physiol. 1990;259:H1822–1825. doi: 10.1152/ajpheart.1990.259.6.H1822. [DOI] [PubMed] [Google Scholar]
- 35.Oh WJ, Jacinto E. mTOR complex 2 signaling and functions. Cell Cycle. 2011;10:2305–2316. doi: 10.4161/cc.10.14.16586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bracho-Valdés I, Moreno-Alvarez P, Valencia-Martínez I, Robles-Molina E, Chávez-Vargas L, Vázquez-Prado J. mTORC1- and mTORC2-interacting proteins keep their multifunctional partners focused. IUBMB Life. 2011;63:896–914. doi: 10.1002/iub.558. [DOI] [PubMed] [Google Scholar]
- 37.Ruvinsky I, Katz M, Dreazen A, Gielchinsky Y, Saada A, Freedman N, Mishani E, Zimmerman G, Kasir J, Meyuhas O. Mice deficient in ribosomal protein S6 phosphorylation suffer from muscle weakness that reflects a growth defect and energy deficit. PLoS One. 2009;4:e5618. doi: 10.1371/journal.pone.0005618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee SB, Kwon IS, Park J, Lee KH, Ahn Y, Lee C, Kim J, Choi SY, Cho SW, Ahn JY. Ribosomal protein S3, a new substrate of Akt, serves as a signal mediator between neuronal apoptosis and DNA repair. J Biol Chem. 2010;285:29457–29468. doi: 10.1074/jbc.M110.131367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kohr MJ, Aponte AM, Sun J, Wang G, Murphy E, Gucek M, Steenbergen C. Characterization of potential S-nitrosylation sites in the myocardium. Am J Physiol Heart Circ Physiol. 2011;300:H1327–1335. doi: 10.1152/ajpheart.00997.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jonassen AK, Sack MN, Mjøs OD, Yellon DM. Myocardial protection by insulin at reperfusion requires early administration and is mediated via Akt and p70s6 kinase cell-survival signaling. Circ Res. 2001;89:1191–1198. doi: 10.1161/hh2401.101385. [DOI] [PubMed] [Google Scholar]
- 41.Vigneron F, Dos Santos P, Lemoine S, Bonnet M, Tariosse L, Couffinhal T, Duplaà C, Jaspard-Vinassa B. GSK-3β at the crossroads in the signalling of heart preconditioning: implication of mTOR and Wnt pathways. Cardiovasc Res. 2011;90:49–56. doi: 10.1093/cvr/cvr002. [DOI] [PubMed] [Google Scholar]
- 42.Park SS, Zhao H, Jang Y, Mueller RA, Xu Z. N6-(3-iodobenzyl)-adenosine- 5′-N-methylcarboxamide confers cardioprotection at reperfusion by inhibiting mitochondrial permeability transition pore opening via glycogen synthase kinase 3 beta. J Pharmacol Exp Ther. 2006;318:124–131. doi: 10.1124/jpet.106.101477. [DOI] [PubMed] [Google Scholar]
- 43.Khan S, Salloum F, Das A, Xi L, Vetrovec GW, Kukreja RC. Rapamycin confers preconditioning-like protection against ischemia-reperfusion injury in isolated mouse heart and cardiomyocytes. J Mol Cell Cardiol. 2006;41:256–264. doi: 10.1016/j.yjmcc.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 44.El-Ani D, Stav H, Guetta V, Arad M, Shainberg A. Rapamycin (sirolimus) protects against hypoxic damage in primary heart cultures via Na+/Ca2+ exchanger activation. Life Sci. 2011;89:7–14. doi: 10.1016/j.lfs.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 45.Shende P, Plaisance I, Morandi C, Pellieux C, Berthonneche C, Zorzato F, Krishnan J, Lerch R, Hall MN, Rüegg MA, Pedrazzini T, Brink M. Cardiac raptor ablation impairs adaptive hypertrophy, alters metabolic gene expression, and causes heart failure in mice. Circulation. 2011;123:1073–1082. doi: 10.1161/CIRCULATIONAHA.110.977066. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.