Abstract
Aim:
Spatial dispersion of bioactive substances in the myocardium could serve as pathological basis for arrhythmogenesis and cardiac impairment by β-adrenoceptor stimulation. We hypothesized that dispersed NADPH oxidase, protein kinase Cε (PKCε), early response gene (ERG), and matrix metalloproteinase 9(MMP-9) across the heart by isoproterenol (ISO) medication might be mediated by the endothelin (ET) – ROS pathway. We aimed to verify if ISO induced spatially heterogeneous distribution of pPKCε, NAPDH oxidase, MMP-9 and ERG could be mitigated by either an ET receptor antagonist CPU0213 or iNOS inhibitor aminoguanidine.
Methods:
Rats were treated with ISO (1 mg/kg sc) for 10 days, and drug interventions (mg/kg) either CPU0213 (30 sc) or aminoguanidine (100 ip) were administered on days 8–10. Expression of NADPH oxidase, MMP-9, ERG, and PKCε in the left and right ventricle (LV, RV) and septum (S) were measured separately.
Results:
Ventricular hypertrophy was found in the LV, S, and RV, in association with dispersed QTc and oxidative stress in ISO-treated rats. mRNA and protein expression of MMP-9, PKCε, NADPH oxidase and ERG in the LV, S, and RV were obviously dispersed, with augmented expression mainly in the LV and S. Dispersed parameters were re-harmonized by either CPU0213, or aminoguanidine.
Conclusion:
We found at the first time that ISO-induced dispersed distribution of pPKCε, NADPH oxidase, MMP-9, and ERG in the LV, S, and RV of the heart, which were suppressed by either CPU0213 or aminoguanidine. It indicates that the ET-ROS pathway plays a role in the dispersed distribution of bioactive substances following sustained β-receptor stimulation.
Keywords: endothelin receptor antagonists, isoproterenol, PKCε, NADPH oxidase, MMP-9, aminoguanidine
Introduction
Many causal factors participate in deteriorating cardiac arrhythmias and cardiac performance, however, what is the proximate precipitator to transform electrophysiologically stable state into one that fibrillated or exacerbating heart failure. Stress has been importantly involved in events causing deterioration of malignant arrhythmias1 and cardiac insufficiency2 resulting from changes in molecular aspects attributed to worsened ischemic myocardium and sympathetic stimulation.
To date, a reduction in risk of sudden cardiac death (SCD) in clinical trials has been found by non-channel drugs such as angiotensin converting enzyme inhibitors4, statins5, and β-blockers6, by relieving the upstream lesion in the myocardium, rather than classic antiarrhythmic agents which possess a direct suppression on ion channel per se1, 7. Thus an attenuation of the consequence to β-adrenoceptor activation is crucial in preventing malignant arrhythmias and relieving cardiac performance as well.
Oxidative stress has been reevaluated as an important etiological event implicated in life threatening arrhythmias8, 9. The genesis of reactive oxygen species (ROS) in the myocardium derives mainly from nicotinamide adenine dinucleotide phosphate-oxidase (NADPH oxidase) and an increased expression of the p47phox and p22phox subunits of NADPH oxidase is significant in the failing hearts and these changes were attenuated by NOX inhibitor apocynin10. H2O2-induces EAD (early after depolarization) in the cardiomyocytes, which is frequently followed by delayed after depolarization (DAD) in response to spontaneous sarcoplasmic reticulum Ca2+ release after repolaryzation8. Abnormal repolaryzation can be caused by excess of ROS as the consequence to β-adrenoceptor stimulation which elicits cardiac oxidative stress9. PKCε (protein kinase Cε) is activated by ROS, associating with phosphorylated Ras/Raf and activated ERK1/2 (the extracellular signal regulated protein kinases) signaling pathway, resulting in myocardial abnormality 11.
An activation of matrix metalloproteinase 9(MMP-9 ), contributing partly to cardiac hypertrophy, requires cAMP/PKA-dependent pathway (the adenosine 3′,5′-cyclic monophosphate-dependent protein kinase A pathway)12, 13 and chronic stimulation of the heart by isoproterenol (ISO) causes excess of ROS generation responsible for cardiac remodeling9. ISO, as we have previously reported, stimulates not only the cAMP-PKA pathway14, but also pPKCε (hyperphosphorylation of PKCε)15 which participates in cardiac remodeling, oxidative stress and arrhythmogenesis16. Furthermore, expression of ERG, which is the α-subunit of KCNH2, is considered as an important target for antiarrhythmic activity17. The relation of change in ERG expression to β-adrenoceptor stimulation and the ET system is interesting, and however, has not been clarified yet18.
Endothelin-1 (ET-1) is a powerful vasoconstrictor, proliferator and cytokine, which promotes cardiac remodeling and oxidative stress19. An endothelin receptor antagonist CPU0213 has been developed possessing a potent blockade on the ETA receptor but a relative low blocking effect on ETB receptor20 and is effective in suppressing both pulmonary artery hypertension21 and ventricular fibrillation18. The ET-ROS (endothelin-reactive oxygen species) pathway influences transcription and expression of genes through the MAPK (mitogen-activated protein kinase) pathway in heart failure and arrhythmia22, 23.
We hypothesized that ISO adversely affected the heart to form spatially dispersed expression of NADPH oxidase, MMP-9, ERG (ether-a-go-go–related gene) and PKCε in different regions of the heart, which were likely mediated by an activated ET-ROS pathway. Thus, the aim of the study was to verify if ISO-induced changes in the heart could be mitigated by an endothelin receptor antagonist CPU0213, compared to that of the antioxidant activity of aminoguanidine.
Materials and methods
Animals
Adult male Sprague-Dawley rats (weighing 220−260 g , from the Experimental Animal Center of Nanjing Medical University) were used. They were housed in a controlled environment and allowed free access to tap water and food. Both animal handling and experimental procedures were in accordance with the animal regulations of the Jiangsu Provincial Government, China.
Experimental protocol
Rats were randomly divided into 4 groups. Rats were injected with ISO 1 mg·kg-1·d-1 sc for 10 d, divided into the untreated (ISO group), treated with CPU0213 30 mg/kg sc (ISO+CPU0213 group) or aminoguanidine 100 mg/kg ip (ISO+AMI group) during the last 3 days. CPU0213 and aminoguanidine were suspended freshly in 0.5% CMC-Na (Sodium carboxymethyl cellulose). The control and untreated ISO groups received an equal volume of CMC-Na.
QTcd and cardiac weight index
On day 11, rats were anesthetized with urethane (1.5 g/kg, ip) and ECG (electrocardiogram) recording (MPA-2000) was performed with a classic 12 lead protocol and QT intervals were measured. The QTc was calculated with Bazett's formula [QTc=QT/(RR)1/2 ms] and QTcd (QTc dispersion ) was defined as the difference between the maximum and minimum of QTc interval in the 12-lead ECG in one rat. The average value of QTc derived from 3 successive beats for each lead was used for analysis.
After ECG measurement, rats were exsanguinated and hearts were harvested and dissected into the left ventricle (LV), septum (S) and right ventricle (RV). The cardiac weight index was separately assessed in the 3 regions of the heart against body weight: the LV weight index (LW/BW), septum weight index (S/BW) and RV weight index (RW/BW). The whole heart weight index was also evaluated by the ratio of the whole heart weight over body weight (HW/BW).
Biochemical assays
Blood samples were collected and centrifuged (1500×g, 4 ºC) for 10 min. Then malondialdehyde (MDA) and glutathione peroxidase (GSH-Px) in serum were measured according to reagent kit instructions (Nanjing Jingcheng Bioengineer Institute, China) as described previously24.
RT-PCR
Total RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. A fraction, 5 mg of RNA, was used to synthesize the first strand of cDNA using SUPERSCRIPT II RNase H-Reverse Transcriptase (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol, and was used as a template in the following PCR reactions24.
mRNA expression of ERG, MMP-9, NADPH p22phox, NADPH p47phox, PKCε and 18S was carried out using sense primers and antisense primers. Sense: 5′-TCTCCATCTGTGCATGCTC-3′ and antisense: 5′-ACCAAGCATGCTGGAAGTAC-3′ for ERG; sense: 5′-CGTGGCCTAGTGACCTATG-3′ and antisense: 5′-GGATAGCTCGGTGGTGTCCT-3′ for MMP-9; sense, 5′-CCCAGCGACAGATTAGAAGC-3′, and antisense, 5′-TGGATTGTCCTTTGAGTCAGG-3′ for p47phox; sense, 5′-GCGGTGTGGACAGAAGTACC-3′, and antisense, 5′-CTTGGGTTTAGGCTCAATGG-3′ for p22phox; sense, 5′-GATGAGCCTCGTTCTCGGTTCTA-3′, antisense, 5′-AGGAGTCCCACAGAAGGTGGTA-3′ for PKCε sense: 5′-GCTGCTGGCACCAGACTT-3′ and antisense: 5′-CGGCTACCACATCCAAGG-3′ for 18S. Products were resolved on 2% agarose gel followed by ethidium bromide staining.
Western blotting
For the quantitative analysis of protein levels of MMP-9, NADPH p67phox and pPKCε in the LV, septum and RV of the heart, an amount (100 mg) of each was homogenized in 4 volumes of extraction buffer and centrifuged at 10 000×g for 10 min. After determination of the protein concentration, the supernatants were stored at -20 °C until use. An aliquot was heated to 98 °C, and size fractionated on 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE). The extracted protein was transferred to a nitrocellulose membrane and blocked with nonfat milk (5% w/v), followed by incubation with first antibody (for MMP-9 from Boster Biological Technology Ltd, Wuhan, China; for NADPH p67phox, pPKCε and β-actin from Upstate Biotechnology, New York, USA) for another 1 h. After 3 washes, the blot was incubated with horseradish peroxidase conjugated goat secondary antibody IgG (Affinity Bioreagents; 1:1000) for an additional 1 h. Antigen was detected with a DAB kit. A linear relationship between the density of blots and the protein load was observed when 20, 40, 60, 80, and 100 μg of membrane protein were used per lane24.
Statistical analysis
SPSS 11.5 (USA) was used to analyze the results. Data were presented as mean±SD. For statistical evaluation one-way analysis of variance was used, following Dunnett's test. The Student Newman Keuls test was performed when the variance was equal, and the Games-Howell test was performed when variance was not equal. A probability value P<0.05 was considered statistically significant.
Results
Myocardial hypertrophy and QTcd
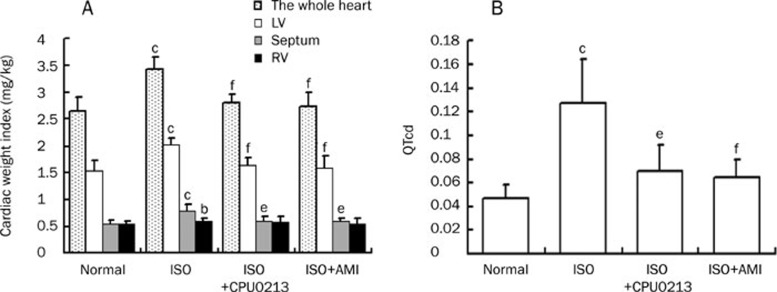
Ten days after ISO administration cardiac hypertrophy was revealed separately by assessing cardiac weight index of the whole heart, LV, septum and RV, relative to body weight. Compared with the normal group, the whole heart weight index of ISO-treated group was increased by 29.3% (P<0.01), and the LV by 31.7% (P<0.01), the septum by 43.0% (P<0.01), and the RV 7.9 % (P<0.05). The drug intervention started on the 8th day when the ventricular hypertrophy has been developed and following three day of drug intervention cardiac hypertrophy was reduced obviously by either CPU0213 or aminoguanidine (P<0.05, P<0.01), with the exception of the RV weight index (Figure 1A).
Figure 1.
Changes in cardiac weight index (the right, septum and left ventricle) and QTcd were found by isoproterenol (ISO) medication for 10 days in rats. These were reversed by 3 d intervention with either an endothelin receptor antagonist CPU0213 or aminoguanidine (AMI). (A) Heart weight index; (B) Dispersion of QTc (QTcd). n=6−10. Mean±SD. bP<0.05, cP<0.01 vs normal; eP<0.05,fP<0.01 vs ISO.
The QTcd was measured as a supplement to cardiac remodeling roughly reflecting changes in bioactive molecules across the heart, which may affect the stability of electrophysiology. An increase in QTcd was found in the ISO-treated group by 170.2% (P<0.01) as compared with the baseline. Medication of CPU0213 and aminoguanidine for three days sufficiently reduced the changes significantly by -44.9% and -48.8% (P<0.01), respectively, relative to the ISO untreated group (Figure 1B).
Oxidative stress and NADPH oxidase
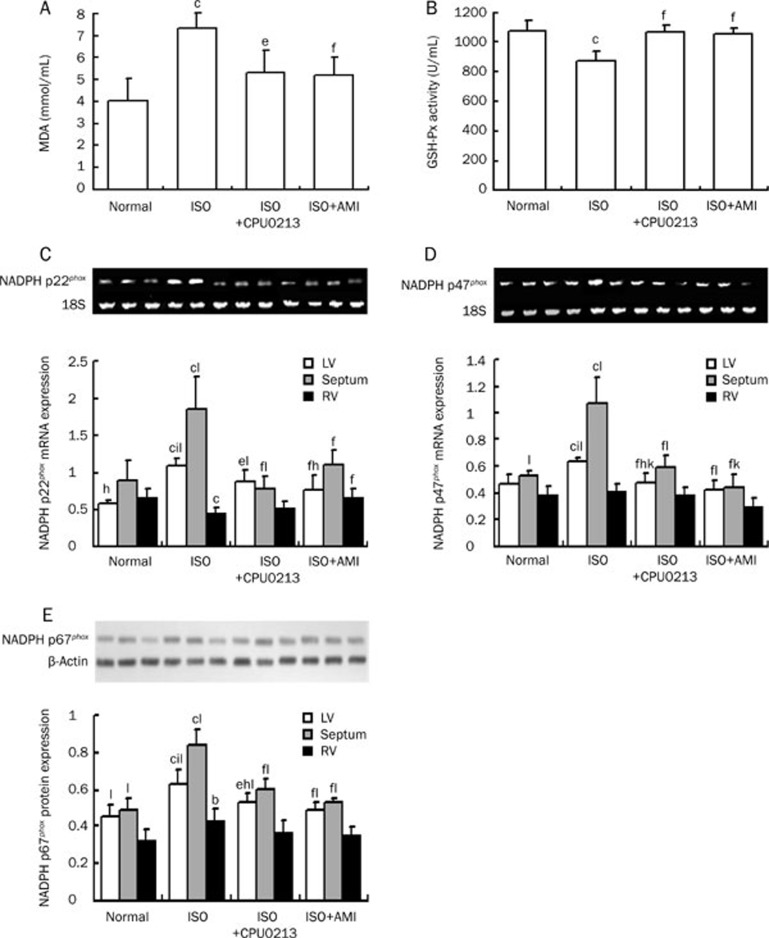
MDA in serum of the ISO-treated group was increased by 80.4 % (P<0.01) relative to control, in consistent with a reduction of serum GSH-Px levels by 18.9 % (P<0.01) (Figure 2A, 2B). Thus, there was a status of oxidative stress established by ISO medication, associating with abnormal distribution of mRNA of the three subtypes of NADPH oxidase of p22phox and p47phox and p67phox protein in regions of the heart. There was a mild difference in expression of mRNA of p22phox and p47phox and protein of p67phox among the three regions in normal rat heart (Figure 2C–2E). Over-expression of gene and over-presentation of protein of NADPH oxidase subtypes were in agreement with a status of oxidative stress. However, differences in mRNA abundance of p22phox and p47phox, and protein of p67phox among the LV, S, and RV were increased obviously in the ISO-treated rat heart (P<0.01), with more prominent in the LV and septum, leading to different pattern of expression of subtypes of NADPH oxidase in the ISO-treated group. Difference among the three regions of the heart was relieved significantly by 3 days of interventions with either CPU0213 or aminoguanidine (Figure 2).
Figure 2.
Changes in MDA, GSH-Px, and dispersed expression of p22phox, p47phox mRNA, and p67phox protein in the LV, septum, and RV were found by ISO (isoproterenol) medication in rats. These were mitigated by CPU0213 or aminoguanidine (AMI). (A) MDA; (B) GSH-Px; (C) p22phox mRNA; (D) p47phox mRNA; (E) p67phox protein. n=6. Mean±SD. bP<0.05, cP<0.01 vs normal; eP<0.05, fP<0.01 vs ISO. hP<0.05, iP<0.01 vs septum; kP<0.05, lP<0.01 vs RV.
Dispersed ERG mRNA
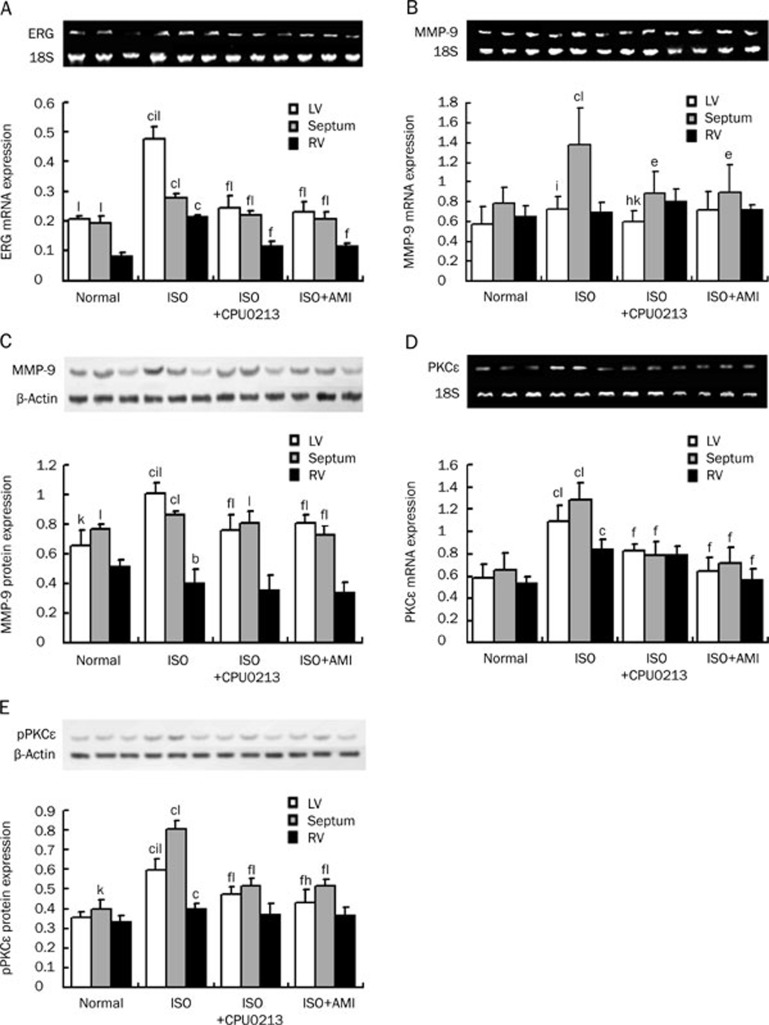
The ERG gene encodes the α-subunit of KCNH2 (IKr channel) and remains a principal target for investigation of arrhythmogenetic property and antiarrhythmic activity of investigated drugs. Thus, it was of interest to reveal whether ERG mRNA expression varied among the different regions in rat hearts following sustained activation of β-adrenoceptors with ISO. In the normal heart, a mild increased expression of ERG was found in the LV and septum, relative to the RV (P<0.01, Figure 3A). Following stimulation with ISO, mRNA expression in the three regions was augmented, with the LV free wall being the most predominant. A recovery of these changes was conspicuous following 3 d of medication of either CPU0213 or aminoguanidine (Figure 3A).
Figure 3.
Dispersed expression and distribution of ERG mRNA, MMP-9 mRNA and protein, and PKCε mRNA and pPKCε protein among the LV, septum, and RV regions were found in ISO medicated rats. These were mitigated by CPU0213 or aminoguanidine (AMI). (A) ERG mRNA; (B) MMP-9 mRNA; (C) MMP-9 protein; (D) PKCε mRNA; (E) pPKCε protein. n=6. Mean±SD. bP<0.05, cP<0.01 vs normal; eP<0.05, fP<0.01 vs ISO. hP<0.05, iP<0.01 vs septum; kP<0.05, lP<0.01 vs RV.
Dispersed MMP-9
Cardiac hypertrophy relates to an increase in extracellular matrix which is governed by the activity of MMPs. Thus, it was of interest to reveal effects of ISO to expand the extracellular matrix consequent to changes in expression of collagenase (MMPs). We measured expression of mRNA of MMP-9 (collagenase IV) to find that its expression in the 3 regions of the rat heart was altered in association with changes in cardiac weight index by ISO. ISO upregulated the expression of MMP-9 gene in the LV and septum (P<0.01), relative to control, but no change in the RV (Figure 3B). A mild increment in protein of MMP-9 in the LV and septum, relative to the RV, was found in the normal heart (Figure 3C) and the magnitude of difference in the ISO treated rats was expanded markedly relative to the RV. CPU0213 and aminoguanidine reduced the upregulation of MMP-9 protein in the LV and septum (P<0.01), as compared with the ISO untreated, but no change in the RV.
Dispersed PKCε
Under physiological conditions either PKCε mRNA or pPKCε protein was differently expressed in the three regions of the heart (Figure 3D, 3E). ISO treatment upregulated the presentation of PKCε mRNA and pPKCε protein obviously in all 3 regions (P<0.01), relative to control and greater upregulation in the LV free wall and septum was noted as compared to the RV (P<0.01). After treatment with CPU0213 and aminoguanidine, the dispersion of mRNA and protein of PKCε in the heart was greatly reduced, but a mild upregulation of pPKCε in the LV and septum remained relative to the RV (Figure 3D, 3E).
Discussion
In the present study we reproduced cardiac hypertrophy and dispersed QTc by ISO medication for 10 days in rats. These changes may indicate that vulnerable substrate of both arrhythmogenesis and impairing cardiac function is formed following sustained β-adrenoceptor stimulation in rats, which may predispose the affected heart to exacerbated VF (ventricular fibrillation) incidence during ischemia/reperfusion episode24. An investigation of biological basis for vulnerability of the heart under β-adrenoceptor stimulation may provide more insights into the molecular mechanisms underlying arrhythmogenesis and exacerbation of cardiac function. Interestingly, with intervention of CPU0213 and aminoguanidine of 3 days, these changes were significantly subsided.
An increase in QTcd is rough evidence indicating an increased heterogeneity of biological substances relating to repolaryzation in the heart, with an increased QTcd the affected heart manifests cardiac electrophysiological instability and an increased risk of life threatening cardiac arrhythmias25. To date, spatial dispersion of repolarization has been considered as an amplification of transmural dispersion of repolarization (TDR) which facilitates commencement of life-threatening ventricular arrhythmias. TDR always associates with known inherited ion channelopathies, those including the LQTS, SQTS, and Brugada syndromes as well as CPVT (catecholaminergic polymorphic ventricular tachycardia)26. Spatial dispersion between the two ventricles reflects heterogeneity of pathological changes in an affected heart leading to appearance of malignant arrhythmias1. Dispersion of repolarization reveals a status of non-homogeneity in the heart, where two sorts of dispersion can be found: special difference in repolarization in the regions either among LV, S, and RV, or between the two chambers1, 27 , or from the apex to the base, and transmural dispersion among the three layers of the LV free wall28. Given the dispersed QTc, what happens to the entity of arrhythmogenetic substrate has not yet been clarified. In this respect oxidative stress and channel abnormality are likely to be constituted in the vulnerable myocardium29.
Spatial dispersion of some bioactive molecules has been found in infarcted rat heart plus ISO medication in our previous report1, manifesting a heterogeneous expression of RyR2 (ryanodine receptor 2), SERCA2a (sarcoplasmic reticulum Ca2+ ATPase) and NCX (Na+/Ca2+ exchanger) between the LV and RV. Along with suppressing VF by propranolol or CPU86017, an antiarrhythmic agent derived from berberine30, dispersed expression of RyR2, SERCA2a, and NCX was completely reduced; indicating that the dispersed calcium handling elements are critically linked with exacerbated VF incidence. In incubated cardiomyocytes, ISO initiates downregulation of FKBP12.6 (calstabin2) in vitro14 that causes the affected heart to be vulnerable to arrhythmogenesis and compromised cardiac performance31 due to calcium leak32. In the present study, ISO injection elicits upregulation of NADPH oxidase subtypes in the myocardium, which presents heterogeneous changes among the LV, septum and RV providing substantial evidence relevant to arrhythmogenesis and cardiac insufficiency. Indeed, abnormal NADPH oxidase may associate with altered calcium handling proteins in the heart treated with ISO, of which cardiomyocytes present calcium leak at diastole15.
KCNH2 is an important target in arrhythmogenesis and antiarrhythmic activity33. An increased current in KCNH2 has been found in SQTS resulting from gain of function mutation, manifesting an increased risk for SCD34. Following ISO medication, upregulation of ERG was found in the LV and S, which are coincided with the behavior of p22phox, p47phox, and p67phox in these regions. It indicates that oxidative reaction may be responsible for such changes of ERG. An upregulation of ERG has been found previously in L-thyroxin induced cardiomyopathy in rats18, 35, in which an exaggerated incidence of VF was associated. Interestingly upregulated ERG is sensitive to CPU0213 and aminoguanidine supporting that the ET-ROS pathway is implicated in ISO induced changes of the IKr channel. The effects regulates the expression of ERG is likely instituted in the mechanisms of its antiarrhythmic activity of endothelin receptor antagonist CPU021318, 35.
Given accumulated data that oxidative lesion is a primary causal factor in inducing arrhythmias36, our data further suggest that dispersed upregulation of expression of NADPH oxidase may intimately participate in arrhythmogenesis and compromised cardiac performance as well37. Thus, heterogeneity in expression of NADPH oxidase between the LV, septum and RV confers a basic entity of vulnerable myocardium produced by sustained β-adrenergic receptor activation. Participation of ROS in arrhythmogenesis may be multifaceted, as known recently, ROS modulates the L-type channel, implicated in cardiac arrhythmias38.
An increase in extracellular matrix by an activated MMP-9 is a causal factor to impair both impulse conduction and cardiac performance. Thus, MMP-9 has been targeted for a relief of cardiac failure39. More significant changes in MMPs are in the LV and S, in agreement with dispersed increase in cardiac weight index, pPKCε and ERG in the two regions, relative to the RV, accounting for basic pathologies of instability of electrophysiology and compromised cardiac pumping function following sustained β-adrenoceptor stimulation.
There are several pathways of ROS affecting transcription in the nucleus, those including PKC, MAPK, and calcineurin40. The activity of the redox system is mediated by MAPK which closely links with ET-141. Upregulation of mRNA of PKCε and pPKCε protein in response to β-stimulation has been previously found15 and is also confirmed in the present study. We speculate that hyperphosphorylation of PKCε is actively implicated in the vulnerable substrate for arrhythmogenesis and impaired cardiac function and its heterogeneous expression reflects trans-signalling pathway not in harmony across the heart.
We have previously suggested that H2O2 stimulates isolated cardiomyocytes to hyperphosphorylate PKCε that is completely reversed by CPU021315. Furthermore, upregulated ETAR, vice versa, can be dramatically reversed by a classic antioxidant, tocopherol. Indeed, ISO-induced hyperphosphorylation of PKCε in cardiomyocytes is completely reversed by either CPU0213 or tocopherol. Our findings in the present study are in line with those mentioned above; we have previously proved further that the presentation of pPKCε in intact rats was heterogeneous across the two ventricles by ISO medication, and these changes were effectively reversed by either CPU0213 or aminoguanidine, an inhibitor of iNOS, attributed to their potent anti-oxidative activity.
It was interesting to find that upregulation of expression of the related genes in the presence of ISO stimulation is mainly located in the LV free wall and septum, indicating that changes in biological substances in these regions are critical in terms of arrhythmogenesis and impaired cardiac performance.
In conclusion, we afforded evidence of different presentation of pPKCε in the LV, septum, and RV following ISO medication, associating with dispersed expression of NADPH oxidase, ERG, and MMP-9. These changes may be related to arrhythmogenesis and impaired cardiac performance under β-receptor stimulation and are, at least in part, mediated by an activation of the ET-ROS pathway, thus, benefits from anti-oxidant activity of an endothelin receptor antagonist and aminoguanidine are obvious.
Author contribution
Yu-si CHENG performed research project, collected and analysed data, and wrote the paper. Dei-zai DAI and Yin DAI designed research project by setting up hypothesis, raising suitable targets for investigation, correcting the manuscript writing and responding queries to referees and editors.
Acknowledgments
We are grateful for the grant by National Natural Foundation of China No 30670760 and the Major State Basic Research Development Program of the People's Republic of China (No 2006CB503807).
We also appreciate Prof David Triggle from The State University of New York for his kind assistance in styling the English writing of the MS.
References
- Wang HL, Dai DZ, Gao E, Zhang YP, Lu F. Dispersion of ventricular mRNA of RyR2 and SERCA2 associated with arrhythmogenesis in rats. Acta Pharmacol Sin. 2004;25:738–43. [PubMed] [Google Scholar]
- Cheng YS, Dai DZ, Dai Y. Stress-induced cardiac insufficiency relating to abnormal leptin and FKBP12.6 is ameliorated by CPU0213, an endothelin receptor antagonist, which is not affected by the CYP3A suppressing effect of erythromycin. J Pharm Pharmacol. 2009;61:569–76. doi: 10.1211/jpp/61.05.0004. [DOI] [PubMed] [Google Scholar]
- Kassotis J, Mongwa M, Reddy CV. Effects of angiotensin-converting enzyme inhibitor therapy on QT dispersion post acute myocardial infarction. Pacing Clin Electrophysiol. 2003;26:843–8. doi: 10.1046/j.1460-9592.2003.t01-1-00148.x. [DOI] [PubMed] [Google Scholar]
- Levantesi G, Scarano M, Marfisi R, Borrelli G, Rutjes AW, Silletta MG, et al. Meta-analysis of effect of statin treatment on risk of sudden death. Am J Cardiol. 2007;100:1644–50. doi: 10.1016/j.amjcard.2007.07.015. [DOI] [PubMed] [Google Scholar]
- Nessler J, Nessler B, Kitliński M, Libionka A, Kubinyi A, Konduracka E, et al. Sudden cardiac death risk factors in patients with heart failure treated with carvedilol. Kardiol Pol. 2007;65:1417–22. [PubMed] [Google Scholar]
- Tomaselli GF, Zipes DP. What causes sudden death in heart failure. Circ Res. 2004;95:754–63. doi: 10.1161/01.RES.0000145047.14691.db. [DOI] [PubMed] [Google Scholar]
- Goette A, Bukowska A, Lendeckel U. Non-ion channel blockers as anti-arrhythmic drugs (reversal of structural remodeling) Curr Opin Pharmacol. 2007;7:219–24. doi: 10.1016/j.coph.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Xie LH, Chen F, Karagueuzian HS, Weiss JN. Oxidative-stress-induced afterdepolarizations and calmodulin kinase II signaling. Circ Res. 2009;104:79–86. doi: 10.1161/CIRCRESAHA.108.183475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang GX, Kimura S, Nishiyama A, Shokoji T, Rahman M, Yao L, et al. Cardiac oxidative stress in acute and chronic isoproterenol-infused rats. Cardiovasc Res. 2005;65:230–8. doi: 10.1016/j.cardiores.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Zhang P, Hou M, Li Y, Xu X, Barsoum M, Chen Y, et al. NADPH oxidase contributes to coronary endothelial dysfunction in the failing heart. Am J Physiol Heart Circ Physiol. 2009;296:H840–6. doi: 10.1152/ajpheart.00519.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter JC, Korzick DH. Age- and sex-dependent alterations in protein kinase C (PKC) and extracellular regulated kinase 1/2 (ERK1/2) in rat myocardium. Mech Ageing Dev. 2005;126:535–50. doi: 10.1016/j.mad.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Zhao P, Li XG, Yang M, Shao Q, Wang D, Liu S, et al. Hypoxia suppresses the production of MMP-9 by human monocyte-derived dendritic cells and requires activation of adenosine receptor A2b via cAMP/PKA signaling pathway. Mol Immunol. 2008;45:2187–95. doi: 10.1016/j.molimm.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Xia QG, Na T, Guo YM, Bi YT, Zhang HY, Dai DZ. Improvement of chronic heart failure by dexamethasone is not associated with downregulation of leptin in rats. Acta Pharmacol Sin. 2007;28:202–10. doi: 10.1111/j.1745-7254.2007.00503.x. [DOI] [PubMed] [Google Scholar]
- Feng Y, Tang XY, Dai DZ, Dai Y. Reversal of isoproterenol-induced downregulation of phospholamban and FKBP12.6 by CPU0213-mediated antagonism of endothelin receptors. Acta Pharmacol Sin. 2007;28:1746–54. doi: 10.1111/j.1745-7254.2007.00650.x. [DOI] [PubMed] [Google Scholar]
- Li N, Jia N, Dai NZ, Dai Y. Endothelin receptor antagonist CPU0213 and vitamin E reverse downregulation of FKBP12.6 and SERCA2a: A role of hyperphosphorylation of PKCepsilon. Eur J Pharmacol. 2008;591:211–8. doi: 10.1016/j.ejphar.2008.06.080. [DOI] [PubMed] [Google Scholar]
- Iwasaki M, Hayashi Y, Kamibayashi T, Yamatodani A, Mashimo T. The antiarrhythmic effect of centrally administered rilmenidine involves muscarinic receptors, protein kinase C and mitochondrial signalling pathways. Br J Pharmacol. 2008;153:1623–30. doi: 10.1038/bjp.2008.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehn J, Thomas D, Karle CA, Schöls W, Kübler W. Inhibitory effects of the class III antiarrhythmic drug amiodarone on cloned HERG potassium channels. N-S Arch Pharmacol. 1999;359:212–9. doi: 10.1007/pl00005344. [DOI] [PubMed] [Google Scholar]
- Feng Y, Dai DZ, Na T, Cui B, Wang T, Zhang Y, et al. Endothelin receptor antagonist CPU0213 suppresses ventricular fibrillation in L-thyroxin induced cardiomyopathy. Pharmacol Rep. 2007;59:306–14. [PubMed] [Google Scholar]
- Chiu J, Xu BY, Chen S, Feng B, Chakrabarti S. Oxidative stress-induced, poly(ADP-ribose) polymerase-dependent upregulation of ET-1 expression in chronic diabetic complications. Can J Physiol Pharmacol. 2008;86:365–72. doi: 10.1139/Y08-033. [DOI] [PubMed] [Google Scholar]
- Dai DZ, Huang M, Ji M, Liu LG. Endothelin receptor antagonist activity and selective blocking the ETA and ETB of compound 0213. J China Pharm Univ. 2004;35:552–7. [Google Scholar]
- Cui B, Cheng YS, Dai DZ, Li N, Zhang TT, Dai Y. CPU0213, a non-selective ETA/ETB receptor antagonist, improves pulmonary arteriolar remodeling of monocrotaline-induced pulmonary hypertension in rats. Clin Exp Pharmacol Physiol. 2009;36:169–75. doi: 10.1111/j.1440-1681.2008.05044.x. [DOI] [PubMed] [Google Scholar]
- Kyoi S, Otani H, Matsuhisa S, Akita Y, Enoki C, Tatsumi K, et al. Role of oxidative/nitrosative stress in the tolerance to ischemia/reperfusion injury in cardiomyopathic hamster heart. Antioxid Redox Signal. 2006;8:1351–61. doi: 10.1089/ars.2006.8.1351. [DOI] [PubMed] [Google Scholar]
- Xia HJ, Dai DZ, Dai Y. Up-regulated inflammatory factors endothelin, NFkappaB, TNFalpha and iNOS involved in exaggerated cardiac arrhythmias in L-thyroxine-induced cardiomyopathy are suppressed by darusentan in rats. Life Sci. 2006;79:1812–9. doi: 10.1016/j.lfs.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Na T, Huang ZJ, Dai DZ, Zhang Y, Dai Y. Abrupt changes in FKBP12.6 and SERCA2a expression contribute to sudden occurrence of ventricular fibrillation on reperfusion and are prevented by CPU86017. Acta Pharmacol Sin. 2007;28:773–82. doi: 10.1111/j.1745-7254.2007.00580.x. [DOI] [PubMed] [Google Scholar]
- Antzelevitch C. Role of spatial dispersion of repolarization in inherited and acquired sudden cardiac death syndromes. Am J Physiol Heart Circ Physiol. 2007;293:H2024–38. doi: 10.1152/ajpheart.00355.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antzelevitch C. Heterogeneity and cardiac arrhythmias: an overview. Heart Rhythm. 2007;4:964–72. doi: 10.1016/j.hrthm.2007.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenmakers M, Ramakers C, van Opstal JM, Leunissen JD, Londoño C, Vos MA. Asynchronous development of electrical remodeling and cardiac hypertrophy in the complete AV block dog. Cardiovasc Res. 2003;59:351–9. doi: 10.1016/s0008-6363(03)00430-9. [DOI] [PubMed] [Google Scholar]
- Opthof T. In vivo dispersion in repolarization and arrhythmias in the human heart. Am J Physiol Heart Circ Physiol. 2006;290:H77–8. doi: 10.1152/ajpheart.00954.2005. [DOI] [PubMed] [Google Scholar]
- Dai DZ. Two patterns of ion channelopathy in the myocardium: perspectives for development of anti-arrhythmic agents. Curr Opin Invest Drugs. 2005;6:289–97. [PubMed] [Google Scholar]
- Dai DZ. CPU86017: a novel class III antiarrhythmic agent with multiple actions at ion channels. Cardiovasc Drug Rev. 2006;24:101–15. doi: 10.1111/j.1527-3466.2006.00101.x. [DOI] [PubMed] [Google Scholar]
- Chelu MG, Wehrens XH. Sarcoplasmic reticulum calcium leak and cardiac arrhythmias. Biochem Soc Trans. 2007;35:952–6. doi: 10.1042/BST0350952. [DOI] [PubMed] [Google Scholar]
- Huang F, Shan J, Reiken S, Wehrens XH, Marks AR. Analysis of calstabin2 (FKBP12.6)-ryanodine receptor interactions: rescue of heart failure by calstabin2 in mice. Proc Natl Acad Sci USA. 2006;103:3456–61. doi: 10.1073/pnas.0511282103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamargo J, Caballero R, Gómez R, Valenzuela C, Delpón E. Pharmacology of cardiac potassium channels. Cardiovasc Res. 2004;62:9–33. doi: 10.1016/j.cardiores.2003.12.026. [DOI] [PubMed] [Google Scholar]
- Cerrone M, Noujaim S, Jalife J. The short QT syndrome as a paradigm to understand the role of potassium channels in ventricular fibrillation. J Intern Med. 2006;259:24–38. doi: 10.1111/j.1365-2796.2005.01582.x. [DOI] [PubMed] [Google Scholar]
- Du RH, Yi HW, Dai DZ, Tang WH, Dai Y. Inflammatory factors that contribute to upregulation of ERG and cardiac arrhythmias are suppressed by CPU86017, a class III antiarrhythmic agent. J Pharm Pharmacol. 2008;60:1089–95. doi: 10.1211/jpp.60.8.0015. [DOI] [PubMed] [Google Scholar]
- Korantzopoulos P, Kolettis TM, Galaris D, Goudevenos JA. The role of oxidative stress in the pathogenesis and perpetuation of atrial fibrillation. Int J Cardiol. 2007;115:135–43. doi: 10.1016/j.ijcard.2006.04.026. [DOI] [PubMed] [Google Scholar]
- Amir O, Paz H, Rogowski O, Barshai M, Sagiv M, Shnizer S, et al. Serum oxidative stress level correlates with clinical parameters in chronic systolic heart failure patients. Clin Cardiol. 2009;32:199–203. doi: 10.1002/clc.20317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hool LC. Evidence for the regulation of L-type Ca2+ channels in the heart by reactive oxygen species: mechanism for mediating pathology. Clin Exp Pharmacol Physiol. 2008;35:229–34. doi: 10.1111/j.1440-1681.2007.04727.x. [DOI] [PubMed] [Google Scholar]
- Moshal KS, Rodriguez WE, Sen U, Tyagi SC. Targeted deletion of MMP-9 attenuates myocardial contractile dysfunction in heart failure. Physiol Res. 2008;57:379–84. doi: 10.33549/physiolres.931221. [DOI] [PubMed] [Google Scholar]
- Adiga IK, Nair RR. Multiple signaling pathways coordinately mediate reactive oxygen species dependent cardiomyocyte hypertrophy. Cell Biochem Funct. 2008;26:346–51. doi: 10.1002/cbf.1449. [DOI] [PubMed] [Google Scholar]
- Cheng TH, Shih NL, Chen CH, Lin H, Liu JC, Chao HH, et al. Role of mitogen-activated protein kinase pathway in reactive oxygen species-mediated endothelin-1-induced beta-myosin heavy chain gene expression and cardiomyocyte hypertrophy. J Biomed Sci. 2005;12:123–33. doi: 10.1007/s11373-004-8168-6. [DOI] [PubMed] [Google Scholar]