Abstract
Aim:
Alcohol, which is predominantly metabolized in the liver, is a major hepatic toxicant that readily induces hepatic steatosis. The expression of CCAAT enhancer binding protein (C/EBP), especially the C/EBP delta variety, is increased in the early phase of adipogenesis. However, the role of C/EBP delta in ethanol-induced hepatosteatosis is unclear.
Methods:
Male C57BL/6J mice were randomized to one of four groups: a control group, a group receiving orally administered ethanol (4 g ethanol/kg body weight) (EtOH), a high-fat-diet (HF) group and an EtOH+HF group. Mice were sacrificed after 5 or 10 weeks for various measurements. The in vitro effect of ethanol on the expression of C/EBP alpha, beta and delta was studied in HepG2 cells.
Results:
By week 5, ethanol treatment had significantly increased liver C/EBP delta and beta protein expression (by 2.3- and 1.4-fold, respectively), which then returned to the control level by week 10. In contrast, the expression of C/EBP alpha was evident only at week 10. The in vitro study shows that C/EBP delta expression was elevated significantly at 24 h but not at 48 or 72 h. C/EBP beta expression was highest at 48 h, whereas C/EBP alpha expression was highest at 72 h. We also found that a low concentration of ethanol plus oleic acid enhanced C/EBP delta expression in HepG2 cells.
Conclusion:
C/EBP delta expression appears to play an important role in the early phase of ethanol-induced hepatosteatosis in mice and in ethanol-treated HepG2 cells. In addition, EtOH+HF enhances the expression of C/EBP delta in HepG2 cells. Thus, C/EBP delta might be a therapeutic target in alcoholic hepatosteatosis.
Keywords: C/EBP delta, ethanol, hepatosteatosis, adipogenesis
Introduction
Acute or chronic alcohol consumption can cause severe liver injury1. Alcohol is known to impair fat oxidation and to stimulate lipogenesis in the liver2, 3. Thus, alcohol consumption can lead to the development of hepatic steatosis. Most studies on alcoholic hepatic steatosis have focused on the ability of ethanol to shift the redox state in the liver and to inhibit fatty acid oxidation3, 4. Indeed, previous studies have shown the repression of some enzymes involved in fatty acid oxidation and induction of lipogenic enzymes in ethanol-fed animals2, 5. The de novo synthesis of fatty acids in the liver was shown to increase with ethanol feeding in conjunction with the induction of adipogenic enzymes, including peroxisome proliferator-activated receptor alpha (PPAR alpha) and/or sterol regulatory element binding protein 1 (SREBP-1)2, 6. However, the mechanisms by which ethanol causes steatosis are multiple and complex.
The CCAAT enhancer binding proteins (C/EBPs) are a family of basic leucine zipper transcription factors involved in the regulation of cellular differentiation and functions7, 8, 9. Six members of the C/EBP family have been described, including C/EBP alpha, beta, gamma, delta, epsilon and zeta10. C/EBP alpha, beta, and delta are important transcription factors in adipose differentiation. C/EBPs may affect adipogenesis by regulation and coordination of a cascade of transcription factors that together lead to the establishment of the differentiated state11. For instance, C/EBP beta and delta have been shown to be expressed early in adipogenesis, whereas C/EBP alpha is expressed much later12, 13. The vital role played by C/EBP beta and delta in adipogenesis was demonstrated by disruption of both genes, which prevents the normal development of adipose tissue14. Furthermore, silencing of C/EBP delta impairs the expression of factor for adipocyte differentiation 49(fad49), which is up-regulated in adipogenesis and appears to play a crucial role early in the process15.
McKnight et al16 have shown that C/EBP delta alone possesses minimal adipogenic activity and C/EBP beta and C/EBP delta play important roles in inducing expression of C/EBP alpha and PPAR gamma. Lai et al17 have demonstrated that induction of C/EBP delta expression transiently activates PPAR gamma 2 transcription, which is involved in adiopocyte-like lipogenesis in HepG2 cells. The temporal pattern of expression of these three C/EBP isoforms during adipose differentiation may reflect the underpinnings of a regulatory cascade that controls the process of terminal cell differentiation18.
Previous studies have examined the effect of ethanol on C/EBPs in animals and in cell cultures, but the results are inconsistent10, 19, 20. In addition, little or no information is available about the effect of ethanol on C/EBP delta. In this study, we conducted both in vivo and in vitro experiments to investigate the changes of C/EBP delta expression in ethanol-induced hepatosteatosis. We postulated that C/EBP delta was an important transcription factor in the early phase of ethanol-induced hepatosteatosis.
Materials and methods
Animals and diet
Experiments were performed on 6-week-old male C57BL/6J mice obtained from the Animal Center of National Cheng Kung University Medical College, Tainan, Taiwan. C57BL/6J mice were used because it has been shown that they consume alcohol more readily than most strains 21. The mice were provided free access to distilled water and either standard laboratory rodent chow (10% fat) or a high-fat diet (HF, 58Y1, TestDiet, Richmond, VA, USA). They were divided into four groups (12 mice in each group): (1) control, oral administration of saline, (2) EtOH, oral administration of ethanol (4 g/kg BW), (3) HF, oral administration of saline+high-fat diet, and (4) EtOH+HF, ethanol (4 g/kg BW)+high-fat diet. Mice were given saline or ethanol via gastric lavage three times/week for 5 or 10 weeks. Six mice in each group were sacrificed at 5 and 10 weeks after treatment. The study protocol was approved by the Institutional Animal Care and Use Committee of the Central Taiwan University of Science and Technology.
Assay of serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT)
The mice were sacrificed following 5 or 10 weeks of treatment. Blood samples were obtained from the orbital vascular plexus of each mouse and centrifuged at 1000×g at 4 °C for 5 min to separate serum. Serum levels of AST and ALT were determined using clinical test kits (AppliedBio assay kits; Hercules, CA, USA).
Liver morphology
Immediately after the mice had been killed, the liver tissue was fixed in 10% neutral buffered formalin. The tissue was then processed with graded alcohol and xylene and embedded in paraffin. Liver sections (3-5 μm thick) were stained with hematoxylin and eosin (HE). Pathological changes were observed by light microscopy.
Cell culture conditions
The human hepatoma HepG2 cell line was purchased from Bioresource Collection and Research Center (Food Industry Research and Development Institute, Hsinchu, Taiwan). These cells were maintained in Dulbecco's Modified Essential Media (DMEM) containing 10% FBS at 37 °C with 5% CO2 and 95% humidity. HepG2 cells were treated with ethanol at different concentrations (10, 100, 200, or 400 mmol/L) as described previously22. Then, the cells were gently sealed with parafilm to avoid the evaporation of ethanol. Control cells not treated with ethanol were sealed in parafilm as well. The cells were then incubated for 24–72 h at 37 °C with 5% CO2. The cells did not show any change in viability following sealing with parafilm.
Induction of steatosis in HepG2 cells was obtained using oleic acid (Sigma-Aldrich Inc, Saint Louis, MO, USA) as described previously23. Oleic acid was added to the culture medium to obtain a final concentration of 25, 50, 100, or 200 μmol/L. The duration of this treatment was 72 h.
Western blot analysis
Expression levels of C/EBP alpha, beta, and delta in HepG2 cells and mouse liver tissues were determined by Western blot techniques. Proteins were extracted using RIPA buffer and separated by SDS-PAGE and then transferred and immobilized on a nitrocellulose membrane. The membrane was blocked with 5% non-fat dry milk in phosphate-buffered saline containing 0.1% Tween 20 (PBS-T) for a 2-h incubation at room temperature. The membrane was then washed in PBS-T and hybridized with primary antibodies diluted in PBS-T for 16 h. Proper dilutions of specific antibodies for C/EBP alpha, beta, delta (1:200) and actin (1:1000) purchased from Santa Cruz Biotechnology Inc (Santa Cruz, CA, USA) were used. Incubation with secondary antibodies and detection of the antigen-antibody complex were performed using the ECL kit (Amersham Biosciences, UK). Densities of the obtained immunoblots were quantified using Image J (NIH).
Statistical analysis
Data are expressed as the means±SD. Statistical significance of the differences between groups means was analyzed by one-way ANOVA followed by Duncan's multiple comparisons, with P<0.05 considered significant.
Results
Ethanol administration and high-fat diet increase liver function abnormality
After 5 weeks of treatment, serum levels of AST and ALT were markedly higher in mice in the EtOH group, the HF group and the EtOH+HF group than in those in the control group (P<0.05). The levels of AST and ALT in these three groups of mice were further increased after 10 weeks of treatment (Table 1).
Table 1. Serum levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) in C57BL/6J mice orally treated with ethanol (4 g/kg BW) with or without a high-fat diet for 5 or 10 weeks. Data are means±SD. n=6. Values not sharing a common alphabet letter are significantly different. bP<0.05 vs control. eP<0.05 vs corresponding group at 5 weeks.
| Group | AST(U/L) | ALT(U/L) | |
|---|---|---|---|
| 5 weeks | Control | 53.3±3 | 24.0±3 |
| EtOH | 125±3b | 65.0±9b | |
| HF | 78.8±9b | 39.3±5b | |
| EtOH+HF | 103±20b | 55.0±3b | |
| 10 weeks | Control | 54.0±15 | 26.3±2 |
| EtOH | 215±41e | 140±3e | |
| HF | 121±6e | 53.8±3e | |
| EtOH+HF | 175±56e | 69.5±9e |
Control mice (Control), ethanol administered mice (EtOH), high-fat diet mice (HF), or ethanol administered combined with high-fat diet (EtOH+HF).
Liver histology of experimental animals
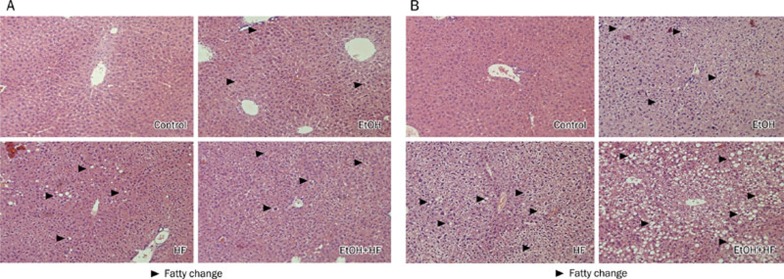
Morphological changes in the liver induced by ethanol with or without the high-fat diet were examined. The HE stain showed a mild fatty degeneration in mice in the EtOH, HF and EtOH+HF groups after 5 weeks, but no such changes in the control mice (Figure 1A). After 10 weeks of treatment, hepatic steatosis was evident in the three intervention groups, but not in the control mice (Figure 1B). Notably, the EtOH+HF group exhibited the most severe fatty change of the intervention groups.
Figure 1.
Histopathological changes of liver from C57BL/6J mice. (A) 5-week treatment; the liver of control mice (Control), ethanol administered mice (EtOH), high-fat diet mice (HF), or ethanol administered combined with high-fat diet (EtOH+HF). (B) 10-week treatment, the liver of control mice (Control), ethanol administered mice (EtOH), high-fat diet mice (HF), or ethanol administered combined with high-fat diet mice (EtOH+HF) (HE stain, ×200).
Protein expressions of C/EBP alpha, beta and delta in C57BL/6J (B6) mice with/without ethanol and with/without a high-fat diet
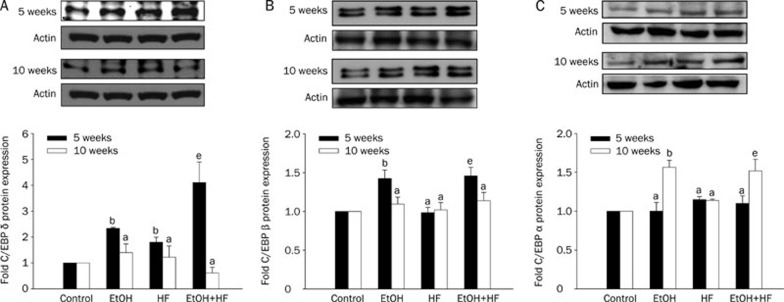
To investigate whether C/EBPs are involved in the progression of ethanol-induced hepatic steatosis, we determined the expression of C/EBP alpha, beta and delta in the mouse liver after treatment with ethanol, with or without HF diet, for 5 weeks and 10 weeks. After 5 weeks of treatment, the hepatic C/EBP delta expression increased significantly (2.3-fold) in ethanol-treated mice, as compared with that in the control mice. Moreover, the hepatic C/EBP delta expression in mice treated with EtOH+HF was higher than that in mice treated with either EtOH or HF alone. However, after 10 weeks of treatment, the hepatic expression of C/EBP delta in mice treated with EtOH, HF and EtOH+HF was restored to that in the control mice (Figure 2A).
Figure 2.
The protein expression of CCAAT enhancer binding protein (C/EBP) delta, beta and alpha in C57BL/6J mice orally treated with ethanol (4 g /kg BW; EtOH) with or without a high-fat (HF) diet for 5 or 10 weeks. The upper panels are western blots of C/EBP delta (A), beta (B) and alpha (C). Densitometric quantification of protein levels (mean±SD, n=3–4); values not sharing a common alphabet letter are significantly different (P<0.05, upper cases for the 5-week experiment and lower cases for the 10-week experiment). aP>0.05, bP<0.05 vs control. eP<0.05 vs HF group or EtOH group alone.
As shown in Figure 2B, the hepatic C/EBP beta expression increased significantly in mice treated with EtOH or with EtOH+HF for 5 weeks (1.4-fold in each case), as compared with that in control mice. However, the hepatic expression of C/EBP beta in mice treated with EtOH and EtOH+HF for 10 weeks was restored to that in the control mice.
After 5 weeks of treatment, the hepatic C/EBP alpha expression in the four groups did not differ significantly. By contrast, after 10 weeks of treatment, the hepatic C/EBP alpha expression increased significantly in mice treated with EtOH and EtOH+HF, as compared with that in control mice (Figure 2C).
Protein expression of C/EBP alpha, beta and delta in HepG2 cells with/without ethanol
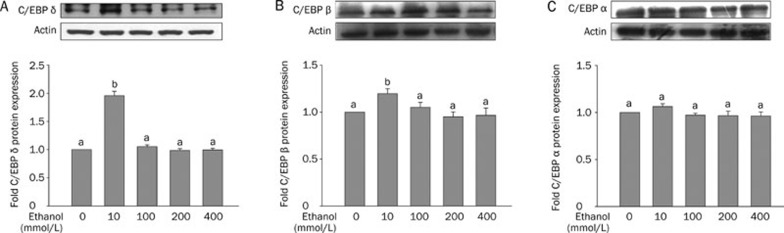
We incubated HepG2 cells with ethanol at different concentrations and determined the protein expression of C/EBP alpha, beta and delta. The expression of C/EBP delta in HepG2 cells incubated with 10 mmol/L ethanol for 24 h was higher than that of C/EBP alpha and beta. Treatment of cells with higher concentrations of ethanol (100–400 mmol/L) decreased the expression of C/EBPs and restored the expression to the control level (Figure 3A, 3B, and 3C).
Figure 3.
The protein expression of CCAAT enhancer binding protein (C/EBP) delta, beta and alpha in HepG2 cells incubated with ethanol. The HepG2 cells were treated with different concentrations of ethanol (0, 10, 100, 200, or 400 mmol/L) for 24 h. The upper panels are Western blots of C/EBP delta (A), beta (B) and alpha (C). Densitometric quantification of protein levels (mean±SD). n=3–4. aP>0.05, bP<0.05 vs control.
We also determined the effect of ethanol on the expression of C/EBP delta mRNA in HepG2 cells by RT-PCR. We found that ethanol (10–400 mmol/L) did not significantly affect the mRNA expression of C/EBP delta (data not shown). The results suggest that the change in C/EBP delta protein expression may occur at the post-transcriptional level.
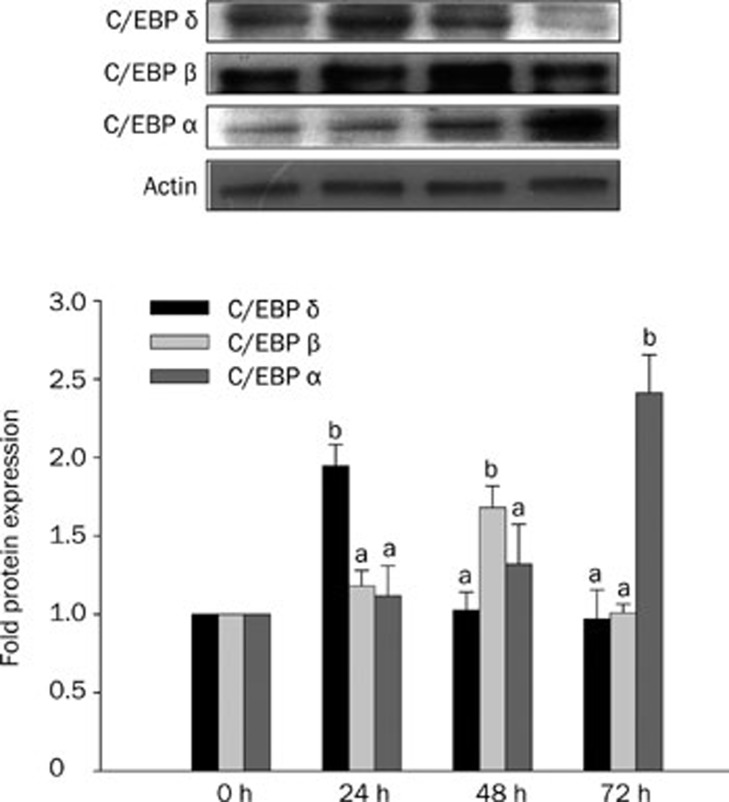
To determine the time course of C/EBP alpha, beta and delta protein expression, cells were exposed to 10 mmol/L ethanol for 24, 48, and 72 h. We found that C/EBP delta expression was significantly elevated at 24 h but not at 48 and or 72 h. By contrast, C/EBP beta expression was highest at 48 h, and the C/EBP alpha expression was highest at 72 h (Figure 4).
Figure 4.
The protein expression of CCAAT enhancer binding protein (C/EBP) delta, beta and alpha in HepG2 cells incubated with/without ethanol. The HepG2 cells were treated with 10 mmol/L ethanol for 24, 48, and 72 h. The upper panels are western blots of C/EBP delta, beta and alpha. Densitometric quantification of protein levels (mean±SD, n=3–4); values not sharing a common alphabet letter are significantly different (P<0.05, lower cases for C/EBP delta; upper cases for C/EBP beta and italic for C/EBP alpha). aP>0.05, bP<0.05 vs control.
Protein expression of C/EBP delta in HepG2 cells with/without oleic acid
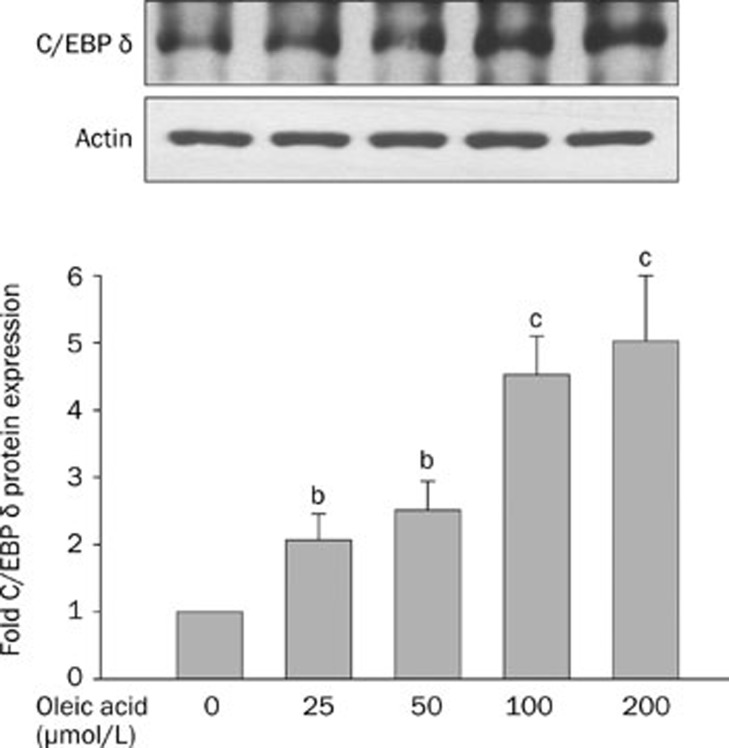
We treated HepG2 cells with different concentrations of oleic acid to mimic adipogenesis in hepatocytes. Oleic acid treatment increased C/EBP delta expression in HepG2 cells in a concentration-dependent manner (Figure 5).
Figure 5.
The protein expression of CCAAT enhancer binding protein (C/EBP) delta in HepG2 cells incubated with oleic acid. The HepG2 cells were treated with different concentrations of oleic acid (0, 25, 50, 100, or 200 μmol/L) for 72 h. The upper panels are western blots of C/EBP delta. Densitometric quantification of protein levels (mean±SD, n=4); values not sharing a common alphabet letter are significantly different. bP<0.05, cP<0.01 vs control.
Protein expression of C/EBP delta in HepG2 cells incubated with ethanol and with/without oleic acid
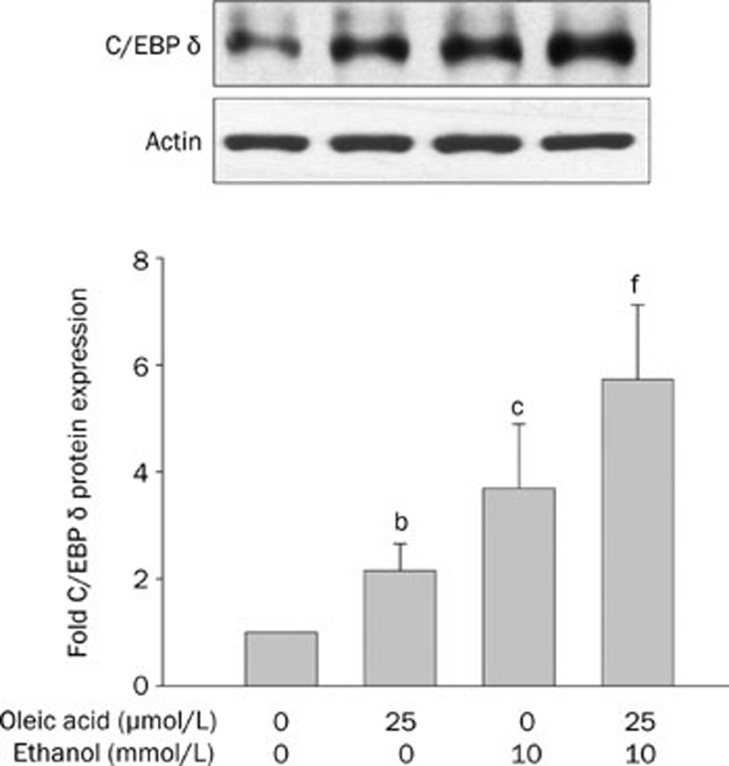
We then treated HepG2 cells with oleic acid (25 μmol/L) and with or without ethanol at a low concentration (10 mmol/L) to avoid ethanol cytotoxicity (Figure 6). We found that the combination of ethanol and oleic acid significantly increased C/EBP delta expression, and that this effect was additive or synergistic, as compared with oleic acid or ethanol alone, according to the following calculation: [(combination – control)/ (oleic acid – control)+(ethanol – control)].
Figure 6.
The protein expression of CCAAT enhancer binding protein (C/EBP) delta in HepG2 cells. The HepG2 cells were treated with 25 μmol/L oleic acid, 10 mmol/L ethanol or 25 μmol/L oleic acid combined with 10 mmol/L ethanol for 24 to 72 h. Protein expression shown in the upper panels are C/EBP delta. Densitometric quantification of protein levels (mean±SD, n=4); values not sharing a common alphabet letter are significantly different. bP<0.05, cP<0.01 vs control. fP<0.01 vs oleic acid or ethanol alone.
Discussion
Steatosis is a critical stage of alcoholic liver disease (ALD), and it has been shown that prevention of steatosis may actually protect against more severe stages of ALD24. C/EBP alpha, beta, and delta are important transcription factors in adipose differentiation. Early expression of C/EBP delta is required for efficient induction of C/EBP alpha and PPAR gamma25. However, little is known whether C/EBPs are involved in the progression of alcoholic hepatosteatosis. In this study, we treated mice with ethanol to induce alcoholic hepatosteatosis, and we found that C/EBP delta expression was elevated in the initial phase of hepatosteatosis, suggesting that C/EBP delta plays a major role in the progression of alcoholic hepatosteatosis.
In the present study, we showed that ethanol administration induced liver injury, as evidenced by increased serum AST and ALT and by pathologic changes, which demonstrated that lipids started to accumulate in hepatocytes of mice treated with ethanol for 5 weeks. Mice treated with ethanol for 10 weeks developed more severe hepatosteatosis than those treated for 5 weeks. ALT was much lower in mice treated with EtOH+HF than in those treated with ethanol alone. We speculate that the high-fat diet may protect against ethanol-induced liver damage, possible by decreasing gastric absorption of ethanol. The more severe hepatosteatotic changes in the livers of mice treated with EtOH+HF was likely due to the combined effect of EtOH and HF, as indicated in the present study by the enhanced expression of C/EBP delta and perhaps other C/EBPs.
We showed that C/EBP delta was involved in the progression of ethanol-induced hepatic steatosis and that its expression was delta greater than that of C/EBP beta at week 5 (2.3- and 1.4-fold increases, respectively), whereas the expression of C/EBP alpha was not evident until week 10. Interestingly, hepatic expression of C/EBP beta and delta returned to the control level by week 10. These results suggest that C/EBP delta may play an important role in the early phase but not in the late phase of hepatosteatosis in this mouse model. By contrast, C/EBP alpha may participate in the late phase of hepatic steatosis in mice.
In HepG2 cells incubated with oleic acid, we observed that C/EBP delta expression was significantly increased. The data support the notion that C/EBP delta is involved in adipogenic differentiation25. The present in vitro study revealed that a low concentration (10 mmol/L) of ethanol not only increased C/EBP delta expression but also was synergistic with oleic acid in the promotion of C/EBP delta expression in HepG2 cells. Surprisingly, C/EBP alpha, beta, and delta expression in HepG2 cells were restored to a level similar to that of the control at higher concentrations of ethanol (200 and 400 mmol/L). It is possible that low and high ethanol concentrations may affect C/EBP expression via different mechanisms. The detailed mechanisms through which C/EBP delta coordinates with other C/EBPs to mediate additional adipogenic factors such as PPARs, adiponectin and SREBPs remain to be investigated.
In conclusion, we have demonstrated an increase in C/EBP delta expression at a relatively early phase of ethanol-induced hepatosteatosis in mice. In addition, our in vitro study shows that ethanol plus oleic acid increases the expression of C/EBP delta in HepG2 cells. These results suggest that the inhibition of C/EBP delta is a potential therapeutic target in alcoholic hepatosteatosis.
Author contribution
Miao-lin HU and Yu-hsuan CHEN designed the study; Yu-hsuan CHEN, Chih-min YANG and Shih-pei CHANG performed the experiment and analyzed the data; Miao-lin HU and Yu-hsuan CHEN wrote the paper.
Acknowledgments
This work was supported by grants from the Central Taiwan University of Science and Technology (CTU-96-23 to Yu-hsuan CHEN and Shih-pei CHANG).
References
- Dey A, Cederbaum AI. Alcohol and oxidative liver injury. Hepatology. 2006;43:S63–74. doi: 10.1002/hep.20957. [DOI] [PubMed] [Google Scholar]
- You M, Crabb DW. Recent advances in alcoholic liver disease II. Minireview: molecular mechanisms of alcoholic fatty liver. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1–6. doi: 10.1152/ajpgi.00056.2004. [DOI] [PubMed] [Google Scholar]
- Donohue TM., Jr Alcohol-induced steatosis in liver cells. World J Gastroenterol. 2007;13:4974–8. doi: 10.3748/wjg.v13.i37.4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers CQ, Ajmo JM, You M. Adiponectin and alcoholic fatty liver disease. IUBMB Life. 2008;60:790–7. doi: 10.1002/iub.124. [DOI] [PubMed] [Google Scholar]
- You M, Crabb DW. Molecular mechanisms of alcoholic fatty liver: role of sterol regulatory element-binding proteins. Alcohol. 2004;34:39–43. doi: 10.1016/j.alcohol.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Crabb D, Sozio MS. Alcohol and lipid metabolism. Am J Physiol Endocrinol Metab. 2008;295:E10–6. doi: 10.1152/ajpendo.00011.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinić SIc S, Mihailović M, Bogojević D, Poznanović G. Expression of C/EBP delta in rat liver during development and the acute-phase response. Gen Physiol Biophys. 2004;23:499–504. [PubMed] [Google Scholar]
- Lane MD, Tang QQ, Jiang MS. Role of the CCAAT enhancer binding proteins (C/EBPs) in adipocyte differentiation. Biochem Biophys Res Commun. 1999;266:677–83. doi: 10.1006/bbrc.1999.1885. [DOI] [PubMed] [Google Scholar]
- Lekstrom-Himes J, Xanthopoulos KG. Biological role of the CCAAT/enhancer-binding protein family of transcription factors. J Biol Chem. 1998;273:28545–8. doi: 10.1074/jbc.273.44.28545. [DOI] [PubMed] [Google Scholar]
- He L, Ronis MJ, Badger TM. Ethanol induction of class I alcohol dehydrogenase expression in the rat occurs through alterations in CCAAT/enhancer binding proteins beta and gamma. J Biol Chem. 2002;277:43572–7. doi: 10.1074/jbc.M204535200. [DOI] [PubMed] [Google Scholar]
- Rosen ED. The transcriptional basis of adipocyte development. Prostaglandins Leukot Essent Fatty Acids. 2005;73:31–4. doi: 10.1016/j.plefa.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Tang QQ, Lane MD. Activation and centromeric localization of CCAAT/enhancer-binding proteins during the mitotic clonal expansion of adipocyte differentiation. Genes Dev. 1999;13:2231–41. doi: 10.1101/gad.13.17.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrup S, Lane MD. Regulating adipogenesis. J Biol Chem. 1997;272:5367–70. doi: 10.1074/jbc.272.9.5367. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Yoshida N, Kishimoto T, Akira S. Defective adipocyte differentiation in mice lacking the C/EBPbeta and/or C/EBPdelta gene. EMBO J. 1997;16:7432–43. doi: 10.1093/emboj/16.24.7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hishida T, Nishizuka M, Osada S, Imagawa M. The role of C/EBPdelta in the early stages of adipogenesis. Biochimie. 2009;91:654–7. doi: 10.1016/j.biochi.2009.02.002. [DOI] [PubMed] [Google Scholar]
- McKnight SL. McBindall — A better name for CCAAT/enhancer binding proteins. Cell. 2001;107:259–61. doi: 10.1016/s0092-8674(01)00543-8. [DOI] [PubMed] [Google Scholar]
- Lai PH, Wang WL, Ko CY, Lee YC, Yang WM, Shen TW, et al. HDAC1/HDAC3 modulates PPARG2 transcription through the sumoylated CEBPD in hepatic lipogenesis. Biochim Biophys Acta. 2008;1783:1803–14. doi: 10.1016/j.bbamcr.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Cao Z, Umek RM, McKnight SL. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 1991;5:1538–52. doi: 10.1101/gad.5.9.1538. [DOI] [PubMed] [Google Scholar]
- Chen X, Sebastian BM, Tang H, McMullen MM, Axhemi A, Jacobsen DW, et al. Taurine supplementation prevents ethanol-induced decrease in serum adiponectin and reduces hepatic steatosis in rats. Hepatology. 2009;49:1554–62. doi: 10.1002/hep.22811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Kunos G, Gao B. Ethanol rapidly inhibits IL-6-activated STAT3 and C/EBP mRNA expression in freshly isolated rat hepatocytes. FEBS Lett. 1999;457:162–8. doi: 10.1016/s0014-5793(99)01031-5. [DOI] [PubMed] [Google Scholar]
- Crabb DW. Alcohol deranges hepatic lipid metabolism via altered transcriptional regulation. Trans Am Clin Climatol Assoc. 2004;115:273–87. [PMC free article] [PubMed] [Google Scholar]
- Mitra SK, Varma SR, Codavarthi A, Nandakumar KS. Liv.52 regulates ethanol induced PPARc and TNF alpha expression in HepG2 cells. Mol Cell Biochem. 2008;315:9–15. doi: 10.1007/s11010-008-9782-9. [DOI] [PubMed] [Google Scholar]
- De Gottardi A, Vinciguerra M, Sgroi A, Moukil M, Ravier-Dall'Antonia F, Pazienza V, et al. Microarray analyses and molecular profiling of steatosis induction in immortalized human hepatocytes. Lab Invest. 2007;87:792–806. doi: 10.1038/labinvest.3700590. [DOI] [PubMed] [Google Scholar]
- Day CP, James OF. Hepatic steatosis: innocent bystander or guilty party. Hepatology. 1998;27:1463–6. doi: 10.1002/hep.510270601. [DOI] [PubMed] [Google Scholar]
- Pantoja C, Huff JT, Yamamoto KR. Glucocorticoid signaling defines a novel commitment state during adipogenesis in vitro. Mol Biol Cell. 2008;19:4032–41. doi: 10.1091/mbc.E08-04-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]