Abstract
Aim:
To examine whether liquiritigenin, a newly found agonist of selective estrogen receptor-β, has neuroprotective activity against β-amyloid peptide (Aβ) in rat hippocampal neurons.
Methods:
Primary cultures of rat hippocampal neurons were pretreated with liquiritigenin (0.02, 0.2, and 2 μmol/L) prior to Aβ25–35 exposure. Following treatment, viability of the cells was measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide analysis and by a lactate dehydrogenase activity-based cytotoxicity assay. Intracellular Ca2+ concentration ([Ca2+]i) and levels of reactive oxygen species (ROS), as well as apoptotic rates, were determined. Our studies were extended in tests of whether liquiritigenin treatment could inhibit the secretion of Aβ1–40 as measured using an ELISA method. In order to analyze which genes may be involved, we used a microarray assay to compare gene expression patterns. Finally, the levels of specific proteins related to neurotrophy and neurodenegeration were detected by Western blotting.
Results:
Pretreated neurons with liquiritigenin in the presence of Aβ25–35 increased cell viability in a concentration-dependent manner. Liquiritigenin treatment also attenuated Aβ25–35-induced increases in [Ca2+]i and ROS level and decreased the apoptotic rate of neurons. Some genes, including B-cell lymphoma/leukemia-2 (Bcl-2), neurotrophin 3 (Ntf-3) and amyloid β (A4) precursor protein-binding, family B, member 1 (Apbb-1) were regulated by liquiritigenin; similar results were shown at the protein level by Western blotting.
Conclusion:
Our results demonstrate that liquiritigenin exhibits neuroprotective effects against Aβ25-35-induced neurotoxicity and that it can decrease the secretion of Aβ1–40. Therefore, liquiritigenin may be useful for further study as a prodrug for treatment of Alzheimer's disease.
Keywords: liquiritigenin, selective ERβ agonist, neuroprotection, Aβ secretion
Introduction
In addition to its classic function as a sex hormone, estrogen plays key regulatory roles in various biological pathways. It has outstanding neuroprotective and neurotrophic activities and has been linked to neurodegenerative diseases, including Alzheimer's disease (AD)1, 2. A large body of work has shown that estrogen can block β-amyloid peptide (Aβ)-induced neuronal cell death and influence Aβ secretion3, 4, 5, 6, 7. However, long-term compliance with estrogen administration is estimated to be no more than 15%–40% because of undesirable side effects8, 9, 10. As a result, several phytoestrogens with fewer side effects and potential neuroprotective effects have been developed as alternative treatment strategies11, 12.
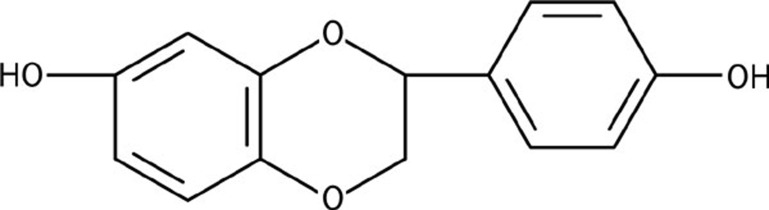
Liquiritigenin (7,4′-dihydroxyflavanone, Figure 1) is a flavonoid extracted from Glycyrrhizae radix that exhibits life-enhancing properties and is frequently used in traditional Oriental medicine to treat injury or swelling and for detoxification. Our interest in liquiritigenin developed as a result of the following observations. First, liquiritigenin was shown to be a highly selective agonist of estrogen receptor-β (ERβ)13, an observation that is consistent with our previous findings (unpublished data). ERβ is expressed in brain centers related to learning and memory and is less likely than ERα to be related to sexual function14, 15. Second, liquiritigenin has been shown in several studies to exert cytoprotective effects in vitro and in vivo16, 17; recent studies demonstrate that liquiritigenin exerts anti-inflammatory effects through the NF-κB pathway18. Third, we previously observed that liquiritigenin did not induce proliferation of MCF-7 and T47D breast cancer cells (data not shown), which is consistent with other studies13. Finally, pharmacokinetic studies from our laboratory have demonstrated that liquiritigenin exhibits good intestinal absorption and blood-brain barrier permeability19. These characteristics suggest that liquiritigenin may have a therapeutic function in the brain.
Figure 1.
Chemical structure of liquiritigenin.
Materials and methods
Cell culture
Primary rat hippocampal neurons were obtained from newborn Wistar rats (postnatal day 0, obtained from the Academy of Military Medical Science, grade SPF, certificate number: SCXK(Jing)2005-0013) as previously described12 with minor modifications. Procedures were carried out in accordance with the Institutional Animal Care Guidelines of the Chinese Society of Laboratory Animals Science. Briefly, after being dissected from the brains of the rats, hippocampi were treated with 0.25% trypsin for no more than 30 min at 37 °C and were dissociated by repeated passage through a series of fire-polished, constricted Pasteur pipettes. Neurons were seeded into poly-L-lysine (100 μg/mL)-coated plates and grown in serum-free DMEM-F12 medium supplemented with 2% B27 (Sigma, USA). This method yielded cell cultures consisting of more than 90% neurons. Cell cultures were routinely observed by phase-contrast inverted microscopy and were maintained for 7 d before treatment.
Aβ is a 39−43 amino acid peptide that is derived from amyloid precursor protein. The toxicity of Aβ25–35 is very similar to that of Aβ1–42. Both peptides are easily amyloidogenic, so Aβ25–35 was used in the present study. Seven-day-old neurons were pretreated with or without 0.02, 0.2, or 2 μmol/L liquiritigenin (synthesized in our institute; purity >95% by HPLC) alone for 24 h; then 10 μmol/L Aβ25–35 (Sigma, USA), the concentration of which was chosen as previously described20, was added together with the liquiritigenin for 72 h. To produce neurotoxicity, Aβ25–35 (diluted in saline) was incubated at 37 °C for 7 d to make fibril formation before use.
Cell viability detection
Seven-day-old neurons were plated into 96-well plates (5×104 cells/well) and treated with liquiritigenin for 24 h and Aβ25–35 for 72 h. Cell viability was measured using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, as previously described21. Absorbance at 540 nm was measured using a microplate reader (ThermoLab, USA). The viability of vehicle-treated control groups (not exposed to Aβ25–35) was defined as 100%.
Lactate dehydrogenase activity-based cytotoxicity assay
Lactate dehydrogenase (LDH) is a stable cytoplasmic enzyme expressed in most cells, including neurons. When the plasma membrane is damaged, LDH is rapidly released into the cell culture supernatant. In the context of this study, we used LDH release as a biochemical index of cytotoxicity. Culture supernatant 100 μL was collected and LDH activity was measured using LDH detection kits (BIOSINO, China) according to the manufacturer's instructions. Colorimetric absorbance at 570 nm was measured using an automatic chemistry analyzer (ELx800, Italy). LDH activity in each group was calculated as percentage of the LDH activity of cells that had been exposed to Aβ25-35 alone, which was defined as 100%.
Measurement of intracellular Ca2+
Intracellular Ca2+ concentration ([Ca2+]i) was determined using the Ca2+-sensitive fluorochrome Fluo-3/acetoxymethyl ester (Fluo-3/AM, Biotium, USA). Fluorescence intensity was analyzed using a fluorescence spectrophotometer (FOLARstar, BMGLABTech, German) with excitation and emission wavelengths of 488 nm and 526 nm; [Ca2+]i was calculated according to the described method22.
Measurement of intracellular reactive oxygen species
The level of intracellular reactive oxygen species (ROS) was determined using 2,7-dichlorodihydro fluorescent diacetate (DCFH-DA, Beyotime, China), following the manufacturer's instructions. The fluorescence intensity of cells was measured with a fluorescence spectrophotometer, with excitation and emission wavelengths of 490 nm and 520 nm, respectively.
Hoechst 33342 staining
The morphology of nuclear chromatin was assessed by staining with the fluorescent dye Hoechst 33342 as described11. Briefly, cells were fixed with 3.7% paraformaldehyde (v/v) and then stained with Hoechst 33342 (10 μg/mL) for 10 min at room temperature. After washing, each field of cells was analyzed using a fluorescence microscope (Zeiss, Germany) and recordings were made using a CCD camera (Apogee). DNA-fragmented cells stained with Hoechst 33342 take on a bright blue.
Flow cytometric detection of apoptotic cells
For detection of apoptotic rate, Annxin V-FITC/PI (Jingmei Biotech, China) staining was performed following the manufacturer's instructions. Cells that could be stained with Annxin V but not with PI were defined as apoptotic. Apoptotic rate was measured with a flow cytometer (FCM, Becton Dickinson, USA) using CellQuest software.
Treatment of cells with liquiritigenin to detect soluble Aβ1–40
Seven-day-old neurons were cultured in 96-well dishes and treated separately in the presence of 0.02, 0.2, 2 μmol/L liquiritigenin or vehicle for 24 h. For each treatment, three wells containing the same number of cells were used. The levels of soluble Aβ1-40 (the predominant form of Aβ peptide) in the conditioned medium were assayed using the sensitive sandwich ELISA detection kit, following the manufacturer's instructions (Uscnlife, USA). Levels of Aβ1-40 were expressed in pg/mL as deduced from the appropriate standard curve run in parallel with the assay.
Microarray assay
In order to understand which genes may be influenced by exposure of neurons to liquiritigenin, a microarray technique was used to explore gene expression profiles. Seven-day-old cells were seeded into 6-well plates at a density of 2×106/well and were treated with/without 2 μmol/L liquiritigenin for 24 h. The Oligo GEArray Rat Neurogenesis and Neural Stem Cell Microarray (ORN-404, Superarray, USA), which includes 263 genes, was chosen because it contains genes related to both neurotrophy and neuroprotection. After treatment, cells were lysed using 1 mL TRIzol (Sigma, USA) and immediately delivered to the manufacturer. The entire microarray procedure, including RNA preparation and reverse transcription PCR, was performed by the manufacturer. The fold change was calculated for each gene as the ratio of liquiritigenin/control. In this experiment, only the high dose of 2 μmol/L liquiritigenin was chosen and the assay was not repeated because of its costliness.
Western blotting
In light of results from microarray and similar studies23, 24, 25, we selected four representative proteins, including B-cell lymphoma/leukemia-2 (Bcl-2), Bcl-2-associated X protein (Bax), neurotrophin 3 (Ntf-3) and amyloid β (A4) precursor protein-binding, family B, member 1 (Apbb-1), for further analysis to validate whether liquiritigenin affects related protein expression. For detection of Bcl-2, Bax and Ntf-3, seven-day-old rat hippocampal neurons were seeded into 6-well plates at a density of 2×106 cells/well. After an overnight incubation, cells were treated with/without liquiritigenin (0.02, 0.2, or 2 μmol/L) for 24 h, followed by addition of 10 μmol/L Aβ25–35 and further incubation with liquiritigenin for 72 h. For targeted detection of Apbb-1, cells were treated with liquiritigenin (0.02, 0.2, or 2 μmol/L) alone for 24 h, then lysed with 4 °C cell lysis buffer (Beyotime, China) as recommended by the manufacturer. SDS-PAGE and Western blotting were performed according to standard protocols22 using 40 μg protein per lane. Primary antibodies were as follows: Bcl-2 (Beyotime, China, 1:500 dilution), Bax (Beyotime, China, 1:500 dilution), Ntf-3 (Chemicon, USA, 1:1000 dilution), Apbb-1 (Bioss, China, 1:200 dilution), β-tubulin (Walterson, China, 1:1000 dilution, as internal control) and secondary antibody (goat anti-rabbit or goat anti-mouse IgG-HRP, Zhongshan, China, 1:2000 dilution). The grey levels were presented as the ratio against β-tubulin and were analyzed using the software Scion Image for Windows (http://www.scioncorp.com).
Statistical analysis
All data, except those of microarray assay, are expressed as mean±SEM; assays were repeated in at least three independent experiments (in the context, “n” represents the number of repetitions), each performed in triplicate. Data comparisons among groups were assessed by one-way ANOVA. Between two groups, Dunnett's test was used; P<0.05 was assumed to indicate statistical significance.
Results
Liquiritigenin protects primary rat hippocampal neurons against Aβ25–35-induced cytotoxicity
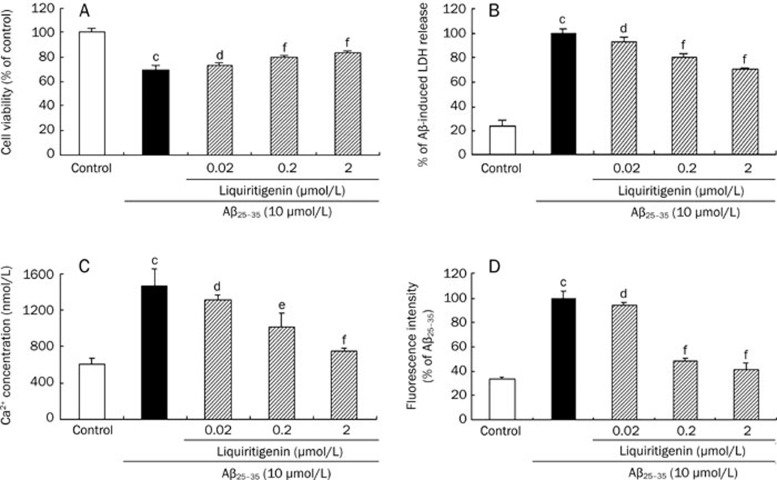
As shown in Figure 2A, Aβ25–35 reduced cell viability to 69.5%±3.8% (P<0.01 vs control, n=3) of control; when cells were pre-incubated with 0.02, 0.2, or 2 μmol/L liquiritigenin, cell viability was 73.5%±1.3% (P>0.05 vs Aβ25–35, n=3), 79.5%±2.0% (P<0.01 vs Aβ25–35, n=3) and 83.4%±1.6% (P<0.01 vs Aβ25–35, n=3), respectively, compared with control.
Figure 2.
Liquiritigenin exhibits neuroprotective activity against Aβ25–35-induced damage in primary rat hippocampal neuron cells. Cells were pretreated with various concentrations of liquiritigenin for 24 h prior to insult by 10 μmol/L Aβ25–35 for another 72 h. (A) Liquiritigenin increases cell viability as determined by MTT assay. (B) LDH leakage into the supernatant was reduced by pretreating cells with liquiritigenin. (C) Liquiritigenin inhibits Aβ25–35-induced up-regulation of [Ca2+]i detected with Fluo-3/AM fluorescent dye, the intensity of which increases in parallel with concentration of cytoplasmic free Ca2+. (D) Effect of liquiritigenin on Aβ25–35-induced increase of intracellular ROS level measured by DCFH-DA fluorescent dye, the intensity of which increases with cytosolic ROS level. Bars represent mean±SEM (n=3). aP>0.05, bP<0.05, cP<0.01 vs control; dP>0.05, eP<0.05, fP<0.01 vs Aβ25–35 alone.
Liquiritigenin reduces cell membrane permeability
Our results (Figure 2B) show that Aβ25–35 increased 4.3-fold of LDH leakage compared with control (P<0.01 vs control, n=3); when cells were treated with liquiritigenin, LDH leakage decreased to 80.3%±2.7% (0.2 μmol/L, P<0.01 vs Aβ25–35, n=3) and 70.1%±1.1% (2 μmol/L, P<0.01 vs Aβ25–35, n=3) that of cells treated with Aβ25–35 alone. However, 0.02 μmol/L liquiritigenin had no effect on cell permeability.
Liquiritigenin inhibits Aβ25–35-induced increases in Ca2+ influx
The Ca2+-sensitive fluorescent probe Fluo-3/AM was used to monitor alterations in [Ca2+]i by spectrofluorometry. As illustrated in Figure 2C, cells that were incubated with Aβ25-35 alone had a much higher [Ca2+]i than control cells (1468.6±184.7 nmol/L vs 601.0±65.6 nmol/L, P<0.01, n=3); [Ca2+]i was significantly decreased by pretreatment with 0.2 μmol/L (1014.3±142.1 nmol/L, P<0.05 vs Aβ25–35, n=3) or 2 μmol/L (748.6±34.3 nmol/L, P<0.01 vs Aβ25–35, n=3) liquiritigenin, but not by 0.02 μmol/L liquiritigenin.
Liquiritigenin decreases Aβ25–35-induced upregulation of cellular ROS
DCFH-DA, the fluorescence of which increases along with intracellular ROS, was used for detection of cellular ROS. As shown in Figure 2D, Aβ25–35 increased 3.0-fold of intracellular ROS level compared with control (P<0.01 vs control, n=3); when 0.2 or 2 μmol/L liquiritigenin was added before Aβ25–35, intracellular ROS levels decreased to 48.2%±2.8% (P<0.01 vs Aβ25–35, n=3) and 41.3%±5.9% (P<0.01 vs Aβ25–35, n=3), respectively, of ROS levels in cells treated with Aβ25–35 alone.
Liquiritigenin protects neurons against Aβ25–35-induced apoptosis
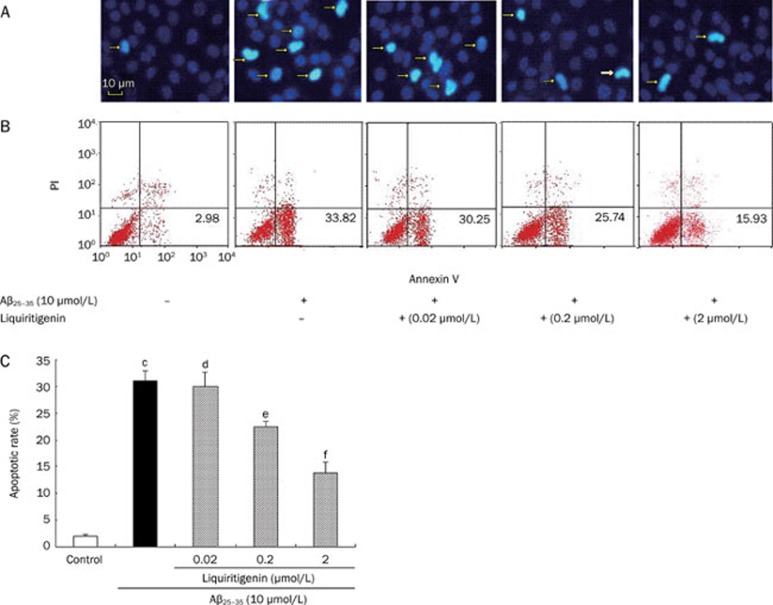
Nuclear morphology was evaluated using the membrane-permeable blue dye Hoechst 33342. As shown in Figure 3A, the content of DNA-fragmented cells increased after Aβ25–35 treatment. There were fewer DNA-fragmented cells after incubation of the neurons with 0.2 or 2 μmol/L liquiritigenin.
Figure 3.
Liquiritigenin protects neuron cells against Aβ25–35-induced apoptosis. (A) Nuclei were stained with fluorescent dye Hoechst 33342 for assessment of apoptosis; arrowheads indicate apoptotic cells. Magnification: ×200. (B) Representative FCM histograms stained by Annexin V-FITC/PI. Annexin V+ and PI− cells were designed as apoptotic. (C) Apoptotic rate of cells, determined by FCM. Bars represent mean±SEM (n=4). aP>0.05, bP<0.05, cP<0.01 vs control; dP>0.05, eP<0.05, fP<0.01 vs Aβ25–35 alone.
Annexin V-FITC/PI staining gave similar results; Figure 3B shows typical histograms from FCM. As shown in Figure 3C, the apoptotic rate of cells treated with 10 μmol/L Aβ25-35 alone was much higher than control (31.27%±1.76% vs 1.92%±0.36%, P<0.01, n=4). Pretreatment with 0.2 or 2 μmol/L liquiritigenin decreased the apoptotic rate significantly; it decreased to 21.94%±0.90% and 13.95%±1.79%, respectively, of cells treated with Aβ25–35 alone (P<0.01 vs Aβ25–35, n=4).
Liquiritigenin regulates secretion of soluble Aβ1–40
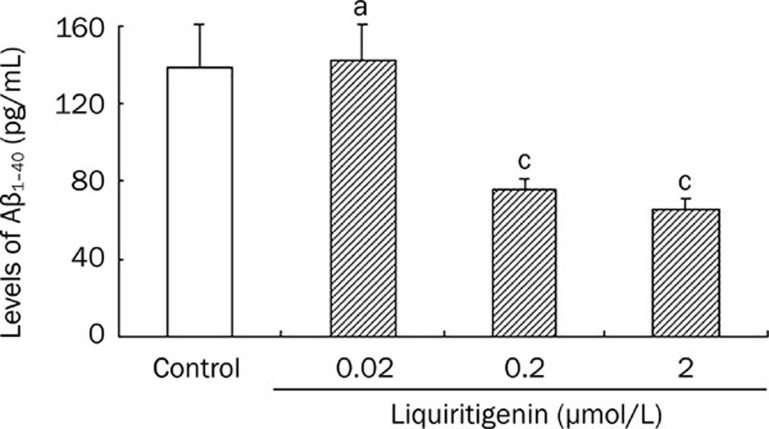
Hippocampal neurons were treated with liquiritigenin and the concentration of Aβ1–40 in the supernatant was measured using ELISA. Figure 4 shows that incubation of neurons with 0.2 or 2 μmol/L liquiritigenin decreased Aβ1–40 levels significantly (0.2 μmol/L: 73.5±5.0 pg/mL, 2 μmol/L: 65.2±2.9 pg/mL vs control: 138.5±21.8 pg/mL, P<0.01, n=5). However, 0.02 liquiritigenin did not show any effects.
Figure 4.
Liquiritigenin reduces the release of Aβ1–40 into the medium in primary rat hippocampal neuron cells. Cells were cultured in the presence of liquiritigenin (0, 0.02, 0.2, 2 μmol/L) for 24 h and were assayed for Aβ1–40 with ELISA (mean±SEM, n=5). aP>0.05, cP<0.01 vs control.
Effects of liquiritigenin on some genes involved in neuroprotection and neurotrophy
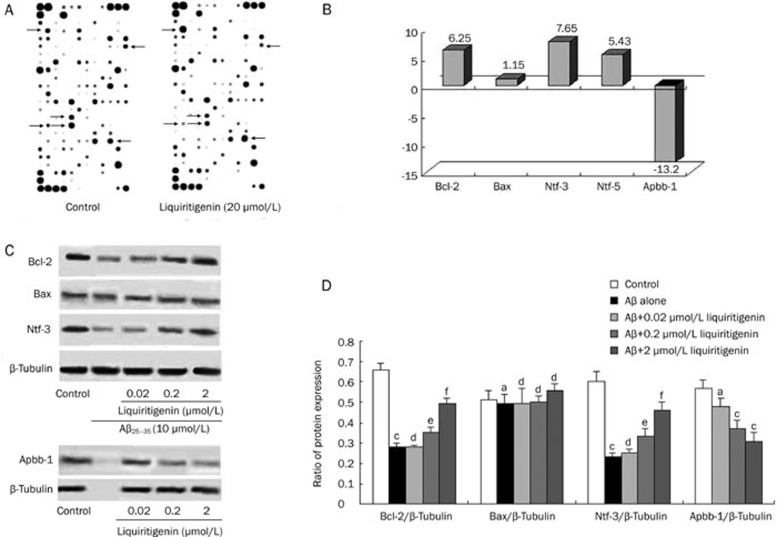
Using the rat neurogenesis and neural stem cell microarray assay, we could determine differential gene expression in liquiritigenin-treated and untreated cells through a simple side-by-side hybridization experiment. If the fold change is greater than 1, the result is reported as a fold up-regulation. If the fold change is less than 1, the negative inverse of the result is reported as a fold down-regulation. Figure 5A shows the results of the microarray assay; from it, we can see that some genes were up-regulated whereas others were down-regulated.
Figure 5.
Effect of liquiritigenin on expression of genes Bcl-2, Bax, Ntf-3, Ntf-5, Apbb-1 and relative protein expression in rat primary neurons. (A) Figures of microarray assay. Each marking represents a type of gene; arrowheads indicate some genes that were regulated by 2 μmol/L liquiritigenin treatment. (B) Changes in gene expression pattern determined by microarray assay. The fold change was calculated for each gene as the ratio of liquiritigenin/control (n=1). (C) Representative photos of Western blots. For detection of Bcl-2, Bax, and Ntf-3, cells were pretreated with various concentrations of liquiritigenin for 24 h prior to insult by 10 μmol/L Aβ25–35 for 72 h. For examination of Apbb-1 levels, cells were treated with liquiritigenin alone. (D) Quantitative densitometric analysis. Ratio is expressed as percentage of grey level of β-tubulin (mean±SEM, n=3). aP>0.05, bP<0.05,cP<0.01 vs control; dP>0.05, eP<0.05, fP<0.01 vs Aβ25–35 alone.
Figure 5B shows quantitative analysis of the microarray assay, which indicating that the expression level of Bcl-2, the most important anti-apoptotic gene, was increased by 6.25-fold (n=1), while the expression level of Bax was similar to that of control (n=1). Ntf-3 and Ntf-5 were up-regulated by 7.65 and 5.43-fold, respectively (n=1). Furthermore, the expression of Apbb-1, which is related to amyloidosis, was sharply decreased by 13.2-fold (n=1) after liquiritigenin treatment.
Changes in related protein expression
The proteins Bcl-2, Bax, Ntf-3 and Apbb-1 were chosen for further analysis. Figure 5C shows a representative result of Western blotting. From the quantitative analysis (Figure 5D) we can see that Aβ25–35 treatment reduced Bcl-2 expression significantly compared with control (0.28±0.02 vs 0.66±0.03, P<0.01, n=3); when 0.2 μmol/L or 2 μmol/L liquiritigenin was added, the ratio of protein expression increased markedly (0.2 μmol/L: 0.35±0.01, P<0.05 vs Aβ25–35; 2 μmol/L: 0.49±0.03, P<0.01 vs Aβ25–35, n=3). There was no significant difference in Bax expression among the groups. Ntf-3 expression in cells treated with Aβ25–35 alone was reduced to less than half of control (0.23±0.04 vs 0.60±0.03, P<0.01, n=3); it could be up-regulated by liquiritigenin treatment in a dose-dependent manner (0.2 μmol/L: 0.33±0.02, P<0.05 vs Aβ25–35; 2 μmol/L: 0.46±0.04, P<0.05 vs Aβ25-35, n=3). The expression of Apbb-1 was reduced by 0.2 μmol/L (0.37±0.04, P<0.01 vs control, n=3) and 2 μmol/L liquiritigenin (0.31±0.04, P<0.01 vs control, n=3), respectively, compared with control, but not by 0.02 μmol/L liquiritigenin (0.48±0.06, P>0.05 vs control, n=3).
Discussion
In patients with AD, brain Aβ aggregates into clumps called oligomers that can accumulate and form deposits called amyloid plaques, which are thought to be a major pathologic mechanism of AD. Aβ-induced neurotoxicity has been attributed in various studies to Ca2+ influx, generation of ROS, induction of apoptosis, and other causes. The present study confirms that Aβ25–35 can cause neural cell death, an increase of Ca2+ influx and generation of ROS, as previously evidenced in other studies20. In addition, we have shown that Aβ25–35 treatment can decrease the expression of Ntf-3 protein, an important neurotrophic factor; such a decrease in Ntf-3 protein may also contribute to the neuronal death induced by Aβ25–35.
The present study provides evidence that liquiritigenin at a dosage in the range of 0.2–2 μmol/L can prevent Aβ25–35-induced injury to rat hippocampal neurons, as shown by cell viability measurements using MTT and LDH detection assays. In a previous study, Kim et al showed that liquiritigenin exerted anti-inflammatory effects against lipopolysaccharide in Raw264.7 cells at a concentration ranging from 3 to 30 μmol/L18. We reckon that the differences in effective dose observed in the two studies may be due to the use of different cells and/or different toxic factors.
It has been reported that ROS generation is a consequence of Ca2+ accumulation26. However, in many experiments, free radicals are responsible for the increase in [Ca2+]i. ROS-induced membrane damage causes further Ca2+ influx, and resultant accentuated Ca2+ influx will in turn induce the generation of further ROS 27. In the present study, Aβ25–35 elicited significant [Ca2+]i increase and ROS accumulation, both of which contributed to the excitatory neurotoxicity of Aβ25–35, as previously reported20. Both [Ca2+]i increase and ROS accumulation were blocked by liquiritigenin to some extent. However, we have not clarified whether liquiritigenin suppresses ROS generation through the inhibition of [Ca2+]i increase, or conversely, whether decreases in ROS generation prevents increases in [Ca2+]i. Elucidation of the variety of events occurring downstream of neuronal Ca2+ overloading and/or increases in cellular levels of ROS is still a matter for further research.
In the present study, cultured hippocampal neurons exposed to Aβ25–35 for more than 72 h showed increased chromatin condensation, a typical feature of apoptotic cell death. Bcl-2 family proteins are a critical regulatory factor in cellular response to apoptosis through mitochondrial pathways. In most instances, anti-apoptotic and pro-apoptotic factors in the Bcl-2 family have synergistic effects and play alternating roles. In the present study, it was shown that liquiritigenin exerted its anti-apoptotic role in the presence of Aβ25–35 at least in part through increasing the expression of Bcl-2, suggesting that liquiritigeinin could regulate mitochondrial function and thereby inhibit neuronal apoptosis. With regard to the underlying cellular mechanism, we previously observed that an ER antagonist, ICI 182 780, could partially block the anti-apoptotic effect of liquiritigenin in our culture system (unpublished data). Since liquiritigenin has a 20-fold higher affinity for ERβ than for ERα13, it is likely that its neuroprotective ability is mediated by ERβ. Meanwhile, the partial blocking effect of ICI 182 780 also suggests that the protective effect may be mediated by multiple pathways, including that of mitogen-activated protein kinase (MAPK)3. The results of the microarray assay and Western blotting in the present study indicate that liquiritigenin probably has some neurotrophic actions, including causing an increase in the expression of Ntf-3 at both the genetic and protein levels. Since we observed that Aβ25–35 treatment inhibited the expression of Ntf-3, whereas liquiritigenin treatment could attenuate this effect, the action of liquiritigenin on Ntf-3 may account to some extent for its neuroprotective effect. This deduction is consistent with previous findings showing that Ntf-3 protects primary cortical neurons against Aβ toxicity by limiting caspase-8, caspase-9 and caspase-3 cleavage and that it can also induce an up-regulation of neuronal apoptosis inhibitory protein-1 expression in neurons, thereby promoting the inhibition of Aβ-induced neuronal apoptosis28.
Targeting generation and initial formation of amyloid assemblies is a preferred approach for therapeutic intervention in amyloidosis, and studies have reported that estrogen could be used for this purpose4, 6, 7. Most Aβ is composed of a peptide designated Aβ40, Aβ1–40, or, in some cases, Aβx–40. Because Aβ1–40 is a main form of Aβ, our demonstration that liquiritigenin inhibits its secretion is meaningful. The sensitive sandwich ELISA was used in this experiment because the expression level of Aβ1–40 was so low that it could not be detected using Western blotting or other methods. Herein, we have also shown that Apbb-1, a peptide that forms the extracellular amyloid fibrils of Alzheimer senile plaques, was down-regulated by liquiritigenin. This finding could explain why liquiritigenin inhibited the accumulation of Aβ, but more research is needed to verify this hypothesis.
In pioneer studies in the field of AD treatment using SERMs, Carroll et al found that both PPT (a selective ERα agonist) and DPN (a selective ERβ agonist) could reduce Aβ accumulation in the brain in 3×Tg-AD mice29. The present research reports that a newly found ERβ agonist, liquiritigenin, showed satisfactory effects against Aβ25–35-induced insults in primary neurons. Liquiritigenin may therefore serve as a named NeuroSERM30, 31, in other words, an estrogen alternative that selectively targets and activates estrogen mechanisms of action in the brain while avoiding activation of estrogen receptors peripheral to the brain, particularly in reproductive organs. Furthermore, our results are consistent with the finding that the expression of the ERβ gene is positively correlated with increased levels of learning and memory ability in some mouse models of AD, while ERα shows almost no relationship to learning and memory improvements32.
Our data confirm the potential of NeuroSERMs in protecting against AD neuropathology and support continued development and investigation in this field. An important qualification is that we have examined the effects of liquiritigenin only in vitro; follow-up studies that address the application of liquiritigenin in vivo and its potential therapeutic value are needed. From a signaling standpoint, there is a significant need for targeted research to better elucidate the genomic/non-genomic signaling pathways through which liquiritigenin exerts its effects. Future research should be aimed at rapidly providing answers to these key questions.
Author contribution
Qiu-jun LU and Li-bo ZOU designed the research; Rui-ting LIU performed the research; Qiu-jun LU contributed new reagents and analytic tools; Rui-ting LIU analyzed data; Rui-ting LIU wrote the paper; Qiu-jun LU and Li-bo ZOU revised the paper.
Abbreviations
Alzheimer's disease (AD); β-amyloid peptide (Aβ); estrogen receptor- β (ERβ); 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide(MTT); lactate dehydrogenase (LDH); intracellular Ca2+ concentration ([Ca2+]i); reactive oxygen species (ROS); flow cytometer (FCM); B-cell lymphoma/leukemia-2 (Bcl-2), Bcl-2-associated X protein (Bax); neurotrophin (Ntf) and amyloid β (A4) precursor protein-binding, family B, member 1 (Apbb-1).
Acknowledgments
This project was supported by the Academy of Military Medical Science and Genova (Beijing) Biopharmaceutical Research Institute.
References
- Brann DW, Dhandapani K, Wakade C, Mahesh VB, Khan MM. Neurotrophic and neuroprotective actions of estrogen: basic mechanisms and clinical implications. Steroids. 2007;72:381–405. doi: 10.1016/j.steroids.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickelgren I. Estrogen stakes claim to cognition. Science. 1997;276:675–8. doi: 10.1126/science.276.5313.675. [DOI] [PubMed] [Google Scholar]
- Mize AL, Shapiro RA, Dorsa DM. Estrogen receptor-mediated neuroprotection from oxidative stress requires activation of the mitogen-activated protein kinase pathway. Endocrinology. 2003;144:306–12. doi: 10.1210/en.2002-220698. [DOI] [PubMed] [Google Scholar]
- Greenfield JP, Leung LW, Cai D, Kaasik K, Gross RS, Rodriguez-Boulan E, et al. Estrogen lowers Alzheimer beta-amyloid generation by stimulating trans-Golgi network vesicle biogenesis. J Biol Chem. 2002;277:12128–36. doi: 10.1074/jbc.M110009200. [DOI] [PubMed] [Google Scholar]
- Kim H, Bang OY, Jung MW, Ha SD, Hong HS, Huh K, et al. Neuroprotective effects of estrogen against beta-amyloid toxicity are mediated by estrogen receptors in cultured neuronal cells. Neurosci Lett. 2001;302:58–62. doi: 10.1016/s0304-3940(01)01659-7. [DOI] [PubMed] [Google Scholar]
- Xu H, Wang R, Zhang YW, Zhang X. Estrogen, beta-amyloid metabolism/trafficking, and Alzheimer's disease. Ann N Y Acad Sci. 2006;1089:324–42. doi: 10.1196/annals.1386.036. [DOI] [PubMed] [Google Scholar]
- Zheng H, Xu H, Uljon SN, Gross R, Hardy K, Gaynor J, et al. Modulation of A(beta) peptides by estrogen in mouse models. J Neurochem. 2002;80:191–6. doi: 10.1046/j.0022-3042.2001.00690.x. [DOI] [PubMed] [Google Scholar]
- Beresford SA, Weiss NS, Voigt LF, McKnight B. Risk of endometrial cancer in relation to use of oestrogen combined with cyclic progestagen therapy in postmenopausal women. Lancet. 1997;349:458–61. doi: 10.1016/S0140-6736(96)07365-5. [DOI] [PubMed] [Google Scholar]
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer Lancet 19973501047–59. [PubMed] [Google Scholar]
- Ravnikar VA. Compliance with hormone replacement therapy: are women receiving the full impact of hormone replacement therapy preventive heath benefits. Womens Health Issues. 1992;2:75–80. doi: 10.1016/s1049-3867(05)80275-0. [DOI] [PubMed] [Google Scholar]
- Bang OY, Hong HS, Kim DH, Kim H, Boo JH, Huh K, et al. Neuroprotective effect of genistein against beta amyloid-induced neurotoxicity. Neurobiol Dis. 2004;16:21–8. doi: 10.1016/j.nbd.2003.12.017. [DOI] [PubMed] [Google Scholar]
- Zhao L, Chen Q, Brinton DR. Neuroprotective and neurotrophic efficacy of phytoestrogens in cultured hippocampal neurons. Exp Biol Med (Maywood) 2002;227:509–19. doi: 10.1177/153537020222700716. [DOI] [PubMed] [Google Scholar]
- Mersereau JE, Levy N, Staub RE, Baggett S, Zogric T, Chow S, et al. Liquiritigenin is a plant-derived highly selective estrogen receptor beta agonist. Mol Cell Endocrinol. 2008;283:49–57. doi: 10.1016/j.mce.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson JA. What pharmacologists can learn from recent advances in estrogen signaling. Trends Pharmacol Sci. 2003;24:479–85. doi: 10.1016/S0165-6147(03)00229-3. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–25. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Kim SC, Byun SH, Yang CH, Kim CY, Kim JW, Kim SG. Cytoprotective effects of Glycyrrhizae radix extract and its active component liquiritigenin against cadmium-induced toxicity (effects on bad translocation and cytochrome c-mediated PARP cleavage) Toxicology. 2004;197:239–51. doi: 10.1016/j.tox.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Kim YW, Ki SH, Lee JR, Lee SJ, Kim CW, Kim SC, et al. Liquiritigenin, an aglycone of liquiritin in Glycyrrhizae radix, prevents acute liver injuries in rats induced by acetaminophen with or without buthionine sulfoximine. Chem Biol Interact. 2006;161:125–38. doi: 10.1016/j.cbi.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Kim YW, Zhao RJ, Park SJ, Lee JR, Cho IJ, Yang CH, et al. Anti-inflamatory effects of liquiritigenin as a consequence of the inhibition of NF-kappaB-dependent iNOS and proinflammatory cytokines production. Br J Pharmacol. 2008;154:165–73. doi: 10.1038/bjp.2008.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Bian GX, Wen LQ, Ren JP, Lu QJ.Properties of the intestinal absorption and in vitro blood-brain barrier permeability of liquiritigenin Chin J New Drugs 20088661–5.Chinese. [Google Scholar]
- Ban JY, Cho SO, Koh SB, Song KS, Bae K, Seong YH. Protection of amyloid beta protein (25–35)-induced neurotoxicity by methanol extract of Smilacis chinae rhizome in cultured rat cortical neurons. J Ethnopharmacol. 2006;106:230–7. doi: 10.1016/j.jep.2005.12.034. [DOI] [PubMed] [Google Scholar]
- Yu JH, Wang HJ, Li XR, Tashiro S, Onodera S, Ikejima T. Protein tyrosine kinase, JNK, and ERK involvement in pseudolaric acid B-induced apoptosis of human breast cancer MCF-7 cells. Acta Pharmacol Sin. 2008;29:1069–76. doi: 10.1111/j.1745-7254.2008.00835.x. [DOI] [PubMed] [Google Scholar]
- Zhang L, Yu H, Sun Y, Lin X, Chen B, Tan C, et al. Protective effects of salidroside on hydrogen peroxide-induced apoptosis in SH-SY5Y human neuroblastoma cells. Eur J Pharmacol. 2007;564:18–25. doi: 10.1016/j.ejphar.2007.01.089. [DOI] [PubMed] [Google Scholar]
- Adams MM, Shah RA, Janssen WG, Morrison JH. Different modes of hippocampal plasticity in response to estrogen in young and aged female rats. Proc Natl Acad Sci USA. 2001;98:8071–6. doi: 10.1073/pnas.141215898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JM, Romeo RD, Brake WG, Bethea CL, Rosenwaks Z, McEwen BS. Estradiol increases pre- and post-synaptic proteins in the CA1 region of the hippocampus in female rhesus macaques (Macaca mulatta) Endocrinology. 2003;144:4734–8. doi: 10.1210/en.2003-0216. [DOI] [PubMed] [Google Scholar]
- Zhao L, Wu TW, Brinton RD. Estrogen receptor subtypes alpha and beta contribute to neuroprotection and increased Bcl-2 expression in primary hippocampal neurons. Brain Res. 2004;1010:22–34. doi: 10.1016/j.brainres.2004.02.066. [DOI] [PubMed] [Google Scholar]
- Ekinci FJ, Linsley MD, Shea TB. Beta-Amyloid-induced calcium influx induces apoptosis in culture by oxidative stress rather than tau phosphorylation. Brain Res Mol Brain Res. 2000;76:389–95. doi: 10.1016/s0169-328x(00)00025-5. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Pike CJ, Copani A. Beta-Amyloid neurotoxicity: a discussion of in vitro findings. Neurobiol Aging. 1992;13:587–90. doi: 10.1016/0197-4580(92)90060-b. [DOI] [PubMed] [Google Scholar]
- Lesne' S, Gabriel C, Nelson DA, White E, Mackenzie ET, Vivien D, et al. Akt-dependent expression of NAIP-1 protects neurons against amyloid-β toxicity. J Biol Chem. 2005;280:24941–7. doi: 10.1074/jbc.M413495200. [DOI] [PubMed] [Google Scholar]
- Carroll JC, Pike CJ. Selective estrogen receptor modulators differentially regulate Alzheimer-like changes in female 3xTg-AD mice. Endocrinology. 2008;149:2607–11. doi: 10.1210/en.2007-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton RD. Requirements of a brain selective estrogen: advances and remaining challenges for developing a NeuroSERM. J Alzheimers Dis. 2004;6 Suppl:27–35. doi: 10.3233/jad-2004-6s607. [DOI] [PubMed] [Google Scholar]
- Zhao L, Neill K, Brinton DR. Selective estrogen receptor modulators (SERMs) for the brain: current status and remaining challenges for developing NeuroSERMs. Brain Res Brain Res Rev. 2005;49:472–93. doi: 10.1016/j.brainresrev.2005.01.009. [DOI] [PubMed] [Google Scholar]
- An SJ.The study of the relationship between hippocampal ERs gene expression and brain degeneration and learning and memory function [dissertation] Beijing: Acad Milit Med Sci 2002. Chinese.