Abstract
Aim:
Statin disposition and response are greatly determined by the activities of drug metabolizing enzymes and efflux/ uptake transporters. There is little information on the regulation of these proteins in human cells after statin therapy. In this study, the effects of atorvastatin and simvastatin on mRNA expression of efflux (ABCB1, ABCG2 and ABCC2) and uptake (SLCO1B1, SLCO2B1 and SLC22A1) drug transporters in Caco-2 and HepG2 cells were investigated.
Methods:
Quantitative real-time PCR was used to measure mRNA levels after exposure of HepG2 and Caco-2 cells to statins.
Results:
Differences in mRNA basal levels of the transporters were as follows: ABCC2>ABCG2>ABCB1>SLCO1B1>>>SLC22A1>SLC O2B1 for HepG2 cells, and SLCO2B1>>ABCC2>ABCB1>ABCG2>>>SLC22A1 for Caco-2 cells. While for HepG2 cells, ABCC2, ABCG2 and SLCO2B1 mRNA levels were significantly up-regulated at 1, 10 and 20 μmol/L after 12 or 24 h treatment, in Caco-2 cells, only the efflux transporter ABCB1 was significantly down-regulated by two-fold following a 12 h treatment with atorvastatin. Interestingly, whereas treatment with simvastatin had no effect on mRNA levels of the transporters in HepG2 cells, in Caco-2 cells the statin significantly down-regulated ABCB1, ABCC2, SLC22A1, and SLCO2B1 mRNA levels after 12 or 24 h treatment.
Conclusion:
These findings reveal that statins exhibits differential effects on mRNA expression of drug transporters, and this effect depends on the cell type. Furthermore, alterations in the expression levels of drug transporters in the liver and/or intestine may contribute to the variability in oral disposition of statins.
Keywords: simvastatin, atorvastatin, Caco-2 cells, HepG2 cells, drug transporters
Introduction
One of the major difficulties for the effective oral drug delivery is the poor drug absorption or rapid excretion via bile. Although it has long been recognized that most drugs are absorbed through the gastrointestinal epithelium by a simple diffusion mechanism depending on their lipophilicity, more recent research has demonstrated the occurrence of a transporter-mediated absorption. Most drug transporters belong to two super-families, ABC (ATP-binding cassette) and SLC (solute-linked carrier), including both cellular uptake and efflux transporters, being expressed in apical or basolateral membranes of different cells1. The membrane in which the drug transporter is located is critical in determining the net transcellular transport and, governing the pharmacokinetics profiles of substrates in the body.
Statins are 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors used for treatment of hypercholesterolemia. Intestinal absorption appears to have an important role in disposition of statins and other hypolipidemic agents. After an oral ingestion, for example, the fraction of atorvastatin absorbed is only 30%, and its oral bioavailability is of 14%2. This may be due to extensive first-pass metabolism in the gut wall, as well as the fact that atorvastatin is substrate of efflux transporters, and it can inhibit transporter-mediated efflux3, 4, 5. In a recent study of our group, we demonstrated that atorvastatin inhibited the activity of the efflux transporter ABCB1, in vitro, and it down-regulated ABCB1 expression in mononuclear peripheral blood cells of hypercholesterolemic individuals, which was negatively correlated with plasma total cholesterol reductions after statin therapy6.
Breast cancer resistance protein (BCRP/ABCG2) and multidrug resistance associated protein 2 (MRP2/ABCC2) are efflux transporters that belong to the ABC family. These transporters are expressed in the apical membrane of many cells including tissues of gastrointestinal tract and canalicular membrane of liver7, 8, 9 where it has been shown to limit the oral absorption through efflux from the intestinal mucosa to gut lumen of some drugs as digoxin3. Efflux transporters affect drug disposition, predominantly, of class 2 compounds, which exhibit low solubility and high permeability (Biopharmaceutics System Classification) such as atorvastatin10. They affect the extent of oral bioavailability and the rate of absorption. However, in the intestine and liver are also localized biotransformation enzymes such as CYP3A4, thus changes in expression (inhibition/induction) of efflux transporters affect intestinal and hepatic metabolism of drugs that are substrates for these enzymes11.
Uptake transporters such as organic anion transporting polypeptides (OATPs) and organic cation transporters (OCTs) may be also important factors for statins absorption and disposition10. OATP1B1 and OATP2B1 (SLCO1B1 and SLCO2B1) and OCT1 (SLC22A1) are uptake transporters, members of the SLC family, being involved in hepatic and intestinal drug uptake, facilitating its translocation inside the cell12. OATP1B1 is primarily localized in liver12 and it has been characterized as a transporter of statins such as pravastatin and rosuvastatin13, 14, 15. Recently, Chen et al (2005)4 have demonstrated that atorvastatin acid is an inhibitor of OATP1B1 transport and polymorphisms in SLCO1B1 gene have been associated with statin efficacy16.
OATP2B1 is localized at apical membrane of enterocytes in human small intestine17 and basolateral membrane of hepatocytes18. Atorvastatin was shown to be a high-affinity substrate for OATP2B1 in vitro, and it is capable of inhibiting estrone-3-sulfate transport in OATP2B1-overexpressing Madin-Darby canine kidney II cells19. OCT1 is highly expressed in the liver and is also expressed in the intestine1, 20, where it is localized on the basolateral membrane of enterocytes, facilitating the elimination of its substrates from circulating blood into the intestinal lumen as demonstrated in Oct1 knockout mice 21. Whether statins regulate SLC22A1 gene or are substrates for this transporter remains to be determined.
Little is known about statins regulation of drug transporters. In the present study, we examined time and concentration dependent effects of two commons statins, atorvastatin and simvastatin, on the mRNA expression of efflux (ABCB1, ABCG2 and ABCC2) and uptake (SLCO1B1, SLCO2B1 and SLC22A1) drug transporters in enterocytes (Caco-2) and hepatocytes (HepG2) cell lines. The findings of the present study are relevant since these statins has to be first absorbed from the intestine to be then taken up by the liver.
Materials and methods
Chemicals
Atorvastatin was kindly provided by Pfizer Pharmaceuticals Ltd (Guarulhos, SP, Brazil). Simvastatin, Triton-X-100 and glutamine were purchased from Sigma (St Louis, MO, USA). Dulbeccós modified Eagle's medium (DMEM), penicillin, TRIzol® reagent, streptomycin, SuperScript II reverse transcriptase and random hexamers were purchased from Invitrogen (Carlsbad, CA, USA). Trypsin-versene mixture containing trypsin (0.2%) and versene (0.02%) was obtained from Adolfo Lutz Institute (Sao Paulo, SP, Brazil). Propidium iodide was obtained from ICN Biomedicals (Costa Mesa, CA, USA). Citrate was purchased from Merck (Frankfurt/Main, Hessen, Germany) and sodium bicarbonate from Labsynth products (Diadema, SP, Brazil). HepG2 and Caco-2 cell lines were obtained from the Cell Bank of Rio de Janeiro (Rio de Janeiro, RJ, Brazil). Primers and probes for TaqMan® real-time PCR were purchased from Applied Biosystem (Foster City, CA, USA).
Caco-2 and HepG2 cell cultures
Caco-2 (human colorectal adenocarcinoma) and HepG2 (human hepatocellular carcinoma) cells were maintained in DMEM supplemented with 10% fetal bovine serum, 2 mmol/L glutamine, 44 mmol/L sodium bicarbonate, 10 000 U/mL streptomycin and 10 000 UI/mL penicillin. Cells were grown at 37 oC in a humidified atmosphere, containing 5% CO2. Culture medium was replaced twice a week and cells were trypsinized and subcultured every 7 days.
Cell treatments
Atorvastatin was dissolved in methanol. Simvastatin was dissolved in ethanol and the following procedure was used to activate it. NaOH 0.1 mol/L was added to the solution and subsequently incubated at 50 oC for 2 h. The pH was brought to 7.0 by HCl, and the final concentration was adjusted to 5.6 mmol/L. The solution was kept at 4 oC.
The final concentration of methanol or ethanol in the culture medium did not exceed 0.1% and 0.2%, respectively. Preliminary experiments with these concentrations of methanol or ethanol did not show cytotoxicity. For cytotoxicity assays, four concentrations of atorvastatin were tested, starting with vehicle control (0 μmol/L), and 0.1 μmol/L as the lowest concentration up to a maximum of 20 μmol/L for 24 h, and for simvastatin we have tested from 0.01 to 10 μmol/L.
For mRNA expression measurements, Caco-2 cells were seeded at a density of 1.0×106 cells per 75 cm2, cultured for three days, and then treated with atorvastatin or simvastatin (0–1 μmol/L) for 12 or 24 h. HepG2 cells were seeded at 2.5×106 cells per 75 cm2, cultured for 24 h, and treated with atorvastatin (0–20 μmol/L) or simvastatin (0–10 μmol/L) for the same time.
Cell viability and DNA fragmentation
Toxicity of atorvastatin and simvastatin treatment was evaluated by measuring the percentage of cells with loss of membrane integrity and DNA fragmentation. The percentage of viable Caco-2 and HepG2 cells treated with the statins was determined by flow cytometry using propidium iodide solution (100 μg/mL in phosphate buffer saline) to detect membrane integrity of the cells. Propidium iodide is a highly water-soluble fluorescent compound that cannot pass through intact membranes being generally excluded from viable cells. It binds to DNA by intercalating between the bases with little or no sequence preference.
DNA fragmentation was also analyzed by flow cytometry after DNA staining with propidium iodide. The membrane of the cells was lyzed to allow binding of propidium iodide to DNA22. Briefly, cells (5×105) were gently resuspended in 200 μL hypotonic solution containing 50 μg/mL propidium iodide, 0.1% sodium citrate, and 0.1% Triton X-100. The cells were then incubated overnight at 4 oC. Fluorescence was measured and analyzed by flow cytometry.
Flow cytometric analysis
Cells (numbering 10 000) were analyzed in a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA, USA) using an argon-ion laser (15 mW) with incident beam at 488 nm. Red (propidium iodide) fluorescence was evaluated using 585 nm filter. Data were acquired and analyzed using the FACS/Cell Quest software (Becton Dickinson, San Jose, CA). Results were expressed as mean of the fluorescence intensity.
Reference gene selection
Five reference genes (GAPDH, HRPT1, SDHA, UBC, and HMBS) were selected in order to determine the most stable one in our study (Table 1). Primer sequences for GAPDH were gently provided by Dr Nancy Amaral REBOUÇAS (Department of Physiology and Biophysics, Institute of Biomedical Sciences, University of Sao Paulo). Primer sequences for all other reference genes were used as described by Vandesompele et al23. The MGBTM probes for each primer pairs were designed using the software Primer Express 3.0 (Applied Biosystems, Foster City, CA, USA).
Table 1. Ranking of the reference genes as determined using the geNorm software tool.
| Caco-2 | HepG2 |
|---|---|
| HMBS/HRPT1 | GAPDH/HMBS |
| SDHA | UBC |
| UBC | HPRT1 |
| GAPDH | SDHA |
The genes are ranked in order of their expression stability under the influence of atorvastatin decreasing from top to bottom. In bold are indicated the most stable. GAPDH, Glyceraldehyde-3-phosphate dehydrogenase; SDHA, succinate dehydrogenase complex, subunit A, flavoprotein (Fp); UBC, ubiquitin C; HPRT1, hypoxanthine phosphoribosyltransferase 1 (Lesch-Nyhan syndrome); HMBS, hydroxymethylbilane synthase.
Real-time qPCR
RNA was extracted from Caco-2 and HepG2 (0.5 to 1×107) cells using TRIzol® Reagent following the manufacturer's protocol. RNA was dissolved in DEPC-treated water and the concentration and purity of each sample was obtained from A260/A280 measurements. cDNA was produced from 4 μ g total RNA by SuperScript II reverse transcriptase and ABCB1, ABCG2, ABCC2, SLCO1B1, SLCO2B1 and SLC22A1 mRNA levels were determined by TaqMan® quantitative polymerase chain reaction (PCR) assay that provides more precision and greater dynamic range than endpoint PCR24.
The primers and probes were designed to span exon/exon junctions in order to avoid amplification of eventually contaminating genomic DNA (Table 2). The PCR assays were carried out in 96-well plates using a 7500 Real-Time PCR system (Applied Biosystems, Foster City, CA, USA). The thermal cycler protocol consisted of an initial activation step at 50 °C for 2 min and 95 °C for 10 min and 40 cycles of denaturation at 95 °C for 15 s and annealing/extension at 60 °C for 1 min.
Table 2. Primers and probes sequences used for TaqMan® real-time PCR.
| Gene | Primers and probes* | Sequence 5′ ← 3′ | Product size (bp) | Primers efficiency |
|---|---|---|---|---|
| ABCG2 | Forward | CCATTGCATCTTGGCTGTCA | 66 | 2.00 |
| Reverse | GCAAAGCCGTAAATCCATATCG | |||
| Probe | FAM-CAGTACTTCAGCATTCC-NFQ | |||
| ABCC2 | Forward | AGAGAACAGCTTCGTCGAACAC | 59 | 1.97 |
| Reverse | TCAGATGCCTGCCATTGGA | |||
| Probe | FAM-TAGCCGCAGTTCTAG-NFQ | |||
| SLCO1B1 | Forward | AGCAGAGGCACAACCTTCAGA | 82 | 2.05 |
| Reverse | GCTGAGTGACAGAGCTGCCA | |||
| Probe | FAM-CAATGGATTGAAGATGTT-NFQ | |||
| SLCO2B1 | Forward | TCAGCCCTTGGCATCTCC | 91 | 1.98 |
| Reverse | CATCATGGTCACTGCAAACAGG | |||
| Probe | FAM-ACAACAGCAACTCGC-NFQ | |||
| SLC22A1 | Forward | TGGACCACATCGCTCAAAAG | 69 | 2.15 |
| Reverse | CCTCTTCGAGGGAAAGCATCTT | |||
| Probe | FAM-ATGGGAAGTTGCCTCCT-NFQ | |||
| GAPDH | Forward | GGAAGGTGAAGGTCGGAGTCA | 230 | 1.96 |
| Reverse | CTGGAAGATGGTGATGGGATTC | |||
| Probe | VIC-TCAGCCTTGACGGTGC-NFQ | |||
| HMBS | Forward | GGCAATGCGGCTGCAA | 64 | 1.95 |
| Reverse | GGGTACCCACGCGAATCAC | |||
| Probe | VIC-CGGAAGAAAACAGCC-NFQ |
*MGB: Minor groove binding DNA; NFQ: Nonfluorescent quencher
Sample cycle threshold (CT) values were determined from plots of normalized fluorescence versus PCR cycle number during exponential amplification. Standard curves for all primer amplifications were generated by plotting average CT values against the logarithm starting quantity of target template molecules.
The relative quantification value of each target gene was analyzed using a comparative CT method25. The following formula was used to calculate the relative amount of the transcript in the sample and normalized to the appropriated endogenous reference: 2−ΔΔCT. For baseline mRNA expression of drug transporters, no calibrator was used; the formula was basically 2−ΔCT.
Statistical analysis
Each set of experiments was repeated at least four times in cells pertaining to different passages. Results are reported as means±SEM. Differences among the means were analyzed by one-way analysis of variance (ANOVA) followed by the Bonferroni post-test comparing each treatment column to control column (0 μmol/L). All statistical analysis was performed using Prism (Graph Pad Software, Inc, San Diego, CA, USA). Statistical significance was set for P<0.05.
Results
Cytotoxicity of statins treatment
Twenty-four hours treatment of HepG2 cells with atorvastatin at different concentrations (0–20 μmol/L) did not affect membrane integrity of the cells nor increased the DNA fragmentation. Loss of cell viability and DNA fragmentation were found in 4% and 8%, respectively, of the cells. On the other hand, Caco-2 cells treated for 24 h with atorvastatin presented loss of membrane integrity in 15% of the cells from 10 μmol/L. Based on these results, Caco-2 cells were treated with atorvastatin at 0–1 μmol/L, whereas HepG2 cells were treated with 0–20 μmol/L for 12 or 24 h. For simvastatin acid, none of the concentrations tested in HepG2 (0–10 μmol/L) or Caco-2 (0–1 μmol/L) cells induced loss of cell viability or DNA fragmentation after 24-h treatment.
GeNorm analysis
In order to determine the most stable reference genes in our study, the results were analyzed using GeNorm software24. The ranking of the candidate reference genes according to their expression stability under the influence of both statins on HepG2 and Caco-2 cells was established (Table 1). Based on this ranking, the most stable genes were GAPDH and HMBS; so they were chosen for normalization of gene expression in HepG2 and Caco-2 cells, respectively.
Basal expression of drug transporters in Caco-2 and HepG2 cells
The mRNA expression of the membrane transporters was measured by TaqMan® real-time PCR. Linear regression of plots generated from cDNA serial dilutions (ranging from 12.5 to 200 ng cDNA) indicated amplification linearity (R2>0.96) and adequate slope values for membrane transporters (-3.00 to -3.40) and the reference genes GAPDH (-3.42) and HMBS (-3.44). Efficiencies are shown in Table 2.
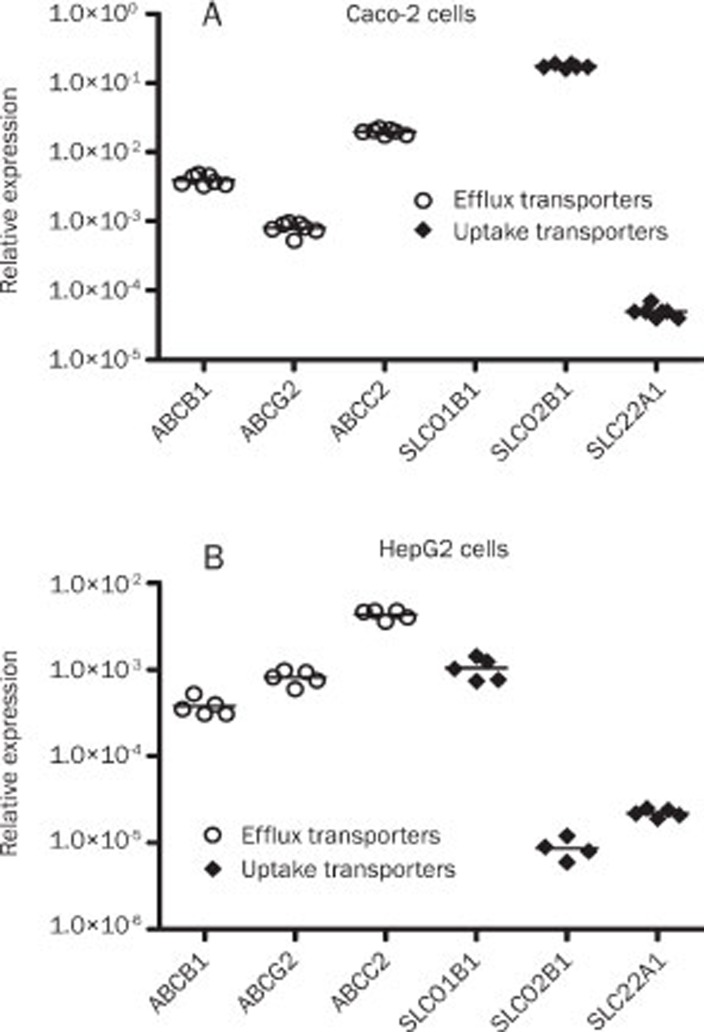
Baseline gene expression of drug transporters was evaluated in Caco-2 and HepG2 cells (Figure 1). The target genes had the following mean CT values (Caco-2/HepG2 cells): ABCB1, 25/27; ABCC2, 23/24; ABCG2, 27/26; SLC22A1, 31/31, SLCO2B1, 19/32, and SLCO1B1, not detected/28. Differences in mRNA levels among the transporters were observed, and the uptake transporter SLCO2B1 was the most prevalent transcript in Caco-2 cells (Figure 1A), being followed by ABCC2, which was 9-fold lower. The expression of the uptake transporter SLC22A1 was extremely low; 3500-fold lower compared to SLCO2B1. Among the efflux transporters, ABCC2 was highly different from ABCG2 (>10-fold) (Figure 1A).
Figure 1.
Relative gene expression pattern of the drug transporters in Caco-2 (A) and HepG2 (B) cells. Analysis of mRNA expression of each transporter was carried out by real-time PCR and normalized with GAPDH (HepG2 cells) or HMBS (Caco-2 cells). Each line represents the mean value of five or four (HepG2 cells) or seven (Caco-2 cells) experiments.
In HepG2 cells, the efflux transporter ABCC2 was the most prevalent transcript, being followed by ABCG2, which was 5-fold lower (Figure 1B). SLCO1B1 mRNA levels were only found to be expressed in HepG2 cells, and it was the most expressed uptake transporter being followed by SLC22A1, whose mRNA levels were 2-fold higher than SLCO2B1 expression (Figure 1B).
Effects of statins on mRNA expression of the efflux transporters
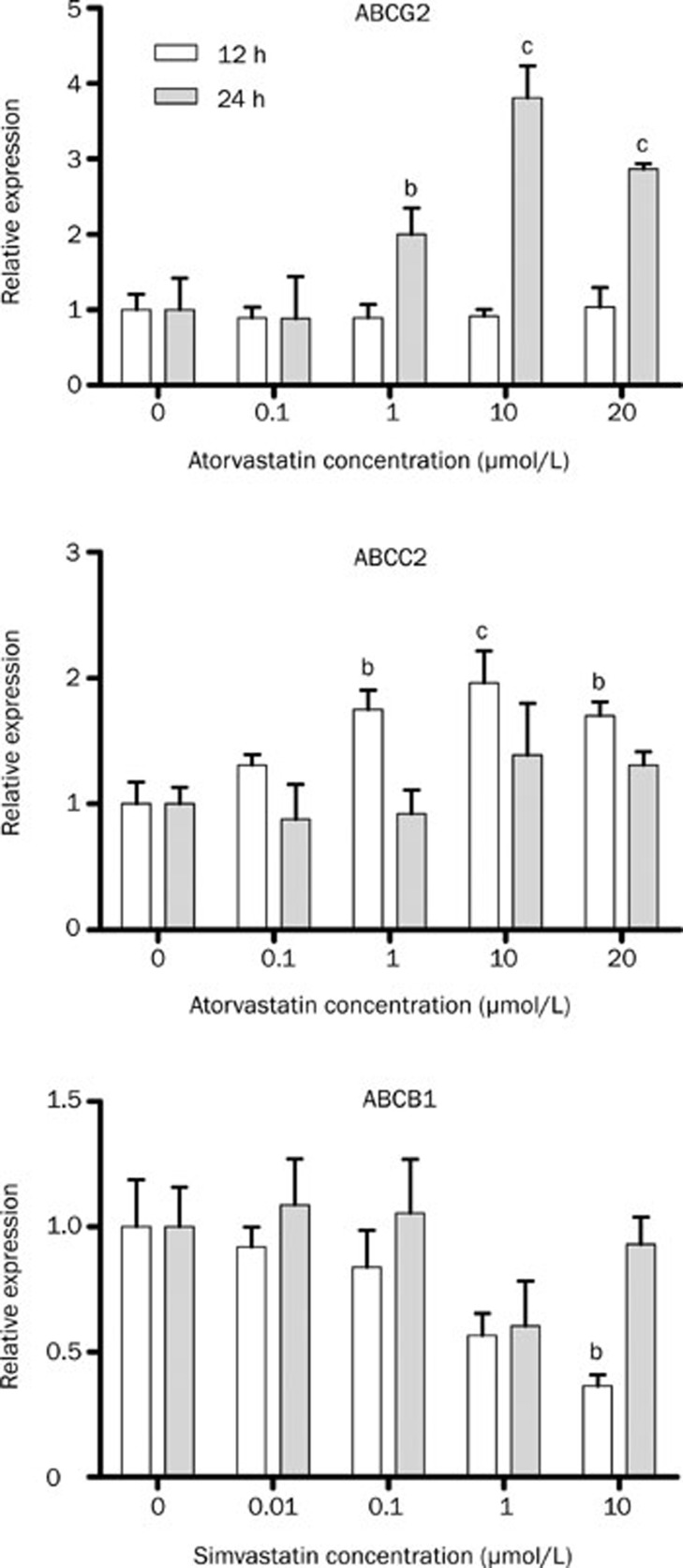
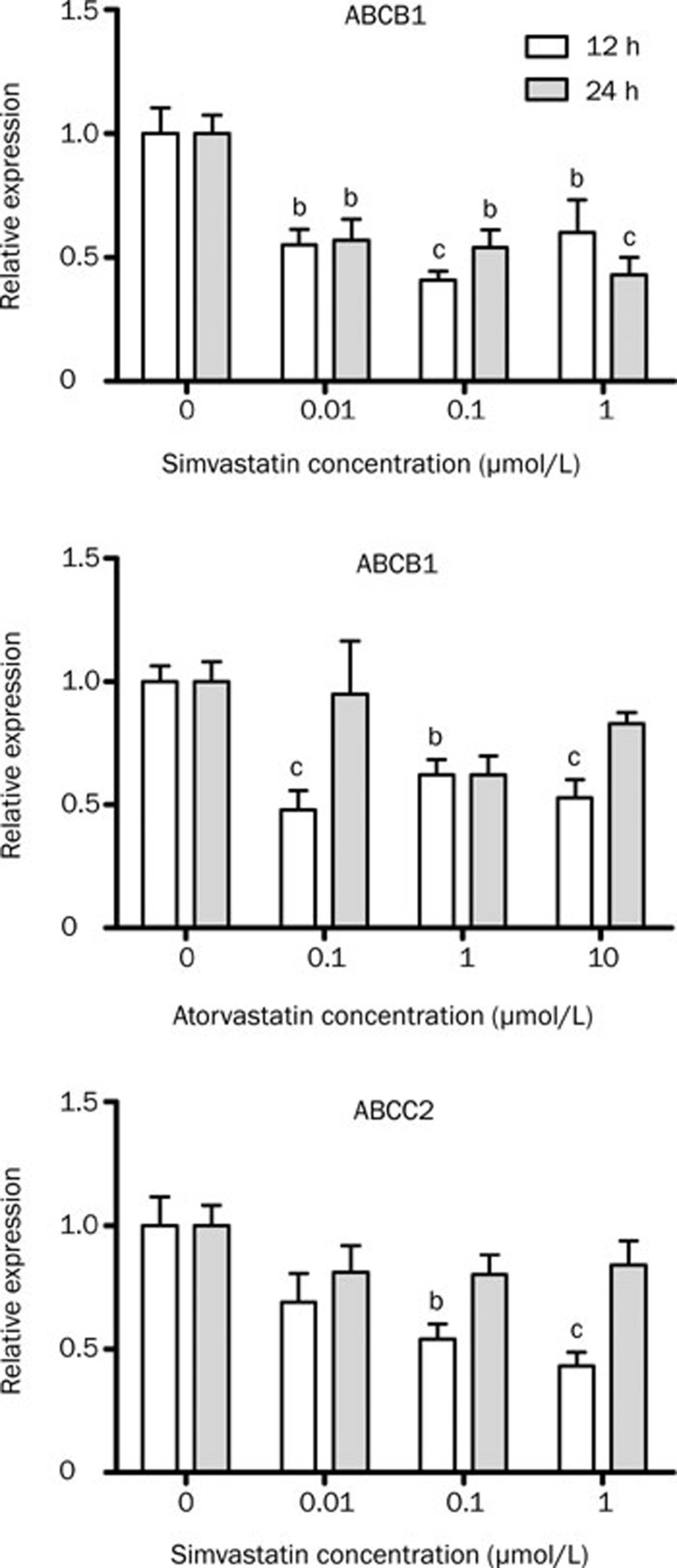
Atorvastatin treatment of HepG2 cells increased ABCG2 and ABCC2 expression (Figure 2); in these experiments dose-dependent and time-dependent effects were observed. ABCG2 transcript levels were increased after 24-h treatment at 1 (2.00±0.35 vs 1.00 ± 0.13, P<0.05), 10 (3.81±0.43 vs 1.00±0.13, P<0.001) and 20 μmol/L (2.87±0.07 vs 1.00±0.13, P<0.001). After 12 h treatment, ABCC2 mRNA levels were significantly increased also at 1 (1.75 ±0.16 vs 1.00±0.17), 10 (1.96±0.26 vs 1.00± 0.17) and 20 μmol/L (1.70±0.11 vs 1.00±0.17). Simvastatin treatment only reduced ABCB1 mRNA expression after 10 μmol/L (0.36±0.09 vs 1.00±0.38, Figure 2). In contrast, simvastatin treatment of Caco-2 cells induced a decrease around 50% of ABCB1 and ABCC2 mRNA levels (Figure 3). Atorvastatin treatment of Caco-2 cells also reduced ABCB1 mRNA level after 12-h treatment (Figure 3).
Figure 2.
Relative expression of the efflux transporters after treatment of HepG2 cells with atorvastatin or simvastatin. Real-time PCR was performed using total RNA extracted from 12–24 h atorvastatin-treated (0 to 20 μmol/L), simvastatin (0 to 10 μmol/L), and vehicle control (0 μmol/L) cells. n=4 (12 h) or 5 (24 h). Values represent mean±SEM. bP<0.05, cP<0.01 as compared to 0 μmol/L atorvastatin or simvastatin as indicated by one-way ANOVA and Bonferroni test.
Figure 3.
Relative expression of efflux transporters after treatment of Caco-2 cells with atorvastatin or simvastatin. Real-time PCR was performed using total RNA extracted from 12–24 h atorvastatin-treated, simvastatin and vehicle control (0 μmol/L) cells. n=3. Values represents mean±SEM. bP<0.05, cP<0.01 compared to 0 μmol/L atorvastatin or simvastatin as indicated by one-way ANOVA and Bonferroni test.
Effects of atorvastatin on mRNA expression of the uptake transporters
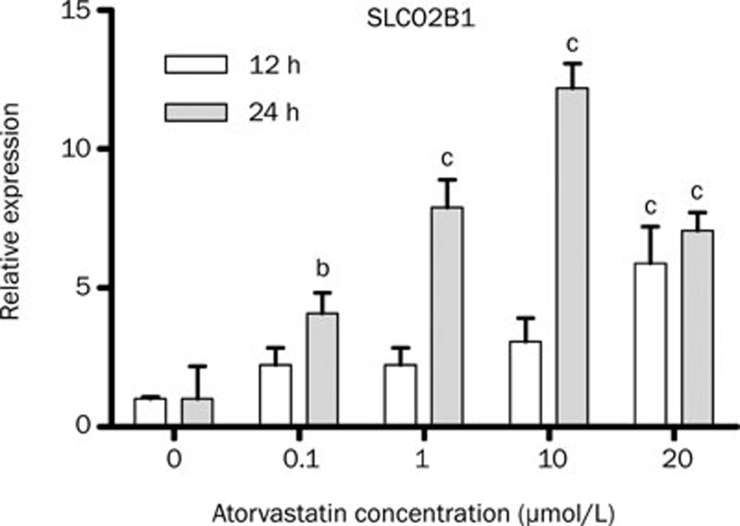
SLCO1B1 mRNA expression was not modulated by atorvastatin or simvastatin treatment in HepG2 cells (data not shown). After 12 h exposure to 20 μmol/L atorvastatin, there was a five-fold increase in SLCO2B1 expression. Treatment of HepG2 cells for 24-h pronounced the increase in SLCO2B1 transcript levels, being dose-dependently regulated (0.1 μmol/L: four-fold; 1 μmol/L: eight-fold; 10 μmol/L: twelve-fold; 20 μmol/L: seven-fold) (Figure 4). Neither atorvastatin nor simvastatin affected SLC22A1 mRNA expression in HepG2 cells (data not shown).
Figure 4.
Relative expression of SLCO2B1 after treatment of HepG2 cells with atorvastatin. Real-time PCR was performed using total RNA extracted from 12–24 h atorvastatin-treated (0 to 20 μmol/L) and vehicle control (0 μmol/L) cells. n=4 (12 h) or 5 (24 h). Values represents mean±SEM. bP<0.05, cP<0.01 compared to 0 μmol/L atorvastatin as indicated by one-way ANOVA and Bonferroni test.
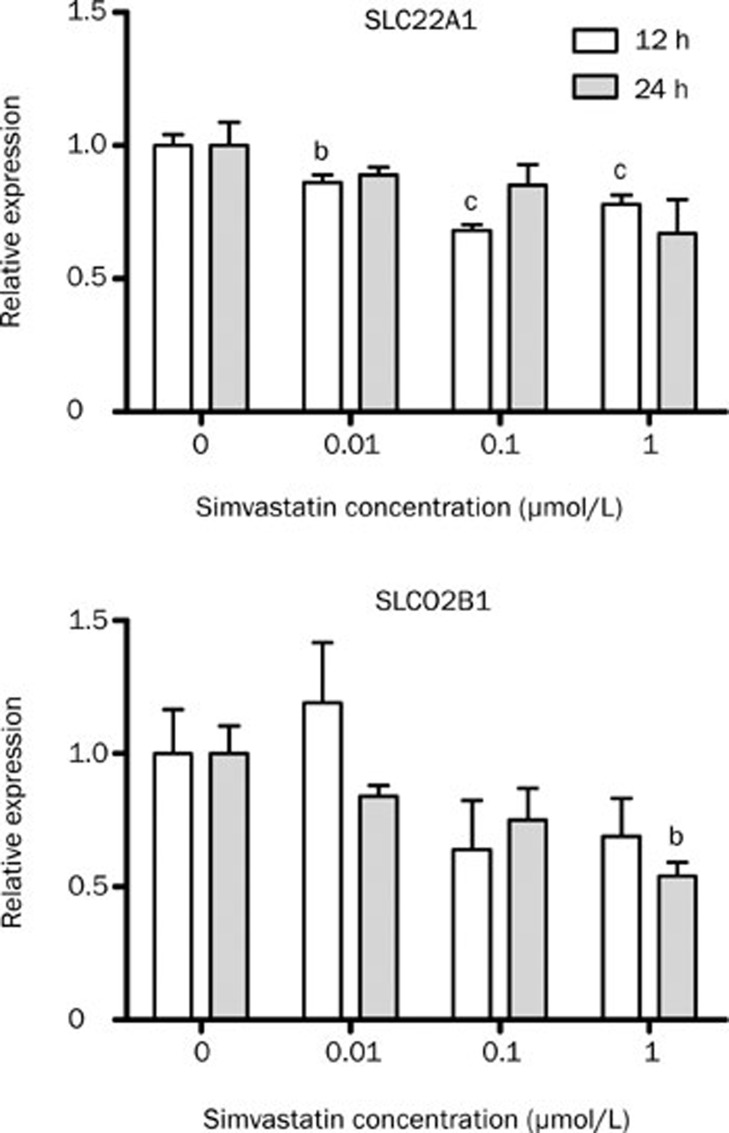
In Caco-2 cells, SLCO2B1 mRNA levels were not changed after atorvastatin treatment (data not shown). Simvastatin decreased SLCO2B1 only at the higher concentration (1 μmol/L) after 24 h treatment. SLC22A1 mRNA expression was around 20% decreased after doses of 0.01 to 1 μmol/L within 12 h (Figure 5). However, no differences were observed in SLC22A1 after prolonged time of exposure to atorvastatin.
Figure 5.
Relative expression of the uptake transporters after treatment of Caco-2 cells with simvastatin. Real-time PCR was performed using total RNA extracted from 12–24 h simvastatin (0 to 1 μmol/L), and vehicle control (0 μmol/L) cells. n=4. Values represent mean±SEM. bP<0.05, cP<0.01 compared to 0 μmol/L simvastatin as indicated by one-way ANOVA and Bonferroni test.
Discussion
The effects of statins on activity and/or expression levels of drug transporters can be extremely important not only to obtain adequate knowledge on drug pharmacokinetics but also to predict adverse drug interactions. The effect of statins on expression of genes involved in its own pharmacokinetics is poorly understood. The modulating effect of atorvastatin and simvastatin acid on the expression of efflux (ABCB1, ABCG2, and ABCC2) and uptake (SLCO1B1, SLCO2B1, and SLC22A1) transporters in human immortalized cell lines, HepG2 (hepatocyte) and Caco-2 (enterocyte), was investigated.
Firstly, the pattern of gene expression of the drug transporters was evaluated in Caco-2 and HepG2 cells. Differences in mRNA levels among the transporters were observed, as follows: ABCC2>ABCG2>ABCB1>SLCO1B1 >>>SLC22A1>SLCO2B1 for HepG2 and SLCO2B1>>ABCC2> ABCB1>ABCG2>>>SLC22A1 for Caco-2 cells. The presence of SLCO1B1 transcripts in HepG2 cells only is consistent with its primary localization in the liver12. Basically, we could observe that the major difference between hepatocytes and enterocytes was the expression of the uptake transporter SLCO2B1, which is the major transcript in enterocytes and the minor in hepatocytes.
Other investigators have measured transcript levels of the drug transporters in Caco-2 cells, and they also compared expression of transporters between Caco-2 cells and human intestine20, 27, 28. In spite of the fact that mRNA expression of some transporters is differently expressed in undifferentiated and fully differentiated Caco-2 cells27, similar results were obtained when mRNA expression was measured in Caco-2 cells after growing for 21 days in filters that ensure full cell differentiation20. The rank order was SLCO2B1∼ABCC2>ABCB1>ABCG2>>SLC22A1. However, Englund et al (2006) observed that Caco-2 expression pattern was clearly distinguishable from that found in human small intestine20.
Although Caco-2 cells have proven to be a suitable model for studying carrier-mediated transport in human intestines29, the expression of specific transporter and ion channel genes may differ substantially as previously discussed. Therefore the results reported herein may be not fully applied to in vivo conditions.
Rank drug transporter expression was also studied by others in HepG2 cells and was compared with primary hepatocytes30. Le Vee et al (2006)30 reported that the efflux transporter ABCC2 mRNA levels in HepG2 cells were detected at levels closed to those found in cultured primary hepatocytes, and ABCC2 was also the most expressed in the present study. With respect to uptake transporters, Le Vee et al30 failed to find SLC22A1 mRNA transcripts, whereas we found low levels. Low levels of SLC22A1 in HepG2 cells were also found by others31, 32. Expression of several drug transporters in this cell line poorly correlated with expression in the liver31, 32. Thus, it has to be used with caution in drug transport studies.
We have previously described that atorvastatin down-regulates ABCB1 gene6. We studied herein atorvastatin and simvastatin effects on mRNA expression of ABCC2, ABCG2, SLCO1B1, SLCO2B1, and SLC22A1. We have also evaluated the effect of simvastatin on ABCB1 mRNA levels, in order to confirm if the reduction of ABCB1 mRNA levels is an effect of the class of the HMG-CoA reductase inhibitors. Thus, this is the first evidence that statin modulates the expression of these drug transporters in human hepatoma and adenoma cell lines. Various concentrations of the statins, in the range of blood levels reached after an oral dose of 10 to 80 mg/d for atorvastatin, and 5 to 80 mg/d for simvastatin33, were tested. In Caco-2 cells, expression of ABCB1, ABCC2, SLC22A1, and SLCO2B1 was significantly down-regulated by simvastatin treatment. Conversely, in HepG2 cells, expression of ABC transporters and SLCO2B1 was up-regulated following atorvastatin treatment, and no effect was observed after simvastatin treatment. These results are very interesting, and are an evidence for the fact that transporters are differently regulated in the liver and intestine. Furthermore, indicates that with the exception of ABCB1, the other transporters evaluated are differently regulated by the statins, which might be related to the efficacy of these statins.
Reduction of the expression of ABCB1 and ABCC2 by simvastatin in Caco-2 cells is significant because simvastatin is frequently combined with ezetimibe, an inhibitor of the cholesterol uptake transporter Niemann-Pick C1-like protein (NPC1L1), for the treatment of hypercholesterolemia. As well as this, it has been shown that ABC transporters intestinal expression is crucial for ezetimibe disposition34. In fact, up-regulation of intestinal ABCB1 and ABCC2 by rifampin reduces the sterol-lowering effect of ezetimibe, due to reduced plasma levels. Our results indicates that simvastatin decreases ABCC2 and ABCB1 expression, and these data suggest a mechanism for the beneficial interaction between simvastatin and ezetimibe in the cholesterol-lowering therapy. At least for ABCB1, we have previously established that mRNA expression positively correlates with the activity6. Conversely, atorvastatin treatment of Caco-2 cells only affected ABCB1 expression after 12-h treatment. This might suggest that atorvastatin could be less effective when combined to ezetimibe. Actually, in a multicenter study performed by Pearson et al (2005)35 which determined the extend of reduction in LDL-C after addition of ezetimibe to ongoing statin therapy of hypercholesterolemic subjects, they could observe that simvastatin was the only drug that had a dose dependent effect when combined with ezetimibe, and a dose of 80 mg/day was more effective than the maximum dose for other statins. Disagreeing with our data, simvastatin treatment did not influence the expression of duodenal ABCC2 expression in healthy individuals36. However, the discrepancy between our results and the above is that the expression of ABC transporters in Caco-2 cells, as previously discussed, is more similar to the colon than to the small intestine.
In relation to atorvastatin, reduced statin systemic exposure has been associated with higher hepatic expression of ABCC237. Our in vitro observations confirm these findings, as atorvastatin up-regulates ABCC2 expression in HepG2 cells.
OCT1 (SLC22A1) facilitates the excretion of its substrates from circulating blood into the intestinal lumen21. Gene expression of SLC22A1 was down-regulated in Caco-2 cells after simvastatin treatment.
Other studies have shown a down-regulation for this transporter. After bile duct ligation in the rat, SLC22A1 mRNA was profoundly decreased38. In addition, SLC22A1 gene was demonstrated to be regulated by cholesterol treatment in HepG2 cells39.
In spite of the fact that OATP1B1 has been involved in statins disposition40, no results are available on modulation of its mRNA expression in the liver. Otherwise, SLCO2B1 has been demonstrated to be down-regulated in HepG2 cells after 72 h treatment with atorvastatin19. However, the time fixed by Grube et al19 (72 h) is cytotoxic for HepG2 cells. Preliminary experiments were performed to determine the toxicity of atorvastatin in HepG2 cells. We have found an increase in DNA fragmentation, as well as an antiproliferative effect after 48 h treatment with atorvastatin at 4 to 20 μmol/L. So, we can not exclude the possibility that reduction in mRNA levels is a consequence of cell toxicity.
The mechanism involved in statins regulation of drug transporters is currently being investigated. However, some lines of evidence suggest that statin-mediated changes may occur at transcriptional level. Statins are presumably activators of the nuclear receptors constitutive androstane receptor (CAR) and pregnane X receptor (PXR)41, 42, that regulate expression of drug transporters. For example, ABCC2 has been shown to be regulated by CAR, PXR, and farnesoid X-activated receptor (FXR) in human and rat hepatocytes43, 44 and induction of ABCG2 was associated to aryl hydrocarbon receptor (AhR) and nuclear factor E2-related factor 2 (Nrf2) activation, and PXR45, 46, 47. SLCO2B1 was found to be repressed by CAR and AhR activator in human hepatocytes48, and Sp1, a transcription factor, was required for constitutive expression of SLCO2B1 in the liver and small intestine48.
The effect of statins on mRNA levels of ABC transporters, such as ABCA1 and ABCB1, have been studied. The expression of both genes is modulated by the cellular cholesterol content. The crosstalk between cholesterol homeostasis and drug metabolism is probably mediated by nuclear receptors, activating target genes in response to endogenous and exogenous ligands. Stedman et al (2005)49, using a bile duct ligation model of cholestasis, have shown that PXR and CAR have crucial roles in the regulation of lipid and bile acid homeostasis. CAR and PXR knockout attenuated the expected increase in total cholesterol in both genotypes and increased HDL cholesterol levels49. Thus, we believe that activation/repression of CAR and PXR could result in the regulation of drug transporters, as it has been reported in the promoter of almost all the genes herein studied.
In summary, these findings reveal that atorvastatin and simvastatin exhibit differential effects on mRNA expression of drug transporters in intestinal and liver cells, which may be related to the efficacy of these statins. Furthermore, alterations in the expression levels of drug transporters in the liver and/or intestine may contribute to the variability in oral disposition of statins, and in the selection of statin when starting combined therapy with ezetimibe.
Author contribution
Alice Cristina RODRIGUES designed research, performed research, analysed data, and wrote the paper; Fabiana Dalla Vecchia GENVIGIR performed the research; Rui CURI, Mario Hiroyuki HIRATA, and Rosario HIRATA helped write the paper.
Acknowledgments
This work is supported by grants from FAPESP (2007/ 00347-6). AC Rodrigues, and FDV Genvigir are recipient fellowships from FAPESP R Curi, MH Hirata and RDC Hirata are recipient fellowships from CNPq.
References
- Ito K, Suzuki H, Horie T, Sugiyama Y. Apical/basolateral surface expression of drug transporters and its role in vectorial drug transport. Pharm Res. 2005;22:1559–77. doi: 10.1007/s11095-005-6810-2. [DOI] [PubMed] [Google Scholar]
- Corsini A, Bellosta S, Baetta R, Fumagalli R, Paoletti R, Bernini F. New insights into the pharmacodynamic and pharmacokinetic properties of statins. Pharmacol Ther. 1999;84:413–28. doi: 10.1016/s0163-7258(99)00045-5. [DOI] [PubMed] [Google Scholar]
- Boyd RA, Stern RH, Stewart BH, Wu X, Reyner EL, Zegarac EA, et al. Atorvastatin coadministration may increase digoxin concentrations by inhibition of intestinal P-glycoprotein-mediated secretion. J Clin Pharmacol. 2000;40:91–8. doi: 10.1177/00912700022008612. [DOI] [PubMed] [Google Scholar]
- Chen C, Mireles RJ, Campbell SD, Lin J, Mills JB, Xu JJ, et al. Differential interaction of 3-hydroxy-3-methylglutaryl-Coa reductase inhibitors with ABCB1, ABCC2, and OATP1B1. Drug Metab Dispos. 2005;33:537–46. doi: 10.1124/dmd.104.002477. [DOI] [PubMed] [Google Scholar]
- Wu X, Whitfield LR, Stewart BH. Atorvastatin transport in the Caco-2 cell model: contributions of P-glycoprotein and the proton–monocarboxylic acid co-transporter. Pharm Res. 2000;17:209–15. doi: 10.1023/a:1007525616017. [DOI] [PubMed] [Google Scholar]
- Rodrigues AC, Curi R, Britto LR, Rebbechi IM, Hirata MH, Bertolami MC, et al. Down–regulation of ABCB1 transporter by atorvastatin in a human hepatoma cell line and in human peripheral blood mononuclear cells. Biochim Biophys Acta. 2006;1760:1866–73. doi: 10.1016/j.bbagen.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Fromm MF, Kauffmann HM, Fritz P, Burk O, Kroemer HK, Warzok RW, et al. The effect of rifampin treatment on intestinal expression of human MRP transporters. Am J Pathol. 2000;157:1575–80. doi: 10.1016/S0002-9440(10)64794-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maliepaard M, Scheffer GL, Faneyte IF, Van Gastelen MA, Pijnenborg AC, Schinkel AH, et al. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res. 2001;61:3458–64. [PubMed] [Google Scholar]
- Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, Willingham MC. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci USA. 1987;84:7735–8. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CY, Benet LZ. Predicting drug disposition via application of BCS: transport/absorption/elimination interplay and development of a biopharmaceutics drug disposition classification system. Pharm Res. 2005;22:11–23. doi: 10.1007/s11095-004-9004-4. [DOI] [PubMed] [Google Scholar]
- Benet LZ, Cummins CL, Wu CY. Transporter-enzyme interactions: implications for predicting drug-drug interactions from in vitro data. Curr Drug Metab. 2003;4:393–8. doi: 10.2174/1389200033489389. [DOI] [PubMed] [Google Scholar]
- Chandra P, Brouwer KL. The complexities of hepatic drug transport: current knowledge and emerging concepts. Pharm Res. 2004;21:719–35. doi: 10.1023/b:pham.0000026420.79421.8f. [DOI] [PubMed] [Google Scholar]
- Hsiang B, Zhu Y, Wang Z, Wu Y, Sasseville V, Yang WP, et al. A novel human hepatic organic anion transporting polypeptide (OATP2). Identification of a liver-specific human organic anion transporting polypeptide and identification of rat and human hydroxymethylglutaryl-CoA reductase inhibitor transporters. J Biol Chem. 1999;274:37161–8. doi: 10.1074/jbc.274.52.37161. [DOI] [PubMed] [Google Scholar]
- Nakai D, Nakagomi R, Furuta Y, Tokui T, Abe T, Ikeda T, et al. Human liver-specific organic anion transporter, LST-1, mediates uptake of pravastatin by human hepatocytes. J Pharmacol Exp Ther. 2001;297:861–7. [PubMed] [Google Scholar]
- Bolego C, Poli A, Cignarella A, Catapano AL, Paoletti R. Novel statins: pharmacological and clinical results. Cardiovasc Drugs Ther. 2002;16:251–7. doi: 10.1023/a:1020656607497. [DOI] [PubMed] [Google Scholar]
- Couvert P, Giral P, Dejager S, Gu J, Huby T, Chapman MJ, et al. Association between a frequent allele of the gene encoding OATP1B1 and enhanced LDL-lowering response to fluvastatin therapy. Pharmacogenomics. 2008;9:1217–27. doi: 10.2217/14622416.9.9.1217. [DOI] [PubMed] [Google Scholar]
- Kobayashi D, Nozawa T, Imai K, Nezu J, Tsuji A, Tamai I. Involvement of human organic anion transporting polypeptide OATP-B (SLC21A9) in pH-dependent transport across intestinal apical membrane. J Pharmacol Exp Ther. 2003;306:703–8. doi: 10.1124/jpet.103.051300. [DOI] [PubMed] [Google Scholar]
- Kullak-Ublick GA, Ismair MG, Stieger B, Landmann L, Huber R, Pizzagalli F, et al. Organic anion-transporting polypeptide B (OATP-B) and its functional comparison with three other OATPs of human liver. Gastroenterology. 2001;120:525–33. doi: 10.1053/gast.2001.21176. [DOI] [PubMed] [Google Scholar]
- Grube M, Kock K, Oswald S, Draber K, Meissner K, Eckel L, et al. Organic anion transporting polypeptide 2B1 is a high-affinity transporter for atorvastatin and is expressed in the human heart. Clin Pharmacol Ther. 2006;80:607–20. doi: 10.1016/j.clpt.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Englund G, Rorsman F, Ronnblom A, Karlbom U, Lazorova L, Grasjo J, et al. Regional levels of drug transporters along the human intestinal tract: co-expression of ABC and SLC transporters and comparison with Caco-2 cells. Eur J Pharm Sci. 2006;29:269–77. doi: 10.1016/j.ejps.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Jonker JW, Wagenaar E, Mol CA, Buitelaar M, Koepsell H, Smit JW, et al. Reduced hepatic uptake and intestinal excretion of organic cations in mice with a targeted disruption of the organic cation transporter 1 (Oct1 [Slc22a1]) gene. Mol Cell Biol. 2001;21:5471–7. doi: 10.1128/MCB.21.16.5471-5477.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:271–9. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes Genome Biol 20023RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD, Zakrajse BA, Mills AG, Gorn V, Singer MJ, Reed MW. Quantitative reverse transcription-polymerase chain reaction to study mRNA decay: comparison of endpoint and real-time methods. Anal Biochem. 2000;285:194–204. doi: 10.1006/abio.2000.4753. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Cilla DD, Jr, Whitfield LR, Gibson DM, Sedman AJ, Posvar EL. Multiple-dose pharmacokinetics, pharmacodynamics, and safety of atorvastatin, an inhibitor of HMG-CoA reductase, in healthy subjects. Clin Pharmacol Ther. 1996;60:687–95. doi: 10.1016/S0009-9236(96)90218-0. [DOI] [PubMed] [Google Scholar]
- Anderle P, Rakhmanova V, Woodford K, Zerangue N, Sadee W. Messenger RNA expression of transporter and ion channel genes in undifferentiated and differentiated Caco-2 cells compared to human intestines. Pharm Res. 2003;20:3–15. doi: 10.1023/a:1022282221530. [DOI] [PubMed] [Google Scholar]
- Calcagno AM, Ludwig JA, Fostel JM, Gottesman MM, Ambudkar SV. Comparison of drug transporter levels in normal colon, colon cancer, and Caco-2 cells: impact on drug disposition and discovery. Mol Pharm. 2006;3:87–93. doi: 10.1021/mp050090k. [DOI] [PubMed] [Google Scholar]
- Hilgers AR, Conradi RA, Burton PS. Caco-2 cell monolayers as a model for drug transport across the intestinal mucosa. Pharm Res. 1990;7:902–10. doi: 10.1023/a:1015937605100. [DOI] [PubMed] [Google Scholar]
- Le Vee M, Jigorel E, Glaise D, Gripon P, Guguen-Guillouzo C, Fardel O. Functional expression of sinusoidal and canalicular hepatic drug transporters in the differentiated human hepatoma HepaRG cell line. Eur J Pharm Sci. 2006;28:109–17. doi: 10.1016/j.ejps.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Hilgendorf C, Ahlin G, Seithel A, Artursson P, Ungell AL, Karlsson J. Expression of thirty-six drug transporter genes in human intestine, liver, kidney, and organotypic cell lines. Drug Metab Dispos. 2007;35:1333–40. doi: 10.1124/dmd.107.014902. [DOI] [PubMed] [Google Scholar]
- Kanebratt KP, Andersson TB. Evaluation of HepaRG cells as an in vitro model for human drug metabolism studies. Drug Metab Dispos. 2008;36:1444–52. doi: 10.1124/dmd.107.020016. [DOI] [PubMed] [Google Scholar]
- Mohammadi A, Macri J, Newton R, Romain T, Dulay D, Adeli K. Effects of atorvastatin on the intracellular stability and secretion of apolipoprotein B in HepG2 cells. Arterioscler Thromb Vasc Biol. 1998;18:783–93. doi: 10.1161/01.atv.18.5.783. [DOI] [PubMed] [Google Scholar]
- Oswald S, Haenisch S, Fricke C, Sudhop T, Remmler C, Giessmann T, et al. Intestinal expression of P-glycoprotein (ABCB1), multidrug resistance associated protein 2 (ABCC2), and uridine diphosphate–glucuronosyltransferase 1A1 predicts the disposition and modulates the effects of the cholesterol absorption inhibitor ezetimibe in humans. Clin Pharmacol Ther. 2006;79:206–17. doi: 10.1016/j.clpt.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Pearson T, Ballantyne C, Sisk C, Shah A, Veltri E, Maccubbin D. Comparison of effects of ezetimibe/simvastatin versus simvastatin versus atorvastatin in reducing C-reactive protein and low-density lipoprotein cholesterol levels. Am J Cardiol. 2007;99:1706–13. doi: 10.1016/j.amjcard.2007.01.062. [DOI] [PubMed] [Google Scholar]
- Bernsdorf A, Giessmann T, Modess C, Wegner D, Igelbrink S, Hecker U, et al. Simvastatin does not influence the intestinal P-glycoprotein and MPR2, and the disposition of talinolol after chronic medication in healthy subjects genotyped for the ABCB1, ABCC2 and SLCO1B1 polymorphisms. Br J Clin Pharmacol. 2006;61:440–50. doi: 10.1111/j.1365-2125.2006.02599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemi M, Arnold KA, Backman JT, Pasanen MK, Godtel-Armbrust U, Wojnowski L, et al. Association of genetic polymorphism in ABCC2 with hepatic multidrug resistance-associated protein 2 expression and pravastatin pharmacokinetics. Pharmacogenet Genomics. 2006;16:801–8. doi: 10.1097/01.fpc.0000230422.50962.91. [DOI] [PubMed] [Google Scholar]
- Denk GU, Soroka CJ, Mennone A, Koepsell H, Beuers U, Boyer JL. Down-regulation of the organic cation transporter 1 of rat liver in obstructive cholestasis. Hepatology. 2004;39:1382–9. doi: 10.1002/hep.20176. [DOI] [PubMed] [Google Scholar]
- Dias V, Ribeiro V. The expression of the solute carriers NTCP and OCT-1 is regulated by cholesterol in HepG2 cells. Fundam Clin Pharmacol. 2007;21:445–50. doi: 10.1111/j.1472-8206.2007.00517.x. [DOI] [PubMed] [Google Scholar]
- Pasanen MK, Fredrikson H, Neuvonen PJ, Niemi M. Different effects of SLCO1B1 polymorphism on the pharmacokinetics of atorvastatin and rosuvastatin. Clin Pharmacol Ther. 2007;82:726–33. doi: 10.1038/sj.clpt.6100220. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Yamanaka Y, Iwazaki N, Nakajo I, Hosokawa M, Negishi M, et al. Identification of HMG-CoA reductase inhibitors as activators for human, mouse and rat constitutive androstane receptor. Drug Metab Dispos. 2005;33:924–9. doi: 10.1124/dmd.104.002741. [DOI] [PubMed] [Google Scholar]
- Kocarek TA, Dahn MS, Cai H, Strom SC, Mercer-Haines NA. Regulation of CYP2B6 and CYP3A expression by hydroxymethylglutaryl coenzyme A inhibitors in primary cultured human hepatocytes. Drug Metab Dispos. 2002;30:1400–5. doi: 10.1124/dmd.30.12.1400. [DOI] [PubMed] [Google Scholar]
- Dussault I, Lin M, Hollister K, Wang EH, Synold TW, Forman BM. Peptide mimetic HIV protease inhibitors are ligands for the orphan receptor SXR. J Biol Chem. 2001;276:33309–12. doi: 10.1074/jbc.C100375200. [DOI] [PubMed] [Google Scholar]
- Kast HR, Goodwin B, Tarr PT, Jones SA, Anisfeld AM, Stoltz CM, et al. Regulation of multidrug resistance-associated protein 2 (ABCC2) by the nuclear receptors pregnane X receptor, farnesoid X-activated receptor, and constitutive androstane receptor. J Biol Chem. 2002;277:2908–15. doi: 10.1074/jbc.M109326200. [DOI] [PubMed] [Google Scholar]
- Ebert B, Seidel A, Lampen A. Identification of BCRP as transporter of benzo[a]pyrene conjugates metabolically formed in Caco-2 cells and its induction by Ah-receptor agonists. Carcinogenesis. 2005;26:1754–63. doi: 10.1093/carcin/bgi139. [DOI] [PubMed] [Google Scholar]
- Jigorel E, Le Vee M, Boursier-Neyret C, Parmentier Y, Fardel O. Differential regulation of sinusoidal and canalicular hepatic drug transporter expression by xenobiotics activating drug-sensing receptors in primary human hepatocytes. Drug Metab Dispos. 2006;34:1756–63. doi: 10.1124/dmd.106.010033. [DOI] [PubMed] [Google Scholar]
- Oscarson M, Zanger UM, Rifki OF, Klein K, Eichelbaum M, Meyer UA. Transcriptional profiling of genes induced in the livers of patients treated with carbamazepine. Clin Pharmacol Ther. 2006;80:440–56. doi: 10.1016/j.clpt.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Maeda T, Hirayama M, Higashi R, Sato M, Tamai I. Characterization of human OATP2B1 (SLCO2B1) gene promoter regulation. Pharm Res. 2006;23:513–20. doi: 10.1007/s11095-006-9572-6. [DOI] [PubMed] [Google Scholar]
- Stedman CA, Liddle C, Coulter SA, Sonoda J, Alvarez JG, Moore DD, et al. Nuclear receptors constitutive androstane receptor and pregnane X receptor ameliorate cholestatic liver injury. Proc Natl Acad Sci USA. 2005;102:2063–8. doi: 10.1073/pnas.0409794102. [DOI] [PMC free article] [PubMed] [Google Scholar]