Abstract
Aim:
Neutropenic individuals are at high risk for invasive pulmonary aspergillosis (IPA), a life-threatening infection. To evaluate the therapeutic potential of antioxidants, IPA was induced in neutropenic mice and the effect of N-acetyl-l-cysteine (NAC) on oxidative stress levels and lung injury was analyzed.
Methods:
Mice were pretreated with three daily intraperitoneal injections of 150 mg/kg cyclophosphamide, followed by intratracheal inoculation with 4.5×106 conidia of Asperǵillus fumiǵatus. The infected mice were then randomly assigned to an amphotericin B (AMB) group, an AMB plus NAC group, or an untreated control (C) group. In each group, the duration of treatment was 24, 48, or 72 h, and activities such as appearance, feeding, and dermal temperature were observed throughout the experiment. Sera and lung tissues were collected and analyzed by quantitative enzyme-linked immunosorbent assay (ELISA) for total protein, superoxide dismutase (SOD), malondialdehyde (MDA), tumor necrosis factor-α (TNF-α), and interleukin-10 (IL-10) levels. The wet/dry weight ratio of the lung was also calculated and lung sections were stained with hematoxylin-eosin for pathological examination and with methenamine silver stain for fungus detection.
Results:
Compared with the mice untreated with NAC, mice in the AMB plus NAC group had increased SOD and reduced MDA levels both systemically and locally at 24, 48, and 72 h after inoculation with conidia. NAC treatment also decreased the pulmonary protein content at 48 and 72 h and the lung wet/dry weight ratio at 24 and 48 h. Additionally, NAC enhanced pulmonary production of TNF-α and IL-10 at 24 h and 48 h.
Conclusion:
In combination with antifungal therapy, NAC treatment can alleviate oxidative stress and lung injury associated with IPA in neutropenic mice.
Keywords: invasive pulmonary aspergillosis, neutropenia, amphotericin B, N-acetyl-l-cysteine, oxidant/antioxidant therapy
Introduction
Aspergillus fumigatus (A fumigatus) is the most common species among Aspergillus, accounting for 90% of all respiratory infections. The infectious agent of A fumigatus is its airborne conidium, which may develop 45° dichotomous branching of hyphae 2–5 μm in diameter that are able to invade tissue1. Invasive pulmonary aspergillosis (IPA) is a rapidly progressing, often fatal disease that commonly affects immunocompromised patients and is characterized by necrotic tissue containing abundant hyphae. Because of the widespread use of antibiotics, corticosteroids, antitumor drugs, and immunosuppressive drugs, the morbidity of IPA is currently increasing. IPA is particularly prevalent in intensive care units (ICUs)2, 3, 4, 5 and in patients with neutropenia, the most common and best-characterized risk factor for IPA6. Mortality due to IPA ranges from 30% to 90%7, and even in conjunction with antifungal therapy, the mortality rate exceeds 60%8. In the ICU, the incidence of Aspergillus infection increased from 0.3% to 5.8%. In a 3-year study of ICU in Belgium, the observed mortality was up to 80%9. In neutropenic patients with IPA, treatment with amphotericin B is customary but often ineffective, with reported response rates of less than 55% in leukemia patients and bone marrow recipients6. Therefore, insight into IPA pathogenesis during antifungal treatment may aid the development of safer, more effective treatment regimens, such as combination therapy using caspofungin and liposomal amphotericin B (AMB)10.
An imbalance between oxidants and antioxidants has been observed in many pulmonary diseases11. In immunocompetent hosts, inhaled A fumigatus conidias are phagocytosed by alveolar macrophages, which kill the fungus using powerful reactive oxygen intermediates (ROIs). If conidial germination occurs before or after phagocytosis, neutrophils migrate to the site, degranulate after contact, and release ROIs that eliminate the hyphae. Several studies have found that oxidative mechanisms kill conidias, whereas oxidative-burst and non-oxidative mechanisms kill hyphae12, 13. Oxidative stress in the lung is induced by the oxidant-antioxidant imbalance, but excessive ROIs may directly damage tissues and induce inflammation. The current study was based on the hypothesis that combining antioxidants with classical anti-aspergillosis treatment could alleviate ROI-induced tissue damage and thus improve the drugs' therapeutic effects.
N-acetyl-l-cysteine (NAC) is a natural derivative of L-aminothiopropionic acid. Once administered orally or by injection, it can be rapidly absorbed and transformed into aminothiopropionic acid by deacetylation14. By generating aminothiopropionic acid, the substrate of the endocellular antioxidant reduced glutathione hormone (GSH), NAC eventually complements intracellular GSH levels, elevates the ratio of GSH/GSSG, and promotes the antioxidative effects of GSH. NAC can also inhibit white-cell activation and indirectly modulate the production of inflammatory cytokines such as IL-10 and IL-1215. More specifically, an in vitro model of lung cell stretch showed that intracellular GSH, acting as a scavenger of ROS, regulates lung cell stretch-induced cytokine production16.
To evaluate the therapeutic potential of antioxidants during antifungal treatment, IPA was induced in neutropenic mice and the effects of NAC on pulmonary oxidative stress and injury were observed.
Materials and methods
IPA model in neutropenic mice
Male BALB/c mice (8–10 weeks old, about 20 g) were obtained from the animal center of the Shanghai Medical School of Fudan University. The animals were housed, six mice per standard cage, in a specified pathogen-free environment. These mice were maintained on a 12-h light/dark cycle and received water and food ad libitum. All experiments were approved by the Experimental Animal Ethics Committee of Fudan University.
Neutropenia was induced by intraperitoneal (ip) administration of 150 mg·kg−1·d−1 cyclophosphamide17 (Hengrui Pharmaceutical Factory, Jiangsu Province, China) 3 days before fungal inoculation. Animals were kept under strict hygienic conditions and were observed daily until the end of the study.
A strain of Aspergillus fumigatus was isolated from an immunocompromised patient with IPA. The fungus was cultured on Sabouraud agar and conidia were harvested in sterile saline. Preparation of a microscopically confirmed, hyphae-free conidial suspension was performed as previously described18. A suspension of 1×109 conidia/mL was then prepared prior to inoculation. Each inoculum consisted of 0.025 mL of suspension (106 conidia) administered intratracheally using a micro-amount syringe (Micro Fine 0.025 mL, Commerce and Trade Gaoge Company Ltd, Shanghai, China) after blunt dissection of the subcutaneous tissue.
Pharmaceutical intervention
The neutropenic mice were randomly assigned to an AMB treatment (AMB), an AMB plus NAC treatment (AMB plus NAC), or a control (C) group. Weight, behavior, feeding, and other activities and characteristics were observed before inoculation with A fumigatus conidia (0 h) as well as 24, 48, and 72 h after inoculation. For the AMB group, AMB (New Pioneer Pharmaceutical Company Ltd, Shanghai, China) was diluted in 5% dextrose and administered ip at a dose of 1 mg/kg per day6. Treatment was started at 24 h after fungal inoculation and was administered for 3 consecutive days. For the AMB plus NAC group, beyond AMB treatment, mice received an injection of NAC at 150 mg/kg per day before infection (0 h) and every 24 h over the 3 days following inoculation19.
Blood was collected immediately after sacrifice. Three hundred microliters of each sample was dissolved in 570 μL of glacial acetic acid to obtain a white blood cell count. The sera were separated by centrifugation at 2000 r/min for 10 min at 4 °C for further testing.
The superior lobe of the right lung was homogenized in 1.0 mL of cold phosphate-buffered saline (PBS) and incubated in an ice bath before use. Lung homogenates were centrifuged at 12000 r/min for 15 min at 4°C. The sediment of the homogenates was then cultured on Sabouraud agar at 37 °C. The supernatants and sera mentioned above were stored at −70 °C until analysis.
Oxidation evaluation
Superoxide dismutase (SOD) and malondialdehyde (MDA) levels in the sera and lung homogenates were quantified using commercially available kits according to the manufacturer's instructions (Nanjing Jiancheng Co Ltd). Results were normalized to the total amount of protein measured by Bradford protein assay (Beyotime Institute of Biotechnology).
Cytokine determination
TNF-α and IL-10 levels in the sera and lung homogenates were determined using commercially available enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer's instructions (Shanghai Senxiong Corp). Results were normalized to total amount of protein.
Lung pathology and injury analysis
Pulmonary vascular permeability and wet/dry weight ratio were measured as described previously20. The left lung was obtained and weighed immediately and 72 h after storage at 60 °C to determine the wet/dry weight ratio of the lung tissue. Meanwhile, the pulmonary permeability index was determined as the ratio of protein levels in the bronchoalveolar lavage fluid to levels in the plasma. The total amount of protein in the supernatants of the lung homogenates and sera was measured using the Bradford protein assay.
The inferior lobe of the right lung was fixed in 4% neutral buffered formaldehyde, embedded in paraffin, and cut into sections. These sections were stained with hematoxylin-eosin for histological examination and with methenamine silver for fungus detection21. A trained pathologist evaluated lung sections in a blinded fashion and graded the degree of lung injury using a previously described scoring system22, 23.
Statistical analysis
All data were expressed as means and standard errors of the means (SEMs). One-way analysis of variance (ANOVA) was used to perform comparisons among three or more groups, and Student's t test was used for comparing the two groups. Data were analyzed using the SPSS 13.0 software. P<0.05 was considered significant.
Results
Induction of IPA in neutropenic mice
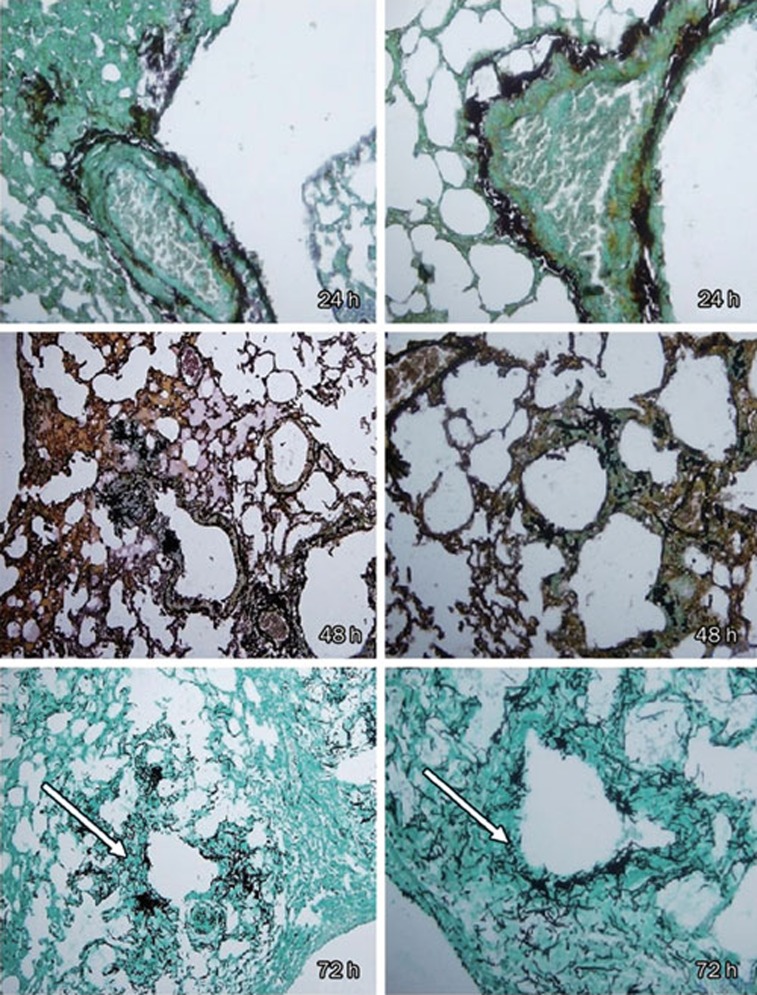
A neutropenic mice model was successfully established, as evidenced by a median white blood cell count of (2.42±1.02)×108/L (<5×108/L), as compared with 4.0×109-12.0×109/L in normal mice24. Successful IPA induction was verified by histological analysis using hexamethylene tetramine silver staining, which revealed pulmonary dissemination and infiltration of hyphae in the control mice (Figure 1).
Figure 1.
Methenamine silver stain of lung infected with A fumigatus. (All from the placebo group) magnification, ×400 (left) and ×800 (right), a lot of hyphae within the lesions of necrosis can be observed (arrows). Hexamethylene tetramine silver staining exhibited considerable hyphae invasion into pulmonary alveoli and interstitium at 72 h. Only few conidia were found at 72 h.
After conidia inoculation, the infected control mice exhibited depression, lower activity levels, reduced feeding, decreased dermal temperature, and slower responses. These mice tended to gather and shiver with hair erect. The deceased animals usually exhibited mass conjunctival secretion and gummed eyelids. Meanwhile, mice in both treatment groups displayed typical behavior, and no remarkable behavioral difference was noted between the two groups.
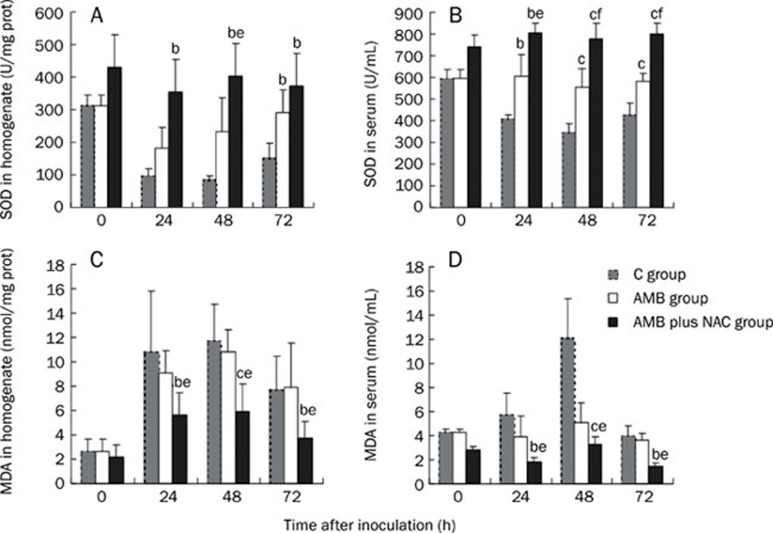
NAC treatment blocked inhibition of SOD production in both lung and serum
As shown in Figure 2A, the pulmonary SOD levels of the AMB plus NAC group were significantly higher than those of the C group at 24, 48, and 72 h. However, pulmonary SOD levels of the AMB group were significantly higher than those of the C group only at 72 h.
Figure 2.
Influences of different interventions on the oxidative stress of neutropenic mice of invasive pulmonary aspergillosis. White bar represents data for AMB group which was treated with amphotericin b (AMB), and black bar for AMB plus NAC group which was treated with AMB combined with n-acetylgalactosamine (NAC) while grey bar for the control group. (A) The concentration of SOD in homogenates. (B) The concentration of SOD in sera. (C) The concentration of MDA in homogenates. (D) The concentration of MDA in sera of mice. bP<0.05, cP<0.01 vs C group. eP<0.05, fP<0.01 vs AMB group.
The serum SOD levels of the AMB and AMB plus NAC groups were significantly higher than the C group's levels. Additionally, the AMB plus NAC group's serum SOD concentration was statistically different from that of the AMB group, especially at 48 h (779.46±71.89 U/mL vs 554.24±86.81 U/mL, P<0.01) and 72 h (799.36±49.09 U/mL vs 580.45±38.58 U/mL, P<0.01; Figure 2B).
NAC treatment decreased MDA levels in both lung and serum
The MDA levels in the lung homogenates of the AMB plus NAC group were reduced compared with those of the C group, whereas the AMB group's MDA levels were similar to the C group's levels (Figure 2C). The serum MDA levels of the AMB plus NAC group were also noticeably lower than those of C group, in accordance with the lung homogenate data (Figure 2D).
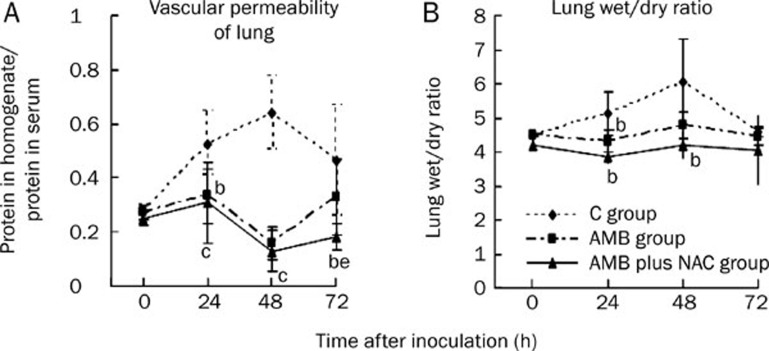
NAC treatment changed the indices of lung injury at both 48 and 72 h
The vascular permeability and wet/dry weight ratio of the lung were utilized as indices of tissue injury. Pulmonary vascular permeability was expressed as the ratio of protein levels in the bronchoalveolar lavage fluid to levels in the plasma, and pulmonary edema was estimated from the wet/dry lung weight ratio. In this study, AMB plus NAC intervention improved vascular permeability at 48 and 72 h and lung edema at 24 and 48 h. Moreover, compared with the AMB group, AMB plus NAC treatment improved permeability more noticeably at 72 h (0.18±0.05 vs 0.323±0.14, P<0.05; Figure 3A). The latter treatment also significantly decreased lung edema at 48 h, as compared with the C group (4.19±0.41 vs 6.05±1.29, P<0.05), whereas no statistical difference was noted between the AMB and C groups (Figure 3B).
Figure 3.
Influences of different interventions on the indices of lung injury of neutropenic mice of invasive pulmonary aspergillosis. Triangles and continuous line represent data for CT group which was treated with amphotericin b (AMB) combined with n-acetylgalactosamine (NAC), squares and broken line represent data for AMB group which was treated only with AMB, and diamond and broken line for the control group. (A) Vascular permeability of lung: the protein of lung homogenates/protein in sera; (B) Lung wet/dry ratio. bP<0.05, cP<0.01 vs C group. eP<0.05 vs AMB group.
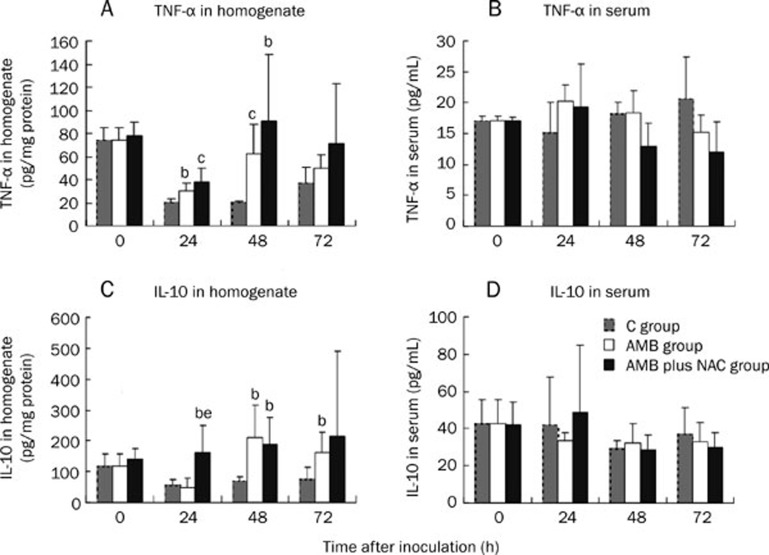
TNF-α and IL-10 levels in lungs and serum
The serum concentrations of TNF-α and IL-10 did not differ significantly among the three groups of mice. AMB and AMB plus NAC treatments both resulted in elevated TNF-α levels in the pulmonary homogenates at 24 and 48 h. The pulmonary IL-10 levels of the AMB plus NAC group were also higher than those of the C group at 24 and 48 h. In contrast, the AMB group's IL-10 levels were higher than the C group's levels at 48 and 72 h (Figure 4C).
Figure 4.
Influences of different interventions on the cytokines in sera and lung homogenates of mice of invasive pulmonary Aspergillosis. White bar represents data for AMB group which was treated with amphotericin b (AMB), and black bar for AMB plus NAC group which was treated with AMB combined with N-acetylgalactosamine (NAC) while grey bar for the control group. TNF-α and IL-10 contents in the right lungs and in serum of the mice were determined by ELISA. (A) The concentration of TNF-α in homogenates; (B) The concentration of TNF-α in sera; (C) The concentration of IL-10 in homogenates; (D) The concentration IL-10 in sera. bP<0.05, cP<0.01 vs C group. eP<0.05 vs AMB group.
Histology
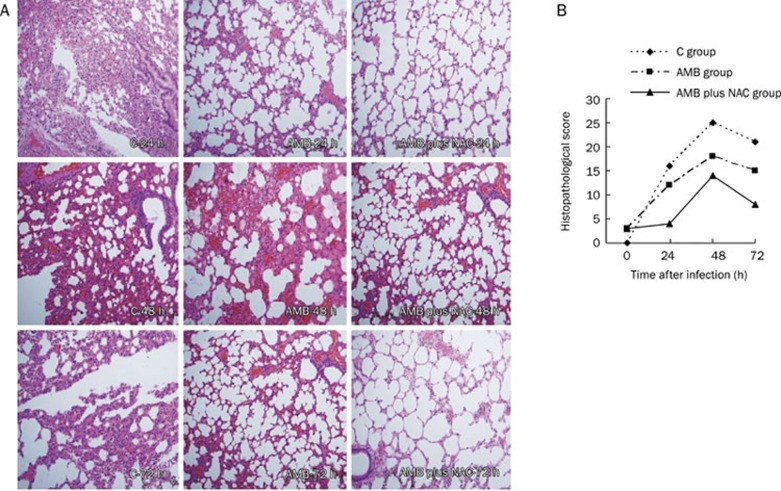
The results of histopathological examination are shown in Figure 5. At 24 h post-infection in the C group, the inflammatory cells accumulated in the alveolar septum and around the bronchi. Next, at 48 h, the alveolar septum was markedly widened and filled with neutrophils. However, tissue damage seemed be somewhat alleviated at 72 h. Yet, when treated, tissue damage had been repaired to some extent at each time point. These results were in accordance with the quantitative scoring of histopathological damage (Figure 5B).
Figure 5.
Lung histological features of mice infected with A fumigatus, which were treated with AMB, or AMB plus NAC, or untreated (Control)(A). Histopathological examination was carried out and showed that inflammatory cells increased in alveolar septum and around bronchi at 24 h after injection. At 48 h, alveolar septum was obviously widened under microscopes, filled with massive neutrophils. It seemed that the histopathological findings were improved somehow at 72 h. When being treated, the tissue damage had been repaired to some extent at each time point. Heamatoxylin-eosin staining. Magnification,×400. It also told the same tendency from the quantifying score of histopathological damage (B, n=3).
Discussion
Murine models of aspergillosis have been used extensively in the study of antifungal drug efficacy25, 26, 27, 28. Intratracheal inoculation best mimics the natural route of infection in humans, in whom the lung is the primary target organ following conidia inhalation, especially during immunosuppression29, 30. In the current study, IPA pathogenesis was induced by this mode of infection. Intratracheal instillation after blunt dissection was adopted because intranasal inoculation led to variability in inhaled conidia count, often a result of damp nostrils or sneezing. This new method was 100% successful in inducing infection, as confirmed by the results of histopathological examination. The alveolar septum was obviously widened and filled with inflammatory cells, both of which are characteristic of IPA. Moreover, hexamethylene tetramine silver staining revealed a considerable number of hyphae but few conidia in the pulmonary alveoli and interstitium at 72 h after inoculation (Figure 1), which is typical of IPA in mice with chemotherapy-induced neutropenia31.
SOD content reflects the host's ability to quench free radicals. Treatment of IPA with AMB plus NAC markedly elevated SOD levels in mice both in local tissue and in blood serum in comparison with the other two groups. Moreover, the AMB plus NAC treatment markedly reduced MDA levels, which are an indicator of oxidative damage. Thus, NAC increased the antioxidant capability of infected mice and ameliorated tissue damage caused by free radicals during IPA progression.
During IPA pathogenesis, A fumigatus conidia are phagocytosed by macrophages and polymorphonuclear cells (PMNs) and circulating monocytes damage escaping hyphae by secreting microbicidal oxidative metabolites and nonoxidative compounds, thus preventing the establishment of invasive disease5, 28, 32. While PMNs are essential for pathogen eradication, excessive release of oxidants and proteases may be responsible for organ injury as well as fungal sepsis33, 34, 35, 36. A powerful antioxidant and an effective preventive and therapeutic agent in diseases was related to inflammation and oxidative stress16, NAC would be likely to alleviate damage caused by excessive oxidants. NAC was previously reported to significantly reduce lung injury in mice with Pseudomonas aeruginosa-induced pneumonia37 and was here observed to effectively alleviate pulmonary damage in IPA mice. NAC's efficacious reduction of oxidative stress was supported by histopathological data and pathological scoring as well as by the lung injury indices.
No remarkable differences in TNF-α and IL-10 serum content were observed among the three groups of mice. This finding may be attributed to the fact that the systemic reaction to IPA was not noticeable. Meanwhile, significant changes in the cytokine levels of the lung homogenates were recorded among all three groups, with markedly elevated levels of both TNF-α and IL-10 at 24, 48, and 72 h with AMB plus NAC treatment. Studies of the IPA murine model have demonstrated that resistance to infection is associated with the production of Th1 cytokines, whereas Th2-cytokine release was correlated with disease progression32, 38, 39. Aihara et al previously reported that NAC could increase Th1/Th2 cytokine levels in vitro40. TNF-α is a pro-inflammatory Th1 cytokine that is important to the host response to infectious agents, including fungal pathogens41. Phagocytosis of hyphae induces release of IL-10, a Th2 cytokine that regulates the inflammatory response by impairing the antifungal activity of phagocytes and secretion of proinflammatory cytokines by macrophages, T-cell, neutrophils and dendritic cells31, 35. In this study, NAC elevated pulmonary TNF-α levels, strengthening resistance to IPA infection. This finding was in accordance with other data pertaining to alleviation of lung injury. The rise in IL-10 due to NAC, however, is more difficult to explain. Further work is necessary to elucidate the influences of NAC on inflammation, and specifically on cytokine production.
Conclusion
In combination with antifungal therapy, NAC treatment can alleviate oxidative stress both in local tissue and in blood serum and lung injury associated with IPA in neutropenic mice. Additionally, combined treatment with NAC improved vascular permeability and palliated lung edema. Antioxidants in conjunction with antifungal treatment may thus be a more effective means of treating IPA in neutropenic hosts.
Since the complexity of cytokine production during IPA treatment with antioxidants is still not well understood, further studies using additional time points and examining multiple Th1/Th2 cytokines will be required.
Author contribution
Jie-ming QU, Peng XU, and Hui-jun ZHANG designed research; Peng XU performed research; Hong-ni JIANG contributed new analytical tools and reagents; Peng XU analyzed data; Peng XU, Jin-fu XU, and Jing ZHANG wrote the paper.
Acknowledgments
This project was supported by the National Natural Science Foundation of China (No 30571542) and the Shanghai Leading Academic Discipline Project (Project No B 115).
References
- Reichenberger F, Habicht JM, Gratwohl A, Tamm M. Diagnosis and treatment of invasive pulmonary aspergillosis in neutropenic patients. Eur Respir J. 2002;19:743–55. doi: 10.1183/09031936.02.00256102. [DOI] [PubMed] [Google Scholar]
- Meersseman W, Vandecasteele SJ, Wilmer A, Verbeken E, Peetermans W, Wijngaerden E. Invasive aspergillosis in critically ill patients without malignancy. Am J Respir Crit Care Med. 2004;170:621–5. doi: 10.1164/rccm.200401-093OC. [DOI] [PubMed] [Google Scholar]
- Garnacho-Montero J, Amaya-Villar R, Ortiz-Leyba C, Ortiz-Leyba C, León C, Álvarez-Lerma F. Isolation of Aspergillus spp from the respiratory tract in critically ill patients: risk factors, clinical presentation and outcome. Crit Care. 2005;9:R191–199. doi: 10.1186/cc3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandewoude K, Blot S, Depuydt P, Benoit D, Temmerman W, Colardyn F. Clinical relevance of Aspergillus isolation from respiratory tract samples in critically ill patients. Crit Care. 2006;10:R31. doi: 10.1186/cc4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan T, Kaur S, Saxena S, Singh M, Kishore U, Thiel S. Role of collectins in innate immunity against Aspergillosis. Med Mycol. 2005;43 Suppl 1:155–63. doi: 10.1080/13693780500088408. [DOI] [PubMed] [Google Scholar]
- Becker MJ, de Marie S, Fens MH, Verbrugh HA, Bakker-Woudenberg IA. Effect of amphotericin B treatment on kinetics of cytokines and parameters of fungal load in neutropenic rats with invasive pulmonary Aspergillosis. J Antimicrob Chemother. 2003;52:428–34. doi: 10.1093/jac/dkg367. [DOI] [PubMed] [Google Scholar]
- Brakhage AA. Systemic fungal infections caused by Aspergillus species: epidemiology, infection process and virulence determinants. Curr Drug Targets. 2005;6:875–86. doi: 10.2174/138945005774912717. [DOI] [PubMed] [Google Scholar]
- Saxena S, Bhatnagar PK, Ghosh PC, Usha Sarma P. Effect of amphotericin B lipid formulation on immune response in Aspergillosis. Int J Pharm. 1999;188:19–30. doi: 10.1016/s0378-5173(99)00200-8. [DOI] [PubMed] [Google Scholar]
- Vandewoude KH, Vogelaers D, Blot SI. Aspergillosis in the ICU — the new 21st century problem. Med Mycol. 2006;44 Suppl:71–6. doi: 10.1080/13693780600919262. [DOI] [PubMed] [Google Scholar]
- Segal BH, Walsh TJ. Current approaches to diagnosis and treatment of invasive Aspergillosis. Am J Respir Crit Care Med. 2006;173:707–17. doi: 10.1164/rccm.200505-727SO. [DOI] [PubMed] [Google Scholar]
- Morcillo EJ, Estrela J, Cortijo J. Oxidative stress and pulmonary inflammation: pharmacological intervention with antioxidants. Pharmacol Res. 1999;40:393–402. doi: 10.1006/phrs.1999.0549. [DOI] [PubMed] [Google Scholar]
- Bellocchio S, Moretti S, Perruccio K, Fallarino F, Bozza S, Montagnoli C, et al. TLRs govern neutrophil activity in Aspergillosis. J Immunol. 2004;173:7406–15. doi: 10.4049/jimmunol.173.12.7406. [DOI] [PubMed] [Google Scholar]
- Chauhan N, Latgé JP, Calderone R. Signalling and oxidant adaptation in Candida albicans and Aspergillus fumigatus. Nat Rev Microbiol. 2006;4:435–44. doi: 10.1038/nrmicro1426. [DOI] [PubMed] [Google Scholar]
- Dekhuijzen PN. Antioxidant properties of N-acetylcysteine: their relevance in relation to chronic obstructive pulmonary disease. Eur Respir J. 2004;23:629–36. doi: 10.1183/09031936.04.00016804. [DOI] [PubMed] [Google Scholar]
- Huang XL, Ling YL. The antioxidant therapy of N-acetylcysteine to pulmonary injury. Int J Respir. 2001;21:64–9. [Google Scholar]
- Cotgreave IA. N-acetylcysteine: pharmacological considerations and experimental and clinical applications. Adv Pharmacol. 1997;38:205–27. [PubMed] [Google Scholar]
- Sbaraglia G, D'Errico P, Serafini S, Vecchiarelli L, Perito S. Pathogenicity of various species of Candida in mice immunodepressed with cyclophosphamide. Boll Soc Ital Biol Sper. 1984;60:1421–6. [PubMed] [Google Scholar]
- Leenders AC, de Marie S, ten Kate MT, Bakker-Woudenberg IA, Verbrugh HA. Liposomal amphotericinB (AmBisome) reduces dissemination of infection as compared with amphotericin B deoxycholate (Fungizone) in a rat model of pulmonary Aspergillosis. J Antimicrob Chemother. 1996;38:215–25. doi: 10.1093/jac/38.2.215. [DOI] [PubMed] [Google Scholar]
- Wang AL, Wang JP, Wang H, Chen YH, Zhao L, Wang LS, et al. A dual effect of N-acetylcysteine on acute ethanol-induced liver damage in mice. Chia J Dis Control Prev. 2006;10:593–6. doi: 10.1016/j.hepres.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Liu KX, Wu WK, He W, Liu CL. Ginkgo biloba extract (Egb 761) attenuates lung injury induced by intestinal ischemia/reperfusion in rats: roles of oxidative stress and nitric oxide. World J Gastroenterol. 2007;13:299–305. doi: 10.3748/wjg.v13.i2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grocott RG. A stain for fungi in tissue section and smears using Gomori's methenamine-silver nitrate technic. Am J Clin Pathol. 1955;25:975–9. doi: 10.1093/ajcp/25.8_ts.0975. [DOI] [PubMed] [Google Scholar]
- Murao Y, Loomis W, Wolf P, Hoyt DB, Junger WG. Effect of dose of hypertonic saline on its potential to prevent lung tissue damage in a mouse model of hemorrhagic shock. Shock. 2003;20:29–34. doi: 10.1097/01.shk.0000071060.78689.f1. [DOI] [PubMed] [Google Scholar]
- Kotanidou A, Loutrari H, Papadomichelakis E, Glynos C, Magkou C, Armaganidis A, et al. Inhaled activated protein C attenuates lung injury induced by aerosolized endotoxin in mice. Vascul Pharmacol. 2006;45:134–40. doi: 10.1016/j.vph.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Nemzek JA, Bolgos GL, Williams BA, Remick DG. Differences in normal values for murine white blood cell counts and other hematological parameters based on sampling site. Inflamm Res. 2001;50:523–7. doi: 10.1007/PL00000229. [DOI] [PubMed] [Google Scholar]
- Chiller TM, Sobel RA, Capilla Luque J, Clemons KV, Stevens DA. Efficacy of amphotericin B or itraconazole in a murine model of central nervous system Aspergillus infection. Antimicrob Agents Chemother. 2003;47:813–5. doi: 10.1128/AAC.47.2.813-815.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemons KV, Stevens DA. Comparative efficacies of four amphotericin B formulations-Fungizone, amphotec (Amphocil), AmBisome, and Abelcet — against systemic murine aspergillosis. Antimicrob Agents Chemother. 2004;48:1047–50. doi: 10.1128/AAC.48.3.1047-1050.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybill JR, Bocanegra R, Gonzalez GM, Najvar LK. Combination antifungal therapy of murine aspergillosis: liposomal amphotericin B and micafungin. J Antimicrob Chemother. 2003;52:656–62. doi: 10.1093/jac/dkg425. [DOI] [PubMed] [Google Scholar]
- Lewis RE, Albert ND, Kontoyiannis DP. Comparison of the dose-dependent activity and paradoxical effect of caspofungin and micafungin in a neutropenic murine model of invasive pulmonary Aspergillosis. J Antimicrob Chemother. 2008;61:1140–4. doi: 10.1093/jac/dkn069. [DOI] [PubMed] [Google Scholar]
- Latgé JP. The pathobiology of Aspergillus fumigatus. Trends Microbiol. 2001;9:382–9. doi: 10.1016/s0966-842x(01)02104-7. [DOI] [PubMed] [Google Scholar]
- Pasqualotto A. Differences in pathogenicity and clinical syndromes due to Aspergillus fumigatus and Aspergillus flavus. Med Mycol. 2008. pp. S1–S10. [DOI] [PubMed]
- Balloy V, Huerre M, Latgé JP, Chignard M. Differences in patterns of infection and inflammation for corticosteroid treatment and chemotherapy in experimental invasive pulmonary aspergillosis. Infect Immun. 2005;73:494–503. doi: 10.1128/IAI.73.1.494-503.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luther K, Rohde M, Sturm K, Kotz A, Heesemann J, Ebel F. Characterisation of the phagocytic uptake of Aspergillus fumigatus conidia by macrophages. Microbes Infect. 2008;10:175–84. doi: 10.1016/j.micinf.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Latgé JP. Aspergillus fumigatus and Aspergillosis. Clin Microbiol Rev. 1999;12:310–50. doi: 10.1128/cmr.12.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves EP, Nagl M, Godovac-Zimmermann J, Segal AW. Reassessment of the microbicidal activity of reactive oxygen species and hypochlorous acid with reference to the phagocytic vacuole of the neutrophil granulocyte. J Med Microbiol. 2003;52:643–51. doi: 10.1099/jmm.0.05181-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellocchio S, Moretti S, Perruccio K, Fallarino F, Bozza S, Montagnol C, et al. TLRs govern neutrophil activity in Aspergillosis. J Immunol. 2004;173:7406–15. doi: 10.4049/jimmunol.173.12.7406. [DOI] [PubMed] [Google Scholar]
- Hohl TM, Marta Feldmesser EC. Aspergillus fumigatus: principles of pathogenesis and host defense. Eukaryot Cell. 2007;6:1953–63. doi: 10.1128/EC.00274-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu JF, Qu JM. The effect of antioxidant treatment in pneumonia of granulocytopenic rats. Chin J Internal Med. 2004;43:852–6. [PubMed] [Google Scholar]
- Sainz J, Hassan L, Perez E, Romero A, Moratalla A, López-Fernández E, et al. Interleukin-10 promoter polymorphism as risk factor to develop invasive pulmonary Aspergillosis. Immunol Lett. 2007;109:76–82. doi: 10.1016/j.imlet.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Stevens DA. Th1/Th2 in aspergillosis. Med Mycol. 2006;44 Suppl:229–35. [Google Scholar]
- Aihara M, Dobashi K, Akiyama M, Naruse I, Nakazawa T, Mori M. Effects of N-acetylcysteine and ambroxol on the production of IL-12 and IL-10 in human alveolar macrophages. Respiration. 2000;67:662–71. doi: 10.1159/000056297. [DOI] [PubMed] [Google Scholar]
- Sambatakou H, Pravica V, Hutchinson IV, Denning DW. Cytokine profiling of pulmonary Aspergillosis. Int J Immunogenet. 2006;33:297–302. doi: 10.1111/j.1744-313X.2006.00616.x. [DOI] [PubMed] [Google Scholar]