This paper reports transgenesis by genetic modification of gametes in the domestic cat. The approach is used to generate transgenic cats expressing a virus restriction factor from rhesus macaque.
Supplementary information
The online version of this article (doi:10.1038/nmeth.1703) contains supplementary material, which is available to authorized users.
Subject terms: Genetic engineering, Transgenic organisms, Antivirals
Abstract
Studies of the domestic cat have contributed to many scientific advances, including the present understanding of the mammalian cerebral cortex. A practical capability for cat transgenesis is needed to realize the distinctive potential of research on this neurobehaviorally complex, accessible species for advancing human and feline health. For example, humans and cats are afflicted with pandemic AIDS lentiviruses that are susceptible to species-specific restriction factors. Here we introduced genes encoding such a factor, rhesus macaque TRIMCyp, and eGFP, into the cat germline. The method establishes gamete-targeted transgenesis for the first time in a carnivore. We observed uniformly transgenic outcomes, widespread expression, no mosaicism and no F1 silencing. TRIMCyp transgenic cat lymphocytes resisted feline immunodeficiency virus replication. This capability to experimentally manipulate the genome of an AIDS-susceptible species can be used to test the potential of restriction factors for HIV gene therapy and to build models of other infectious and noninfectious diseases.
Supplementary information
The online version of this article (doi:10.1038/nmeth.1703) contains supplementary material, which is available to authorized users.
Main
Felis catus has been domesticated for over 9,000 years and presently numbers 0.5–1.0 billion worldwide. Medical surveillance of this most common companion animal is extensive, and over 250 hereditary pathologies common to both cats and humans are known1. The F. catus genome was recently sequenced at light (1.9×) coverage and a 10× assembly is imminent2. Over 90% of identified cat genes have a human homolog, and compared with the mouse there are fewer genomic rearrangements. Intermediate size, prolific breeding capacity, similarity of systems to humans, abundance, modest costs and the neurobehavioral complexity of a Carnivoran make the cat of value in experimental settings ranging from neurobiology to diverse genetic, ophthalmologic and infectious diseases. These include conditions in which mice or rats are not useful on the basis of disease susceptibility, organ size or other factors1. Cat transgenesis is thus of interest for both human and cat health research and potentially for developing ways to confer protection from epidemic pathogens to free-ranging feline species, all 36 of which now face the threat of extinction3.
The world has two AIDS pandemics, one in domestic cats and the other in humans. The causative lentiviruses, feline immunodeficiency virus (FIV) and HIV-1, are highly similar in genome structure, disease manifestations and host cell dependency factor use4,5. The differences between these lentiviruses are also informative and potentially exploitable. For example, species-specific lentiviral restriction factors such as TRIM and APOBEC3 proteins6 restrict FIV and HIV-1 with distinctive patterns7,8,9,10. These genes have not been studied in a controlled manner at the systemic and species levels by introduction into the genome of an AIDS virus–susceptible species (Old World primates or felids). Given the challenges inherent to macaque transgenesis, the AIDS virus–susceptible cat would be singularly positioned for such studies if it can be accessed by genetic approaches used in mice. In contrast to primates, feline species lack antiviral TRIM5α genes11 but have potently restrictive APOBEC3 proteins9,10, which sets up intriguing possibilities for testing such genes at the whole-animal level, for conferring gene-based immunity with them or engineered variants12,13, and potentially for HIV-1 disease model development10.
To realize the potential of the species for virology and nonvirology models, a means for practical cat genome modification is needed. Somatic cell nuclear transfer (SCNT) was recently used to generate cats that express fluorescent proteins14,15. However, the efficiency of animal cloning is extremely low16, and SCNT results in faulty epigenetic reprogramming in most embryos17. Cloned mammals with apparently normal gross anatomy can have many abnormalities resulting from failure to erase and reprogram epigenetic memory completely17.
The two key approaches for generating transgenic mice are DNA injection into fertilized embryo pronuclei and injection of genetically modified embryonic stem cell (ESC) lines into blastocysts. However, in nonrodent mammals, pronuclear injection is very inefficient, and the second method is blocked by the lack of germline-competent ESCs. Transgenesis with germline transmission has been achieved in some mammals by microinjecting lentiviral vectors into oocytes or single-cell zygotes18. This has not been achieved in any carnivore species. Here we performed oocyte-targeted lentiviral transgenesis in the domestic cat.
Results
Multi-transgenic, nonmosaic cat embryo generation
We optimized reagents, gamete collection, microinjection parameters, embryo culture and recipient queen preparation to establish an optimal cat transgenesis protocol (Fig. 1a). We obtained gametes from both sexes without additional animal procedures by microdissecting gonads discarded after spaying or neutering.
Figure 1. Transgenic feline embryo generation.

(a) Optimized transgenesis protocol. PMSG, pregnant mare serum gonadotropin; HCG, human chorionic gonadotropin; IU, international units; IVM, in vitro maturation; IVC, in vitro culture. (b) Transgene expression in hatching feline blastocysts developed in vitro after pre-IVF lentiviral vector microinjection (top left) of feline oocytes. Living GFP-transgenic cat blastocyst (bottom left) developed from oocyte transduced before IVF with TSinG. Confocal images (right) of fixed transgenic (TBDmGpT) and control (product of untransduced oocytes) blastocysts subjected to immunolabeling show HA-tagged rhTRIMCyp signal (HA); GFP fluorescence; DAPI staining for nuclear DNA and merged images. Scale bars, 100 μm (black bars) and 50 μM (white bars).
In experiments summarized in Supplementary Table 1, we subjected 195 in vitro–matured grade I and II domestic cat oocytes to perivitelline space microinjection (PVSMI) with lentiviral vector TSinG5; we performed injection 10–12 h before or 10–12 h after in vitro fertilization (IVF) (Supplementary Fig. 1). Then we cultured these embryos until blastocyst stage (day 7). Comparisons of embryo development rates (Supplementary Tables 1 and 2) and enhanced GFP (referred to as GFP throughout) expression (Fig. 1b) showed that transgenesis rates were high (>75%) and the process was well tolerated, as cleavage and blastocyst formation rates did not differ substantially between PVSMI and control embryos (Supplementary Table 1). There were no differences in morphology or total cell number and no preference for vector injection timing before or after IVF (Supplementary Table 1). However, mosaicism scored by nonuniform fluorescent protein expression in the blastocyst was negligible when we injected vectors before IVF but was substantial with injection after IVF (Supplementary Table 1).
To investigate whether more than one transgene could be expressed in cat embryos in a single step by PVSMI, we microinjected 418 oocytes with single- or dual-transgene lentiviral vectors. Transgene assemblages were genes encoding GFP, GFP plus RFP, or GFP plus rhesus macaque TRIMCyp (Supplementary Fig. 1). The latter combination was expressed from either a dual promoter or as a single 2A peptide-linked preprotein. After microinjection we performed IVF with cat sperm 10 h later. We consistently observed embryo-pervasive, abundant expression of both proteins encoded by dual gene vectors in cat blastocysts when we injected lentiviral vector before IVF (Fig. 1b and Supplementary Table 2). We observed no detrimental effects of dual expression on embryo development or GFP expression irrespective of transgene combination (Supplementary Table 2). In addition, the 2A peptide or the dual promoter were each effective for simultaneous expression.
Generation of GFP and restriction factor transgenic cats
The process from oocyte collection to fallopian tube transfer took 3–4 d (Fig. 1a). We randomly selected embryos for implantation from cleaved oocytes that had been subjected to IVF and transferred them into surgically exposed fallopian tubes at 48–72 h after lentiviral vector transduction. We carried out no preselection for transgene expression after microinjection (embryos were in any case not reliably fluorescent by the time of transfer). We performed transfers into hormonally synchronized queens prepared by a 14–10 h light-dark environment. We administered to queens pregnant mare serum gonadotropin on day −4 and human chorionic gonadrotropin on day −1 with respect to lentiviral vector transduction, and mated them ad lib from the day of human chorionic gonadrotropin injection until the day before embryo transfer with a vasectomized, azoospermia-verified tomcat to induce ovulation and corpus luteum formation. During surgery we punctured follicles with a needle if not naturally ovulated.
Twenty-two embryo-transfer procedures resulted in five pregnancies (labeled A–E), five births and three live kittens (Table 1). We achieved a high rate of transgenesis, with 10 of 11 testable live-born or fetal offspring found to be transgenic (a twelfth, spontaneously miscarried 10 d preterm, was consumed by the surrogate mother and could not be tested). Three male and two female transgenic cats, named TgCat1–5, were born by spontaneous vaginal deliveries at term and all five were transgenic (Fig. 2, Table 1 and Supplementary Fig. 2). TgCat1 (male), TgCat2 (male) and TgCat3 (female) survived, whereas the fourth and fifth cats died perinatally from obstetrical complications (Table 1). TgCats1–3 were vigorous from birth, fed, played, developed and socialized normally and were healthy, with the exception that TgCat2 is unilaterally cryptorchid. He also has intermittent pruritic dermatitis, which may be due to a food allergy. In the first year he developed a ventral abdominal hernia and a lower eyelid irritation (entropion), both of which we cured surgically. Although we cannot exclude vector-insertion genotoxicity in TgCat2, the conditions do not constitute a recognizable syndrome.
Table 1.
Cat transgenesis: founder pregnancies and outcomes
| Transgenic cat namea | Vector | Total embryos transferred per vector | Transfers per vector | Pregnancy | Transgenic status | Product of unique oocyte | Sex, age of transgenic kitten |
|---|---|---|---|---|---|---|---|
| Cats | |||||||
| TgCat1 | TBDmGpT | 346 | 9 | A | Yes | Yes | Male, 27 months |
| TgCat2 | TBDmGpT | A | Yes | Yes | Male, 27 months | ||
| TgCat3 | TSinT2AG | 325 | 8 | B | Yes | Yes | Female, 12 months |
| TgCat4 | TSinG | 128 | 3 | D | Yes | Yes | Male, died at birth |
| TgCat5 | TSinT2AG | b | b | E | Yes | Yes | Female, stillbornc |
| TBDmGpR | 97 | 2 | None | ||||
| Pre-term | |||||||
| TgPre1 | TSinG | d | d | C | Yes | Yes | |
| TgPre2 | TSinG | d | d | C | Yes | Yes | |
| TgPre3 | TSinG | d | d | C | Yes | Yes | |
| TgPre4 | TSinG | d | d | C | Yes | Yes | |
| TgPre5 | TSING | d | d | C | Unknowne | Unknowne | |
| Pre6 | TSinT2AG | b | b | B | Nof | Yes | |
| TgPre7 | TSinT2AG | b | b | E | Yes | Yes | |
| Totals | 996 | 22 | 5 | 10/11 | 11/11 |
aFifteen to twenty-five embryos, each a product of a microinjected oocyte, were transferred per fallopian tube for a total of 30–50 per transfer; 22 such transfers resulted in pregnancies (A–E). Ages are as of July 2011.
bIncluded in totals for vector TSinT2AG above.
cTgCat5 was stillborn after placental abruption occurred, though it was fully developed and ultrasound examination the day before birth showed a normal heartbeat; TgPre7 was not viable ultrasonographically and was developmentally arrested at about day 50 of gestation.
dIncluded in totals for vector TSinG above.
eDay 45 radiography in pregnancy C showed five fetuses. They were born about 10 d prematurely, on 51–53 d of gestation, with morphology and size appropriate for this late stage. TgPre5 was consumed by the surrogate mother and could not be analyzed.
fAn underdeveloped, non-transgenic fetus delivered 6 h after TgCat3. No pregnancies resulted from two transfers of TBDmGpR vector-transduced embryos into queens.
Figure 2. Transgenic kittens.
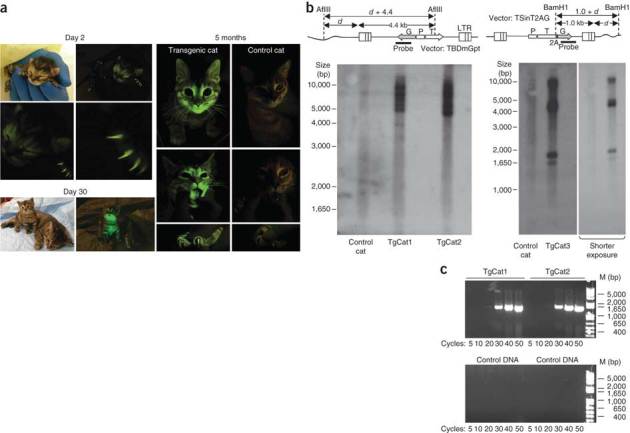
(a) Ambient light– and 485 nM light–illuminated images showing GFP signal at indicated times after birth for TgCat3. In the 30 d and 5 month images TgCat3 was photographed with a non-transgenic control cat (right). Coat, claw, whisker, nose, tongue and oropharyngeal mucosa fluorescence are evident; fluorescence was relatively quenched in dark fur. (b) Southern blotting of genomic DNA from TgCat1, TgCat2 and TgCat3. Southern junction blot designs are shown. d, distance from vector-host DNA junction to nearest genomic AflIII or BamH1 site in base pairs; P, promoter; LTR, long terminal repeat; G, eGFP; T, TRIMCyp. Genomic DNA from tail tips was digested with AflIII (left blot). Genomic DNA from peripheral blood mononuclear cells was digested with BamH1 (right blot). After electrophoresis and Southern blot transfer, membranes were probed for integrated vector DNA as indicated. (c) Amplicons from semiquantitative PCR amplifications of kitten genomic DNA using primers for the rhTRIMCyp sequence. M, marker. Cycles, number of PCR amplification cycles. Quantitative PCR showed that TgCat1 and TgCat2 had 15.2 ± 2.1 and 4.38 ± 0.2 GFP gene copies per cell equivalent respectively, using a value of 6.3 pg genomic DNA per diploid cell and normalizing to the signal obtained with GAPDH primers.
Southern blotting on restriction enzyme–digested genomic DNA from the three living transgenic kittens, from TgCat4 and from four miscarried fetuses showed that all eight were transgenic, with 6–12 insertions per cat (Fig. 2b). PCR assays on genomic DNA confirmed the high level of genomic transduction (Fig. 2c). Southern blot hybridization bands were specific, as all were (i) absent from control cat DNA, (ii) different from cat to cat and (iii) of greater than the predicted minimum size determined by the distance from restriction site to end of the vector provirus (Fig. 2b). Sequencing of proviral genomic DNA junctions (n = 4) from two cats was performed and each was a bona fide retroviral integration junction, with the genomic sequences mapping to the cat genome (Supplementary Table 3).
Transgene expression and phenotypes
TgCat3, in which transgene expression was driven by the standard (0.52-kilobase) human cytomegalovirus (hCMV) promoter of vector TSinT2AG, was brightly and stably green fluorescent in integument and oropharyngeal mucosa surfaces (Fig. 2a), but surface tissue expression was less bright for TgCat1 and TgCat2 (vector TBDmGpT). For the live kittens, we collected cells for protein analyses by oral mucosa scrapings (which showed GFP-expressing squamous epithelial cells), and blood and semen collection. Both transgenes were expressed in activated peripheral blood mononuclear cells (PBMCs) but with notable variation (Fig. 3a,b). Percentages of GFP-positive cells as determined by FACS were 15–80% in TgCat1, TgCat2 and TgCat3 and increased gradually as the kittens aged (Fig. 3b,c). TgCat2 had the most GFP-positive cells in the PBMC compartment, being about 65% GFP-positive early in life and then over 70–75% later (Fig. 3a–c and Supplementary Fig. 3). Several specific aspects here are interesting for developing models that will depend on lymphocyte or monocyte lineage expression. First, irrespective of promoter used, FACS and immunoblot detection of GFP and rhTRIMCyp in PBMCs in living cats required activation by phytohemagglutin-E (PHA-E) and interleukin 2 (IL-2), and GFP expression increased steadily with time in culture (Fig. 3c). Fluorescence intensity was variable (Fig. 3b and Supplementary Fig. 3a). Second, driving GFP expression from a minimal CMV (mCMV) promoter element adjacent to the PGK promoter was effective in TgCat2, but we observed only low GFP expression with the same vector in TgCat1, although even in this cat GFP expression increased steadily from rare positives to 14.8% by 20 months (Fig. 3b,c). Third, all three cats expressed hemagglutinin epitope (HA)-tagged rhTRIMCyp in the bulk PBMC population as detected by immunoblotting (Fig. 3a). TgCat1 consistently expressed more rhTRIMCyp than the other two living cats by quantitative western blot analysis. However, this protein was more difficult to detect than GFP, and was clearly visualized by immunofluorescence, using an antibody to the HA tag, in only a fraction of the cells (Supplementary Fig. 3). Even so, rhTRIMCyp transgenic cat PBMCs displayed resistance to FIV replication, with the greatest resistance to replication seen in cells from the cat that expressed the most rhTRIMCyp (TgCat1; Fig. 3d). The resistance to FIV replication was partial, as predicted for cell populations that express such a restriction factor partially19.
Figure 3. Immunoblotting and FIV challenge of transgenic PBMCs.
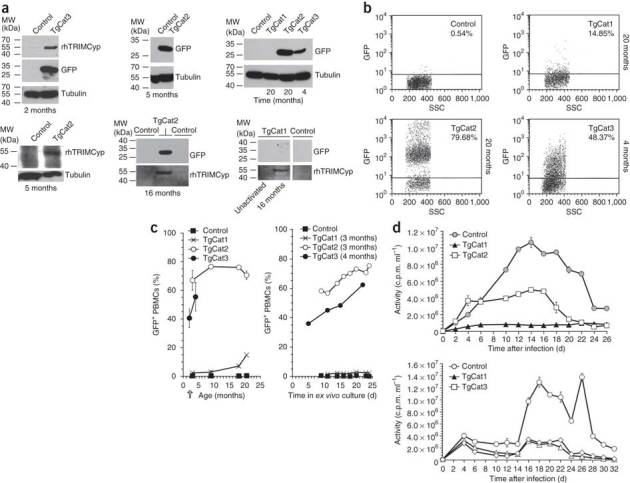
(a) Representative immunoblots for GFP and HA-tagged rhTRIMCyp in PBMCs isolated from transgenic and control cats. All PBMC are activated (PHA-E) except for the TgCat1 sample labeled 'unactivated'. (b) Flow cytometry analysis of GFP expression in activated PBMCs. Percentages of cells that are GFP-positive are indicated. (c) GFP expression in PBMCs versus cat age (left) and GFP expression in PBMCs from a single time point, as a function of days in ex vivo culture; sampling here was at 3–4 months of age (arrow). (d) PBMCs from cat were infected with 105 Crandell feline kidney cells (CrFK) cell-infectious units of FIV on day 0, washed on day 1 and then followed by sampling for supernatant reverse transcriptase activity determination every 48 h as shown. RT, reverse transcriptase; SSC, side scatter.
Fertility, germline transmission and F1 transgene expression
Washed swim-up purified sperm from the two males had normal motility and strongly expressed the transgene as determined by PCR (Fig. 4). Consistent with this result and with the lack of embryo mosaicism when IVF was done after vector microinjection (Supplementary Table 1) germline transmission was readily achieved by direct mating, with all progeny being transgenic. Therefore, the transgenesis procedure preserves fertility, and the germline is transduced. Transgene expression persisted in the F1 offspring of transgenic F0 parents, indicating that silencing did not occur (Fig. 4). Matings of TgCat1 with three nontransgenic queens produced five additional kittens from three pregnancies. Similar to the sire, they were less surface green-fluorescent but were strongly 'PCR- and Southern blot-positive' (data not shown); of these one died perinatally owing to dystocia associated with a hypocontractile uterus. Thus, all F1 cats were transgenic; 8 of 9 were alive and healthy.
Figure 4. Germline transmission and expression in F1 progeny.
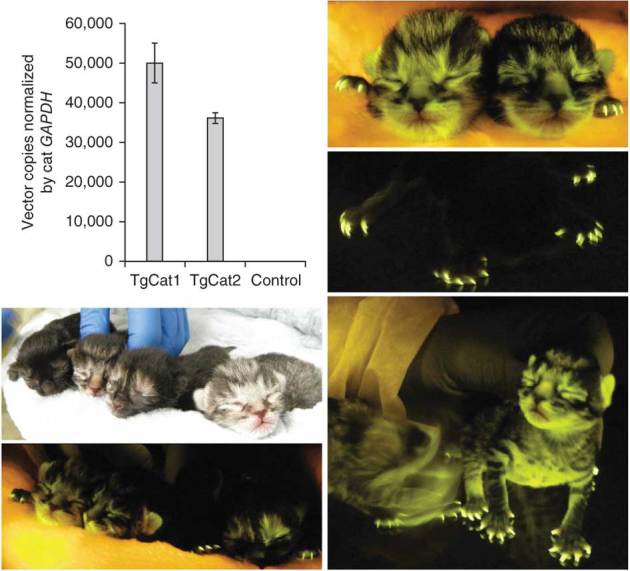
Sperm from the two males (20 months) and a control non-transgenic cat was filtered, pelleted, washed and then purified by the swim-up technique. Sperm genomic DNA was subjected to real time quantitative PCR with primers that amplify the GFP sequence. Images show four F1 progeny of a mating of TgCat1 and TgCat3, imaged for GFP expression; dark fur quenches such that in the black cat only claws were visibly green fluorescent (middle, right).
Whole-body analyses show widespread gene expression
TgCat4 was born after an uncomplicated singleton pregnancy at a normal gestation time (65 d). It was morphologically normal but died during or shortly after parturition from an apparent obstetrical accident involving aspiration, although a precise cause could not be determined at autopsy. This cat provided the opportunity to study all tissues (Fig. 5a). Detailed organ examination and histology did not identify abnormalities. TgCat4 is the product of an oocyte transduced by the TSinG vector, in which GFP was driven by the hCMV promoter, and had ∼10 vector insertions (Fig. 5). As was TgCat3, the kitten was brightly green fluorescent in fur and skin, and immunoblotting revealed abundant GFP expression in all tissues tested: brain, spinal cord, heart, spleen, skin, muscle, liver, kidney, small intestine and stomach (Fig. 5a and Supplementary Fig. 4). Solid viscera were visibly green fluorescent at the gross level, as were adipose tissues (for example, all omental and pericardial fat) and antibody labeling of fixed tissue showed uniform expression in all cells (Fig. 5c). When fresh tissue was sectioned and imaged directly for GFP by epifluorescence microscopy, pervasive expression was similarly evident (Fig. 5d).
Figure 5. Whole body analyses of TgCat4 and late developmental stage fetuses.
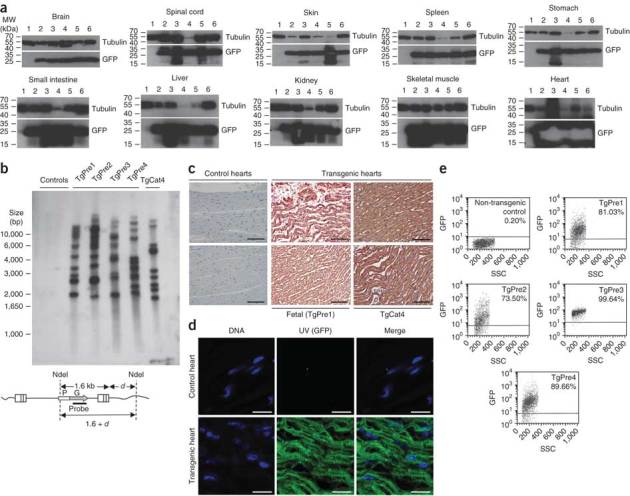
(a) Immunoblotting on lysates from indicated organs from non-transgenic control cat (lanes 1); preterm fetal tissues (lanes 2–5; and TgCat4 (lanes 6). Uncropped versions of these films are available in Supplementary Figure 4. These are minimal (<1 s) film exposures of the immunoblots; the central white-out in the heart GFP band is a result of heavy GFP expression causing artifactual exhaustion of chemiluminescent substrate. (b) Southern blotting for integrated vector DNA. Genomic DNA from heart tissue was digested to completion with NdeI and 5 μg were loaded per lane. Specific bands for intact integrated vector are predicted to be ≥ 1.6 kb. Feline T cell line (FetJ) (left control); control cat; TgPre1–4 from pregnancy C; and TgCat4 are shown. (c) Cardiac muscle from a control cat, TgPre1 and TgCat4 was subjected to indirect immunofluorescence with a monoclonal antibody to GFP. (d) GFP imaged directly in fresh thin sections of TgPre1 myocardium by epifluorescence microscopy. (e) FACS analyses of fetalPBMCs. Scale bars, 100 μm (black bars) and 20 μM (white bars).
A fourth pregnancy (C; Table 1), for which we identified five well-formed, appropriately sized fetal skeletons by X-ray analysis at day 45 of gestation (Supplementary Fig. 2d), ended in serial miscarriages between days 51 and 53 (∼10 d before term). We recovered four of these preterm cats (named TgPre1–4; Table 1) for gross and molecular autopsy. Dissection did not identify birth defects. As for TgCat1–4, Southern blotting showed that TgPre1–4 were each amply transgenic, with 10–13 genomic TSinG vector insertions (Fig. 5b) and GFP expression was similarly found in all tissues tested (Fig. 5a,c). We also probed rhTRIMCyp expression (Supplementary Fig. 5) using organs from a cat that was stillborn after a placental abruption (Table 1; TgCat5), and observed that rhTRIMCyp expression was similarly widespread, including in the main lymphoid organs (lymph node, thymus and spleen). Consistent with the immunoblotting data, tissues of individual organs were green fluorescent at the gross level. FACS of fetal PBMCs from TgPre1–4 showed that 74–100% were GFP-positive (Fig. 5e). Southern blots of genomic DNA from the products of non-singleton pregnancies (Figs. 2b and 5b), showed also that each was the genetically unique product of a different transduced oocyte, and none were a product of twinning after transduction.
Discussion
Our results indicate that transgenic cats may be used as experimental animals for biomedical research. The approach enables transgenesis by germ cell genetic modification for the first time in this species and in any carnivore. Notably, we achieved uniformly transgenic outcomes, which reduce screening cost and time. A second implication of the high efficiency and the copy numbers achieved is that it should be possible to titrate vector dose down or to microinject a mix of vectors into one oocyte to produce complex multi-transgenics. The approach is accessible: feline oocytes competent for efficient transgenesis are readily obtained noninvasively and without added animal procedures from ovaries discarded during routine spaying (laparoscopic or ultrasound-guided percutaneous oocyte retrieval is also feasible). In vitro blastocyst development rates were higher than had been seen previously with SCNT-developed transgenic embryos (19–20% versus 3%)14. We prevented mosaicism by microinjection before IVF and observed germline transmission. The persistence of transgene expression in F1 cats is encouraging for establishing useful transgenic lines. The lack of multiple inbred strains of cat, a current limitation, could be addressed in a focused breeding project.
Introducing a lentiviral restriction factor(s) into the genome of the cat has specific potential because this species is naturally susceptible to lentiviral infection (and AIDS) whereas mice, unmodified or transgenic, are not. Several questions can therefore now be addressed. First, it is unknown whether introducing a single active restriction factor into the genome of an AIDS virus–susceptible species can protect it, and if so, at which of three broadly considered levels: transmission, establishment of sustained viremia and disease development. When antiviral genes are interrogated at the whole animal level by transgenesis in a natural host, results can be surprising and informative. For example, a recent transgenic intervention against influenza in chickens prevented secondary virus transmission to transgenic and nontransgenic contacts, but it had no effect on mortality after primary virus challenge20. Because species-specific lentiviral restriction factors have not been tested by controlled experimental introduction into an animal, the most fundamental question directly answerable with the approach is whether restriction factor transgenesis can mimic natural experiments that normally take place over large expanses of evolutionary time, with selection by viral culling, and render a species genetically immune to its own lentivirus. It is not possible to make clear predictions. For example, there are natural macaque and sootey mangabey TRIM5 alleles that do not block simian immunodeficiency virus transmission to animals that carry them but appear to constrain extent of replication in vivo and to exert selection pressure on the capsid21. When breeding expansion is completed with our present restriction factor transgenic cats, FIV challenges can be done.
Whether or not more than one restriction factor will be needed to achieve antiviral protection, the concept of using them for this purpose in gene therapy has stimulated efforts to devise non-immunogenic human and feline versions12,13. Both of these recently bioengineered TRIMCyps restrict FIV and can be tested in our system. Indeed, FIV is unique among lentiviruses in being restricted by both Old and New World monkey TRIMCyps. We speculate that feline transgenesis with host defense molecules could also confer protection from viral pathogens to wild feline species, all of which face accelerating extinction threats and which are among the most charismatic, ecosystem-iconic taxa in the Carnivora.
Cat transgenesis could have additional impact. As we recently proposed, the domestic cat may have potential for modeling HIV-1 disease itself because, except for entry receptors, the cat genome can supply the dependency factors needed for HIV-1 replication10. This is a fundamental difference compared to the mouse22. Gene knockdowns and targeting are foreseeable by combining our approach with current technologies. Furthermore, transgenesis in this accessible, abundant species with intermediate size and complex neurobehavioral repertoire will permit other human-relevant models in areas such as neurobiology, where the cat is already a paramount model. Studies in the cat have revealed much of the present knowledge on organization of the mammalian brain, in particular the visual cortex23,24,25,26,27; work in this area has been critical to unraveling the neural mechanisms of vision. Although transgenesis in this species will not be as common as in rodents, the creation of a small number of lines with genetic tools could build on the large knowledge base in the species to dramatically alter capability for understanding the cerebral cortex.
Transgenic mice have many advantages, but fundamental differences with human physiology limit their utility in many ways. Many diseases cannot be modeled in mice or rats, with size alone being sometimes intrinsically limiting. Transgenesis has been performed in marmosets28, and, so far without demonstrated germline transmission, in macaques29. These two primate models have clear promise, but limitations arise from scarcity, expense, longer gestation times and, for macaques, prolonged time to sexual maturity (4–8 years) and the requirement to shield handlers from casually transmitted cercopithecine herpesvirus 1. For the purpose of AIDS-relevant work, New World monkeys such as marmosets are not susceptible to any lentivirus.
Even with a generic viral promoter we observed transgene expression in 16 of 16 cat organs tested. We observed rhTRIMCyp expression in the main AIDS-relevant lymphoid tissues (lymph node, spleen and thymus). Mature circulating hematopoietic lineages have notoriously specialized transcriptional environments, but 15–80% of PBMCs in the living cats were GFP-positive in culture. Variation may reflect genome positional effects. Whereas tissue-specific or alternative promoter or enhancer elements can be used, cats with partial PBMC expression profiles also provide a good experimental opportunity because they allow the question of virus-mediated cell lineage selection in vivo30 to be addressed, modeling a realistic cell-based therapy situation, for example, gene therapy for HIV-1 disease. One important issue is whether FIV infection will result in long-term selection of a virus-refractory lymphocyte population as has been observed in nonobese diabetic severe combined immune deficiency (NOD-SCID) IL2Rγnull mice transplanted with CCR5−/− human CD34 cells30. Conversely, if systemic viral replication occurs, we can determine whether escape mutations arise.
Methods
General.
All animal procedures were approved by the Mayo Clinic Institutional Animal Care and Use and Institutional Biosafety Committees. The studies involved specific pathogen-free (SPF) cats (Liberty Research) that were individually housed and provided food and water ad libitum. Vendor tests to exclude specific pathogens were for feline herpesvirus (rhinotracheitis), feline leukemia virus, feline calicivirus, feline coronavirus, feline panleukopenia virus, feline immunodeficiency virus, feline infectious peritonitis, rabies, feline chlamydia and toxoplasmosis. Vaccines given in our facility were: rabies virus feline herpesvirus, calcivirus, panleukopenia virus, Chlamydia psittaci.
The domestic cat is seasonally polyestrous and positively photoperiodic, with seasonality controlled by the duration of light31. Manipulation of day length is used to induce estrus32 and a 14 h light and 10 h dark diurnal cycle was maintained in the facility, with light onset at 06:00. A vasectomized male, verified to be azoospermic, was provided to embryo-recipient females for ad lib mating as shown in Figure 1a.
Ooctye in vitro maturation (IVM).
Gametes used for embryo formation were obtained from gonads discarded after routine elective sterilization. Oocyte-cumulus complexes (COCs) were recovered within 6 h by repeated fine slicing of ovarian tissue in modified phosphate-buffered saline (mPBS) supplemented with 4 mg ml−1 bovine serum albumin (BSA) and 50 μg ml−1L gentamicin. Only grade I and II oocytes33 were used. Selected COCs were washed and matured in modified TCM-199 (Gibco) containing 1 IU ml−1 human chorionic gonadrotropin (HCG), 0.5 IU ml−1 pregnant mare serum gonadotropin (PMSG), 10 μg ml−1 epidermal growth factor (EGF), and 4 mg ml−1 BSA in a humidified atmosphere of 5% CO2 in air at 38 °C for 28 h.
In vitro fertilization and in vitro culture.
Twenty-eight hours after IVM, cooled spermatozoa were washed twice in Brackett-Oliphant medium supplemented with 137 μg ml−1 sodium pyruvate, 4 mg ml−1 BSA, and 50 μg ml−1 gentamicin by centrifugation at 1800 rpm for 5 min. The supernatant was removed and sperm pellet was diluted in 500 μL fertilization medium (G-IVF Plus, Vitrolife), and placed in the incubator to allow sperm swim-up for 30 min. The spermatozoa concentration was adjusted to 2 × 106 ml−1. Ten oocytes were transferred into each of 100 μl sperm microdroplets under mineral oil and co-cultured for 12 h, after which presumptive zygotes were removed from sperm with a small-bore pipette, washed, and cultured in a modified Earl's balanced salt solution (MK-1) supplemented with 4 mg ml−1 BSA and 50 μg ml−1 gentamicin for 3 d. Three days after sperm exposure, cleaved embryos were selected for transfer or subsequently cultured in MK-1 medium supplemented with 5% (v/v) FBS (FBS, Hyclone laboratories) and 50 μg ml−1 gentamicin for a further 4 days to evaluate developmental capacities to morula and blastocyst stages.
Transgenic embryo production.
Before to lentiviral vector microinjection, cumulus cells were mechanically removed from the oocytes 18–20 h after incubation in maturation medium (pre-IVF injection group) or from presumptive zygotes at 12–14 h post-incubation in fertilization medium (post-IVF injection group). A volume of ∼100 pl vector was injected directly into the oocyte perivitelline space 12 h before or 12 h after IVF using a finely pulled glass capillary (Femtotips, Eppendorf) connected to a microinjector (Eppendorf FemtoJet) adjusted for injection and compensation pressure with an injection time of 12 s. After microinjection, the oocytes were washed and returned to culture in IVM medium until hour 28 of maturation when they were used for IVF. For post-IVF injection, zygotes were washed and subsequently cultured in MK-1 medium. With the conditions developed, oocytes were modified at high rates without apparent toxicity to the zygotes early development and timing microinjection before fertilization created reliably non-mosaic embryos.
Embryo transfer, pregnancy detection, parturition and photography.
Healthy 2–3-year-old SPF queens were the recipients for embryo transfer. They were induced with 150 IU PMSG injected intramuscularly at 96–120 h before IVF, followed by injection of 100 IU of HCG 72 h after the PMSG. In addition, ad lib mating with a vasectomized male was done from the day of HCG injection until the day before embryo transfer.
The females were anesthetized on the day of transfer with ketamine (5 mg kg−1), medetomidine (0.03 mg/kg) and buprenorphine (0.01 mg kg−1) administered intramuscular and maintained with 1–3% isoflurane gas. Prior to abdominal incision the medetomidine was reversed with an intramuscular injection of atipamezole to minimize any effects the alpha-2 agonist may have on transfer success. An approximately 2 cm ventral midline incision was made and ovaries and fallopian tube exteriorized. Each ovary was examined for evidence of ovulation. If no corpus hemorrhagicum or corpus luteum was visualized, follicles were punctured with a needle to artificially induce ovulation. Then, a transmural puncture of the fallopian tube was performed with a 28 gauge needle and this was replaced with a fine hand-pulled glass transfer pipette, through which fifteen to twenty-five pre-loaded embryos (transduced, cleaved, >4 cell stage) in 10-20 μl MK-1 medium were transferred per fallopian tube under microscopic visualization using gentle positive mouth-controlled pressure. The pipette was withdrawn and the incision was closed in three layers.
Pregnancy status was determined with a canine Relaxin kit (Synbiotics) on day 30 after transfer and by film radiography on day 45. Pregnant recipients were monitored daily until delivery of term kittens which occurred by un-assisted spontaneous vaginal birth at term. All control and transgenic animal photographs were taken with a Nikon camera at the same time using identical lighting, filter, and camera settings, with GFP imaged under blue light illumination with a long pass filter. Supplementary Figure 2 contains additional images.
Immunofluorescence microscopy and immunohistochemistry.
Blastocysts (Fig. 1c) were attached to a slide with BD-Cell Tak, cell and tissue adhesive, fixed and permeabilized for 15 min at room temperature in PBS supplemented with 4% (w/v) paraformaldehyde and 1% (v/v) Triton X-100 and blocked with 1% BSA in PBS for 15 min. Transduced and control blastocysts and activated PBMCs were imaged by confocal microscopy with GFP fluroescence imaged directly and HA-tagged rhTRIMCyp detected using primary anti-HA (high affinity anti-HA rat monoclonal, Roche, used at 1:1000 dilution), with incubation for 1 h at RT, washed, followed by incubation with Cy3-conjugated goat anti-rat IgG secondary (1:500 dilution, Chemicon International) for 1 h. Controls with each protein alone verified no signal cross-reception between channels and blastocysts derived from untransduced ova were negative as shown. Following three 5 min washing steps in PBS and mounting with addition of Prolong Gold anti-fade reagent with DAPI (Invitrogen) for nuclear DNA staining, the embryos were analyzed by laser confocal microscopy (Axiovert 100M; Carl Zeiss MicroImaging).
Animal tissues were fixed with 4% paraformaldehyde and paraffin-embedded. Serial 10 μm sections were made. Immunohistochemistry was performed using a DAKO Envision Plus kit. Sections were dewaxed in xylene and rehydrated in alcohol. Endogenous peroxidase activity was blocked with 0.03% hydrogen peroxide. Sections were incubated with a 1:200 diluted primary mouse monoclonal antibody (Clontech, JL8, 1:5000) for 2 h. Dako Envision anti-mouse secondary antibody (1:200) was then applied for 30 min. The sections were mounted using Prolong Gold anti-fading reagent and observed by light microscopy.
Vectors and FIV infections.
All vectors and vector sequences are available from the authors upon request. Lentiviral vectors were HIV-1-based to permit PCR-based tracking of infectious FIV in future experiments. GFP is the enhanced version (eGFP). TSiN series lentiviral vectors were previously described5, and were prepared using 293T transfection in Nunc Cell Factories and concentrated by ultracentrifugation using established methods34,35,36. The transfer vectors have cPPT-CTS and WPRE elements and are U3-deleted. Dual gene vectors with rhesus (Macaca mulatta) TRIMcyp8 and eGFP utilize either a porcine teschovirus 2A peptide37 expressing a single pro-protein (human cytomegalovirus immediate early gene (hCMV)-promoted rhTRIMCyp-P2A-GFP) or a bi-directional promoter kindly provided by Amendola et al.38 with tandemly arranged phosophglycerate kinase (PGK) and minimal CMV (mCMV, 0.16 kb) promoter elements driving rhTRIMcyp and GFP respectively on opposite strands. VSV-G-pseudotyped vectors were produced in two-chamber Cell Factories (CF2) and concentrated by ultracentrifugation over a sucrose cushion as described5,36. Vectors were titrated on feline kidney cell line (CrFK) cells using flow cytometry for GFP expression. Reverse transcriptase activities were used to normalize preparations36. PBMCs were cultured in RPMI with 10% FCS, rhIL-2 and antibiotics and were activated with 10 μg ml−1 PHA-E. For FIV infection of PBMCs, 50,000 feline PBMCs were infected with 2 × 106 RT activity units (10 μl) of FIV 34TF1039 generated by 293T cell transfection of pCT5orfArep, a version of pCT540 in which we repaired the premature ORF-A stop codon by overlap extension PCR to enable PBMC replication. Supernatants were collected approximately every 2 d thereafter and assayed for reverse transcriptase activity as described above.
Immunoblotting.
Transfected cell lysate or minced tissue samples were homogenized in RIPA (150 mM NaCL, 0.5% deoxycholate, 0.1% sodium dodecyl sulfate, 1% NP-40, 150 mM Tris-HCl, pH 8.0) supplemented with protease inhibitors (complete-Mini, Boehringer). Fractions and lysates were boiled in Laemmli supplemented with β-Mercaptoethanol for 10 min, separated by gel electrophoresis, transferred onto PVDF membranes (immobilon-P, Millipore), and blocked in mPBS containing 2 mg ml−1 BSA and 1% Tween 20 for 1 h at room temperature (22–25 °C). Blots were treated with primary antibodies against: GFP (JL8, 1:5000, Clontech), α-tubulin (mouse monoclonal antibody 1:8,000, Sigma), HA (high affinity anti-HA antibody, rat monoclonal, 1:1,000, Roche, cat # 11867423001) for 1 h at room temperature. After washing, secondary antibodies were applied: alkaline phosphatase-conjugated goat anti-mouse IgG (Calbiochem) diluted 1:10,000, and alkaline phosphatase-conjugated goat anti-rat IgG (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) diluted 1:1000. Membranes were then incubated with ECL reagent (Thermo Scientific) and exposed to film.
Sperm collection and storage.
Epidydymi were separated by dissection within 6 h and repeatedly finely sliced in mPBS supplemented with 4 mg ml−1 BSA and 50 μg ml−1 gentamicin to release spermatozoa. The medium was filtered with a 70 μM Cell Strainer (BD Falcon) and centrifuged at 18,000 rpm for 5 min. Sperm pellets were resuspended in 500 μl TEST yolk buffer (Refrigeration Medium, Irving Scientific) in a 1.5-ml microcentrifuge tube at room temperature and gradually cooled to 4 °C. The samples were kept at 4 °C until use, or cryopreserved in liquid nitrogen. Sperm of transgenic males was obtained by electroejaculation.
Southern blotting.
Genomic DNA of newborn and spontaneously aborted kittens was analyzed by Southern blot hybridization and PCR. Total DNA was isolated from blood, tail tips and heart using the DNeasy blood and tissue kit (Qiagen). Five micrograms DNA was digested with AflIII, BamH1 or NdeI as indicated. DNA fragments were separated by electrophoresis on 0.8% agarose gel and transferred by capillary action to a Nytran Supercharge membrane (Schleicher & Schuell Bioscience). DNA was cross-linked to the membrane using a UV Crosslinker (UVC500; Hoefer). Blots were then hybridized overnight at 42 °C in ULTRAhyb (Ambion) containing an 32P-labeled eGFP probe. After washing at 60 °C with 0.5% SDS, 2× SSC followed by 0.5% SDS, 0.1× SSC, the blots were exposed to the Kodak BioMax MS X-ray film (Sigma-Aldrich) with intensifying screen at -80 °C and developed. Bands in Figure 5b and the right blot of Figure 2b are more widely spaced than bands in the left blot of Figure 2b because NdeI and BamHI cleave, on average, every 4,096 bp apart, while AflIII cuts on average every 1024 nt bp.
Quantitative RT-PCR and semi-quantitative PCR.
Transgenic and control genomic DNA samples (PBMC, tail tip and organs) were analyzed by real-time quantitative PCR using the Roche FastStart DNA Master SYBR Green Kit I. Samples were quantified against a serially-diluted plasmid standard for total GFP using the Roche LightCycler and Roche LCDA software. Initial denaturation was at 95 °C for 10 min and a melting step after amplification (40–95 °C, temperature transition rate = 0.05 °C s−1). GFP was amplified using 300 nM each sense primer 5′-AGAACGGCATCAAGGTGAAC-3′ and antisense primer 5′-TGCTCAGGTAGTGGTTGTCG-3′. PCR amplification and analysis was performed as follows; 95 °C for 10 s, 62 °C for 10 s, 72 °C for 10 s, × 35 cycles, temperature transition rate = 5 °C s. As a loading control feline GAPDH was quantified using 300 nM each sense primer 5′-ACCACAGTCCATGCCATCAC-3′ and antisense primer 5′-TCCACCACCCGGTTGCTGTA-3′. PCR amplification and analysis was performed using a Roche Lightcycler as follows: 95 °C for 10 s, 54 °C for 10 s, 72 °C for 18 s, × 35 cycles, temperature transition rate = 5 °C s. Semiquantitative analysis for rhesus TRIMCyp was performed using Phusion Hot Start High-Fidelity DNA Polymerase (Finnzymes) in a standard thermocycler. The entire rhesus TRIMCyp transgene (1.4 kb) was amplified using 500 nM each sense primer 5′-ATGTACCCATACGATGTTCC-3′ and antisense primer 5′-GCCGCTTATTCGAGTTGCC-3′. The program included an initial denaturation step at 98 °C for 30 s. PCR amplification was performed as follows; 98 °C for 7 s, 60 °C for 20 s, 72 °C for 30 s. A final extension step at 72 °C for 7 min concludes the program. Reactions proceeded to either 5, 10, 20, 30, 40 or 50 cycles. PCR products were analyzed on a 1% agarose gel and compared to amplified transfer construct plasmid.
Supplementary information
Supplementary Figures 1–5 and Supplementary Tables 1–3 (PDF 2718 kb)
Acknowledgements
Funding from US National Institutes of Health grants AI47536 and EY14411 assisted prior key technology developments. We thank the Helen C. Levitt Foundation for initial pilot funding and A. Keller for coordinating it, members of our laboratory for helpful discussions and assistance, H. Fadel for assisting with site-directed mutagenesis, members of our transgenic mouse core for sharing microinjection equipment, G. Towers (University College London) for a rhTRIMCyp cDNA, and Mayo Clinic veterinary staff for advice and surgical assistance.
Author Contributions
All authors designed experiments, analyzed data and critiqued the manuscript. E.P. conceived the project and recruited P.W. and T.O. E.P. and T.O. oversaw the project. P.W. and D.S. produced vector and retrieved gametes; P.W. microinjected vector and did embryo cultures. P.W. transfered embryos with assistance from T.R. and E.P. with surgery. P.W., D.S and T.R. monitored cats, did cell and tissue assays and virology. P.W., D.S. and E.P. wrote the manuscript.
Competing interests
The authors declare no competing financial interests.
References
- 1.Menotti-Raymond, M. & O'Brien, S.J. The domestic cat, Felis catus, as a model of hereditary and infectious disease. in Sourcebook of Models for Biomedical Research (ed., Conn, P.M.) 221–232 (Humana Press, 2008).
- 2.O'Brien SJ, et al. State of cat genomics. Trends Genet. 2008;24:268–279. doi: 10.1016/j.tig.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meli ML, et al. Feline leukemia virus and other pathogens as important threats to the survival of the critically endangered Iberian lynx (Lynx pardinus) PLoS ONE. 2009;4:e4744. doi: 10.1371/journal.pone.0004744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Willett BJ, et al. Shared usage of the chemokine receptor CXCR4 by the feline and human immunodeficiency viruses. J. Virol. 1997;71:6407–6415. doi: 10.1128/jvi.71.9.6407-6415.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Llano M, et al. An essential role for LEDGF/p75 in HIV integration. Science. 2006;314:461–464. doi: 10.1126/science.1132319. [DOI] [PubMed] [Google Scholar]
- 6.Huthoff H, Towers GJ. Restriction of retroviral replication by APOBEC3G/F and TRIM5alpha. Trends Microbiol. 2008;16:612–619. doi: 10.1016/j.tim.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saenz DT, Teo W, Olsen JC, Poeschla E. Restriction of feline immunodeficiency virus by Ref1, LV1 and primate TRIM5a proteins. J. Virol. 2005;79:15175–15188. doi: 10.1128/JVI.79.24.15175-15188.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson SJ, et al. Independent evolution of an antiviral TRIMCyp in rhesus macaques. Proc. Natl. Acad. Sci. USA. 2008;105:3557–3562. doi: 10.1073/pnas.0709003105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Münk C, et al. Functions, structure, and read-through alternative splicing of feline APOBEC3 genes. Genome Biol. 2008;9:R48. doi: 10.1186/gb-2008-9-3-r48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stern MA, et al. Productive replication of Vif-chimeric HIV-1 in feline cells. J. Virol. 2010;84:7378–7395. doi: 10.1128/JVI.00584-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McEwan WA, et al. Truncation of TRIM5 in Feliformia explains the absence of retroviral restriction in cells of the domestic cat. J. Virol. 2009;16:8270–8275. doi: 10.1128/JVI.00670-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dietrich I, et al. Potent lentiviral restriction by a synthetic feline TRIM5 cyclophilin A fusion. J. Virol. 2010;84:8980–8985. doi: 10.1128/JVI.00858-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neagu MR, et al. Potent inhibition of HIV-1 by TRIM5-cyclophilin fusion proteins engineered from human components. J. Clin. Invest. 2009;119:3035–3047. doi: 10.1172/JCI39354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yin XJ, et al. Generation of cloned transgenic cats expressing red fluorescence protein. Biol. Reprod. 2008;78:425–431. doi: 10.1095/biolreprod.107.065185. [DOI] [PubMed] [Google Scholar]
- 15.Gomez MC, et al. Generation of domestic transgenic cloned kittens using lentivirus vectors. Cloning Stem Cells. 2009;11:167–176. doi: 10.1089/clo.2008.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meissner A, Jaenisch R. Mammalian nuclear transfer. Dev. Dyn. 2006;235:2460–2469. doi: 10.1002/dvdy.20915. [DOI] [PubMed] [Google Scholar]
- 17.Yamanaka S, Blau HM. Nuclear reprogramming to a pluripotent state by three approaches. Nature. 2010;465:704–712. doi: 10.1038/nature09229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pfeifer A. Lentiviral transgenesis–a versatile tool for basic research and gene therapy. Curr. Gene Ther. 2006;6:535–542. doi: 10.2174/156652306777934856. [DOI] [PubMed] [Google Scholar]
- 19.Richardson MW, et al. Mode of transmission affects the sensitivity of human immunodeficiency virus type 1 to restriction by rhesus TRIM5alpha. J. Virol. 2008;82:11117–11128. doi: 10.1128/JVI.01046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lyall J, et al. Suppression of avian influenza transmission in genetically modified chickens. Science. 2011;331:223–226. doi: 10.1126/science.1198020. [DOI] [PubMed] [Google Scholar]
- 21.Lim SY, et al. TRIM5alpha modulates immunodeficiency virus control in Rhesus monkeys. PLoS Pathog. 2010;6:e1000738. doi: 10.1371/journal.ppat.1000738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bieniasz PD, Cullen BR. Multiple blocks to human immunodeficiency virus type 1 replication in rodent cells. J. Virol. 2000;74:9868–9877. doi: 10.1128/JVI.74.21.9868-9877.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hubel DH, Wiesel TN. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J. Physiol. (Lond.) 1962;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hubel DH, Wiesel TN. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J. Physiol. (Lond.) 1970;206:419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blakemore C, Van Sluyters RC. Innate and environmental factors in the development of the kitten's visual cortex. J. Physiol. (Lond.) 1975;248:663–716. doi: 10.1113/jphysiol.1975.sp010995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blakemore C, Van Sluyters RC, Peck CK, Hein A. Development of cat visual cortex following rotation of one eye. Nature. 1975;257:584–586. doi: 10.1038/257584a0. [DOI] [PubMed] [Google Scholar]
- 27.Douglas RJ, Martin KA. Neuronal circuits of the neocortex. Annu. Rev. Neurosci. 2004;27:419–451. doi: 10.1146/annurev.neuro.27.070203.144152. [DOI] [PubMed] [Google Scholar]
- 28.Sasaki E, et al. Generation of transgenic non-human primates with germline transmission. Nature. 2009;459:523–527. doi: 10.1038/nature08090. [DOI] [PubMed] [Google Scholar]
- 29.Yang SH, et al. Toward a transgenic model of Huntington's disease in a non-human primate. Nature. 2008;453:921–924. doi: 10.1038/nature06975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holt N, et al. Human hematopoietic stem/progenitor cells modified by zinc-finger nucleases targeted to CCR5 control HIV-1 in vivo. Nat. Biotechnol. 2010;28:839–847. doi: 10.1038/nbt.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pineda, M.H. Reproductive patterns of cats. in McDonald's Veterinary Endocrinology and Reproduction (eds. Pineda, M.H. & Dooley, M.P.) 505–522 (Iowa State Press, 2003).
- 32.Leyva H, Madley T, Stabenfeldt GH. Effect of light manipulation on ovarian activity and melatonin and prolactin secretion in the domestic cat. J. Reprod. Fertil. Suppl. 1989;39:125–133. [PubMed] [Google Scholar]
- 33.Wood TC, Wildt DE. Effect of the quality of the cumulus-oocyte complex in the domestic cat on the ability of oocytes to mature, fertilize and develop into blastocysts in vitro. J. Reprod. Fertil. 1997;110:355–360. doi: 10.1530/jrf.0.1100355. [DOI] [PubMed] [Google Scholar]
- 34.Loewen N, Poeschla EM. Lentiviral vectors. Adv. Biochem. Eng. Biotechnol. 2005;99:169–191. doi: 10.1007/10_007. [DOI] [PubMed] [Google Scholar]
- 35.Saenz, D.T., Barraza, R., Loewen, N., Teo, W. & Poeschla, E. Production and Use of Feline Immunodeficiency Virus (FIV)-based lentiviral vectors. in Gene Transfer: A Cold Spring Harbor Laboratory Manual (eds. Rossi, J. & Friedman, T.) 57–74 (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 2006).
- 36.Miest T, Saenz D, Meehan A, Llano M, Poeschla EM. Intensive RNAi with lentiviral vectors in mammalian cells. Methods. 2009;47:298–303. doi: 10.1016/j.ymeth.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Szymczak AL, Vignali DA. Development of 2A peptide-based strategies in the design of multicistronic vectors. Expert Opin. Biol. Ther. 2005;5:627–638. doi: 10.1517/14712598.5.5.627. [DOI] [PubMed] [Google Scholar]
- 38.Amendola M, Venneri MA, Biffi A, Vigna E, Naldini L. Coordinate dual-gene transgenesis by lentiviral vectors carrying synthetic bidirectional promoters. Nat. Biotechnol. 2005;23:108–116. doi: 10.1038/nbt1049. [DOI] [PubMed] [Google Scholar]
- 39.Talbott RL, et al. Nucleotide sequence and genomic organization of feline immunodeficiency virus. Proc. Natl. Acad. Sci. USA. 1989;86:5743–5747. doi: 10.1073/pnas.86.15.5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poeschla E, Wong-Staal F, Looney D. Efficient transduction of nondividing cells by feline immunodeficiency virus lentiviral vectors. Nat. Med. 1998;4:354–357. doi: 10.1038/nm0398-354. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1–5 and Supplementary Tables 1–3 (PDF 2718 kb)


