Abstract
Aim:
To investigate the effect of N-acetylcysteine (NAC), a potent antioxidant, on neuron differentiation of cultured mouse embryonic stem cells (ESCs) induced by retinoic acid (RA) in vitro. Superior cervical ganglion (SCG) neurons were used to study the effect of NAC on neuritogenesis.
Methods:
Immunoblotting was performed to detect the expression of microtubule-associated protein 2 (MAP2). MTT assays were used to determine cell viability. Cell death was estimated with trypan blue exclusion and Hoechst 33342 staining. Immunocytochemical analysis was carried out to identify neurons.
Results:
We obtained a high percentage of MAP2-positive neurons derived from embryoid bodies (EBs) induced by RA by administering 1 mmol/L NAC at differentiation day 0. On differentiation day 8, the expression of MAP2 protein was strongly upregulated in the presence of NAC. NAC promoted neuron differentiation of ES cells in a dose- and time-dependent manner. Notably, NAC suppressed cell death caused by RA during neuron differentiation. In addition, neurite extension of SCG neurons was greatly stimulated in the presence of NAC.
Conclusion:
These results show that NAC enhanced both neuron differentiation and neuritogenesis, suggesting that it may be used in the development of novel therapeutic approaches targeting neuron loss and neurite dystrophy in neurodegenerative diseases.
Keywords: N-acetylcysteine, neuron differentiation, neuritogenesis, ES cells, superior cervical ganglion
Introduction
N-acetylcysteine (NAC), a precursor of glutathione, is a potent antioxidant and free radical scavenger that effectively reduces free radical species and other oxidants1. The neuroprotective effect of NAC has been demonstrated in ischemia and ischemia/reperfusion models2, 3, 4, 5, and in traumatic injury of the nervous system6. NAC is also thought to play a possible beneficial role in age-associated neurodegenerative diseases7. However, the effect of NAC on neuron differentiation and neuritogenesis is poorly understood.
Embryonic stem cells (ESCs), derived from the inner cell mass of the blastula, can maintain self-renewal capability and pluripotency through exogenous application of leukemia inhibitory factor (LIF) or feeder cells. Under appropriate conditions, ESCs are able to differentiate spontaneously into specialized cell types8, including neurons9, 10, 11, 12; however, the efficiency of neuron differentiation is typically low. In the current study, mouse ESCs were differentiated into a high percentage of neurons via the application of NAC, suggesting a potential role for NAC in chemically modifying the neuron differentiation of ESCs.
Since neurites of neuron-like cells derived from ESCs grow slowly and display a neuronal-network formation, it is difficult to test neurite elongation. We therefore extended our studies to tissue cultures of sympathetic neurons, in which neurites grow rapidly without overlap. An explant culture of superior cervical ganglion (SCG) neurons provides a simple model for investigating neurite degeneration after transection (termed “Wallerian degeneration”)13, nerve growth factor (NGF) withdrawal14, amyloid-β peptide (Aβ) toxicity15, neurite regeneration after injury16 and neurite outgrowth17. After the application of NAC, increased neurite extension was observed, implicating NAC promotes neuritogenesis.
Materials and methods
ES cell culture and differentiation
Undifferentiated mouse ES-D3 cells (passages 10–20) were maintained on 0.1% gelatin-coated dishes in DMEM medium (GIBCO), supplemented with 15% FCS (fetal calf serum), 0.1 mmol/L β-mercaptoethanol (Sigma), penicillin (100 U/mL), and streptomycin (0.1 mg/mL). Routine cultures of ESCs were grown in the presence of LIF (1000 U/mL, GIBCO). Medium was changed every 2 days. For neuron differentiation, cells were induced to generate embryoid bodies (EBs) by omitting LIF from medium and allowing aggregation in Agar-coated Petri dishes18 at a density of 5×105 cells/mL. Two days later, EBs with similar diameters were plated onto 3.5 cm gelatin-coated dishes in ESC medium supplemented with retinoic acid (RA) for differentiation. Subsequently, cells were either harvested for Western blot or fixed for immunocytochemistry. The day of RA application was defined as differentiation day 0.
Tissue culture of SCG
Explant cultures of SCG were prepared from postnatal 1-day-old mice (C57BL/6) as described previously19. Briefly, dissected SCGs were plated on collagen-coated 12-well plates. One ganglion was plated for each well. Cultures were maintained in AM50 medium: Eagle's minimum essential medium (MEM) supplemented with 5% fetal bovine serum (FBS) (Trace Bioscience, Ltd, Sydney, Australia), 50 ng/mL NGF, 10 μmol/L fluorodeoxyuridine, 10 μmol/L uridine, 50 U/mL penicillin, and 50 μg/mL streptomycin. Cultures were grown in a humidified atmosphere of 5% CO2/95% air at 36 °C. The cultures were treated with 5 μmol/L aphidicolin (Sigma) at DIV (day in vitro) 1 after dissection for two days to reduce the number of non-neuronal cells.
Determination of cell proliferation
To evaluate cell proliferation, the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was performed. Each data point represented results from 3 independent experiments, and each treatment was performed in sextuplicate. EBs with similar diameter were plated onto 96-well plates, one EB per well. On differentiation day 0, cells were incubated with different concentrations of NAC in combination with RA. After the appropriate incubation time (0, 4, and 8 days), 15% volume of MTT (5 mg/mL) was added to each well. After another 4 h of incubation at 37 °C, the medium was removed and 100 μL dimethyl sulfoxide (DMSO) was added to each well to resuspend the MTT metabolic product. The absorbance of the dissolved formazan was measured at 570 nm (A570) with a microplate reader (Versa Max, Molecular Devices). The percentage of cell proliferation was calculated with the formula: MTT activity (%)=100–[(A570, Control–A570, NAC)/A570, Control]×100.
Immunoblotting
Proteins were extracted from cells using radioimmunoprecipitation assay (RIPA) buffer. Protein extracts were separated on SDS-polyacrylamide gel, transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore, Burlington, MA), blocked with 5% skim milk in Tris-buffered saline containing 0.1% Tween (TBS-T), and then probed with monoclonal anti microtubule-associated protein 2 (MAP2) (1:50, Sigma-Aldrich) at 4 °C overnight. Secondary horseradish peroxidase (HRP)-conjugated anti-mouse IgG was used at a dilution of 1:2000 for 1 h at room temperature. Visualization of immunoreactive bands was performed with an enhanced chemiluminescence detection kit (ECL; Amersham Biosciences, Piscataway, NJ). Anti-actin (1:2000, Santa Cruz Biotechnology) was used for normalization. Western blot analysis was performed in triplicate.
Evaluation of cell survival
Cell viability was evaluated by the Trypan-Blue dye exclusion method as described previously20. On differentiation day 8, cells were stained with 0.2% Trypan Blue for 15 min followed by fixation with 4% paraformaldehyde (PFA) in PBS for 30 min at room temperature. The percentage of viable cells that were capable of excluding the dye was calculated from the total number of cells in each area. At least 15 photomicrographs were evaluated randomly per treatment, and each treatment was performed in triplicate.
Hoechst 33342 staining
Apoptotic cell death was identified by nuclear staining with bis-benzimide (Hoechst 33342). On differentiation day 8, cells were fixed with PFA in PBS for 15 min at room temperature, then washed three times with PBS and exposed to 10 mg/L Hoechst 33342 at 37 °C in the dark for 15 min. After washing with PBS, nuclei were visualized under UV illumination with fluorescence microscope (Olympus). The percentage of apoptotic cells was estimated by counting the cells with condensed and/or fragmented nuclei. At least 15 photomicrographs were evaluated randomly per treatment, and each treatment was performed in triplicate.
Immunofluorescence analysis
Immunostaining with mouse anti-MAP2 (1:50) and Alexa Flour 488-conjugated anti-mouse IgG secondary antibody (1:100, Molecular Probes, Inc, Eugene, OR, USA) was performed as described previously21. In brief, cells plated on gelatin-coated glass coverslips were left untreated or treated with 1 mmol/L NAC and then fixed with 4% PFA, permeabilized with 0.05% Triton X-100 and double stained to detect nuclear and MAP2. Images were visualized with confocal laser scanning microscopy.
Measurement of neurite elongation
Photomicrographs were taken using an Olympus phase-contrast microscope equipped with a digital camera. Neurite outgrowth was assessed by measuring the distance between the edge of a ganglion and the tips of neurites. At least 10 ganglia were analyzed for each experimental group, and each treatment was performed in triplicate.
Statistical analysis
The data were plotted as mean±SD from three independent experiments. Determinations of statistically significant differences were made using one-way ANOVA followed by Tukey's post-hoc tests. P<0.01 was considered statistically significant.
Results
Enhancement of RA-induced neuron differentiation of ESCs by application of NAC
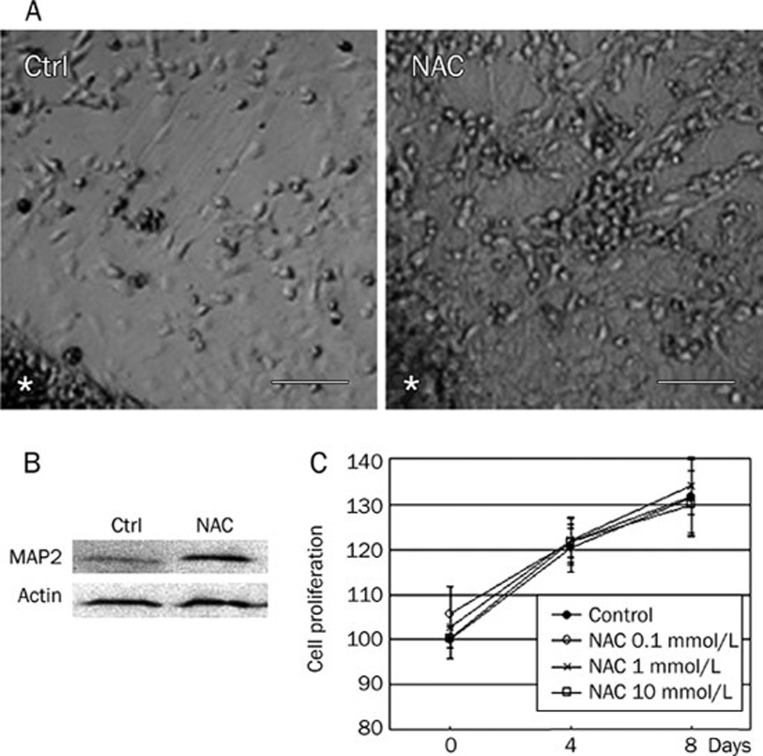
To assess the effect of NAC on neuron differentiation, ESCs were first induced to form EBs by suspension culture. EBs with similar diameters were then plated onto dishes and differentiated with RA in the presence or absence of 1 mmol/L NAC. On differentiation day 8, many neuron-like cells with small cell bodies and long processes were generated around EBs in the presence of NAC (Figure 1A). MAP2 has been recognized as a major agent responsible for promoting assembly and preservation of dendritic microtubules22, 23, and has been extensively used as a somatodendritic marker for all dendrites and the proximal part of axons24. Neurons derived from ESCs were cultured for 8 days with or without NAC (1 mmol/L) and then cell extracts were subjected to immunoblotting analysis with anti-MAP2 antibody. As Figure 1B shows, MAP2 protein was strongly upregulated in the presence of NAC, suggesting that NAC enhaces RA-induced neuron differentiation of ESCs. To rule out the possibility that NAC promotes neuron differentiation by increasing cell proliferation, the MTT assay was used. EBs were differentiated by RA with or without NAC. On differentiation days 0, 4, and 8, cell proliferation was determined. As shown in Figure 1C, no significant difference was detected in cells with NAC treatment as compared with control, implying that NAC has no effect in promoting cell proliferation.
Figure 1.
RA-induced neuron differentiation of ESCs was enhanced in the presence of NAC. (A) EBs were induced to differentiate into neurons by RA with (right) or without (left) 1 mmol/L NAC from differentiation day 0. Phase-contrast photomicrographs were taken on day 8. * indicate the location of EBs. Scale bars, 85 μm. (B) Cells were then lysed and subjected to immunoblotting with anti-MAP2 antibody. The upregulation of MAP2 expression was detected in the presence of NAC. Actin immunoreactive bands were used for normalization. (C) EBs with the similar diameter were picked up, plated onto 96-well plates, and differentiated by RA with or without NAC. On differentiation day 0, 4, and 8, cell proliferation was determined by MTT assay.
NAC promotes ESC neuron differentiation in a dose- and time- dependent manner
To further investigate the effect of NAC on promoting ESC neuron differentiation, the extension of dendrite-like processes was analyzed by immunocytochemistry, using the anti-MAP2 antibody. The average nuclear area of the cells derived from EBs in the presence of NAC was similar to that of controls as identified by propidium iodide (PI) staining (Figure 2Aa, d). However, the number of differentiated cells expressing neuronal-specific protein MAP2 was significantly increased in NAC-treated cultures (Figure 2Ae) compared with untreated control (Figure 2Ab). Enhanced dendritic networks could also be observed after NAC treatment (Figure 2Ae, f). In addition, the positive effect of the antioxidant NAC on neuron differentiation of ESCs was tested using doses ranging from 0.1 to 10 mmol/L. NAC increased the number of MAP2-positive cells derived from EBs in a dose-dependent manner (Figure 2B). However, minor toxicity was observed with doses over 10 mmol/L (data not shown). Cultures at different stages of differentiation (Days 0, 4, and 8) were exposed to 1 mmol/L NAC for 2 days. On Day 12, MAP2-positive cells derived from EBs were counted. As shown in Figure 2C, immature cultures (Day 0) responded to NAC with increased numbers of MAP2-positive cells differentiated from ESCs, whereas more highly differentiated cultures (Day 8) had only a moderate response to NAC stimulation. This result indicates that NAC-driven promotion of ESC neuron differentiation depends on the developmental stage.
Figure 2.
NAC promotes ESCs neuron differentiation in a dose- and time-dependent manner. (A) Immunocytochemical analysis of MAP2-positive neurons in differentiated ESCs. PI nuclear staining (red) was used as the marker of total number of cells. Note that the average nuclear area was similar, while MAP2-positive (green) neurons with dendritic processes were significantly increased in the presence of NAC (1 mmol/L), compared with untreated control. Scale bars, 50 μm. (B) NAC promoted neuron differentiation in a dose-dependent manner. Different concentrations of NAC were added into culture medium on differentiation day 0. Eight days later, cells were subjected to immunocytochemical analysis with anti-MAP2 antibody. The number of MAP2-positive cells was counted per area and was presented as a percentage of the total number of cells. At least 15 photomicrographs were evaluated randomly per treatment and each treatment was performed in triplicate. cP<0.01 compared with cultures without NAC treatment. (C) NAC enhanced neuron differentiation in a time-dependent manner. Cultures of different differentiation stage (differentiation day 0, 4, and 8) were exposed to 1 mmol/L NAC for two days. On day 12, neuron differentiation was estimated as described in (B). Cultures without NAC treatment were used as control (Ctrl). cP<0.01 compared with control.
NAC suppressed cell death caused by RA during neuron differentiation
Given the fact that NAC enhanced RA-induced neuron differentiation of ESCs in a dose- and time-dependent manner, we asked whether NAC relies on RA for promoting neuron differentiation. For this purpose, ESCs were first induced to form EBs by incubating them for 2 days in suspension culture. Subsequently, EBs were plated onto gelatin-coated dishes for differentiation under different conditions. On differentiation day 8, cells were subjected to immunocytochemical analysis with the anti-MAP2 antibody. As shown in Figure 3A, about 5% of MAP2-positive cells could be observed in cultures that were not supplemented. The percentage of MAP2-positive cells increased to over 20% with the application of RA on differentiation day 0. Consistent with previous results (Figure 2B), when cells were exposed to RA accompanied with 1 mmol/L NAC on differentiation day 0, significant enhancement of neuron differentiation was observed. However, NAC itself had no ability to induce ESC neuron differentiation, implying that NAC relies on RA for promoting neuron differentiation. In addition, cell survival estimated by trypan blue exclusion showed that NAC prevented cell death caused by RA during differentiation (Figure 3B). Similarly, apoptotic cell death was decreased in cultures with NAC treatment compared with that of cells treated with RA only (Figure 3C). Collectively, these results indicate that NAC suppressed cell death caused by RA during neuron differentiation.
Figure 3.
NAC suppressed cell death caused by RA during differentiation. (A) NAC relied on RA in promoting neuron differentiation of ESCs. ESCs were induced to form EB formation by two days of suspension culture in Agar-coated dishes. EBs were then plated onto dishes for differentiation in culture medium supplemented with or without either RA or 1 mmol/L NAC as indicated. On differentiation day 8, MAP2-positive cells were counted and presented as percentages of total cells. (B) Cell viability was assessed by trypan blue exclusion test as described in “Materials and Methods”. (C) Apoptotic cell death was determined by Hoechst 33342 staining. The percentage of apoptotic cells was estimated by counting the number of cells with condensed and/or fragmented nuclei. cP<0.01 compared with cultures without any supplementary materials.
Neurite extension was significantly stimulated by NAC application in SCGs
Given the evidence that NAC enhanced the number of neurons derived from ESCs, we asked whether NAC could promote neurite extension. To answer this question, we took advantage of explant SCGs to further investigate the effect of NAC on neurite elongation. After dissection, ganglia were cultured on collagen-coated 12-well plates with or without NAC (1 mmol/L). On the following day, ganglion cells were observed to send out processes. As shown in Figure 4A, treatment with NAC resulted in significant neurite elongation compared with untreated control. Neurite outgrowth was determined by measuring neurite length starting from the ganglia to the distal ends, and the average length of neurites was counted every 2 days as described in “Materials and methods” (Figure 4B). The results suggest that NAC is capable of promoting neuritogenesis.
Figure 4.
NAC promoted neurite extension in SCG neurons. NAC 1 mmol/L was added to explant SCG culture on the day when ganglia were planted on tissue dishes. (A) Phase-contrast photomicrographs of cultures with or without NAC treatment were taken on DIV 4 after dissection. Scale bar, 300 μm. (B) Average neurite length in control or NAC-treated cultures from day 0 to day 8. cP<0.01 compared with control.
Discussion
Results of the present study indicate that NAC promoted neuron differentiation and suppressed cell death caused by RA in ESCs. After NAC treatment, the number of MAP2-positive cells derived from EBs was greatly increased. Moreover, neuritogenesis in cultured SCG neurons was significantly enhanced by the application of NAC.
NAC, a glutathione precursor, is known to be an excellent source of sulfhydryl groups and is converted into metabolites that are capable of stimulating glutathione synthesis, acting directly as free radical scavengers25, 26, 27, 28. It has been shown to directly reduce the level of reactive oxygen species (ROS) as well as acrolein. In addition to its antioxidant properties, NAC has the capacity to inhibit several inflammatory elements related to oxidant stress and is involved in the pathophysiology of inflammation29, 30. Because of the possible role of NAC in suppressing RA-induced oxidative stress during neuron differentiation of ESCs, we added NAC with RA to the culture medium. Notably, in the presence of NAC, the number of neurons derived from EBs was significantly increased. To further investigate the effect of NAC on ESC neuron differentiation, immunoblotting analysis was performed to measure the expression level of MAP2, a somatodendritic marker for all dendrites and the proximal part of axons24. Upregulation of MAP2 protein expression was observed on differentiation day 8 in the presence of NAC. This result was also confirmed by immunocytochemistry in which the percentage of MAP2-positive cells was significantly increased compared with untreated cultures. In addition, the application of NAC on differentiation day 0 showed the most powerful effect on promoting neuron differentiation, suggesting that NAC may regulate neuron differentiation in a manner dependent on the developmental stage.
Although the powerful role of RA in ESC neuron differentiation has been widely accepted, it should be noted that RA induces oxidative stress during EB formation, leading to cell death31. Oxidative stress, which can lead to various disorders, is thought to be caused by two kinds of compounds: 1) ROS, such as superoxide anion radical O2·–, hydrogen peroxide (H2O2) and hydroxyl radical (·OH), and 2) unsaturated aldehydes, such as acrolein and hydroxynonenal32, 33. There are many reports concerning the roles of ROS and unsaturated aldehydes in cell damage, and it is thought that ROS play a predominant role in cell damage. To confirm the possible role of NAC in suppressing RA-induced cell death, EBs were exposed to different culture media for differentiation. On differentiation day 8, cells treated with RA alone showed increased cell death as determined by trypan blue exclusion and Hoechst 33342 staining. However, NAC remarkably prevented cell death (Figure 3B, C).
The results raised the question as to whether NAC can induce neurite promotion. However, neuron-like cells differentiated from ESCs exhibited network formation, hindering the test of neurite extension. Thus, we conducted a study using a primary culture of SCG neurons, in which the rate of neurite extension is rapid and relatively easy to estimate. Tissue cultures of sympathetic neurons have been widely used to study neurite degeneration13, 14, 15 and neurite outgrowth17. In the current study, 1 mmol/L NAC efficiently enhanced neurite extension in SCG neurons. However, we can not rule out the possibility that NAC attenuated the ability of neurites to adhere to the substratum and thus facilitated neurite extension.
Altogether, these results show that NAC enhances both neuron differentiation and neuritogenesis. Therefore, it is likely that NAC would be a beneficial agent for targeting neuron loss and neurite dystrophy in neurodegenerative diseases.
Author contribution
Hao-ran QIAN designed and performed research and wrote the paper. Yi YANG analyzed the data.
References
- Moldeus P, Cotgreune IA, Berggren M. Lung protection by a thiol containing antioxidant N-acetylcysteine. Respiration. 1986;50:531–42. doi: 10.1159/000195086. [DOI] [PubMed] [Google Scholar]
- Knuckey NW, Palm D, Primiano M, Epstein MH, Johanson CE. N-acetylcysteine enhances hippocampal neuronal survival after transient forebrain ischemia in rats. Stroke. 1995;26:305–10. doi: 10.1161/01.str.26.2.305. [DOI] [PubMed] [Google Scholar]
- Cakir O, Erdem K, Oruc A, Kilinc N, Eren N. Neuroprotective effect of N-acetylcysteine and hypothermia on the spinal cord ischemia-reperfusion injury. Cardiovasc Surg. 2003;11:375–9. doi: 10.1016/S0967-2109(03)00077-2. [DOI] [PubMed] [Google Scholar]
- Sekhon B, Sekhon C, Khan M, Patel SJ, Singh I, Singh AK. N-Acetyl cysteine protects against injury in a rat model of focal cerebral ischemia. Brain Res. 2003;971:1–8. doi: 10.1016/s0006-8993(03)02244-3. [DOI] [PubMed] [Google Scholar]
- Khan M, Sekhon B, Jatana M, Giri S, Gilg AG, Sekhon C, et al. Administration of N-acetylcysteine after focal cerebral ischemia protects brain and reduces inflammation in a rat model of experimental stroke. J Neurosci Res. 2004;76:519–27. doi: 10.1002/jnr.20087. [DOI] [PubMed] [Google Scholar]
- Hicdonmez T, Kanter M, Tiryaki M, Parsak T, Cobanoglu S. Neuroprotective effects of N-acetylcysteine on experimental closed head trauma in rats. Neurochem Res. 2006;31:473–81. doi: 10.1007/s11064-006-9040-z. [DOI] [PubMed] [Google Scholar]
- Banaclocha MM. Therapeutic potential of N-acetylcysteine in age-related mitochondrial neurodegenerative diseases. Med Hypotheses. 2001;56:472–7. doi: 10.1054/mehy.2000.1194. [DOI] [PubMed] [Google Scholar]
- Wobus AM, Boheler KR. Embryonic stem cells as developmental model in vitro. Cells Tissues Organs. 1999;165:129–30. doi: 10.1159/000016692. [DOI] [PubMed] [Google Scholar]
- Bain G, Kitchens D, Yao M, Huettner JE, Gottlieb DI. Embryonic stem cells express neural properties in vitro. Dev Biol. 1995;168:342–57. doi: 10.1006/dbio.1995.1085. [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Mizuseki K, Nishikawa S, Kaneko S, Kuwana Y, Nakanishi S, et al. Induction of midbrain dopaminergic neurons from ES cells by stromal cell derived inducing activity. Neuron. 2000;28:31–40. doi: 10.1016/s0896-6273(00)00083-0. [DOI] [PubMed] [Google Scholar]
- Tropepe V, Hitoshi S, Sirard C, Mak TW, Rossant J, van der Kooy D. Direct neural fate specification from embryonic stem cells: a primitive mammalian neural stem cell stage acquired through a default mechanism. Neuron. 2001;30:65–78. doi: 10.1016/s0896-6273(01)00263-x. [DOI] [PubMed] [Google Scholar]
- Zhang SC, Wernig M, Duncan ID, Brüstle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol. 2001;19:1129–33. doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]
- Zhai Q, Wang J, Kim A, Liu Q, Watts R, Hoopfer E, et al. Involvement of the ubiquitin-proteasome system in the early stages of Wallerian degeneration. Neuron. 2003;39:217–25. doi: 10.1016/s0896-6273(03)00429-x. [DOI] [PubMed] [Google Scholar]
- MacInnis BL, Campenot RB. Regulation of Wallerian degeneration and nerve growth factor withdrawal-induced pruning of axons of sympathetic neurons by the proteasome and the MEK/Erk pathway. Mol Cell Neurosci. 2005;28:430–9. doi: 10.1016/j.mcn.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Song MS, Saavedra L, de Chaves EI. Apoptosis is secondary to non-apoptotic axonal degeneration in neurons exposed to Aβ in distal axons. Neurobiol Aging. 2006;27:1224–38. doi: 10.1016/j.neurobiolaging.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Shoemarker SE, Sachs HH, Vaccariello SA, Zigmond RE. A conditioning lesion enhances sympathetic neurite outgrowth. Exp Neurol. 2005;194:432–43. doi: 10.1016/j.expneurol.2005.02.023. [DOI] [PubMed] [Google Scholar]
- Sondell M, Lundborg G, Kanje M. Vascular endothelial growth factor has neurotrophic activity and stimulates axonal outgrowth, enhancing cell survival and schwann cell proliferation in peripheral nervous system. J Neurosci. 1999;19:5731–40. doi: 10.1523/JNEUROSCI.19-14-05731.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt FM, Hogan BL. Out of Eden: stem cells and their niches. Science. 2000;287:1427–30. doi: 10.1126/science.287.5457.1427. [DOI] [PubMed] [Google Scholar]
- Ikegami K, Koike T. Non-apoptotic neurite degeneration in apoptotic neuronal death: pivotal role of mitochondrial function in neurites. Neuroscience. 2003;122:617–26. doi: 10.1016/j.neuroscience.2003.08.057. [DOI] [PubMed] [Google Scholar]
- Yang Y, Kawataki T, Fukui K, Koike T. Cellular Zn2+ chelators cause ''Dying-Back'' neurite degeneration associated with energy impairment. J Neurosci Res. 2007;85:2844–55. doi: 10.1002/jnr.21411. [DOI] [PubMed] [Google Scholar]
- Yang Y, Fukui K, Koike T, Zheng XX. Induction of autophagy in neurite degeneration of mouse superior cervical ganglion neurons. Eur J Neurosci. 2007;26:2979–88. doi: 10.1111/j.1460-9568.2007.05914.x. [DOI] [PubMed] [Google Scholar]
- Maccioni RB, Cambiazo V. Role of microtubule-associated proteins in the control of microtubule assembly. Physiol Rev. 1995;75:835–64. doi: 10.1152/physrev.1995.75.4.835. [DOI] [PubMed] [Google Scholar]
- Sanchez C, Diaz-Nido J, Avila J. Phosphorylation of microtubule-associated protein 2 (MAP2) and its relevance for the regulation of the neural cytoskeleton function. Prog Neurobiol. 2000;61:133–68. doi: 10.1016/s0301-0082(99)00046-5. [DOI] [PubMed] [Google Scholar]
- Caceres A, Banker G, Steward O, Binder L, Payne M. MAP2 is localized to the dendrites of hippocampal neurons which develop in culture. Brain Res. 1984;315:314–8. doi: 10.1016/0165-3806(84)90167-6. [DOI] [PubMed] [Google Scholar]
- Hazinedaroglu SM, Dulger F, Kayaoglu HA, Pehlivan M, Serinsoz E, Canbolat O, et al. N-acetylcysteine in intestinal reperfusion injury: an experimental study in rats. ANZ J Surg. 2004;74:676–8. doi: 10.1111/j.1445-1433.2004.03111.x. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Mazzon E, Costantino G, Serraino I, De Sarro A, Caputi AP. Effects of N-acetylcysteine in a rat model of ischemia and reperfusion injury. Cardiovasc Res. 2000;47:537–48. doi: 10.1016/s0008-6363(00)00018-3. [DOI] [PubMed] [Google Scholar]
- Yildirim Z, Kotuk M, Iraz M, Kuku I, Ulu R, Armutcu F, et al. Attenuation of bleomycin-induced lung fibrosis by oral sulfhydryl containing antioxidants in rats: erdosteine and N-acetylcysteine. Pulm Pharmacol Ther. 2005;18:367–73. doi: 10.1016/j.pupt.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Cotgreave IA, Moldeus P. Lung protection by thiol-containing antioxidants. Bull Eur Physiopathol Respir. 1987;23:275–7. [PubMed] [Google Scholar]
- Blackwell TS, Blackwell TR, Holden EP, Cristman BW, Cristman JW. In vivo antioxidant treatment supresses nuclear factor-kappa B activation and neutrophilic lung inflammation. J Immunol. 1996;157:1630–7. [PubMed] [Google Scholar]
- Blesa S, Cortijo J, Mata M, Serrano A, Closa D, Santangelo F, et al. Oral N-acetylcysteine attenuates the rat pulmonary inflammatory response to antigen. Eur Respir J. 2003;221:394–400. doi: 10.1183/09031936.03.00039602. [DOI] [PubMed] [Google Scholar]
- Castro-Obregon S, Covarrubias L. Role of retinoic acid and oxidative stress in embryonic stem cell death and neuronal differentiation. FEBS Lett. 1996;381:93–7. doi: 10.1016/0014-5793(96)00088-9. [DOI] [PubMed] [Google Scholar]
- Hensley K, Robinson KA, Gabbita SP, Salsman S, Floyd RA. Reactive oxygen species, cell signaling, and cell injury. Free Radic Biol Med. 2000;28:1456–62. doi: 10.1016/s0891-5849(00)00252-5. [DOI] [PubMed] [Google Scholar]
- Kehrer JP, Biswal SS. The molecular effects of acrolein. Toxicol Sci. 2000;57:6–15. doi: 10.1093/toxsci/57.1.6. [DOI] [PubMed] [Google Scholar]