Abstract
Aim:
Previous studies have demonstrated that glycine (GLY) markedly reduces lipopolysaccharide (LPS)-induced myocardial injury. However, the mechanism of this effect is still unclear. The present study investigated the effect of GLY on cytosolic calcium concentration ([Ca2+]c) and tumor necrosis factor-α (TNFα) production in cardiomyocytes exposed to LPS, as well as whether the glycine-gated chloride channel is involved in this process.
Methods:
Neonatal rat cardiomyocytes were isolated, and the [Ca2+]c and TNFα levels were determined by using Fura-2 and a Quantikine enzyme-linked immunosorbent assay, respectively. The distribution of the GLY receptor and GLY-induced currents in cardiomyocytes were also investigated using immunocytochemistry and the whole-cell patch-clamp technique, respectively.
Results:
LPS at concentrations ranging from 10 ng/mL to 100 μg/mL significantly stimulated TNFα production. GLY did not inhibit TNFα production induced by LPS at concentrations below 10 ng/mL but did significantly decrease TNFα release stimulated by 100 μg/mL LPS and prevented an LPS-induced increase in [Ca2+]c, which was reversed by strychnine, a glycine receptor antagonist. GLY did not block the isoproterenol-induced increase in [Ca2+]c, but did prevent the potassium chloride-induced increase in [Ca2+]c in cardiomyocytes. Strychnine reversed the inhibition of the KCl–stimulated elevation in [Ca2+]c by GLY. In chloride-free buffer, GLY had no effect on the dipotassium hydrogen phosphate-induced increase in [Ca2+]c. Furthermore, GLY receptor α1 and β subunit-immunoreactive spots were observed in cardiomyocytes, and GLY-evoked currents were blocked by strychnine.
Conclusion:
Cardiomyocytes possess the glycine-gated chloride channel, through which GLY prevents the increase in [Ca2+]c and inhibits the TNFα production induced by LPS at high doses in neonatal rat cardiomyocytes.
Keywords: cardiomyocytes, lipopolysaccharides, intracellular calcium, tumor necrosis factor, glycine-gated chloride channel
Introduction
Glycine (GLY), a nonessential amino acid, is well recognized as an inhibitory neurotransmitter in the central nervous system and functions via a GLY-gated chloride channel1. Recent studies have demonstrated that GLY has a wide spectrum of pharmacological effects, including suppressing endotoxin-induced fever, antiinflammatory effects and direct cytoprotective actions2, 3. Dietary GLY can improve rat survival in endotoxin shock and significantly reduce the lipopolysaccharide (LPS)-induced elevation in serum tumor necrosis factor-α (TNFα) levels, hepatic necrosis and lung injury4. Furthermore, Froh et al demonstrated that macrophages and leukocytes expressed a GLY-gated chloride channel with molecular and pharmacological properties similar to those of the GLY receptor in the central nervous system5. GLY stimulates the GLY-gated chloride channel, resulting in chloride influx and hyperpolarization of the membrane, which prevents Kupffer cell activation and consequently blunts the increase in cytosolic calcium concentration ([Ca2+]c) and in TNFα production in response to LPS5, 6. Most recently, we found positive immunohistochemical staining for the GLY receptor in the rat heart7, and we also demonstrated that GLY markedly improved the monophasic action potential in the isolated rat heart exposed to LPS and reduced LPS-induced myocardial injury8. However, the mechanism of this effect is still unclear. Although the mechanisms underlying LPS-induced myocardial depression remain elusive, increasing evidence has shown that upregulation of TNFα and abnormal calcium cycling in cardiomyocytes are associated with LPS-induced myocardial dysfunction9, 10. These data suggest that it is possible for GLY to interfere with LPS-induced [Ca2+]c alterations and TNFα production in cardiomyocytes via the GLY receptor. To date, there has been no direct pharmacological or electrophysiological evidence for the existence of GLY-gated chloride channels in cardiomyocytes. Therefore, the purpose of this study was to observe the effects of GLY on [Ca2+]c and TNFα production in cardiomyocytes treated with LPS and to provide direct pharmacological and electrophysiological evidence to determine whether the glycine-gated chloride channel was involved in this process.
Materials and methods
Animals and reagents
Neonatal Sprague-Dawley rats were obtained from the center of laboratory animals in the Guangdong province of China. The experimental protocol was approved by the Experimental Animal Care and Use Committee of Ji-nan University and conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No 85–23, revised 1996). Quantikine enzyme-linked immunosorbent assay (ELISA) kits specific for rat TNFα were purchased from R&D Systems (Minneapolis, MN, USA), and Dulbecco's modified Eagle's medium (DMEM)–F12 was purchased from Gibco-BRL (USA). Trypsin, Fura-2/AM and lipopolysaccharide (LPS, from E coli serotype 055:B5) were purchased from Sigma (St Louis, MO, USA). Modified Hanks' balanced salt solution (mHBSS) was prepared as described previously11 and was composed of (in mmol/L ) 110 NaCl, 0.3 Na2HPO4, 5 KCl, 0.4 KH2PO4, 0.8 MgSO4·7H2O, 5.6 glucose, 4 NaHCO3, 1.26 CaCl2 and 15 HEPES (pH 7.4). Chloride-free buffer was produced by substitution of sodium chloride with sodium gluconate in mHBSS. For immunostaining of the glycine receptor subunits, rabbit polyclonal antibodies to the GLY receptor α1 (ab23809) and β (H-170, sc-20134) subunits and fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit IgG were obtained from Abcam (Cambridge, UK), Santa Cruz Biotechnology, Inc (CA, USA) and KPL Inc (Gaithersburg, MD, USA), respectively.
Primary culture of neonatal rat cardiomyocytes
Neonatal rat cardiomyocytes were isolated by previously described techniques12. Briefly, three- to four-day-old Sprague-Dawley rats were anesthetized and sacrificed, and the ventricles of the heart were harvested and cut into approximately 1-mm pieces. Then, the tissues were digested with a 0.125% trypsin solution (0.2 g/L EDTA, Ca2+/Mg2+-free Hanks' buffered salt solution) for 10 min at 37 °C. The first digestion solution was removed, and the subsequent supernatant fractions were harvested and centrifuged at 1000 r/min for 5 min. The cell pellet was resuspended in DMEM–F12 medium with 10% fetal bovine serum, 100 μg/mL streptomycin and 100 U/mL penicillin. The cell suspensions were incubated in a 5% carbon dioxide and 95% oxygen humidified incubator (Thermo Forma Co, USA) at 37 °C for 3 h. The supernatant fractions rich in cardiomyocytes were carefully collected, and the cell density was adjusted to 5×105 cells/mL. At the same time, 0.1 mmol/L BrdU was used to reduce the contamination of non-muscle cells. The cardiomyocytes began beating after being cultured for 48 h. In addition, cardiomyocyte viability was determined using trypan blue exclusion.
TNFα ELISA
A complex, multiphasic dose dependency of LPS-stimulated TNFα release in cardiomyocytes was observed in previous studies10, 13. Therefore, LPS at concentrations ranging from 1 ng/mL to 100 μg/mL were used in this study. Neonatal rat cardiomyocytes, at a concentration of 5×105 cells/mL, were incubated at 37 °C for 6 h with LPS alone or in combination with 0.5, 1, or 2 mmol/L GLY. TNFα levels in the supernatant were determined using a rat TNFα-specific ELISA kit according to the manufacturer's instructions. The absorbance values were measured at 450 nm in a microtiter plate reader.
Measurement of [Ca2+]c
[Ca2+]c was determined using the calcium-sensitive fluorescent indicator Fura-2 as reported previously14. Neonatal rat cardiomyocytes (5×105 cells/mL) cultured for 2–3 h were loaded with 5 μmol/L Fura-2/AM for 30 min in the dark, and the cells were then washed with Hanks' solution three times to remove the extracellular Fura-2/AM. The cell pellet was resuspended in mHBSS or chloride-free buffer, and the ratio of fluorescence was determined with a fluorescence spectrophotometer (Shimadzu RF-5000, Japan) using excitation wavelengths of 340 and 380 nm and an emission wavelength of 510 nm. The measurement was performed in a water-jacketed cuvette at 37 °C. After equilibration of the fluorescence to a stable baseline, the cardiomyocytes were stimulated with 50 mmol/L KCl or dipotassium hydrogen phosphate; 1, 10, or 100 μg/mL LPS; and/or 50 μmol/L strychnine along with 2 mmol/L GLY. Basal and agent-evoked changes in the fluorescent ratio of Fura-2 (340/380 nm) were then measured continuously. Maximum (Rmax) and minimum (Rmin) fluorescence values were determined by adding 50 μL Triton X-100 (10%) and 100 mmol/L EGTA, respectively. Then, the average cardiomyocyte [Ca2+]c was calculated by means of a computer. In previous studies, it was found that 1-10 μmol/L strychnine partially inhibited the effect of GLY, and high-dose strychnine (1 mmol/L) mimicked the effects of glycine as a partial agonist to the glycine-gated chloride channel15, 16. We found that 50 μmol/L strychnine almost completely inhibited the effect of 2 mmol/L glycine. Therefore, in the present study, strychnine at concentration of 50 μmol/L was used.
Immunohistochemistry
For immunofluorescence, cells were grown on glass slides for 3–4 days. Slide cultures of cardiomyocytes were washed with phosphate-buffered saline solution (PBS), fixed in 95% ethanol at room temperature for 10 min, and then permeabilized in 0.5% Triton-X 100. After being blocked with 5% normal goat serum for 15 min at room temperature, the cardiomyocytes were incubated with the primary antibodies, anti-GLY α1 subunit antibody (diluted 1:500) or anti-GLY β subunit antibody (diluted 1:50), at 37 °C for 1 h, then washed with PBS and further incubated with the secondary antibody, FITC-conjugated goat anti-rabbit IgG, for 30 min at 37 °C. Slides were washed with PBS three times and double stained with 50 μg/mL propidium iodide (PI) at room temperature for another 5 min. The labeled cardiomyocytes were examined with a laser scanning confocal microscope (LSM510META, Zeiss, Germany).
Whole-cell patch clamp recording
Whole-cell currents were recorded using a EPC-7 patch–clamp amplifier (List Electronic, Germany) at room temperature (20–24 °C). Patch pipettes were pulled from glass capillaries with a tip resistance of 5–10 MΩ. The cardiomyocytes cultured for 20–24 h were superfused continuously with the following external solution (osmolarity 300 mOsm/L, pH 7.4) containing (in mmol/L): 70 NaCl, 0.5 MgCl2, 2 CaCl2, 10 HEPES, and 140 D-mannitol. The patch pipette solution contained (in mmol/L) 70 N-methyl-D-glucamine chloride (NMDG-Cl), 1.2 MgCl2, 10 HEPES, 1 EGTA, 140 D-mannitol and 2 ATP and had a final pH of 7.25. Inward current responses were measured at a holding membrane potential of −70 mV, digitized using CED 1401 (Cambridge, UK) and analyzed using EPC software (CED, Cambridge, UK).
Statistical evaluation
All results were expressed as mean±SEM and analyzed using statistical software (SPSS for Windows 13.0). Mean values for groups were compared by Student's t-tests and one-way ANOVA with the post-hoc least significant difference (LSD) or Dunnett test. Differences were considered to be statistically significant at P less than 0.05.
Results
Effect of GLY on LPS-stimulated TNFα release from neonatal rat cardiomyocytes
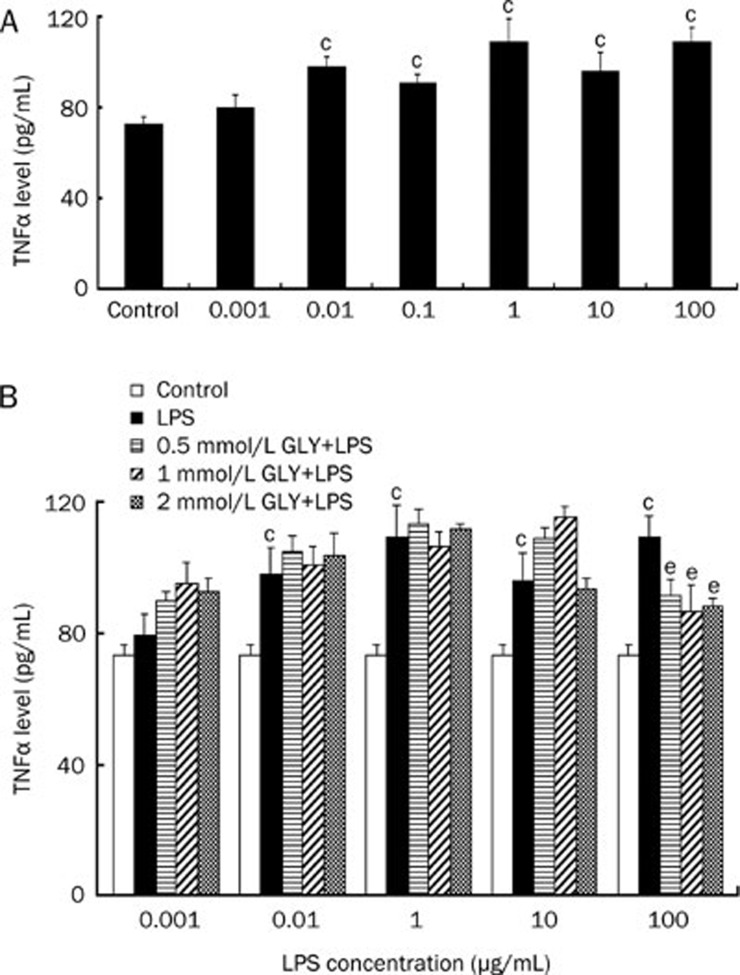
In the presence of 10% fetal bovine serum, control cardiomyocytes released TNFα at a basal level of 73.1±3.4 pg/mL. Consistent with the previous study10, LPS at concentrations of 0.01, 0.1, 1, 10, and 100 μg/mL significantly stimulated TNFα release from the cardiomyocytes at a level of 97.9±8.3, 91.3±3.6, 109.2±10.0, 96.2±8.3, and 109.4±6.6 pg/mL, respectively, which were all higher than that of controls (P<0.05, Figure 1A). However, 0.001 μg/mL LPS did not induce a significant increase in TNFα production in this condition. As mentioned above, 0.001, 0.01, and 1 μg/mL LPS stimulated TNFα release in a dose-dependent manner. For the GLY inhibition of the LPS-induced TNFα production, an LPS concentration of 0.1 μg/mL was not used. When the cardiomyocytes were treated with 0.5, 1, and 2 mmol/L GLY in combination with 0.001, 0.01, 1, and 10 μg/mL LPS, the TNFα levels were not different from those in the LPS-only treatment group (Figure 1B, P>0.05). However, 0.5, 1, and 2 mmol/L GLY significantly attenuated 100 μg/mL LPS-induced TNFα release from the cardiomyocytes (Figure 1B).
Figure 1.
The effect of glycine (GLY) on LPS-induced TNFα release from cultured neonatal rat cardiomyocytes. The cardiomyocytes were incubated with LPS and/or GLY for 6 h. (A) TNFα levels in the media of cardiomyocytes exposed to LPS at concentrations ranging from 1 ng/mL to 100 μg/mL. (B) GLY inhibited the 100 μg/mL LPS-induced TNFα release. n=4. cP<0.01 vs control. eP<0.05 vs LPS group.
Influence of GLY on the LPS-induced increase in [Ca2+]c in cardiomyocytes
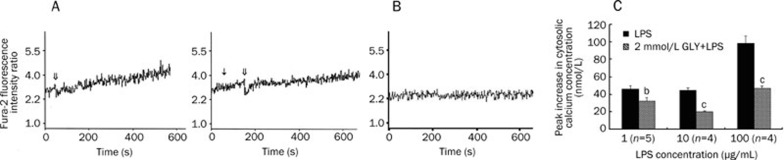
The effects of GLY on the LPS-induced elevation in [Ca2+]c in cardiomyocytes are shown in Figure 2. LPS (1, 10, and 100 μg/mL) caused a marked increase in [Ca2+]c within 600 s in a dose-dependent manner. Trypan blue exclusion showed that LPS at any concentration used in the experiment was not toxic to the cardiomyocytes. Pretreatment with 2 mmol/L GLY significantly — but not completely — inhibited the increases in [Ca2+]c due to LPS at the above concentrations. On average, 100 μg/mL LPS increased [Ca2+]c in cardiomyocytes by 98.2±8.6 nmol/L. In the presence of 2 mmol/L GLY and 100 μg/mL LPS, [Ca2+]c in cardiomyocytes increased by only 47.3±1.9 nmol/L.
Figure 2.
The effect of GLY on the LPS-induced cytosolic calcium concentration increase in cultured neonatal rat cardiomyocytes. Panel A shows the changes in the ratio of Fura-2 fluorescence intensities at excitation wavelengths of 340/380 nm in Fura-2-loaded cardiomyocytes after 2 mmol/L GLY and 100 μg/mL LPS stimulation. ↓ represents the addition of GLY. ⇓ indicates LPS stimulation. Panel B shows changes in the ratio of Fura-2 fluorescence intensities at excitation wavelengths of 340/380 nm in Fura-2-loaded cardiomyocytes without stimulation. Panel C shows changes in the peak cytosolic calcium concentration above the basal concentration. bP<0.05, cP<0.01 vs LPS group.
GLY did not block the isoproterenol-evoked increase in [Ca2+]c in cardiomyocytes
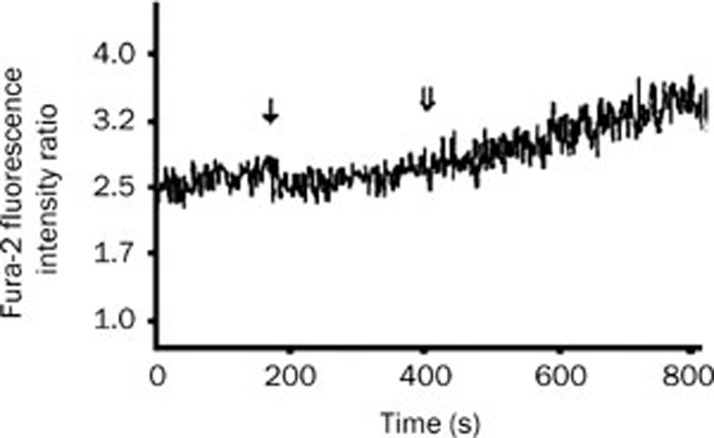
It is well known that isoproterenol can elicit Ca2+ release from the sarcoplasmic reticulum. In order to demonstrate whether GLY inhibits intracellular calcium release, the effect of GLY on the isoproterenol-evoked intracellular calcium release was investigated. As described in Figure 3, GLY did not prevent the isoproterenol-evoked increase in [Ca2+]c in cardiomyocytes. In the presence of 2 mmol/L GLY, 2.5 μg/mL isoproterenol increased [Ca2+]c in cardiomyocytes from 56.4±4.2 nmol/L at the baseline to 111.2±3.6 nmol/L (n=4, P<0.01). In addition, GLY alone did not significantly affect basal [Ca2+]c in cardiomyocytes (data not shown).
Figure 3.
Changes in the ratio of Fura-2 fluorescence intensities at excitation wavelengths of 340/380 nm in Fura-2-loaded cardiomyocytes after 2 mmol/L GLY and 2.5 μg/mL isoproterenol stimulation. ↓ represents the addition of GLY. ⇓ represents isoproterenol stimulation.
GLY almost completely prevented the potassium chloride (KCl)-induced increase in [Ca2+]c in cardiomyocytes
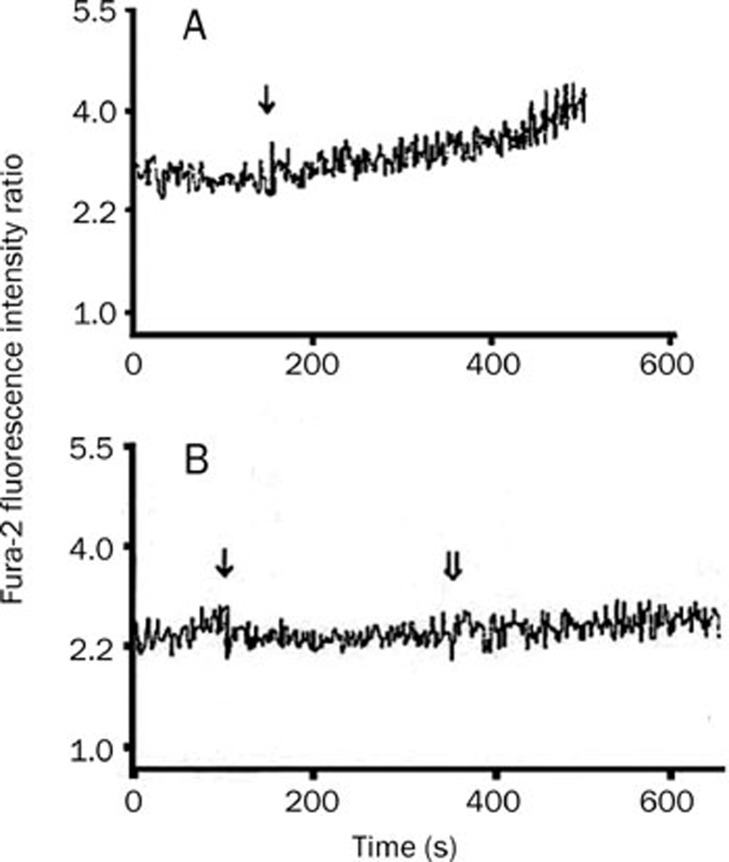
KCl causes Ca2+ influx through the sarcolemmal membrane by depolarization17, and GLY stimulates the GLY-gated chloride channel, resulting in chloride influx and hyperpolarization of the membrane, which in turn blunts the increase in [Ca2+]c in leukocytes5. Thus, it is necessary to examine the effect of GLY on Ca2+ influx in cardiomyocytes. In the present study, we found that basal [Ca2+]c in cardiomyocytes (63.1±3.2 nmol/L) significantly increased to 118.9±7.9 nmol/L after exposure to 50 mmol/L KCl for about 300 s (Figure 4A, n=4, P<0.01). In the presence of 2 mmol/L GLY, stimulation with 50 mmol/L KCl did not produce a marked increase in [Ca2+]c in cardiomyocytes (55.0 ± 6.4 nmol/L vs 70.3±6.5 nmol/L, n=4, P>0.05, Figure 4B). GLY alone did not markedly alter basal [Ca2+]c in cardiomyocytes (data not shown).
Figure 4.
Changes in the ratio of Fura-2 fluorescence intensities at excitation wavelengths of 340/380 nm in Fura-2-loaded cardiomyocytes. Panel A: 50 mmol/L KCl treatment, ↓ represents KCl stimulation; Panel B: 2 mmol/L GLY and 50 mmol/L KCl stimulation, ↓ and ⇓ represent the addition of GLY and KCl, respectively.
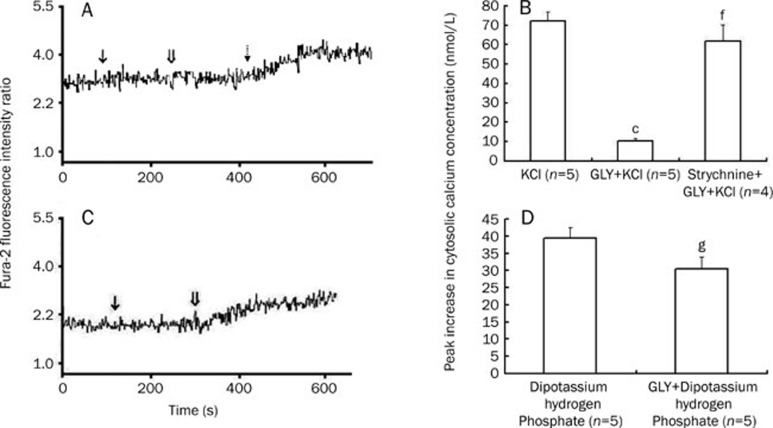
Strychnine reversed the inhibition of the KCl and LPS–stimulated elevations in [Ca2+]c by GLY
As shown above, 50 mmol/L KCl induced a significant increase in [Ca2+]c in cardiomyocytes, which was nearly completely inhibited by GLY. In contrast, addition of the GLY receptor antagonist strychnine about 200 s before GLY treatment antagonized the inhibitory action of GLY on the KCl-evoked increase in [Ca2+]c in cardiomyocytes (Figure 5A, 5B). Strychnine alone did not significantly alter [Ca2+]c in cardiomyocytes (data not shown). Similarly, when strychnine at a final concentration of 50 μmol/L was added to the cardiomyocyte suspension about 3 min before GLY, the inhibitory effect of 2 mmol/L GLY on the 100 μg/mL LPS-induced elevation in [Ca2+]c was completely prevented such that the increased [Ca2+]c value was 95.8±13.0 nmol/L, which was not different from that induced by LPS alone (98.2±8.6 nmol/L, n=4, P>0.05).
Figure 5.
The effects of strychnine (50 μmol/L) and/or GLY (2 mmol/L) on 50 mmol/L KCl (Panel A, B) or dipotassium hydrogen phosphate (Panel C, D)-induced cytosolic calcium concentration increases in cultured neonatal rat cardiomyocytes. Panels A and C represent changes in the ratio of Fura-2 fluorescence intensities at excitation wavelengths of 340/380 nm in Fura-2-loaded cardiomyocytes. ↓ represents the addition of strychnine (panel A) or GLY (panel C); ⇓ represents the addition of GLY (panel A) or dipotassium hydrogen phosphate (panel C); indicates 50 mmol/L KCl stimulation. The representative original tracings for changes in the ratio of Fura-2 fluorescence intensities in Fura-2-loaded cardiomyocytes stimulated with KCl, dipotassium hydrogen phosphate alone or GLY plus KCl are not shown. In panels C and D, the cultured neonatal rat cardiomyocytes were maintained in chloride-free buffer. cP<0.01 vs KCl group. fP<0.01 vs GLY+KCl group. gP>0.05 vs dipotassium hydrogen phosphate treatment group.
Influence of GLY on the dipotassium hydrogen phosphate-evoked increase in [Ca2+]c in chloride-free buffer
GLY functions via chloride influx in macrophages. If GLY receptors exist on cardiomyocytes, extracellular chloride depletion should prevent GLY action. Dipotassium hydrogen phosphate 50 mmol/L produced an increase in [Ca2+]c in cardiomyocytes in chloride-free buffer; however, pretreatment with 2 mmol/L GLY for 3 min did not prevent the dipotassium hydrogen phosphate-induced increase in [Ca2+]c in cardiomyocytes in chloride-free buffer (Figure 5C, 5D).
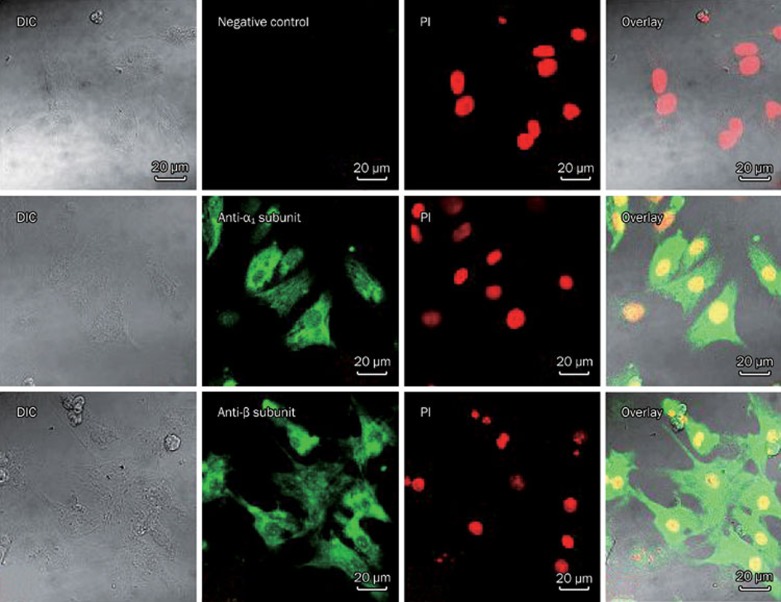
Immunocytochemical staining of the GLY receptor α1 and β subunits in cardiomyoctyes
Labeling patterns of the antibodies against the GLY receptor α1 and β subunits in cardiomyocytes are shown in Figure 6. GLY receptor α1 and β subunit-immunoreactive spots were observed on the membrane and in the cytoplasm of cardiomyocytes, but no fluorescent spots were found when the primary antibodies were omitted.
Figure 6.
Immunocytochemical staining of GLY receptor α1 and β subunits in neonatal rat cardiomyocytes. GLY receptor α1 and β subunit-immunoreactive spots are observed on the membrane and in the cytoplasm of cardiomyocytes. Scale bar represents 20 μm. DIC: differential interference contrast.
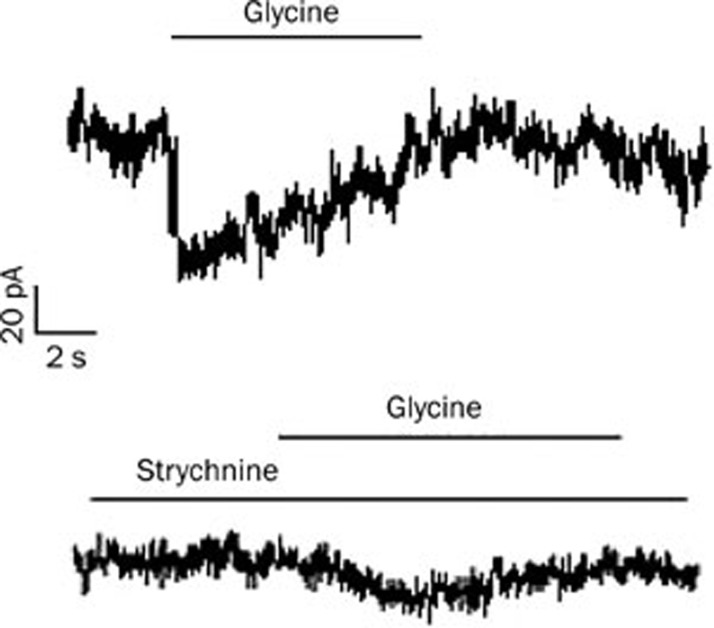
Glycine-induced currents in cardiomyoctyes
For electrophysiological recordings, cardiomyocytes cultured for 22–24 h were chosen. As shown in Figure 7, the application of glycine at a concentration of 2 mmol/L invoked an inward current (89.7%±14.7% pA, n=6), and the current was blocked by the glycine receptor antagonist strychnine (50 μmol/L).
Figure 7.
The effects of strychnine on the glycine (GLY)-activated current in cardiomyocytes. The cell was voltage-clamped at −70 mV. GLY induced an inward current recorded from a single cardiomyocyte (top), which was blocked by the application of the GLY receptor antagonist strychnine (bottom). The duration of GLY and strychnine treatments are indicated by the horizontal bar above the traces.
Discussion
Recently, we observed that GLY attenuated LPS-induced myocardial dysfunction8. In order to investigate the mechanisms of GLY action, we further examined the effect of GLY on the LPS-induced increase in [Ca2+]c and in TNFα production in cardiomyocytes. The cultured neonatal rat cardiomyocytes were spontaneously beating and generated spontaneous Ca2+ transients. In this study, we used neonatal rat cardiomyocytes cultured for 2–3 h in the cytosolic calcium concentration determination. Under these conditions, the cultured neonatal rat cardiomyocytes did not spontaneously beat, which diminished the spontaneous Ca2+ transients. The results showed that GLY partly inhibited the increase in [Ca2+]c induced by LPS at concentrations of 1, 10, and 100 μg/mL. In addition, strychnine, a GLY receptor antagonist, given at a final concentration of 50 μmol/L, completely prevented the inhibitory effect of 2 mmol/L GLY on the 100 μg/mL LPS-induced elevation in [Ca2+]c. These data suggest that GLY may inhibit the LPS-induced elevation in [Ca2+]c in cardiomyocytes via the GLY receptor.
It has been established that TNFα production induced by LPS is dependent on the increase in [Ca2+]c and other signal pathways in macrophages. GLY partially prevents the LPS-stimulated increase in [Ca2+]c and in turn decreases TNFα production in macrophages15. Geoghegan-Morphet18 et al demonstrated that 10 μg/mL LPS increased [Ca2+]c and TNFα expression in cardiomyocytes and that pretreatment with the L-type calcium channel inhibitor verapamil partially decreased TNFα expression. This suggests that elevated [Ca2+]c plays a role in TNFα production induced by LPS to a certain extent in cardiomyocytes. In the present study, we found that GLY markedly reduced the TNFα production induced by LPS at concentration of 100 μg/mL but did not inhibit the TNFα production in cardiomyocytes treated with LPS at concentrations of 1 and 10 μg/mL. The cause of this phenomenon is not understood yet but may be a result of the different contribution of Ca2+ influx from the extracellular space in TNFα production induced by LPS at low doses (less than 10 μg/mL) and high dose (100 μg/mL) in cardiomyocytes. It is reported that the [Ca2+]c elevation involves intracellular calcium release and calcium influx15. It is possible that LPS at concentrations less than 10 μg/mL induces an increase in [Ca2+]c mainly via intracellular calcium release. A previous study suggested that GLY inhibited calcium influx mainly from the extracellular space5. Therefore, the inhibitory amplitude of 2 mmol/L GLY on [Ca2+]c elevation in cardiomyocytes exposed to 1 and 10 μg/mL LPS is not enough to inhibit TNFα production. In contrast, 100 μg/mL LPS may stimulate intracellular calcium release and much more calcium influx. Thus, 2 mmol/L GLY inhibited [Ca2+]c elevation to a greater extent in cardiomyocytes treated with 100 μg/mL than in those treated with 1 and 10 μg/mL LPS and markedly reduced TNFα production induced by LPS at a concentration of 100 μg/mL. This requires further investigation.
Since Sun et al reported that GLY antagonized LPS action by changing LPS structure19, it has been very important to exclude the direct effect of GLY on LPS. Therefore, the effect of GLY on calcium influx and intracellular calcium release by potassium and isoproterenol stimulation in cardiomyocytes deserves to be investigated. In the present study, we found that GLY did not block the isoproterenol-induced increase in [Ca2+]c in cardiomyocytes. Furthermore, we investigated the effect of GLY on calcium influx from the extracellular space due to potassium chloride in cardiomyocytes. The results demonstrated that GLY prevented the potassium chloride-induced increase in [Ca2+]c in cardiomyocytes, which was reversed by strychnine. In addition, the inhibitory effect of GLY on the LPS-induced increase in [Ca2+]c in cardiomyocytes was also reversed by strychnine. These results suggest that GLY may block calcium influx from the extracellular space and inhibit the potassium chloride- or LPS-induced increase in [Ca2+]c in cardiomyocytes via the GLY receptor. It is known that the GLY receptor is a ligand-gated chloride channel5. In order to further confirm the presence of GLY-gated chloride channels in cardiomyocytes, potassium chloride was replaced with dipotassium hydrogen phosphate to minimize the chloride concentration in the extracellular space. In chloride-free buffer, GLY did not prevent the dipotassium hydrogen phosphate–induced increase in [Ca2+]c in cardiomyocytes, suggesting that GLY blocks calcium influx from the extracellular space in a chloride-dependent manner. However, the dipotassium hydrogen phosphate–induced increase in [Ca2+]c in cardiomyocytes in chloride-free buffer was smaller than that induced by potassium chloride. The reason for this difference remains to be further examined.
Recent pharmacological and molecular evidence supports the idea that GLY-gated chloride channel exists in non-neuronal cells, including vascular endothelial cells, hepatocytes and macrophages5, 20, 21, 22. It has been demonstrated that the GLY receptor has the typical pentameric structure, consisting of one of the highly homologous ligand-binding α subunits (α1–4 subunits in mice, α1–3 subunits in rats) and a β subunit that forms a chloride-selective transmembrane channel1. Our previous study showed that mRNA and protein for the α1 and β subunits were detected in neonatal cardiomyocytes23. In order to provide direct evidence for the presence of GLY-gated chloride channels in neonatal rat cardiomyocytes, immunocytochemical staining was performed in the present study. The results demonstrated that GLY receptor α1 and β subunit-immunoreactive spots were found not only on the membrane but also in the cytoplasm of cardiomyocytes; this finding is different from that in neurons. Recently, Ruiz-Meana et al found that intracellular glycine exerted an inhibitory effect on mitochondrial permeability transition in cardiomyocytes and that intracellular glycine depletion made the cells more vulnerable to necrotic death during myocardial hypoxia/reoxygenation24. These findings suggest that GLY can act directly on the mitochondria of cardiomyocytes. Therefore, whether GLY binding sites or receptors might occur in the mitochondria and other places in the cytoplasm of cardiomyocytes remains to be further investigated. In order to investigate the function of the GLY receptor, it is very important to identify the GLY receptor in cardiomyocytes using electrophysiological techniques. In the present study, using whole-cell patch–clamp recordings, we found that the administration of GLY induced an inward current at the holding potential of −70 mV in cardiomyocytes, which was blocked by the glycine receptor antagonist strychnine. These observations suggest that GLY can open chloride channels and cause chloride ion efflux under these external and patch pipette solution conditions. Based on these data, we concluded that GLY receptors were expressed and functioned in neonatal rat cardiomyocytes.
Some researchers have demonstrated that macrophages contain a GLY-gated chloride channel. GLY can completely block the LPS-induced increase in [Ca2+]c in macrophages in a chloride-dependent manner, which is reversed by strychnine. GLY also stimulates a dose-dependent chloride influx to induce the hyperpolarization of the plasma membrane and consequently inhibits depolarization due to potassium and LPS in macrophages. Moreover, superoxide and TNFα production in macrophages exposed to LPS were reduced by GLY6, 15. These findings indicate that GLY activates the glycine-gated chloride channel in macrophages, causes chloride influx and hyperpolarizes the membrane and, as a result, prevents an increase in [Ca2+]c and the subsequent production of TNFα and toxic radicals in response to LPS. Thus, based on the results presented above, we concluded that neonatal rat cardiomyocytes possessed the glycine-gated chloride channel through which GLY might open chloride channels and induce chloride influx under conditions of higher extracellular than intracellular chloride concentration and hyperpolarization of the cell membrane. Consequently, glycine inhibits the voltage-dependent opening of calcium channels and prevents the LPS-induced rapid increase in [Ca2+]c in cardiomyocytes.
In summary, the present study provided direct pharmacological and electrophysiological evidence to demonstrate that the GLY-gated chloride channel existed in neonatal rat cardiomyocytes. Through these channels, GLY inhibited the calcium influx evoked by potassium and LPS and reduced the TNFα production induced by high-dose LPS. These findings may provide an explanation for the action of GLY on the heart.
Author contribution
Hua-dong WANG and Da-xiang LU designed research; Hua-dong WANG, Xiu-xiu LÜ, Ren-bin QI, Yan-ping WANG, Yong-mei FU, and Li-wei WANG performed research; Hua-dong WANG and Xiu-xiu LÜ analyzed the data; Hua-dong WANG and Da-xiang LU wrote and revised the paper.
Acknowledgments
This project was supported by grants from the National Natural Science Foundation of China (No 30470718) and the Guangdong Natural Science Foundation (No 04105844).
The authors are grateful to Prof Li-xin CHEN, Dr Lin-yan ZHU, and Mr Lin-jie YANG in the school of medicine, Ji-nan University, for the electrophysiological experiments.
References
- Rajendra S, Lynch JW, Schofield PR. The glycine receptor. Pharmacol Ther. 1997;73:121–46. doi: 10.1016/s0163-7258(96)00163-5. [DOI] [PubMed] [Google Scholar]
- Lu DX, Li CJ, Fu YM, Wang HD. Inhibitory effect of glycine on endotoxin pyrogenicity. Chin J Pathophysiol. 1996;12:235–8. [Google Scholar]
- Zhong Z, Wheeler MD, Li X, Froh M, Schemmer P, Yin M, et al. L-Glycine: a novel antiinflammatory, immunomodulatory, and cytoprotective agent. Curr Opin Clin Nutr Metab Care. 2003;6:229–40. doi: 10.1097/00075197-200303000-00013. [DOI] [PubMed] [Google Scholar]
- Ikejima K, Iimuro Y, Forman DT, Thurman RG. A diet containing glycine improves survival in endotoxin shock in the rat. Am J Physiol Gastrointest Liver Physiol. 1996;271:G97–103. doi: 10.1152/ajpgi.1996.271.1.G97. [DOI] [PubMed] [Google Scholar]
- Froh M, Thurman RG, Wheeler MD. Molecular evidence for a glycine-gated chloride channel in macrophages and leukocytes. Am J Physiol Gastrointest Liver Physiol. 2002;283:G856–63. doi: 10.1152/ajpgi.00503.2001. [DOI] [PubMed] [Google Scholar]
- Ikejima K, Qu W, Stachlewitz RF, Thurman RG. Kupffer cells contain a glycine-gated chloride channel. Am J Physiol Gastrointest Liver Physiol. 1997;272:G1581–6. doi: 10.1152/ajpgi.1997.272.6.G1581. [DOI] [PubMed] [Google Scholar]
- Qi RB, Zhang JY, Lu DX, Wang HD, Wang HH, Li CJ. Glycine receptors contribute to cytoprotection of glycine in myocardial cells. Chin Med J (Engl) 2007;120:915–21. [PubMed] [Google Scholar]
- Qi RB, Lu DX, Wang HD, Zhou H, Wang HH, Li CJ. Effect of glycine on endotoxin-induced myocardial cell injury of the isolated rat heart. Chin Pharmacol Bull. 2005;21:738–41. [Google Scholar]
- Stamm C, Cowan DB, Friehs I, Noria S, del Nido PJ, McGowan FX., Jr Rapid endotoxin-induced alterations in myocardial calcium handling: Obligatory role of cardiac TNF-α. Anesthesiology. 2001;95:1396–405. doi: 10.1097/00000542-200112000-00019. [DOI] [PubMed] [Google Scholar]
- Comstock KL, Krown KA, Page MT, Martin D, Ho P, Pedraza M, et al. LPS-induced TNF-α release from and apoptosis in rat cardiomyocytes: obligatory role for CD14 in mediating the LPS response. J Mol Cell Cardiol. 1998;30:2761–75. doi: 10.1006/jmcc.1998.0851. [DOI] [PubMed] [Google Scholar]
- Wheeler M, Stachlewitz RF, Yamashina S, Ikejima K, Morrow AL, Thurman RG. Glycine-gated chloride channels in neutrophils attenuate calcium influx and superoxide production. FASEB J. 2000;14:476–84. doi: 10.1096/fasebj.14.3.476. [DOI] [PubMed] [Google Scholar]
- Hwang KC, Lim S, Kwon HM, Bae YS, Kang SM, Chung KH, et al. Phospholipase C-δ1 rescues intracellular Ca2+ overload in ischemic heart and hypoxic neonatal cardiomyocytes. J Steroid Biochem Mol Biol. 2004;91:131–8. doi: 10.1016/j.jsbmb.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Yang J, Wang HD, Lu DX, Wang YP, Qi RB, Li J, et al. Effects of neutral sulfate berberine on LPS-induced cardiomyocyte TNF-α secretion, abnormal calcium cycling, and cardiac dysfunction in rats. Acta Pharmacol Sin. 2006;27:173–8. doi: 10.1111/j.1745-7254.2006.00257.x. [DOI] [PubMed] [Google Scholar]
- Shao Q, Saward L, Zahradka P, Dhalla NS. Ca2+ mobilization in adult rat cardiomyocytes by angiotensin type 1 and 2 receptors. Biochem Pharmacol. 1998;55:1413–8. doi: 10.1016/s0006-2952(97)00653-9. [DOI] [PubMed] [Google Scholar]
- Wheeler MD, Thurman RG. Production of superoxide and TNF-α from alveolar macrophages is blunted by glycine. Am J Pysiol Lung Cell Mol Physiol. 1999;277:L952–9. doi: 10.1152/ajplung.1999.277.5.L952. [DOI] [PubMed] [Google Scholar]
- Miller GW, Schnellmann RG. Cytoprotection by inhibition of chloride channels: the mechanism of action of glycine and strychnine. Life Sci. 1993;53:1211–5. doi: 10.1016/0024-3205(93)90539-f. [DOI] [PubMed] [Google Scholar]
- Yu JZ, Quamme GA, McNeill JH. Altered [Ca2+]i mobilization in diabetic cardiomyocytes: responses to caffeine, KCl, ouabain, and ATP. Diabetes Res Clin Pract. 1995;30:9–20. doi: 10.1016/0168-8227(95)01144-7. [DOI] [PubMed] [Google Scholar]
- Geoghegan-Morphet N, Burger D, Lu X, Sathish V, Peng T, Sims SM, Feng Q. Role of neuronal nitric oxide synthase in lipopolysaccharide-induced tumor necrosis factor-alpha expression in neonatal mouse cardiomyocytes. Cardiovasc Res. 2007;75:408–16. doi: 10.1016/j.cardiores.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Sun W, Lu DX, Ding Y, Li CJ. Effects of glycine on the combining rate of endotoxin to monocytes and endotoxic configuration. Chin J Pathophysiol. 1998;14:359–61. [Google Scholar]
- Yamashina S, Konno A, Wheeler MD, Rusyn I, Rusyn EV, Cox AD, et al. Endothelial cells contain a glycine-gated chloride channel. Nutr Cancer. 2001;40:197–204. doi: 10.1207/S15327914NC402_17. [DOI] [PubMed] [Google Scholar]
- Qu W, Ikejima K, Zhong Z, Waalkes MP, Thurman RG. Glycine blocks the increase in intracellular free Ca2+ due to vasoactive mediators in hepatic parenchymal cells. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1249–56. doi: 10.1152/ajpgi.00197.2002. [DOI] [PubMed] [Google Scholar]
- Miller GW, Schnellmann RG. A putative cytoprotective receptor in the kidney: relation to the neuronal strychnine-sensitive glycine receptor. Life Sci. 1994;55:27–34. doi: 10.1016/0024-3205(94)90078-7. [DOI] [PubMed] [Google Scholar]
- Zhang JY, Lu DX, Qi RB, Wang HD, Wang YP, Fu YM, et al. Expression of glycine receptor in rat cardiomyocytes. Chin J Pathophysiol. 2006;22:1070–3. [Google Scholar]
- Ruiz-Meana M, Pina P, Garcia-Dorado D, Rodríguez-Sinovas A, Barba I, Miró-Casas E, et al. Glycine protects cardiomyocytes against lethal reoxygenation injury by inhibiting mitochondrial permeability transition. J Physiol. 2004;558:873–82. doi: 10.1113/jphysiol.2004.068320. [DOI] [PMC free article] [PubMed] [Google Scholar]