Abstract
Regeneration of blood vessels in ischemic neuronal tissue is critical to reduce tissue damage in diseases. In proliferative retinopathy, initial vessel loss leads to retinal ischemia, which can induce either regrowth of vessels to restore normal metabolism and minimize damage, or progress to hypoxia-induced sight-threatening pathologic vaso-proliferation. It is not well understood how retinal neurons mediate regeneration of vascular growth in response to ischemic insults. In this study we aim to investigate the potential role of Sirtuin 1 (Sirt1), a metabolically-regulated protein deacetylase, in mediating the response of ischemic neurons to regulate vascular regrowth in a mouse model of oxygen-induced ischemic retinopathy (OIR). We found that Sirt1 is highly induced in the avascular ischemic retina in OIR. Conditional depletion of neuronal Sirt1 leads to significantly decreased retinal vascular regeneration into the avascular zone and increased hypoxia-induced pathologic vascular growth. This effect is likely independent of PGC-1α, a known Sirt1 target, as absence of PGC-1α in knockout mice does not impact vascular growth in retinopathy. We found that neuronal Sirt1 controls vascular regrowth in part through modulating deacetylation and stability of hypoxia-induced factor 1α and 2α, and thereby modulating expression of angiogenic factors. These results indicate that ischemic neurons induce Sirt1 to promote revascularization into ischemic neuronal areas, suggesting a novel role of neuronal Sirt1 in mediating vascular regeneration in ischemic conditions, with potential implications beyond retinopathy.
Keywords: Acetylation, Hypoxia-induced factor, Ischemic retinopathy, Neovascularization, Retinal ganglion cell, Revascularization, Sirtuin1
Introduction
Ischemic proliferative retinopathies, including retinopathy of prematurity (ROP) and diabetic retinopathy (DR), are leading causes of blindness [1]. Retinopathy occurs in two phases, with a first phase of vessel loss due to prematurity or diabetes, and a second phase of hypoxia-induced abnormal proliferation of pathologic vessels. The morphology of pathologic neovascularization is distinctly different from normal vasculature with increased leakage and associated retinal detachment and blindness. Physiologic revascularization also occurs in the second phase of retinopathy, which repairs initial vessel loss in the ischemic avascular zone to restore finely branched normal vasculature. Current interventions focus on suppression of the late phase proliferative neovessels through surgical ablation of hypoxic retina or by anti-VEGF (vascular endothelial growth factor) therapy, which also can inhibit physiologic revascularization into the avascular zone. However, promoting normal revascularization to repair ischemic neuronal tissue will prevent the ischemic and metabolic stimuli triggering blinding neovessel proliferation [2].
To achieve the goal of preventing neovascularization, it is essential to understand how ischemic neurons respond to vessel loss and metabolic stress to mediate revascularization. Emerging evidence demonstrates a role of neuronal influence in the process of guiding vascular growth in retinopathy [2, 3]. Not only neuronal guidance growth factors but also neuron-derived energy dependent metabolites are implicated in influencing vascular development in retinopathy [2, 4–6], suggesting that the metabolic state of retinal neurons play a significant role in determining vascular response in retinopathy. We hypothesized that Sirtuin1 (Sirt1), a metabolic and nutrient sensitive regulator [7], is involved in this ischemia-induced reparative angiogenic process.
Sirt1 is a NAD+ dependent protein deacetylase activated under stress condition to regulate cell cycles and longevity [8]. Increased levels of Sirt1 in neurons protect against several neuronal degenerative diseases such as Alzheimer’s [9] and Huntington’s disease [10], both of which are precipitated by neuronal ischemia. Deletion of Sirt1 in neurons also affects normal cognitive function [11]. In addition to neuronal expression, Sirt1 in vascular endothelial cells can also directly regulate endothelial cell growth [12, 13]. Little is known, however, about the potential role of neuronal Sirt1 in neurovascular cross-talk in ischemic conditions to direct blood vessel growth to revascularize and restore ischemic tissue, which is the focus of this study.
Here we used a mouse model of oxygen-induced ischemic retinopathy (OIR) to explore the potential role of neuronal Sirt1 in mediating retinal revascularization. OIR is characterized by vessel loss followed by vascular regrowth and hypoxia-induced neovascularization [14]. This is a well-established animal model of ROP and also mimics some aspects of neovascularization of proliferative DR in humans [15]. Mice with neuronal-specific conditional depletion of Sirt1 were generated and analyzed in this model. We found significant upregulation of Sirt1 in the retinal ganglion cell layer of avascular retinal area in OIR. Neuronal depletion of Sirt1 significantly dampened retinal vascular regrowth in OIR, associated with aggravated pathologic neovascularization. These effects are likely mediated through Sirt1 regulation of hypoxia-induced factor (HIF) acetylation and expression of angiogenic growth factors.
Methods
Animals
These studies were approved by the Children’s Hospital Boston Animal Care and Use Committee. Sirt1flox|flox mice were previously described [16]. Nestin-Cre mice (stock# 003771) and PGC-1α−/− (stock# 008597) were obtained from Jackson Laboratory. Both Sirt1 cKO mice and PGC-1α−/− mice were backcrossed to C57BL/6 mice for at least 9 generations.
Oxygen-induced retinopathy, retina dissection and quantification of vaso-obliteration and vaso-proliferation
To induce oxygen-induced retinopathy (OIR), neonatal mice were exposed to 75 % oxygen from P7 to P12 [14]. Eyes were dissected at various ages and retinal blood vessels stained with fluoresceinated Isolectin B4. Quantification of retinal vasculature was carried out as described previously by using Adobe Photoshop or Image J [17–19], with specifics detailed in supplemental methods.
Cell culture
The rat ganglion cell line, RGC-5, was a kind gift from N. Agarwal. These cells were differentiated by incubation with 1 μM staurosporine for 12 h, which maintains them in a post-mitotic neuronal state [20]. Cells were treated with Sirt1 inhibitor Ex527 (1 or 10 mM) or DMSO control for 24 h under hypoxia (2 % O2) and cell lysates were isolated for gene expression, immunoprecipitation or Western blots. For conditioned medium, Ex527-containing medium was removed and replaced with fresh DMEM (Invitrogen) conditioned by stimulated RGC-5 cells for 24 h, at which point the medium was collected, filtered and subsequently used to culture aortic ring explants and for the Epo ELISA assay.
Aortic ring explant
Aortae from C57Bl/6J mice were cut into 1-mm-thick rings and placed in growth factor–reduced Matrigel (BD Biosciences) in 24-well tissue culture plates and cultured for 2 days in EBM-2 medium (Clonetics) [21], replaced by conditioned medium from RGC-5 cell culture treated previously with Sirt1 inhibitor Ex527 for 72 h. Images of individual explants were taken and the microvascular sprouting area was quantified by measuring the area covered by outgrowth of the aortic ring with Adobe Photoshop. Results are expressed as mean ± SEM.
Statistical analysis
Results were presented as mean ± SEM and were analyzed using a 2-tailed Student t test. p values ≤0.05 is considered statistically significant.
Results and discussion
Sirt1 is upregulated in avascular neurons in OIR
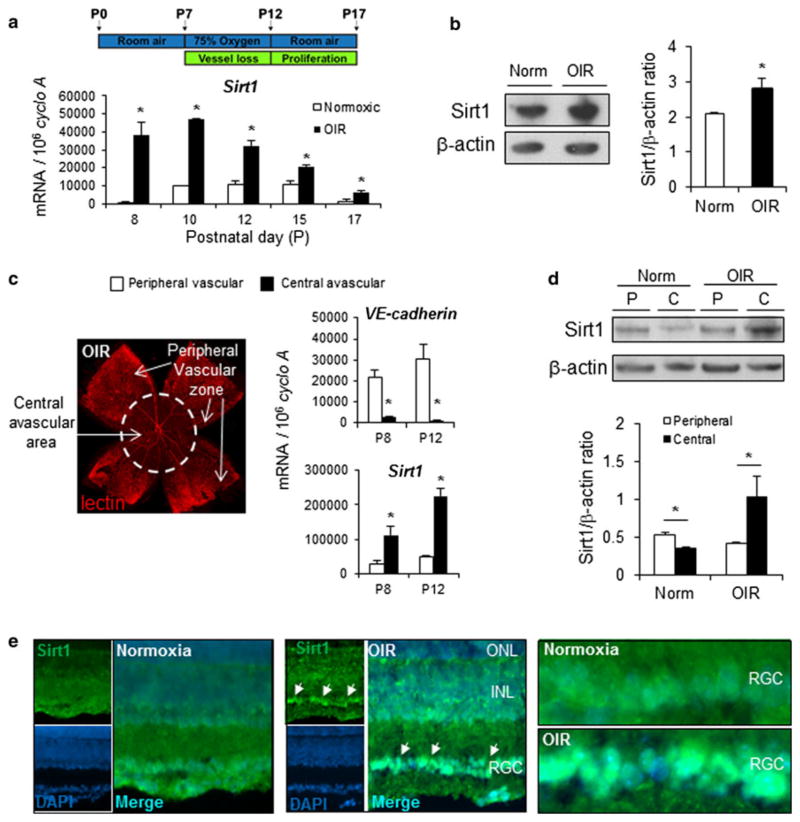
To induce retinopathy, mouse pups with nursing mothers were exposed to 75 % oxygen from postnatal day (P) 7 to day 12, and returned to room air until P17 [14] (Fig. 1a). Central retinal vessel loss is induced during oxygen exposure starting from 8 h after oxygen treatment, and continuing to P12. After returning to room air, revascularization of avascular central retinal area occurs, associated with pathologic neo-vascularization at the border between avascular and vascular zones [18, 22]. We found that Sirt1 mRNA expression is consistently upregulated in retinas with OIR (Fig. 1a). The upregulation of Sirt1 protein is confirmed with quantification of Western blots (Fig. 1b). Next, we used micro-dissection to separate the central avascular zones and the peripheral vascularized regions of OIR-exposed retinas at P8 and P12 (Fig. 1c). Absence of endothelial cell marker VE-cadherin mRNA expression confirmed lack of blood vessels in the samples from the central areas (Fig. 1c). Importantly, Sirt1 mRNA levels are significantly induced in the central avascular zone of the retinas compared to the peripheral vascularized regions (Fig. 1c), suggesting stress-induced upregulated of Sirt1 in retinal neurons from the avascular region. The upregulation of Sirt1 in the central avascular region in OIR is confirmed at protein levels at P12 in micro-dissected samples, compared to peripheral samples (Fig. 1d). In addition, Sirt1 antibody staining shows marked enrichment of staining in the nuclear region of the retinal ganglion cell (RGC) layer (Fig. 1e), suggesting that in OIR, Sirt1 is upregulated in retinal neurons in the avascular region of RGC layer in response to retinal ischemia.
Fig. 1.
Sirt1 is induced in avascular retina in a mouse model of oxygen-induced retinopathy (OIR). a Sirt1 mRNA expression is significantly upregulated in a mouse model of oxygen-induced retinopathy, in which neonatal pups are exposed to 75 % oxygen from postnatal day (P) 7 to P12. b Sirt1 protein levels are upregulated in OIR retinas compared to normoxic controls (Norm) at P8. c At P12 in OIR, retinal vasculature is visualized with lectin staining (red) showing central avascular zone and peripheral vascular zones (arrows), which were separated by micro-dissection. Sirt1 and VE-cadherin mRNA expression levels were quantified with RT-qPCR in peripheral vascular versus central avascular samples isolated at P8 and P12 in OIR. Central avascular retina samples show a lack of vascular endothelial cell marker VE-cadherin, confirming absence of vessels. Sirt1 is significantly up-regulated in central avascular retinal samples compared to samples from peripheral vascular region. d Western blot with Sirt1 antibody shows upregulated Sirt1 protein levels in samples isolated from central avascular regions of retinas in OIR compared with peripheral vascular regions at P12. In room air control retinas (Norm) not exposed to OIR, Sirt1 levels are lowered in the central region (C) compared to peripheral retinal region (P). e Immunohistochemistry staining of retinal cross sections from P17 OIR mouse or room air control shows increased staining of Sirt1 antibody (green) in the retinal ganglion cell layer (arrows), with enrichment in the cellular nucleus region visualized by double staining of DAPI (blue). ONL outer nuclear layer, INL inner nuclear layer, RGC retinal ganglion cell layer. *p ≤ 0.05. (Color figure online)
Depletion of neuronal Sirt1 dampens vascular regrowth in retinopathy
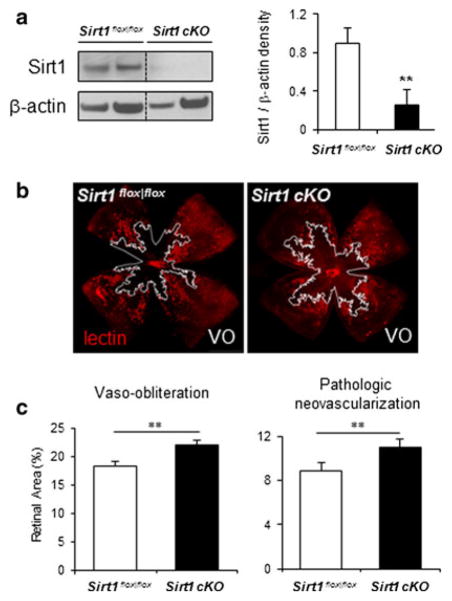
Since systemic Sirt1−/− mice survive infrequently after birth [16], we generated neuronal specific conditional knockout of Sirt1 (Sirt1 cKO) by crossing floxed Sirt1 knockout mice (Sirt1flox|flox) [16] with Nestin-Cre mice expressing Cre recombinase under neuronal specific nestin promoter. Sirt1 cKO mice are live, fertile and grossly normal with significantly decreased levels of Sirt1 protein in the retina compared to littermate control Sirt1flox|flox mice (Fig. 2a). With OIR, Sirt1 cKO retinas show significantly increased levels of vaso-obliteration (VO) at P17 compared with litter mate Sirt1flox|flox controls (22.1 ± 0.9 % of total retinal area in Sirt1 cKO versus 18.3 ± 0.8 % of total retinal area in controls, n = 18–20 per group, p = 0.003, Fig. 2b, c), and are associated with significantly increased levels of pathologic neovascularization (11.0 ± 0.5 % of total retinal area in Sirt1 cKO versus 8.8 ± 0.4 % of total retinal area in controls, p = 0.009, Fig. 2b, c). Vaso-obliteration at P9 and P12 during oxygen exposure is comparable between Sirt1 cKO and Sirt1flox|flox retinas (Fig. S1a), suggesting that the increased vaso-obliteration in Sirt1 cKO retinas at P17 results from dampened revascularization of the avascular retina after returning to room air at P12. There is a ~20 % reduction of revascularization and hence a ~20 % increase in VO areas in Sirt1 cKO retina compared to controls at P17. This marked inhibition of revascularization is associated with aggravated neovascularization. Retinal vascular growth during normal development is not affected in Sirt1 cKO retina (Fig. S1b), indicating that neuronal Sirt1 acts exclusively as a stress response mechanism in diseased retinal conditions but does not impact normal angiogenesis. In addition, we confirmed that the expression of Cre recombinase alone in retinal neurons without a floxed gene (Nes–Cre) in mice does not affect retinopathy outcome compared to littermate wild type (WT) controls (Fig. S2a, b).
Fig. 2.
Depletion of neuronal Sirt1 decreases retinal revascularization in oxygen-induced retinopathy and consequently precipitates pathologic neovascularization. a Western blots show that Sirt1 protein levels are significantly decreased in the retina of neuronal specific conditional Sirt1 knockout mice (Sirt1 cKO) compared to littermate control mice (Sirt1flox|flox). b Representative images of retinal vasculature from P17 Sirt1 cKO mice and littermate control Sirt1flox|flox mice with induced retinopathy (OIR). Blood vessels were stained with lectin (red) with areas of vaso-obliteration highlighted by white line. Quantification of retinopathy in Sirt1 cKO and control retina with OIR shows significantly increased levels of vaso-obliteration (absence of red) and pathologic neovascularization (bright red) (n = 18–20 per group). **p ≤ 0.01. (Color figure online)
The effect of neuronal Sirt1 on retinal vascular growth in OIR is likely independent of PGC1α
Loss of Sirt1 in retinal neurons significantly suppresses retinal levels of PGC-1α (peroxisome proliferator-activated receptor gamma coactivator-1 alpha), a known Sirt1 target important in tissue metabolism [23, 24], and a regulator of vascular endothelial growth factor (Vegf) [25] (Fig. 3a). However, deletion of PGC-1α in KO mice (PGC-1α−/−) does not significantly impact either vaso-obliteration or pathologic neovascularization in OIR compared to WT littermate controls at P17 (n = 10–11 per group, p = 0.80 for VO, p = 0.30 for NV, Fig. 3b, c), although there is a trend of slightly decreased neovascularization in OIR, as reported in a recent study with a smaller number of samples [26]. These data suggest that the noted effects on retinal vasculature secondary to neuronal specific knockout of Sirt1 in OIR is likely mediated by factors other than PGC-1α. We found deletion of PGC-1α also does not affect developmental angiogenesis in the retina at P5 (Fig. S3). It is important to note that approximately half of PGC-1α−/− pups die in the early postnatal period and the surviving PGC-1α−/− mice show decreased body weight compared to WT or heterozygous littermates [27], which we have found impacts retinopathy outcomes [28]. Therefore severely lethargic PGC-1α−/− pups or those with low body weight were excluded in our study [28].
Fig. 3.
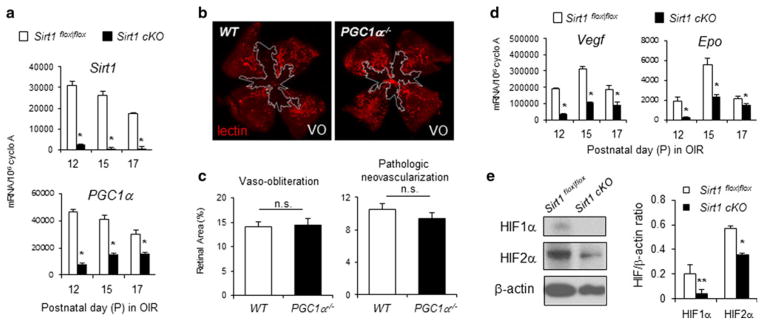
Sirt1 regulation of vascular regrowth in retinopathy is likely mediated via regulation of hypoxia growth factors (HIF) 1α and 2α, other than PGC-1α. a At P12, 15, and 17 in OIR, depletion of neuronal Sirt1 results in significantly decreased mRNA levels of Sirt1 and PGC-1a in the retinas compared to littermate Sirt1flox|flox controls. *p ≤ 0.05. b PGC-1a−/− mice do not show significant differences in vaso-obliteration (outlined in white) and pathologic neovascularization in OIR at P17 compared to littermate WT controls (n = 10–11 per group, n.s. not significant). d OIR exposed Sirt1 cKO retinas show significantly decreased mRNA levels of angiogenic factors Vegf and Epo in the retinas compared to littermate Sirt1flox|flox controls. *p ≤ 0.05. e Western blot with Sirt1 antibody shows decreased levels of HIF1α and 2α protein in Sirt1 cKO retinas compared to littermate Sirt1flox|flox control retinas
Decreased levels of angiogenic factors and hypoxia induced factor (HIF) in Sirt1 cKO retinas
Next, we assessed in Sirt1 cKO retinas the levels of Vegf and erythropoietin (Epo), two hypoxia responsive genes implicated in regulating vascular regeneration in proliferative retinopathy [29, 30]. Both Vegf and Epo are significantly down-regulated in Sirt1 cKO retina exposed to oxygen-induced retinopathy compared to Sirt1flox|flox controls (Fig. 3d). This decrease of Vegf and Epo expression is associated with significantly decreased protein levels of hypoxia-induced factor (HIF)1α and 2α in the Sirt1 cKO retinas compared to Sirt1flox|flox controls (Fig. 3e). These results suggest that endogenous Sirt1 upregulation in the ischemic neurons may thereby potentiate the hypoxia/nutrient deficiency response to promote vascular regeneration into avascular areas in OIR. Lack of growth factors such as Vegf or Epo in Sirt1 cko retinas may directly contribute to the dampen revascularization into avascular zones in OIR, resulting a secondary aggravated pathologic neovascular (NV) response, which is dependent not only on availability of angiogenic factors but also inflammatory response that is exacerbated in the lack of vascular regrowth [31, 32].
Sirt1 mediates acetylation of hypoxia-induced factors (HIF) in RGC-5 cells to regulates vascular growth
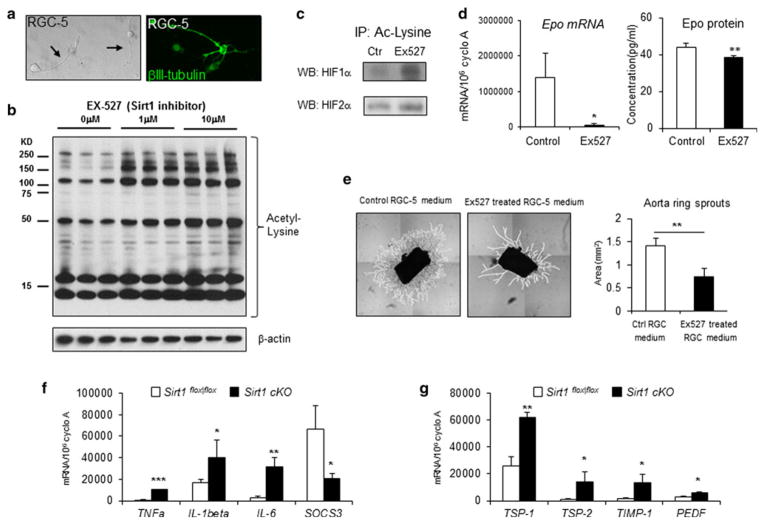
Since Sirt1 is highly enriched in the retinal ganglion cell layer in OIR retinas (Fig. 1e), we used an in vitro cell culture model (RGC-5) of retinal ganglion cell that upon differentiation exhibits characteristic neuronal morphology and the RGC marker β-III-tubulin (Fig. 4a). RGC-5 cells treated with Ex527, a Sirt1 specific inhibitor, showed suppression of Sirt1 deacetylase activity, as evidenced by increased levels of protein acetylation detected by an acetyl-lysine antibody (Fig. 4b). The strong staining around 15–20kd are likely isoforms of histones, which are major endogenous substrates of Sirt1 [7].
Fig. 4.
Sirt1 regulates acetylation of HIF1α and 2α, and expression of Epo in RGC-5 culture. a RGC-5 cells differentiated after incubation with staurosporine show dendrites and axons (arrows) characteristic of neurons. RGC-5 cells also show staining with antibody against βIII-tubulin (green), a RGC marker. b Incubation of RGC-5 cells with Ex527, a specific Sirt1 inhibitor, results in increased levels of protein acetylation detected by an acetyl-lysine antibody. c Inhibition of Sirt1 with Ex527 in RGC-5 culture increased protein acetylation of HIF1α and HIF2α, detected by immunoprecipitation with acetyl-lysine antibody and followed by Western blots against HIF1α or HIF2α. d Ex527 treatment of RGC-5 cells leads to decreased expression level of Epo mRNA measured by RT-qPCR, and decreased Epo protein level measured by ELISA assay. e Aortic ring explants cultured with conditioned medium from RGC-5 culture treated with Sirt1 inhibitor Ex527 shows significantly decreased levels of vascular growth compared with RGC-5 medium treated with vehicle control (vascular sprouts outlined in white, n = 6–10 per group). f Depletion of Sirt1 in neuronal retina leads to increased mRNA expression of inflammatory cytokines TNFα, IL-1β, IL-6 and decreased mRNA level of SOCS3 at P12 in OIR. g Depletion of Sirt1 in neuronal retinal results in increased mRNA expression of anti-angiogenic factors TSP-1, TSP-2, TIMP-1 and PEDF at P12 in OIR. *p ≤ 0.05; **p ≤ 0.01. (Color figure online)
RGC-5 cell lysate treated with Sirt1 inhibitor Ex527 showed increased acetylation of both HIF1α and HIF2α, detected by immunoprecipitation with antibodies against acetyl-lysine (Fig. 4c). This post-translational modification of HIF1α and HIF2α can promote their degradation [33, 34], and consequently results in significant inhibition of HIF-dependent gene Epo expression, as well as significantly decreased Epo protein levels (Fig. 4d). VEGF mRNA and protein levels in RGC-5, however, do not show a significant difference with treatment of Sirt1 inhibitor Ex527 (Fig. S4a, b). This lack of change, compared with in vivo suppression of VEGF in Sirt1 cKO retinas, likely reflects different cellular sources of VEGF production in vivo, including glial cells and other retinal neuronal cells, which are not accounted for in RGC-5 cell culture.
Next conditioned medium from RGC-5 cultures treated with Ex527 were collected and applied to aortic ring explants to evaluate if Sirt1 inhibition in RGC-5 culture can influence vascular growth in vitro. This medium significantly suppressed vascular sprouting of aortic rings compared to conditioned medium from vehicle-treated RGC-5s (Fig. 4e). These results suggest that Sirt1 in RGC-5 regulates HIF acetylation and degradation to influence expression of growth factors including Epo, which may in turn mediate vascular growth. Our results on Sirt1-dependent regulation of HIFs are in agreement with previous studies showing that Sirt1 is essential for HIF1α and HIF2α accumulation and expression of their target genes in liver and tumor cells in response to hypoxia [33, 34].
Altered inflammatory response and anti-angiogenic factors in Sirt1 cKO retinas
While our data demonstrate that Sirt1 mediates HIF acetylation and pro-angiogenic growth factors, these factors might not be the only ones changed in the absence of neuronal Sirt1. Next we analyzed expression of inflammatory cytokines which also impact vascular response in retinopathy. We identified in OIR significantly increased expression of pro-inflammatory cytokines TNFα, IL-1β and IL-6 in Sirt1 cKO retinas compared to controls at P12, as well as significant inhibition of SOCS3 (suppressor of cytokine signaling 3), which is a negative regulator of cytokine signaling with anti-inflammatory functions (Fig. 4f). These inflammatory factors are not significantly altered in RGC-5 cell culture with Sirt1 inhibition (Fig. S4c), since ganglion cells are likely not the major source of inflammatory influences in Sirt1 cKO retinas. These results indicate an enhanced inflammatory response in Sirt1 cKO retinas secondary to neuronal loss of Sirt1, which may also contribute in part to the decreased vaso-regeneration and precipitated neovascularization.
In addition, anti-angiogenic factors were analyzed in the Sirt1 cKO retinas compared to control floxed retinas in OIR. Significantly increased expression of thrombospondin-1 (TSP-1), TSP-2, TIMP-1 and PEDF were observed in Sirt1 cKO retina compared to control retinas at P12 in OIR, suggesting that upregulation of these anti-angiogenic factors may suppress the physiologic revascularization into the avascular zone which starts at P12 (Fig. 4g). These factors are not significantly changed at P15 and P17 in OIR (data not shown), suggesting that they likely do not contribute directly to pathologic neovascularization which usually occurs starting at P15. RGC-5 cell culture with Sirt1 inhibition does not show an upregulation of anti-angiogenic factors as is seen in Sirt1 cKO retinas (Fig. S4d), likely reflecting a retinal cellular source of these factors other than ganglion cells, and/or a lack of critical complex cellular interactions in cell culture conditions. Together these data suggest increased inflammatory response and decreased anti-angiogenic factors in Sirt1 cKO retinas may also contribute in part to the observed decrease in vaso-regeneration.
In summary, our study suggests a novel role of neuronal Sirt1 in mediating vascular growth in a mouse model of oxygen-induced proliferative retinopathy. Ischemic/nutrient deprived retinal neurons in avascular region may induce Sirt1 under metabolically stressed conditions to influence physiologic revascularization of ischemic areas via modulation of HIF signaling and secretion of pro-angiogenic and neuro-protective factors, such as Vegf and Epo, as well as through an altered inflammatory response and expression of anti-angiogenic factors. Although high levels of Vegf and Epo levels directly contribute to the proliferative stage of retinopathy, suppression of both factors below normal levels may also inhibit the physiologic revascularization process that is essential to repair vaso-obliterated retina and restore normal vessels. In the absence of neuronal Sirt1, disruption of these Sirt1 mediated responses may therefore dampen physiologic vascular regeneration and result in secondary exacerbation of subsequent pathologic vaso-proliferation. Our findings may also be relevant for other neurovascular ischemic diseases, such as stroke, in which regeneration of normal vasculature in ischemic neuronal tissue is critical to prevent tissue injury and promote tissue repair.
Supplementary Material
Acknowledgments
We thank Molly R. Seaward, Keirnan L. Willett, Roberta J. Dennison, Nathan M. Krah for their technical assistance. This work was supported by Charles H. Hood Foundation, Boston Children’s Hospital (BCH) Ophthalmology Foundation, BCH Manton Center for Orphan Disease Research, Blind Childrens Center, BrightFocus Foundation, Mass Lions Eye Research Fund Inc. and BCH Career Development Award (to JC), NIH (EY017017, EY022275, PO1 HD18655), V. Kann Rasmussen Foundation, RPB Senior Investigator Award, Alcon Research Institute Award, and the Lowy Medical Research Instiute (LEHS). AS is supported by the Freifrau von Nauendorff Foundation and the German Ophthalmic Society. DAS is supported by the Paul F. Glenn Foundation, NIH (RO1AG019719), JDRF, and the United Mitochondrial Disease Foundation. PS holds a Canada Research Chair in Retinal Cell Biology. PS and JSJ are supported by the Canadian Institutes of Health Research. SM is supported by the Consejo Nacional de Ciencia y Tecnología, Mexico (CONACyT 177819).
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10456-013-9374-5) contains supplementary material, which is available to authorized users.
Conflict of interest DAS is a consultant to and inventor on patents licensed to GlaxoSmithKline and Ovascience.
Contributor Information
Jing Chen, Email: jing.chen@childrens.harvard.edu, Department of Ophthalmology, Boston Children’s Hospital, Harvard Medical School, Boston, MA, USA. Manton Center for Orphan Disease Research, Boston Children’s Hospital, Harvard Medical School, Boston, MA, USA.
Shaday Michan, Instituto Nacional de Geriatría, Institutos Nacionales de Salud, Mexico, Mexico.
Aimee M. Juan, Department of Ophthalmology, Boston Children’s Hospital, Harvard Medical School, Boston, MA, USA. Manton Center for Orphan Disease Research, Boston Children’s Hospital, Harvard Medical School, Boston, MA, USA
Christian G. Hurst, Department of Ophthalmology, Boston Children’s Hospital, Harvard Medical School, Boston, MA, USA
Colman J. Hatton, Department of Ophthalmology, Boston Children’s Hospital, Harvard Medical School, Boston, MA, USA
Dorothy T. Pei, Department of Ophthalmology, Boston Children’s Hospital, Harvard Medical School, Boston, MA, USA
Jean-Sebastien Joyal, Department of Ophthalmology, Boston Children’s Hospital, Harvard Medical School, Boston, MA, USA.
Lucy P. Evans, Department of Ophthalmology, Boston Children’s Hospital, Harvard Medical School, Boston, MA, USA
Zhenghao Cui, Department of Ophthalmology, Boston Children’s Hospital, Harvard Medical School, Boston, MA, USA.
Andreas Stahl, Department of Ophthalmology, Boston Children’s Hospital, Harvard Medical School, Boston, MA, USA. University Eye Hospital Freiburg, Freiburg, Germany.
Przemyslaw Sapieha, Department of Ophthalmology, Boston Children’s Hospital, Harvard Medical School, Boston, MA, USA. Department of Ophthalmology, Maisonneuve-Rosemont Hospital Research Centre, University of Montreal, Montreal, Canada.
David A. Sinclair, Paul F. Glenn Laboratories for the Biological Mechanisms of Aging, Department of Genetics, Harvard Medical School, Boston, MA, USA. Department of Pharmacology, The University of New South Wales, Paddington, Australia
Lois E. H. Smith, Email: lois.smith@childrens.harvard.edu, Department of Ophthalmology, Boston Children’s Hospital, Harvard Medical School, Boston, MA, USA
References
- 1.Antonetti DA, et al. Diabetic retinopathy. N Engl J Med. 2012;366:1227–1239. doi: 10.1056/NEJMra1005073. [DOI] [PubMed] [Google Scholar]
- 2.Sapieha P. Eyeing central neurons in vascular growth and reparative angiogenesis. Blood. 2012;120:2182–2194. doi: 10.1182/blood-2012-04-396846. [DOI] [PubMed] [Google Scholar]
- 3.Akula JD, et al. The neurovascular relation in oxygen-induced retinopathy. Mol Vis. 2008;14:2499–2508. [PMC free article] [PubMed] [Google Scholar]
- 4.Sapieha P, et al. The succinate receptor GPR91 in neurons has a major role in retinal angiogenesis. Nat Med. 2008;14:1067–1076. doi: 10.1038/nm.1873. [DOI] [PubMed] [Google Scholar]
- 5.Fukushima Y, et al. Sema3E-PlexinD1 signaling selectively suppresses disoriented angiogenesis in ischemic retinopathy in mice. J Clin Invest. 2011;121:1974–1985. doi: 10.1172/JCI44900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joyal JS, et al. Ischemic neurons prevent vascular regeneration of neural tissue by secreting semaphorin 3A. Blood. 2011;117:6024–6035. doi: 10.1182/blood-2010-10-311589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guarente L. Franklin H. Epstein Lecture: Sirtuins, aging, and medicine. N Engl J Med. 2011;364:2235–2244. doi: 10.1056/NEJMra1100831. [DOI] [PubMed] [Google Scholar]
- 8.Brunet A, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 9.Donmez G, et al. SIRT1 suppresses beta-amyloid production by activating the alpha-secretase gene ADAM10. Cell. 2010;142:320–332. doi: 10.1016/j.cell.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Jiang M, et al. Neuroprotective role of Sirt1 in mammalian models of Huntington’s disease through activation of multiple Sirt1 targets. Nat Med. 2012;18:153–158. doi: 10.1038/nm.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michan S, et al. SIRT1 is essential for normal cognitive function and synaptic plasticity. J Neurosci. 2010;30:9695–9707. doi: 10.1523/JNEUROSCI.0027-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Potente M, et al. SIRT1 controls endothelial angiogenic functions during vascular growth. Genes Dev. 2007;21:2644–2658. doi: 10.1101/gad.435107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guarani V, et al. Acetylation-dependent regulation of endothelial Notch signalling by the SIRT1 deacetylase. Nature. 2011;473:234–238. doi: 10.1038/nature09917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith LE, et al. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101–111. [PubMed] [Google Scholar]
- 15.Chen J, Smith L. Retinopathy of prematurity. Angiogenesis. 2007;10:133–140. doi: 10.1007/s10456-007-9066-0. [DOI] [PubMed] [Google Scholar]
- 16.Cheng HL, et al. Developmental defects and p53 hyper-acetylation in Sir2 homolog (SIRT1)-deficient mice. Proc Natl Acad Sci USA. 2003;100:10794–10799. doi: 10.1073/pnas.1934713100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Banin E, et al. T2-TrpRS inhibits preretinal neovascularization and enhances physiological vascular regrowth in OIR as assessed by a new method of quantification. Invest Ophthalmol Vis Sci. 2006;47:2125–2134. doi: 10.1167/iovs.05-1096. [DOI] [PubMed] [Google Scholar]
- 18.Connor KM, et al. Quantification of oxygen-induced retinopathy in the mouse: a model of vessel loss, vessel regrowth and pathological angiogenesis. Nat Protoc. 2009;4:1565–1573. doi: 10.1038/nprot.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stahl A, et al. Computer-aided quantification of retinal neovascularization. Angiogenesis. 2009;12:297–301. doi: 10.1007/s10456-009-9155-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frassetto LJ, et al. Kinase-dependent differentiation of a retinal ganglion cell precursor. Invest Ophthalmol Vis Sci. 2006;47:427–438. doi: 10.1167/iovs.05-0340. [DOI] [PubMed] [Google Scholar]
- 21.Sapieha P, et al. 5-Lipoxygenase metabolite 4-HDHA is a mediator of the antiangiogenic effect of {omega}-3 polyunsaturated fatty acids. Sci Transl Med. 2011;3:69ra12. doi: 10.1126/scitranslmed.3001571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stahl A, et al. The mouse retina as an angiogenesis model. Invest Ophthalmol Vis Sci. 2010;51:2813–2826. doi: 10.1167/iovs.10-5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lagouge M, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 24.Rodgers JT, et al. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 25.Arany Z, et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature. 2008;451:1008–1012. doi: 10.1038/nature06613. [DOI] [PubMed] [Google Scholar]
- 26.Saint-Geniez M, et al. PGC-1alpha regulates normal and pathological angiogenesis in the Retina. Am J Pathol. 2012 doi: 10.1016/j.ajpath.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin J, et al. Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1alpha null mice. Cell. 2004;119:121–135. doi: 10.1016/j.cell.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 28.Stahl A, et al. Postnatal weight gain modifies severity and functional outcome of oxygen-induced proliferative retinopathy. Am J Pathol. 2010;177:2715–2723. doi: 10.2353/ajpath.2010.100526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aiello LP, et al. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci USA. 1995;92:10457–10461. doi: 10.1073/pnas.92.23.10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen J, et al. Erythropoietin deficiency decreases vascular stability in mice. J Clin Invest. 2008;118:526–533. doi: 10.1172/JCI33813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kataoka K, et al. The roles of vitreal macrophages and circulating leukocytes in retinal neovascularization. Invest Ophthalmol Vis Sci. 2011;52:1431–1438. doi: 10.1167/iovs.10-5798. [DOI] [PubMed] [Google Scholar]
- 32.Ishida S, et al. Leukocytes mediate retinal vascular remodeling during development and vaso-obliteration in disease. Nat Med. 2003;9:781–788. doi: 10.1038/nm877. [DOI] [PubMed] [Google Scholar]
- 33.Dioum EM, et al. Regulation of hypoxia-inducible factor 2alpha signaling by the stress-responsive deacetylase sirtuin 1. Science. 2009;324:1289–1293. doi: 10.1126/science.1169956. [DOI] [PubMed] [Google Scholar]
- 34.Laemmle A, et al. Inhibition of SIRT1 impairs the accumulation and transcriptional activity of HIF-1alpha protein under hypoxic conditions. PLoS ONE. 2012;7:e33433. doi: 10.1371/journal.pone.0033433. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.