Abstract
Rationale
While clinical studies show maternal consumption of palatable fat-rich diets during pregnancy to negatively impact the children’s behaviors and increase their vulnerability to drug abuse, the precise behavioral and neurochemical mechanisms mediating these phenomena have yet to be examined.
Objective
The study examined in rats whether gestational exposure to a high-fat diet (HFD) can increase the offspring’s propensity to use nicotine and whether disturbances in central nicotinic cholinergic signaling accompany this behavioral effect.
Methods
Rat offspring exposed perinatally to a HFD or Chow diet were characterized in terms of their nicotine self-administration behavior in a series of operant response experiments and the activity of acetylcholinesterase (AChE) and density of nicotinic ACh receptors (nAChRs) in different brain areas.
Result
Perinatal HFD compared to Chow exposure increased nicotine-self administration behavior during fixed-ratio and dose-response testing and caused an increase in breakpoint using progressive ratio testing, while nicotine-seeking in response to nicotine prime-induced reinstatement was reduced. This behavioral change induced by the HFD was associated with a significant reduction in activity of AChE in the midbrain, hypothalamus and striatum and increased density of β2-nAChRs in the ventral tegmental area and substantia nigra and of α7-nAChRs in the lateral and ventromedial hypothalamus.
Conclusions
Perinatal exposure to a HFD increases the vulnerability of the offspring to excessive nicotine use by enhancing its reward potential, and these behavioral changes are accompanied by a stimulation of nicotinic cholinergic signaling in mesostriatal and hypothalamic brain areas important for reinforcement and consummatory behavior.
Keywords: Nicotine self-administration; Perinatal high-fat diet; Sprague-Dawley rats; Acetylcholine; Nicotinic cholinergic receptors, Acetylcholinesterase; Nicotine reinforcement
Introduction
Nicotine is one of the most heavily used and addictive drugs in the U.S., with nearly 70 million Americans age 12 and older using tobacco products at least once a month (NIDA 2012). Despite numerous attempts involving pharmacological and sociological interventions to reduce the use of tobacco products, the number of individuals using and abusing nicotine still remains high. One factor that may contribute to this vulnerability to nicotine abuse may be the increased availability over the past several decades of palatable diets rich in fat (WHO 2003). Clinical studies suggest that childhood weight problems and unhealthy eating patterns can lower the age of onset of nicotine smoking and increase withdrawal symptoms during times of abstinence (Saules et al. 2007). A close relationship between dietary fat and nicotine use is also evident in studies showing that obese individuals have double the risk for developing nicotine addiction (Hussaini et al. 2011) and, conversely, that smokers consume significantly greater amounts of high calorie, fat-rich foods (Dallongeville et al. 1998). Of particular note is evidence demonstrating that maternal consumption of these unhealthy diets during pregnancy, in addition to leading to childhood obesity and hyperlipidemia (Dietz 1998; Hannon et al. 2005), can program behaviors in the offspring that are likely to increase vulnerability to drug abuse. The children of overweight mothers who consume these diets exhibit internalized behaviors, such as anxiety and depression (Rizzo et al. 1997), which promote drug use and increase consumption of and preference for fatty foods, perhaps due to disturbances in major reward circuits (Fisher and Birch 1995). This clinical evidence raises the possibility that maternal consumption of a fat-rich diet may contribute to excessive use of another reinforcing substance, nicotine, in the offspring.
While the effects of dietary fat on nicotine consumption have yet to be examined in animals, recent studies have shown that consumption of a fat-rich diet, in addition to increasing caloric intake and producing obesity-related metabolic abnormalities, can cause disturbances in drug use and related behaviors. For example, in adult rats, binging on fat enhances cocaine-seeking behavior (Puhl et al. 2011) and causes behaviors associated with drug use, such as increased sucrose consumption (Orosco et al. 2002), exploration (Boukouvalas et al. 2008; Soulis et al. 2007), and novelty seeking (Morganstern et al. 2012). Further, animals exposed in utero to a fat-rich diet exhibit an increase in alcohol drinking behavior and sensitivity to amphetamine (Bocarsly et al. 2012; Cabanes et al. 2000), as well as enhanced consumption of and responding for palatable foods (Chang et al. 2008; Naef et al. 2011; Ong and Muhlhausler 2011). They also show increased anxiety (Bilbo and Tsang 2010) and locomotor activity (Raygada et al. 1998), behaviors closely linked to increased drug-taking behaviors (Kabbaj 2006; Piazza et al. 1989) These animal studies further support the possibility that prenatal exposure to dietary fat may increase the vulnerability of offspring specifically toward nicotine-taking behavior.
As for the neurochemical mechanisms that may mediate this behavioral phenomenon, studies to date have focused on reward and consummatory signaling pathways, such as those utilizing dopamine (DA), the opioid peptide enkephalin (ENK) and the orexigenic peptide, orexin (OX). These reports have revealed a stimulatory effect of in utero dietary fat on DA neurotransmission within the mesolimbic reward circuit projecting from the ventral tegmental area (VTA) to the nucleus accumbens (NAc) (Naef et al. 2008; Ong and Muhlhausler 2011), on ENK signaling in regions such as the hypothalamic paraventricular nucleus (PVN) and NAc (Chang et al. 2008; Vucetic et al. 2010), and also on OX levels in the lateral hypothalamus (LH) (Chang et al. 2008). There are no studies examining the effect of maternal fat consumption on nicotininc cholinergic signaling in the offspring, even though this system is known to have a direct role in mediating nicotine reward and consumption (Feduccia et al. 2012). The neurotransmitter acetylcholine (ACh), functioning through two main nicotinic cholinergic receptors (nAChRs), the high affinity β2 subunit-containing nAChRs (β2-nAChRs) and low affinity α7 subunit-containing nAChRs (α7-nAChRs), is found to mediate multiple aspects of nicotine-taking behavior. For example, the activity of nAChRs is important in the NAc and VTA for drug reward (Bruijnzeel and Markou 2004; Gotti et al. 2010; Pons et al. 2008), in the substantia nigra (SN) for hyperactivity associated with drug seeking (Alburges et al. 2007; Wise 2009), and in specific hypothalamic areas for mediating energy balance, arousal, and responses to stress (Imaki et al. 1995; Pasumarthi and Fadel 2010; Ribeiro et al. 2007; Takahashi et al. 1995; Yoburn and Glusman 1984). Nicotinic cholinergic signaling is additionally important in cortical and thalamic regions for cognitive processes related to learning and arousal (Raybuck and Gould 2010) and in the central nucleus of the amygdala (CeA) for anxiety (Zarrindast et al. 2008). This evidence underscores the importance of nicotinic cholinergic signaling in drug use and the need to investigate the effects of maternal fat consumption on this system, which in turn may mediate an increased propensity to use and abuse nicotine. It is noteworthy that adult animals consuming a high-fat diet exhibit an increase in cholinergic activity and densities of nAChRs in different brain areas important for drug use (Kaizer et al. 2004; Morganstern et al. 2012), as well as a stronger propensity to consume excess ethanol (Bocarsly et al. 2012).
The present study examined, first, whether exposure to a high fat diet (HFD) during gestation and lactation increases different aspects of nicotine self-administration behavior, as revealed by fixed ratio (FR), dose-response and breakpoint analysis using progressive ratio testing as well as extinction-reinstatement testing. It then tested whether these behavioral changes in the fat-exposed offspring are accompanied by disturbances in cholinergic neurotransmission in specific hypothalamic and extra-hypothalamic areas, by measuring the activity of ACh esterase (AChE), an enzyme responsible for breaking down ACh, and the binding of both β2 and α7 nAChRs. These experiments were designed to test the specific hypothesis that animals exposed to dietary fat during gestation are more vulnerable to excessive nicotine use, possibly due to heightened nicotinic cholinergic tone.
Methods and Procedures
Animals
Time-pregnant, Sprague-Dawley rats (220–240 g) from Charles River Breeding Laboratories (Hartford, CT) were delivered to the animal facility on embryonic day 4 (E4). The dams were individually housed in plastic cages, in a fully accredited AAALAC facility (22°C, with a 12:12-h light-dark cycle with lights off at noon), according to institutionally approved protocols as specified in the NIH Guide to the Use and Care of Animals and also with approval of the Rockefeller University Animal Care Committee. The rats were maintained ad libitum from E5 on either a HFD with 50% fat or a standard chow diet. For the HFD group, standard lab chow was available for 3 additional days (until E8), while the dams became fully adapted to the mixed diet and consumed little chow. Over the course of the experiments, food intake was measured daily during gestation and 2 times per week during lactation, while body weights of dams and pups were recorded weekly. During gestation, the HFD dams showed hyperphagia compared to the Chow dams (94.9 ±4.1 vs 77.4±2.5 Kcal, p<0.05) with no impact on body weight gain (29.1 ±4.1 vs 24.3±2.5 g).
The litters of the HFD dams were similar to the Chow litters in terms of their size (11.1±1.2 vs 10.8±0.8), body weight at birth (10.6±0.3 vs 9.5±0.4), and female/male ratio (6:7 vs 6:8), with no spontaneous abortions observed in either diet group. On postnatal day 0 (P0), litters studied after birth were culled to n=8, primarily by eliminating the females. After weaning (P22), all rats were switched to a standard chow diet (ad libitum) for the remainder of the experiment, except for the behavior group which was limited to 20 g of chow given to animals immediately after their initial operant training sessions with sucrose. After the sucrose training, all rats were switched back to an ad libitum feeding schedule for the entire study. For the neurochemical experiments, male offspring (1 per litter) in the HFD and Chow groups (n=7–8/group) were examined at P60, and for nicotine self-administration 1–2 male offspring were used starting from P60 (n=12–13). During this time, all rats were maintained on an ad libitum chow diet.
Diets
For the experimental period, rats were either maintained ad libitum on standard rodent chow (12% fat, 3.3 kcal/g) or a HFD (50% fat, 5.2 kcal/g), which was the same as that described in prior publications (Dourmashkin et al. 2006; Leibowitz et al. 2004). It consisted of fat from 75% lard (Armour, Omaha, NE) and 25% vegetable oil (Wesson vegetable oil, Omaha, NE), of carbohydrate from 30% dextrin, 30% cornstarch (ICN Pharmaceuticals, Costa Mesa, CA), and 40% sucrose (Domino, Yonkers, NY), and of protein from casein (Bioserv, Frenchtown, NJ) and 0.03% L-cysteine hydrochloride (ICN Pharmaceuticals). The diet was supplemented with minerals (USP XIV Salt Mixture Briggs; ICN Pharmaceuticals) and vitamins (Vitamin Diet Fortification Mixture; ICN Pharmaceuticals). The macronutrient composition was calculated as percentage of total kcal, with the HFD containing 50% fat, 25% carbohydrate, and 25% protein. This semi-solid diet was stored at 4°C until use. On a daily basis, fresh diet was weighed out in metal bowls and placed into the appropriate animal cages. These diets are nutritionally complete and found to have no detrimental effects on the health of the animals.
Apparatus
Nicotine self-administration was performed in eight standard operant conditioning chambers (Coulbourn Instruments, Allentown, PA). Each chamber was equipped with two levers located 10 cm above the floor, with one lever defined as active and stimulated the infusion pump for 4 sec and the other defined as inactive and produced no scheduled consequences. The chambers also contained a LED house light placed on the wall opposite to the levers and a triple cue light placed right above the active lever. The catheters were attached to an infusion pump (Harvard Apparatus, Natick, MA) through a swivel system and protective metal spring tether. The operant chambers were controlled by a computer using the Graphic State Software package.
Test Procedures
Experiment 1. Nicotine self-administration, extinction and reinstatement
Acquisition of Self-Administration
Prior to surgery, offspring (P42 to P50, n=12/group) from perinatal HFD and Chow dams underwent operant training for 45 mg sucrose pellets (Bioserv, Frenchtown, NJ). Briefly, rats were food deprived overnight and then restricted to 20 g of daily chow, given immediately after the training session, for the remainder of the food training period. There were no visual stimuli presented during food training. All rats were first trained on an FR1 reinforcement schedule, and once the rats earned 100 pellets within an hour, they were switched to an FR2 schedule and then to an FR3 schedule until they were able to earn 100 rewards under each schedule of reinforcement. There were no measured differences between the HFD and Chow rats in their responding during the food training period [active lever presses: FR1 (78±7vs 71±5), FR2 (165±13 vs 173±15) and FR3 (277±18 vs 265±21)] or in the number of days they took to progress from FR1 to FR2 (3.7±1.2 vs 3.1±0.6) and from FR2 to FR3 (2.3±0.7 vs 2.6±1.1) schedules. Following successful training using this protocol, the rats at age of P50 to P55 were surgically implanted with a jugular catheter as described below and allowed to recover for 7 days prior to the start of Experiment 1. During the acquisition phase, animals were trained for 1 h each day during the dark cycle to self-administer nicotine (0.015 mg/kg/infusion over 4 sec in a volume of 100 ul). A training dose of 0.015 mg/kg/infusion was chosen based on published evidence showing that the peak rates of responding and number of infusions on a fixed-ratio schedule are typically obtained at doses ranging from 0.01–0.03 mg/kg/infusion (Donny et al. 1995; Paterson and Markou 2004; Suto et al. 2001) and also on our preliminary data indicating that the highest level of responding occurs at the 0.015 mg/kg/infusion dose. The duration of infusion (4 sec) was chosen based on evidence suggesting that increased infusion times more closely mimic natural smoking conditions, which entail a gradual transit of nicotine from the lungs to the brain (Sorge and Clarke 2009), and on our preliminary data and published evidence (Fowler and Kenny 2011) suggesting that a 3–4 sec infusion time yields optimal responding for nicotine in adult rats. At the beginning of the session, the animals received three infusions of nicotine to fill the lines (300 ul nicotine solution), with the house light on. This volume was precisely calculated, based on the length of the catheter and connection tubing, to fill and not expose the rats to the nicotine. Each active lever press simultaneously activated the cue light as well as the infusion pump, while turning off the house light. After the 4 second infusion, the cue light remained on for an additional 20 seconds, defined as the time out period during which the responses were recorded but had no scheduled consequences. After the time out period, the cue light was turned off, and the house light came back on to signal availability of the drug once again. This paradigm using contingent cue and chamber lights was chosen based on published evidence (Clemens et al. 2010; Donny et al. 1999; Yan et al. 2012) and our preliminary findings showing that rats readily acquire and steadily maintain nicotine self-administration behavior and also reinstate this behavior when primed with a single injection of nicotine.
During the first part of acquisition (10 days), responding was reinforced on an FR1 schedule (1 infusion for each active press), with the reinforcement schedule increasing to FR2 during the next week (day 11–15), FR3 during the next few days (day 16–18) and FR5 during the last week (day 19–23).
Dose-Response Determinations
After the last session on an FR5 schedule, a dose response curve was determined using the following doses: 0.015 mg/kg/infusion, 0.03 mg/kg/infusion and 0.06 mg/kg/infusion, with 4 sessions at each dose and a 1 day return to the training dose (0.015 mg/kg/infusion) between each new dose.
Progressive Ratio Testing
After the dose-response measures were collected, all animals were returned to the training dose (0.015 mg/kg/infusion) for 5 days prior to progressive ratio (PR) testing. PR testing was conducted over 4 days according to procedures previously described (Caille et al. 2012; Shram et al. 2008). The PR schedule was determined using the exponential formula 5Xexp (0.2×infusion), such that the required responses per infusion were as follows: 3, 6, 10, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145 and so forth. PR conditions were identical to the FR session except that the time for each session was extended to 2 hours, and the dose used was 0.03 mg/kg/infusion. Breakpoint was determined when >20 min of inactivity on the active lever passed. During PR testing, all animals reached breakpoint within the 2-hour session and therefore were all used in the analysis.
Extinction Training
Next, the rats were briefly returned to their original training dose (0.015 mg/kg/infusion) on an FR5 schedule for 5 days and subsequently given a 14 day extinction period, during which time the active lever signaled an infusion of 100 ul saline rather than nicotine, with all other parameters remaining identical to the training sessions. Self-administration behavior was considered extinct when the animals responded with fewer than 15 presses on the active lever.
Nicotine-Induced Reinstatement
The day after this extinction period, the reinstatement of nicotine self-administration was examined as previously described (Le et al. 2006; Shaham et al. 1997). Briefly, the rats were first injected with three consecutive daily doses of saline 30 minutes prior to their self-administration sessions, in order to habituate them to the injection procedures and mitigate any stressful reactions to the injection itself. Immediately after habituation, each rat received a priming dose of nicotine (0.15 mg/kg s.c.), and 30 minutes later they were placed in the chambers for measurements of active lever presses within 1 h. Immediately after all the data were collected, catheter patency was confirmed by infusing 0.3 ml methohexital sodium (16.67 mg/ml, IV; Sigma), which produces brief anesthetic effects only when administered IV. The data from 1 of the rats was excluded due to loss of catheter patency and from another 2 animals due to illness during reinstatement testing.
Experiment 2. Acetylcholinesterase activity
In this experiment, the offspring from HFD and Chow consuming dams (n=8/group) were identified as above and sacrificed by rapid decapitation at P60, the same time that behavioral training began. Brain regions of interest were dissected and analyzed for AChE activity, as described below, and trunk blood was collected for measures of circulating levels of free fatty acids (FFA), which are known to be elevated by a HFD and to affect the brain cholinergic system (Ahren et al. 1997; Buettner et al. 2000; Nishizaki et al. 1997; Nishizaki et al. 1999; Vajreswari et al. 2002).
Experiment 3: Nicotinic receptor binding
Experiment 3 was designed to examine the effects of perinatal dietary fat exposure on nAChR binding in specific brain nuclei important for consummatory behavior and drug reward. In an additional set of animals (n=8/group) exposed to Chow or HFD during gestation and lactation, quantitative autoradiography was used to measure the binding of [125I]-epibatidine to β2-nAChRs and [125I]-bungarotoxin (BTX) to α7-nAChRs. Offspring were raised in an identical manner as described above and sacrificed at age P60 to correspond with the time of the behavioral measures and measurements of AChE activity. Shortly after dark onset, animals were sacrificed using rapid decapitation, and their brains were removed, dipped into cold isopentane (−25°C) for 30 s, and then stored at −80°C until further use. Additionally, trunk blood was collected for measurements of circulating FFA.
Jugular Catheterization Surgery
The rats were anesthetized with a combination of ketamine (80 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.), supplemented with ketamine when necessary and prepared surgically with SILASTIC catheters in the right jugular vein as previously published (Corrigall and Coen 1989). The catheter exited in the intra-scapular region and was connected to a 22 gauge cannula attached to a mesh piece, which was implanted subcutaneously in order to hold the catheter in place. Immediately after surgery, the animals were given 0.03 mg/kg of the pain medication bupenepherine (s.c.) and 5 mg/kg of the antibiotic baytril (IV) and then allowed 7–10 days to recover. The rats were flushed daily with 0.9% saline containing heparin (50 units), baytril antibiotic (2.27 mg/kg), and locked with a solution of 50% dextrose containing heparin (200 units).
Drug
(−) Nicotine tartrate (Sigma, St Louis, Mo., USA) was dissolved in isotonic saline and the pH was adjusted to 7.0 with dilute NaOH. The unit doses for the intravenous nicotine self-administration (100 ul over 4 sec) were 0.015, 0.03 and 0.06 mg/kg/infusion (all doses are expressed as free base).
Acetylcholinesterase Activity
Animals were sacrificed using rapid decapitation, and their brains were quickly dissected on ice to remove the entire prefrontal frontal cortex (PFC), striatum (STR), hypothalamus (HYPO), midbrain (MB) and cerebellum (CB) for determinations of AChE activity. Tissue from these brain regions was then individually homogenized at 4°C in filtered 50 mM TrisHCl buffer, pH 7.4 (1:10 w/v) and centrifuged at 1,000 × g for 10 min to remove nuclei and debris. The protein content of each sample’s supernatant was determined using the Bradford Assay (Bradford 1976). AChE activity was then measured using acetylthiocholine iodide and 5,5'-dithiobis-2-nitrobenzoic acid (DTNB), according to the methods of Ellman and colleagues (Ellman et al. 1961). Enzyme activity of AChE is expressed as µmol/min/mg protein at 25°C.
Quantitative Autoradiography
Brains were sectioned (16 µm) at −20°C on a cryostat microtome, thaw mounted on gelatin-coated microscope slides, and desiccated overnight at 4°C using an air vacuum. Multiple sets of adjacent sections were collected for measurements of β2- and α7-nAChRs on sections that had consistent anatomical representation. Brain sections were collected from Bregma 3.2 to 2.7 mm for the medial prefrontal cortex (mPFC); Bregma 1.6 to 2.0 for the NAc shell and core; Bregma −1.8 to −1.88 mm for the PVN and the paraventricular nucleus of the thalamus (PVT), Bregma −2.8 to −3.14 mm for the LH, ventromedial hypothalamus (VMH), zona incerta (ZI) and CeA; and from Bregma −5.6 to −5.8 mm for the VTA and SN pars compacta (SNc) (Paxinos and Watson 1997). All sections, clearly depicting the region of interest, were chosen at the same level for each animal within each group. For each animal, 3 adjacent brain sections were used for the binding assay and averaged for statistical analysis.
The binding of [125I]-epibatidine to β2-nAChRs was measured using a slightly modified method from Tizabi and colleagues (Tizabi and Perry 2000). Briefly, brain sections were pre-incubated at room temperature for 15 min in a 50 mM TrisHCl buffer containing (pH 7.4) 120 mM NaCl, 5 mM KCl, 2.5 mM CaCl2, 1 mM MgCl2 and were then incubated for 60 min at room temperature in the same buffer solution containing 0.165 nM [125I]-epibatidine (2200 Ci/mmol, Perkin-Elmer, Boston, MA,). Nonspecific binding was determined in adjacent sections with the addition of 300 mM nicotine hydrogen tartrate to the incubation buffer. All sections were then rinsed twice (5 min each) in ice-cold buffer, dipped briefly into ice-cold distilled water, and air-dried at room temperature.
The binding of [125I]-BTX to α7-nAChR sites was measured as previously described (Sparks and Pauly 1999) with slight modification. Briefly, slides containing the brain regions of interest were pre-incubated at room temperature for 15 min in a 50 mM TrisHCl (pH 7.4) buffer containing 0.1% BSA and were then transferred to the incubation solution which contained the same buffer solution in addition to 2 nM [125I]-BTX (120 Ci/mmol, Perkin-Elmer, Boston, MA,). In order to determine non-specific binding, adjacent sections were incubated in this same incubation buffer, but with 300 uM nicotine hydrogen tartrate (Sigma Aldrich, St. Louis, MO) added. Sections were allowed to incubate in these two solutions for 2 h at room temperature and then quickly dipped in ice-cold Tris buffer, rinsed 3 times (10 min each) in ice-cold buffer, dipped in distilled water, and finally air-dried at room temperature.
For both binding assays, the air-dried sections were exposed to Kodak BioMax MR (Sigma Aldrich, St. Louis, MO) film along with [125I] standards (American Radiolabeled Chemicals, St. Louis, MO) for 4 h (β2-nAChR) or 5 days (α7-nAChR). After appropriate exposure times, the films were developed using an automatic developer and images scanned and saved for further analysis. Quantitative densitometric analysis of binding was performed using NIH software, Image J v1.43 for Windows. In order to determine specific binding, non-specific binding, which represented less than 5% of the total binding, was subtracted from total binding in adjacent sections. The values of binding were determined using a standard curve constructed from [125I] standards with a known amount of activity and expressed as (fmol/mg brain protein).
Fatty acid determinations
For measurements of non-esterified fatty acids (NEFA), trunk blood collected during the time of sacrifice with an E-Max Microplate Reader using a colorimetric assay kit from WAKO Pure Chemical Industries, Ltd (Osaka, Japan).
Statistical Analyses
The values in the figures are expressed as mean ± SEM. Statistical analyses to compare the effects of perinatal dietary fat to chow on average caloric intake, body weight and serum FFA levels were performed using unpaired Student’s t-tests, with significant differences accepted at p<0.05. Behavioral and neurochemical data were analyzed using appropriate repeated measures ANOVAs (RM-ANOVAs), with day, FR ratio, nicotine dose or brain region as the within subject factor and perinatal treatment (Chow or HFD) as the between subject factor. During progressive ratio testing, measures of the number of rewards earned were analyzed separately using unpaired Student’s t-tests (p<0.05), while the differences in breakpoint (last ratio completed) were analyzed using the nonparametric median and Mann-Whitney U-test to avoid violating the assumption of homogeneity of variance (Richardson and Roberts 1996). For all analyses where significant main effects or interactions were obtained, follow-up pairwise comparisons were made between groups of interest using unpaired two-tailed t-tests, with p<0.05 considered statistically significant. Since we knew a priori that the number of brain regions analyzed in the autoradiography experiments or the number of days during FR training could be a confounding factor in our analyses, follow-up statistical tests to determine significant group differences in specific regions or on specific days were also performed in certain cases where the interaction effect was only a trend. The probability values given in the text or legends to the figures and tables reflect the results of these tests. For the behavioral experiments, data from 2–3 rats/group (out of 12) needed to be excluded when there was a loss of catheter patency or the rat was unable to acquire self-administration behavior.
Results
Litter Parameter
Analysis of the litter parameters produced by the HFD-fed compared to Chow-fed dams is shown in Table 1. There were no significant group differences in the litter size or proportion of male pups born. While body weights were similar at birth for the HFD and Chow offspring, there was a small but significant increase in body weight at weaning (P22) in the HFD pups compared to Chow rats [t(14) = 2.65, p<0.05]. With the HFD rats switched to a Chow diet after weaning, their body weight was normalized by P60. Chow intake was also similar between the HFD and Chow groups at P40. Measurements of circulating fatty acid levels at P60 revealed a significant increase (+60%) in the HFD offspring in levels of these lipids compared to the Chow offspring [t(14) = 4.56, p<0.05].
Table 1.
Effects of Perinatal HFD exposure on physiological measures in the offspring
| Offspring | ||
|---|---|---|
| Litter Parameters | Perinatal Chow | Perinatal HFD |
| Litter Size | 10.8 ± 0.8 | 11.1 ± 1.2 |
| Fraction of Male Pups | 0.57 | 0.53 |
| Body Weight (g) (P0) | 9.5 ± 0.4 | 10.6 ± 0.3 |
| Body Weight (g) (P22) | 68 ± 2.3 | 77 ± 3.1* |
| Caloric Intake (kcal) (P45–P60) | 63 ± 2.7 | 70 ± 3.0 |
| Body Weight (g) (P60) | 350 ± 18.4 | 358 ± 23.1 |
| Free Fatty Acid (mEq/L) (P 60) | 0.13 ± 0.01 | 0.21 ± 0.02* |
p<0.05 perinatal HFD compared to Chow offspring
Experiment 1: The effects of perinatal HFD exposure on nicotine self-administration
Experiment 1a (Acquisition)
This experiment tested whether the offspring from dams fed a HFD exhibited changes in nicotine self-administration behavior during the acquisition phase, as revealed by measurements of their lever pressing behavior over the initial FR training. Figure 1 shows the mean (± SEM) of nicotine infusions (Fig. 1A) and total active and inactive lever presses (Fig. 1B) during the first 23 days of self-administration training under FR1, FR2, FR3 and FR5 reinforcement schedules, using a nicotine dose of 0.015 mg/kg/infusion. Overall, nicotine self-administration was substantially increased in the rats exposed perinatally to a HFD as compared to a control chow diet. Under the FR1 schedule of reinforcement, a significant treatment effect was observed with the measures of total number of infusions [F(1,14) = 8.09, p<0.05] and active lever presses [F(1,14) = 5.32, p<0.05]. While there were no significant interactions between the multiple training days and perinatal treatments, individual group comparisons of HFD to Chow offspring revealed an increase in the total number of infusions and active lever presses on days 2, 3, 4 and 5 (p<0.05). Although these measures showed little group difference when the rats transitioned to the FR2 and FR3 schedules, the FR5 schedule of reinforcement revealed a significant main effect of perinatal treatment, in both the number of infusions [F(1,14) = 5.19, p<0.05] and the active lever presses [F(1,14) = 9.13, p<0.05]. There were no interactions between perinatal treatment and training day but individual group comparisons of HFD to Chow offspring revealed a significant increase in the total number of infusions and active lever presses on days 19, 20 and 22 (p<0.05). In Fig. 1B, active lever presses generally exceeded inactive lever presses during FR1 (F(1,14) = 4.53, p<0.05), FR2 (F(1,14) = 9.57, p<0.05), FR3 (F(1,14) = 9.45, p<0.05) and FR5 (F(1,14) = 10.14, p<0.05) training, while there were no statistically significant differences between perinatal HFD and perinatal Chow rats in terms of their responding on the inactive lever. These results during the acquisition period demonstrate that the HFD offspring on the FR schedules readily acquired the operant task and self-administered more nicotine than the standard chow offspring.
Fig. 1. Nicotine self-administration in perinatal HFD and Chow offspring.
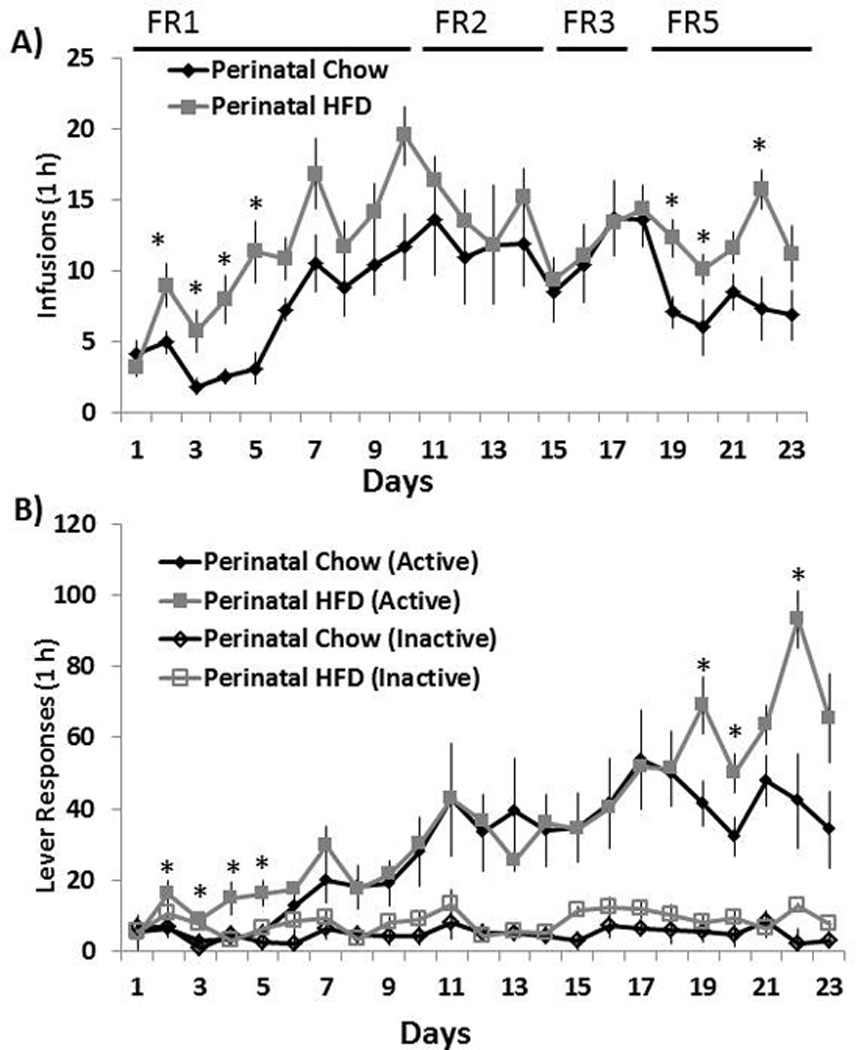
(A) Mean ± SEM number of nicotine infusions and (B) active and inactive lever responses during the first 23 days of nicotine self-administration (0.015 mg/kg per infusion) under FR-1, FR-2, FR-3 and FR-5 reinforcement schedules. * Represents significant differences between HFD and Chow diet animals with active lever presses (p<0.05). Abbreviations: HFD - high-fat diet.
Experiment 1b (Dose-Response)
To evaluate their responding to and intake of increasing doses of nicotine infusions, this experiment compared the dose-response curves for the HFD compared to Chow diet offspring. With measures of the mean (± SEM) number of infusions earned (Fig. 2A) and nicotine intake (Fig. 2B) during the dose-response determination, the perinatal HFD offspring earned an overall greater number of infusions [F(1, 14) = 4.64, p<0.05] and self-administered a greater amount of nicotine [F(1,14) = 5.21, p<0.05] as compared to the Chow offspring. Whereas no interactions were identified with the number of infusions, a significant interaction was obtained with nicotine intake as a function of dose [F(2,22) = 5.43, p<0.05], with the increase in dose of nicotine accompanied by a significant increase in average nicotine intake (p<0.05). Follow-up comparisons demonstrated that the perinatal HFD rats compared to Chow offspring self-administered more nicotine (mg/kg, i.v.) (p<0.05) at the lower two doses (0.015 and 0.03 mg/kg, i.v.) but showed no effect at the highest dose (0.06 mg/kg, i.v.). These effects demonstrate an increase in responding for and intake of nicotine in rats exposed perinatally to a HFD as compared to a Chow diet.
Fig. 2. Dose-response determinations in perinatal HFD and Chow offspring.
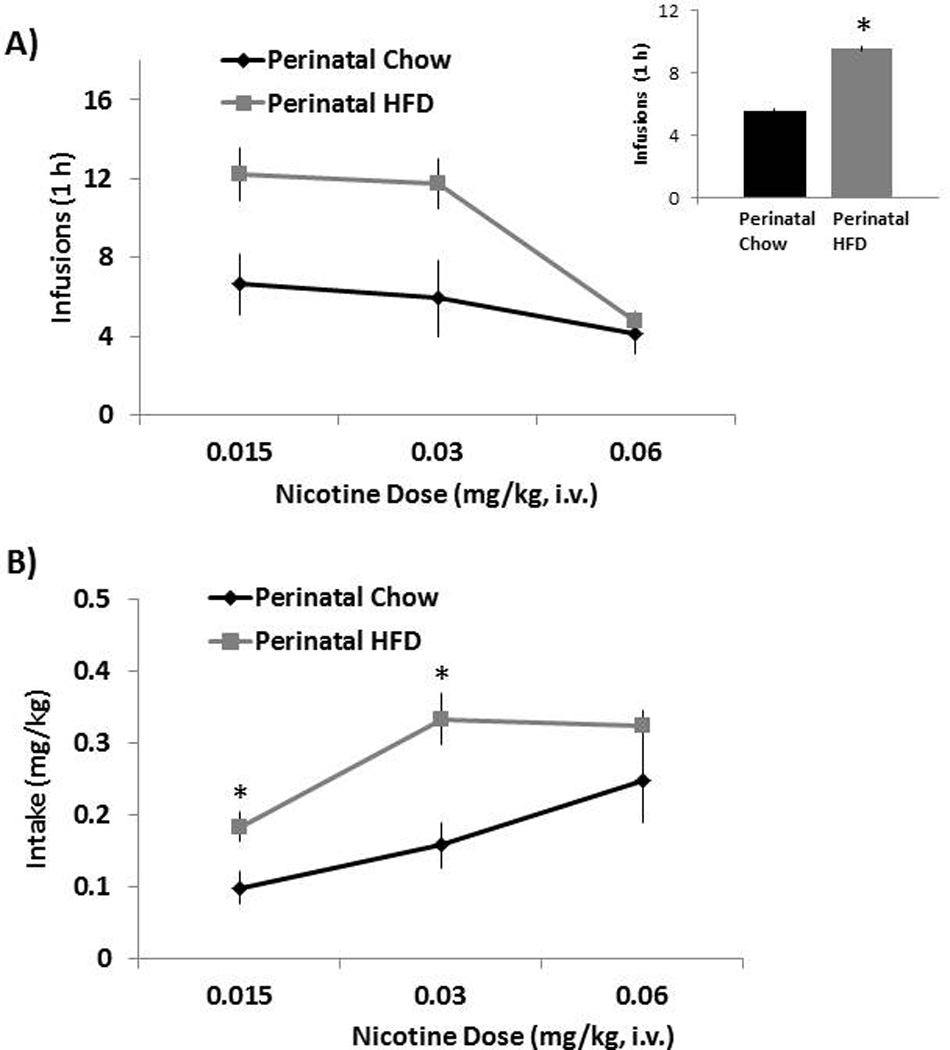
(A) Mean ± SEM number of nicotine infusions with the inset to the right demonstrating the main effects of group and (B) nicotine intake (mg/kg). Data are from the dose-response determination collected during days 24 – 39 of nicotine self-administration training (3 doses). * Represents significant differences between HFD and Chow diet animals (p<0.05). Abbreviations: HFD - high-fat diet.
Experiment 1c (Progressive Ratio Responding)
The progressive ratio responding was additionally measured in the perinatal HFD compared to Chow offspring, to determine if they exhibited differences in the reinforcing properties of nicotine. As depicted in Fig. 3, the progressive ratio analysis revealed significant group differences, with the perinatal HFD rats earning significantly more rewards compared to the perinatal Chow animals [t(14) = 2.45, p<0.05]. Further analysis of the breakpoints indicated that the rats exposed to a HFD show an increase in the median final ratio completed compared to those exposed to a Chow diet (U(14)=9, p< 0.05). These effects, involving an increase in number of rewards earned and greater breakpoints, suggest that the offspring perinatally exposed to a HFD are more motivated to attain nicotine and thus may attribute greater value and reward to this abused drug.
Fig. 3. Progressive ratio testing in perinatal HFD and Chow offspring.
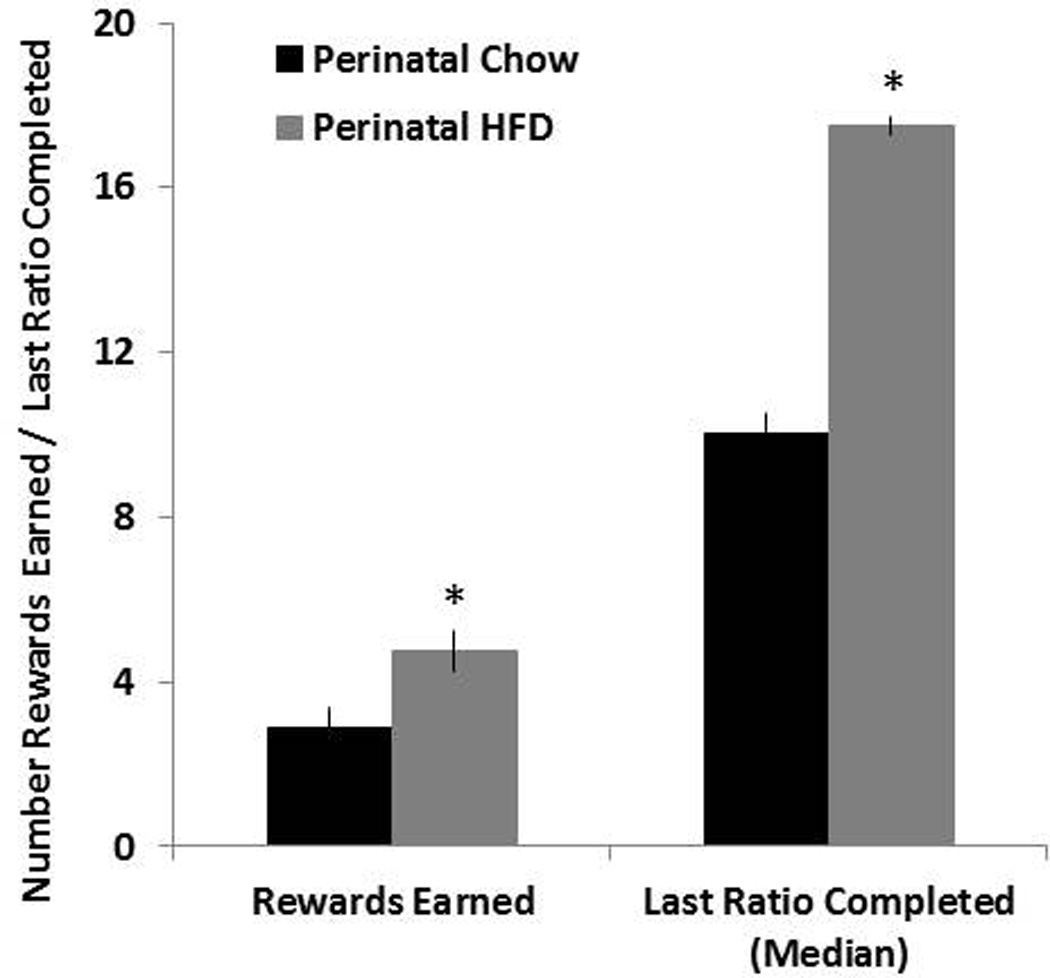
Mean ± SEM number of rewards earned and mean last ratio completed (breakpoint) during progressive ratio testing. Data from the progressive ratio experiment was collected during days 44 – 48 of nicotine self-administration training using a dose of 0.015 mg/kg per infusion. * Represents significant differences between HFD and Chow diet animals (p<0.05). Abbreviations: HFD - high-fat diet.
Experiment 1d (Extinction/Reinstatement)
Through measurements of extinction and reinstatement of nicotine self-administration, this experiment was designed to determine whether the perinatal HFD exposure altered the relapse potential of this drug. With Fig. 4A representing the mean (± SEM) number of active lever responses during 14 sessions of extinction training, statistical analyses showed that the HFD and Chow rats exhibited a similar pattern of nicotine extinction, with no differences observed in the overall number of active lever presses in the absence of nicotine solution or the interaction of active lever presses by extinction day. The reinstatement data, however, showed that a nicotine prime dose (0.15 mg/kg s.c.) was able to reinstate drug-seeking behavior in both the Chow and HFD offspring, as measured by a significant increase in the number of active lever presses [F(1,14) = 4.53, p<0.05] in response to nicotine compared to saline pretreatment. Pairwise comparisons further revealed that the HFD rats showed significantly reduced nicotine prime-induced reinstatement of drug-seeking behavior compared to the Chow offspring (p<0.05). These data demonstrate a clear influence of dietary fat in the reinstatement of nicotine-seeking behavior.
Fig. 4. Extinction and reinstatement of nicotine seeking in perinatal HFD and Chow offspring.
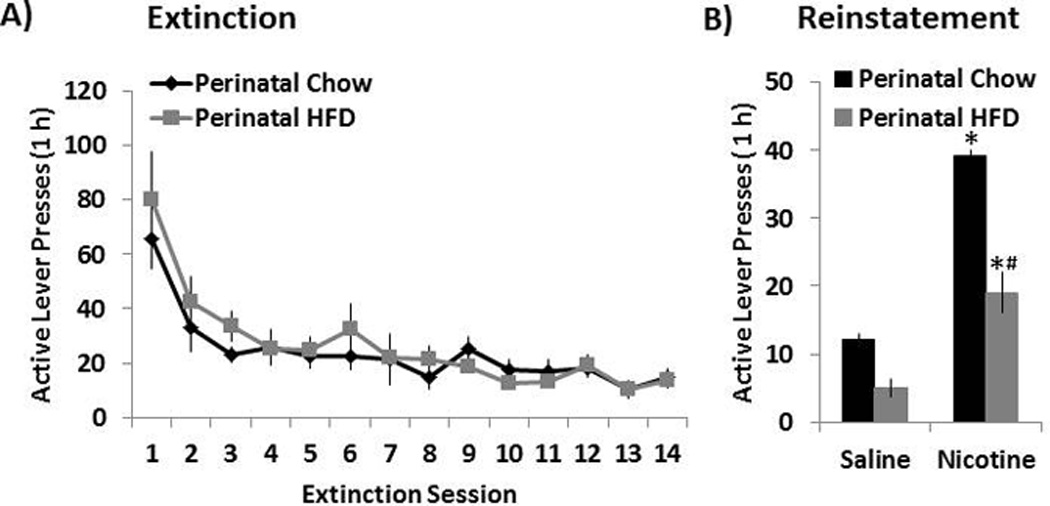
(A) Mean ± SEM number of responses on the previously active lever during the first 14 sessions of the extinction phase. (B) During extinction training, lever responding led to saline infusions and the illumination of the cue light previously paired with nicotine. Mean ± SEM number of responses on the previously active lever during reinstatement training induced by a priming injection of saline or nicotine (0.15 mg/kg, s.c.). During reinstatement training, lever responding was not reinforced with nicotine. * Represents significant differences between saline and nicotine treatment (p<0.05). # Represents significant differences between HFD and Chow diet animals (p<0.05). Abbreviations: HFD - high-fat diet.
Experiment 2: Changes in brain AChE activity induced by perinatal high-fat diet consumption
The question addressed in this experiment is whether the HFD compared to Chow offspring, which exhibit the changes in nicotine self-administration, also show differences in their cholinergic neurotransmission, as indicated by changes in the activity in different brain areas of AChE, an enzyme responsible for the breakdown of ACh. These measurements of AChE activity revealed significant group differences [F(4,40) = 4.56, p<0.05], with the HFD compared to Chow offspring showing markedly reduced activity in the MB, HYP and STR regions (Fig. 2). This change was somewhat larger in the STR (−46%, p<0.05) and HYP (−30%, p<0.05) then the MB (−23%, p<0.05), with no effect apparent in the PFC and CB. With reduced AChE activity normally associated with increased ACh content in the synaptic cleft (Hartmann et al. 2007), these results suggest that ACh levels in the offspring exposed to dietary fat during development may be significantly enhanced in these three brain areas.
Experiment 3 Changes in nAChR binding induced by perinatal high-fat diet consumption
To further examine the effects of perinatal HFD exposure on the cholinergic system, quantitative autoradiography was used to measure the binding of [125I]-epibatidine to β2-nAChRs and of [125I]- bungarotoxin (BTX) to α7-nAChRs in different brain regions. The specific binding of [125I]-epibatidine to β2-nAChRs was dense (binding values of 135–187 fmol/mg brain protein) in the mPFC region, NAc (shell and core), PVT, VTA and SNc, while relatively sparse in the hypothalamus and amygdala. In contrast, the binding of [125I]- BTX to α7-nAChRs was clearly visible (with binding values of 115–198 fmol/mg brain protein) in the different hypothalamic areas (PVN, ZI, LH, VMH) as well as the mPFC and CeA, while the binding was too low for analysis in the NAc, PVT, VTA and SNc regions. Analysis of [125I]-epibatidine binding (fmol/mg brain protein) revealed a trend for an interaction effect of brain area×perinatal diet [F(5,40) = 4.35, p=0.08], which reflected a significant increase in the perinatal HFD compared to Chow rats in the β2-nAChRs in the VTA (+19%, p<0.05) and SNc (+21%, p<0.05), but not in the mPFC, NAc shell (NAc Sh), NAc core (NAc Cr), or PVT (ns) (Table 1). A trend for an interaction between brain area and perinatal diet was also detected with [125I]-BTX binding [F(5,40) = 3.52, p=0.15], with follow-up analyses of binding in specific brain regions of the HFD compared to Chow offspring reflecting a significant increase in α7-nAChR sites in two areas of the hypothalamus, specifically the LH (19%, p<0.05) and VMH (24%, p<0.05), with no group differences detected in the PVN or ZI, as well as the mPFC and CeA (ns) (Table 1). These stimulatory effects of perinatal HFD exposure, specifically on β2-nAChR binding in the VTA and SNc and α7-nAChR binding in the LH and VMH, are illustrated in the autoradiographs of Fig. 6. They indicate that maternal consumption of a HFD can stimulate nAChR binding in the offspring and that the effects observed are both receptor specific and site specific.
Fig. 6. Autoradiographs illustrating the effects of perinatal HFD exposure on nAChR binding.
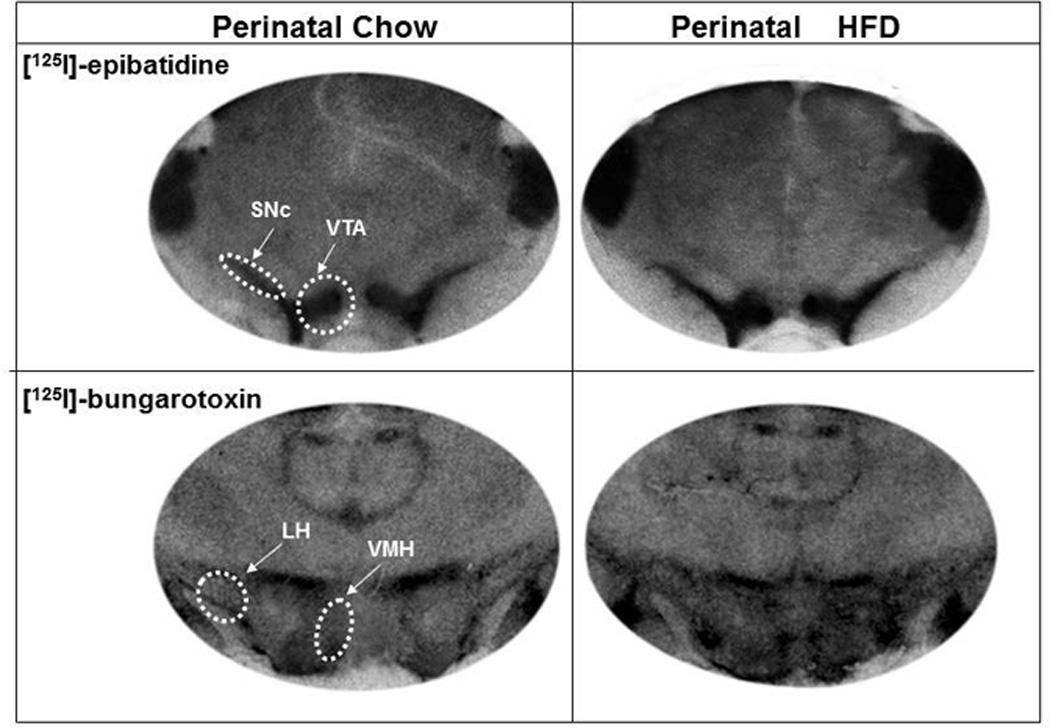
Images depicting a stimulatory effect of perinatal fat on [125I]-epibatidine binding in the VTA and SNc regions (top 2 panels) and [125I]-bungarotoxin binding in the LH and VMH regions (bottom panel). Abbreviations: HFD - high-fat diet; VTA – ventral tegmental area; SNc - substantia nigra pars compacta; LH - lateral hypothalamus and VMH - ventromedial hypothalamus.
Discussion
The present study shows that maternal fat consumption during gestation and lactation can produce significant changes in nicotine self-administration and that these behavioral changes are accompanied by disturbances in nicotinic cholinergic neurotransmission in the brain. When the offspring were examined as adults, those perinatally exposed to dietary fat showed an increase in nicotine-self administration behavior during FR training, in responding and intake during dose-response testing and in breakpoint using progressive ratio testing. During reinstatement training, however, they exhibited reduced nicotine prime-induced drug-seeking behavior. These behavioral changes in the offspring were accompanied by an increase in cholinergic activity in specific brain areas, as indicated by reduced activity of AChE (which breaks down ACh) in the midbrain, hypothalamus and striatum and increased density of specific nAChRs in areas of the midbrain and hypothalamus, suggesting an increase in cholinergic neurotransmission in these brain areas important for reinforcement and consummatory behavior.
Increased nicotine self-administration in offspring exposed to perinatal high-fat diet
The increase in nicotine self-administration behavior in the HFD-exposed offspring is indicated by a variety of measures. The HFD compared to Chow offspring exhibited a general increase in nicotine responding during the training period and dose-response testing and an increase in breakpoint values, indicating that they are more motivated by the rewarding properties of nicotine. While this is the first study to investigate the effect of perinatal HFD on nicotine self-administration and reward, there are other reports relating prenatal and perinatal palatable diet exposure to other drugs of abuse as well as palatable foods. For example, early exposure to fat- and sugar-rich diets is found to increase alcohol consumption in the offspring and enhance their sensitivity to the locomotor-stimulating effects of amphetamine (Bocarsly et al. 2012; Cabanes et al. 2000). These dietary manipulations also enhance consumption of and preference for dietary fat (Chang et al. 2008; Ong and Muhlhausler 2011) and operant responding for fat-enriched rewards (Naef et al. 2011), suggesting an increase in the rewarding properties of these diets similar to drugs of abuse. A behavioral explanation for these results may be found in the ability of prenatal fat exposure to promote behaviors, such as anxiety and locomotor activity (Bilbo and Tsang 2010; Raygada et al. 1998; Sullivan and Grove 2010), which themselves are known to stimulate responding for drugs of abuse (Kabbaj 2006; Piazza et al. 1989). Similar to prenatal exposure to fat, adult consumption of a fat-rich diet can stimulate both cocaine-seeking and sucrose consumption, as well as locomotor activity and novelty-seeking behavior (Boukouvalas et al. 2008; Morganstern et al. 2012; Orosco et al. 2002; Puhl et al. 2011; Soulis et al. 2007). Our finding that circulating FFA are markedly elevated in the HFD-exposed offspring, consistent with other reports (Buettner et al. 2000; Chang et al. 2008), suggests a possible physiological mechanism that may be involved in the effects of dietary fat consumption on nicotine reward. These lipid metabolites have recently been shown to directly mediate nicotine reward in vivo (Panlilio et al. 2012) and to stimulate cholinergic activity in vitro (Tel et al. 2010; Vajreswari et al. 2002). Collectively, these studies support the idea that early exposure to dietary fat, in association with higher circulating FFA levels, is effective in increasing responding and motivation for the drug, nicotine. While it is possible that reinforcement in the present paradigm is related to the contingent cue and chamber lights used in addition to nicotine, as suggested earlier (Caggiula et al. 2002), the pharmacological evidence showing that attenuation of cholinergic nicotinic signaling can reduce nicotine self-administration in this paradigm (Le Foll et al. 2012) suggests that the primary reinforcing effects are related more to the nicotine itself.
Increased cholinergic neurotransmission and nicotinic receptor binding due to perinatal high-fat diet exposure
The results obtained with measurements of the activity of AChE and density of nAChRs in brain areas important for reward and drug consumption demonstrate that the increase in nicotine responding is accompanied by disturbances in cholinergic function. Our findings in the HFD offspring point to a reduction in AChE, the metabolizing enzyme of ACh, and an increase in density of α- and β-nAChR sites in hypothalamic and extra-hypothalamic areas, all of which suggest a stimulation of the cholinergic system in these animals. The possibility that ACh levels in the HFD animals are increased together with this decrease in enzymatic activity is supported by studies showing elevated levels of extracellular ACh in mutant mice lacking the AChE gene and also in mice injected with an AChE antagonist (Hartmann et al. 2007). This change in AChE activity in the perinatal HFD-exposed offspring is consistent with published evidence showing the activity of this enzyme to be reduced in the midbrain, hypothalamus and cortex of adult animals consuming a fat-rich diet (Kaizer et al. 2004; Morganstern et al. 2012) and also in vitro by FFA which are elevated in the HFD offspring (Nishizaki et al. 1997; Nishizaki et al. 1999; Vajreswari et al. 2002). Our finding of increased nAChR density in corresponding brain regions, both within and outside the hypothalamus, may be a direct consequence of such heightened cholinergic tone. This up-regulation of nAChRs with increased cholinergic tone has been demonstrated previously in adult rats with chronic nicotine exposure (Flores et al. 1997; Mugnaini et al. 2002; Parker et al. 2004; Rasmussen and Perry 2006; Schwartz and Kellar 1983) or high-fat diet consumption (Morganstern et al. 2012) and also in offspring exposed prenatally to nicotine (Shacka and Robinson 1998; van de Kamp and Collins 1994). Although there are no prior studies of cholinergic neurotransmission in animals perinatally exposed to dietary fat, there are other investigations showing maternal consumption of junk food or a fat-rich diet to alter the development of the brain reward system in the offspring, producing an overall increase in dopaminergic tone in mesolimbic brain regions (Naef et al. 2011; Naef et al. 2008; Ong and Muhlhausler 2011). Together, the present study and these published reports provide clear evidence for significant HFD-induced changes in baseline nicotinic cholinergic signaling, alterations that may contribute to the enhanced drug responding. In future studies that conduct the behavioral tests and neurochemical measures in the same animals, it will be important to determine whether the nicotine exposure itself, perhaps compounded by the diet-induced differences in cholinergic function, may have contributed to the increase in nACh activity, as suggested by reports of nicotine’s stimulatory effects on signaling in other systems important for drug use (Gotti et al. 2010; Kenny et al. 2009; Moretti et al. 2010).
Role of mesostriatal cholinergic neurotransmission and β2 nAChRs in nicotine reward
The evidence obtained in this study reveals a close relationship between the increased cholinergic activity in mesostriatal regions of the HFD offspring and their enhanced nicotine self-administration behavior. Together with the decrease in AChE activity observed in the striatum and midbrain, an increase in β2-nAChRs was detected specifically in the VTA and SNc, areas where these receptors are located on dopaminergic neurons that project, respectively, to the ventral and dorsal areas of the striatum and have an important role in the behavioral effects of reinforcing drugs (Livingstone and Wonnacott 2009; Pons et al. 2008). Studies have demonstrated that activation of specific β2-nAChRs in the VTA is critical for nicotine as well as ethanol self-administration behavior, with local blockade of this receptor subtype found to significantly reduce the number of nicotine infusions in nicotine self-administration maintenance (Gotti et al. 2010), the self-administration of ethanol (Kuzmin et al. 2009), and the ethanol-associated conditioned reinforcement (Lof et al. 2007). Also, lesions of the VTA and SN are found to increase drug-taking behavior, due perhaps to a reduction in intrinsic nicotine reward (Alderson et al. 2006), and nicotine administered directly into the VTA or SN stimulates the release and turnover of DA in the striatum (Di Matteo et al. 2010; Keath et al. 2007; Teo et al. 2004; Xiao et al. 2009), supporting an additional role for direct ACh - DA interactions in drug-taking behavior. In light of these reports, our findings in the present study, showing reduced ACh break down in the striatum and midbrain together with increased binding of β2-nAChRs in the VTA and SNc regions, suggest the involvement of this increased cholinergic tone in these mesostriatal circuits in mediating the enhanced nicotine self-administration behavior observed in the perinatal HFD exposed offspring.
Role of hypothalamic cholinergic neurotransmission and α7 nAChRs in nicotine consumption
With evidence supporting a role for the hypothalamus in mediating homeostatic processes such as feeding and non-homeostatic processes such as excessive palatable food consumption and drug use (Barson et al. 2011; Suzuki et al. 2012), our findings indicate the additional involvement of this structure and its cholinergic activity in mediating the increased nicotine-taking behavior in perinatal HFD-exposed rats. In the hypothalamus, the HFD offspring exhibited a reduction in the breakdown of ACh, along with a stimulation of α7-nAChR density specifically in the LH and VMH. In these areas where α7-nAChRs are the predominant subtype (Marks et al. 1996), ACh is believed to have a role in mediating both appetite and arousal, which are important components of nicotine intake (Pasumarthi and Fadel 2010; Picciotto and Kenny 2012; Saper et al. 2001). Although studies have long associated nicotine with a reduction in feeding behavior (Miyata et al. 1999; Ramos et al. 2004; Yang et al. 1999), there is a variety of evidence indicating that nicotinic signaling may have a variety of effects on the intake of reinforcing drugs and associated behaviors. For example, direct administration of nicotine in the LH enhances arousal by activating local OX neurons, an effect that may be important for the stimulatory effects of commonly abused drugs on central nervous system arousal, and the inhibition of major cholinergic inputs to the LH reduces arousal and OX neuronal activation (Bayer et al. 2004; Pasumarthi and Fadel 2010). Enhanced nicotinic activity in the LH region may also be directly related to drug reward, since orexin neurons that are stimulated by local or systemic nicotine (Kane et al. 2000; Pasumarthi and Fadel 2010) are found to be activated during the important period of drug seeking (Harris et al. 2005). Although there is little evidence directly supporting the role for ACh in the VMH in drug-taking behavior, the cholinergic system in this area is shown to be important in controlling appetite and arousal, specifically during periods of food anticipation (Ribeiro et al. 2009; Ribeiro et al. 2007), suggesting its involvement in mediating the arousal component of drug-taking behavior. In contrast to the LH and VMH, the perinatal HFD offspring exhibited no change in α7-nAChR binding in the PVN or CeA, where ACh has been associated with the activation of stress responses (Ohmori et al. 1995; Zarrindast et al. 2008; Zhang and Zheng 1997), or in the mPFC, where a7-nAChRs are relatively sparse (Pauly et al. 1991). The site-specificity of this change supports the idea that the increase in nicotine self-administration in HFD offspring is related more specifically to the heightened consummatory behavior triggered by the increased arousal and reward induced by nicotinic cholinergic signaling within the LH and VMH regions.
Lack of perinatal HFD effects on nAChR binding in select brain regions
In contrast to the areas showing significant changes in nicotinic cholinergic signaling, our results identified certain regions as being insensitive to perinatal HFD, including the mPFC, PVT and several hypothalamic nuclei. There are several possible explanations for this, related perhaps to differences in cellular recycling mechanisms, baseline receptor levels, or receptor plasticity after discontinuation of the HFD. Consistent with our results in HFD offspring showing no change in the PVT, there are reports showing nicotine treatment to have no effects on nAChR binding in this nucleus while increasing these receptors in the hypothalamus, specifically the VMH, and the midbrain, specifically the SN region (Kellar et al. 1989; Pauly et al. 1991). The cellular mechanisms responsible for such receptor up-regulation may involve an enhanced rate of synthesis or decrease in degradation of nAChRs, with the unaffected regions showing a more rapid endocytotic turnover of these receptors. It is also likely that the reduced susceptibility of specific brain areas to receptor changes may be related to their lower baseline levels of the nAChRs (Pauly et al. 1991), although our results show changes in nAChR binding specifically in regions that exhibit mid-range values of baseline binding. Another possible explanation for these region-specific effects of dietary fat on nAChR binding may be due to changes in nAChR plasticity, with evidence suggesting that nAChR binding returns to normal levels one week after agonist treatment discontinuation in brain areas such as the cortex, striatum and thalamus, regions not affected in our present study by dietary fat (Miao et al. 1998). Overall, our results indicate that, while β2-nAChRs specifically in the VTA and SN and α7-nAChRs specifically in the VMH and LH are particularly sensitive to the stimulatory effects of in utero dietary fat exposure, there are other regions such as the thalamus, mPFC and specific hypothalamic nuclei that are resistant to such change.
Reinstatement of nicotine seeking behavior in perinatal high-fat diet offspring
While we find the HFD and Chow offspring to reinstate nicotine-taking behavior in response to a prime nicotine dose, it was unexpected to observe a small reduction in nicotine seeking with this priming in the HFD offspring. Whereas the explanation for this requires further investigation, it is possible that the persistent elevated nicotine intake in HFD offspring may have desensitized their nAChR’s, leading to the reduced responding during reinstatement testing. Published studies demonstrate that prolonged exposure to nicotine, while generally up-regulating the density of nAChR’s, can either alter the ratio of α- and β-specific subtypes or lead to an increased number of receptors in the desensitized state within mesostriatal brain areas, thereby changing the functionality of this system (Moretti et al. 2010; Picciotto et al. 2008; Rezvani et al. 2007). Such changes in nAChR function in the HFD offspring may cause them to require a greater priming dose for eliciting optimal responding during reinstatement training, similar to doses that have been used in other studies using this paradigm (Feltenstein et al. 2012; Shaham et al. 1997). Another possible explanation for the reduced responding may be related to the neurotransmitter, DA, which is a critical component of the reinstatement process. Published evidence suggests that perinatal HFD feeding can lead to epigenetic changes in the DA uptake and alter DA signaling post-natally while reducing behavioral sensitization to psychostimulants (Naef et al. 2011; Naef et al. 2008; Vucetic et al. 2010), neurochemical changes that may contribute to reduced reinstatement.
Role of nAChRs in increased vulnerability to nicotine addiction
Increased nAChR signaling can enhance the vulnerability of animals to consume excessive amounts of nicotine. Numerous studies have attributed the reinforcing properties of nicotine to the activation of β2-nAChRs in mesostriatal regions such as the VTA and subsequent release of DA in the striatum (Gotti et al. 2010; Pons et al. 2008). An important role for β2-nAChRs in nicotine reward has been suggested by studies in β2-nAChR knockout mice, which are resistant to the reinforcing properties of nicotine (Besson et al. 2006; Mameli-Engvall et al. 2006; Maskos et al. 2005; Picciotto et al. 1998; Zhou et al. 2001). While studies in α7 nAChR knockout mice have raised some questions regarding the precise role and target regions involved in α7 nAChR-mediated nicotine reinforcement (Pons et al. 2008; Walters et al. 2006), pharmacological evidence supports the function of these receptors in nicotine reward, with systemic injection of a specific and potent α7 nAChR antagonist, MLA, significantly reducing nicotine self-administration behavior in rats (Markou and Paterson 2001). Consistent with evidence indicating that an up-regulation of nAChRs in response to increased cholinergic tone may lead to excessive nicotine use and potential abuse (Slotkin 2002), our findings demonstrate that maternal consumption of dietary fat can produce a subpopulation of offspring with increased motivation to use drugs such as nicotine.
Conclusion
It is concluded that maternal consumption of a diet rich in fat enhances nicotine abuse potential in the offspring by specifically increasing responding and motivation for this drug. These behavioral effects may be attributed to a HFD-induced stimulation of nicotinic cholinergic signaling in select midbrain and hypothalamic regions associated with nicotine reward, and consumption. Thus, the current model of perinatal HFD exposure appears well suited for examining the impact of maternal fat consumption specifically on heightened drug reinforcement, not only with nicotine but perhaps with other commonly abused substances such as cocaine. With the current worldwide trends in the availability and consumption of palatable fat-rich foods, it will be important to identify therapeutic strategies that are effective in preventing such fat-induced disturbances in the programming of nicotinic cholinergic circuits.
Fig. 5. Changes in AChE induced by perinatal HFD exposure.
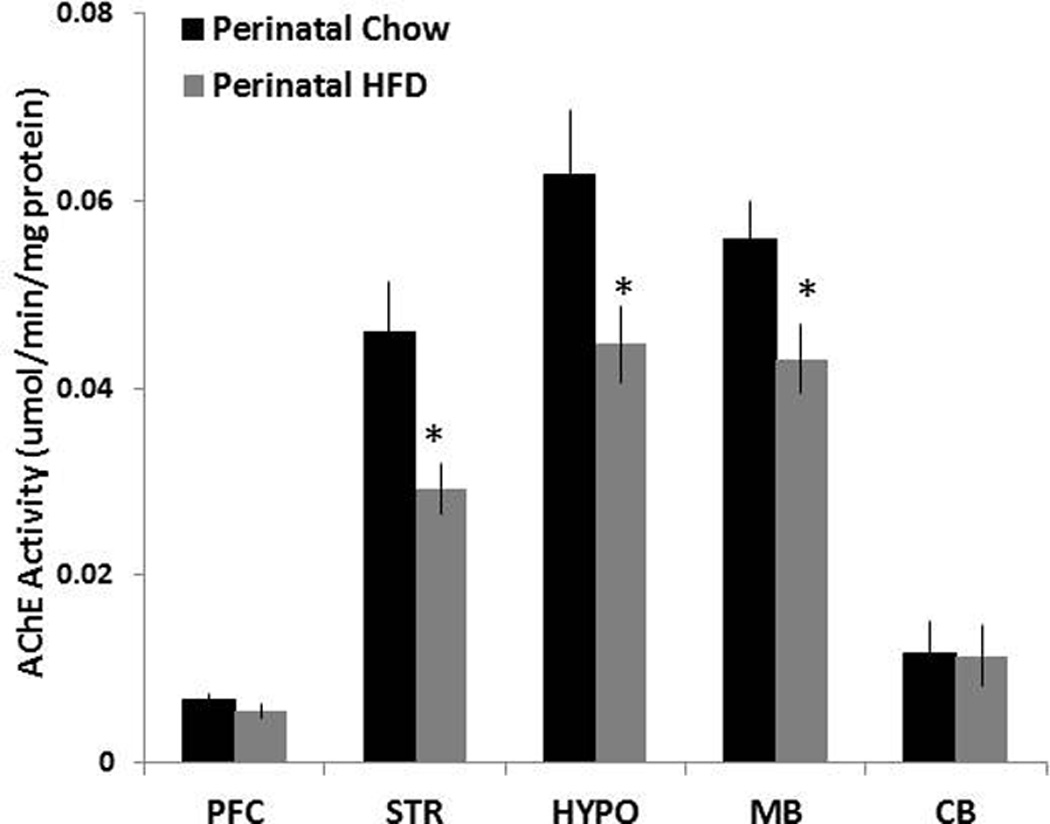
Mean ± SEM AChE activities in homogenized frontal cortex, striatum, hypothalamus, midbrain and cerebellum of rats exposed to a HFD or Chow diet. * Represents significant differences between HFD and Chow diet animals (p<0.05). Abbreviations: HFD - high-fat diet; AChE – acetylcholinesterase; PFC – prefrontal cortex; STR – striatum; HYPO- hypothalamus; MB – midbrain; CB – cerebellum.
Table 2.
Effects of perinatal HFD exposure on the binding of [125I]-epibatidine and [125I]-bungarotoxin in the brain of offspring
| [125I]-epibatidine | [125I] bungarotoxin | |||
|---|---|---|---|---|
| Brain Areas | Perinatal Chow | Perinatal HFD | Perinatal Chow | Perinatal HFD |
| mPFC | 180.2 ± 6.5 | 171.2 ± 4.2 | 117.3 ± 3.1 | 121.9 ± 5.7 |
| NAc Sh | 162.5 ± 4.8 | 159.6 ± 2.3 | ----- | ----- |
| NAc Cr | 170.5 ± 3.1 | 168.1 ± 2.7 | ----- | ----- |
| PVN | ----- | ----- | 170.3 ± 6.1 | 168.0 ± 8.5 |
| PVT | 180.9± 1.2 | 176.6± 4.6 | ----- | ----- |
| Zl | 153.0 ± 14.2 | 174.6 ± 4.5 | ||
| LH | ----- | ----- | 137.8 ± 6.7 | 164.3 ± 7.9* |
| VMH | ----- | ----- | 129.9 ± 9.7 | 159.3 ± 6.5* |
| CeA | ----- | ----- | 177.6 ± 15.7 | 192.2 ± 4.3 |
| VTA | 154.7 ± 12.8 | 184.5 ± 5.8* | ----- | ----- |
| SNc | 145.3± 11.5 | 175.0 ± 8.5* | ----- | ----- |
All values are expressed as fmol/mg brain protein, with an asterisk(*) representing a significant effect of diet on nAChR binding (p<0.05) and dashes representing brain regions where limited or no ligand binding was observed.
Abbreviations: mPFC - medial prefrontal cortex; NAc Sh - nucleus accumbens shell; NAc Cr - nucleus accumbens core; PVN - hypothalamic paraventricular nucleus; PVT - thalamic paraventricular nucleus; ZI - zona incerta; LH - lateral hypothalamus; VMH - vebtromedial hypothalamus; CeA - central nucleus of the amygdale; VTA - ventral tegmental area; and SNc - substantia nigra pars compacta.
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Jessica Barson for her guidance with the statistical analysis of the behavioral and neurochemical data within this manuscript and Sophia Sutcliffe, Svetlana Bagdasarov and Marisa VanBrakle for their help with animal handling and the enzymatic assays used in these experiments. This research was supported by USPHS Grant DA21518.
Footnotes
Statement of conflicts of interest: The authors have no conflicts of interest to declare.
References
- Ahren B, Simonsson E, Scheurink AJ, Mulder H, Myrsen U, Sundler F. Dissociated insulinotropic sensitivity to glucose and carbachol in high-fat diet-induced insulin resistance in C57BL/6J mice. Metabolism. 1997;46:97–106. doi: 10.1016/s0026-0495(97)90175-x. [DOI] [PubMed] [Google Scholar]
- Alburges ME, Hoonakker AJ, Hanson GR. Nicotinic and dopamine D2 receptors mediate nicotine-induced changes in ventral tegmental area neurotensin system. Eur J Pharmacol. 2007;573:124–132. doi: 10.1016/j.ejphar.2007.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderson HL, Latimer MP, Winn P. Intravenous self-administration of nicotine is altered by lesions of the posterior, but not anterior, pedunculopontine tegmental nucleus. Eur J Neurosci. 2006;23:2169–2175. doi: 10.1111/j.1460-9568.2006.04737.x. [DOI] [PubMed] [Google Scholar]
- Barson JR, Morganstern I, Leibowitz SF. Similarities in hypothalamic and mesocorticolimbic circuits regulating the overconsumption of food and alcohol. Physiol Behav. 2011;104:128–137. doi: 10.1016/j.physbeh.2011.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer L, Serafin M, Eggermann E, Saint-Mleux B, Machard D, Jones BE, Muhlethaler M. Exclusive postsynaptic action of hypocretin-orexin on sublayer 6b cortical neurons. J Neurosci. 2004;24:6760–6764. doi: 10.1523/JNEUROSCI.1783-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson M, David V, Suarez S, Cormier A, Cazala P, Changeux JP, Granon S. Genetic dissociation of two behaviors associated with nicotine addiction: beta-2 containing nicotinic receptors are involved in nicotine reinforcement but not in withdrawal syndrome. Psychopharmacology (Berl) 2006;187:189–199. doi: 10.1007/s00213-006-0418-z. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Tsang V. Enduring consequences of maternal obesity for brain inflammation and behavior of offspring. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2010;24:2104–2115. doi: 10.1096/fj.09-144014. [DOI] [PubMed] [Google Scholar]
- Bocarsly ME, Barson JR, Hauca JM, Hoebel BG, Leibowitz SF, Avena NM. Effects of perinatal exposure to palatable diets on body weight and sensitivity to drugs of abuse in rats. Physiol Behav. 2012 doi: 10.1016/j.physbeh.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukouvalas G, Antoniou K, Papalexi E, Kitraki E. Post weaning high fat feeding affects rats' behavior and hypothalamic pituitary adrenal axis at the onset of puberty in a sexually dimorphic manner. Neuroscience. 2008;153:373–382. doi: 10.1016/j.neuroscience.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Markou A. Adaptations in cholinergic transmission in the ventral tegmental area associated with the affective signs of nicotine withdrawal in rats. Neuropharmacology. 2004;47:572–579. doi: 10.1016/j.neuropharm.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Buettner R, Newgard CB, Rhodes CJ, O'Doherty RM. Correction of diet-induced hyperglycemia, hyperinsulinemia, and skeletal muscle insulin resistance by moderate hyperleptinemia. Am J Physiol Endocrinol Metab. 2000;278:E563–E569. doi: 10.1152/ajpendo.2000.278.3.E563. [DOI] [PubMed] [Google Scholar]
- Cabanes A, de Assis S, Gustafsson JA, Hilakivi-Clarke L. Maternal high n-6 polyunsaturated fatty acid intake during pregnancy increases voluntary alcohol intake and hypothalamic estrogen receptor alpha and beta levels among female offspring. Developmental neuroscience. 2000;22:488–493. doi: 10.1159/000017480. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF. Environmental stimuli promote the acquisition of nicotine self-administration in rats. Psychopharmacology (Berl) 2002;163:230–237. doi: 10.1007/s00213-002-1156-5. [DOI] [PubMed] [Google Scholar]
- Caille S, Clemens K, Stinus L, Cador M. Modeling nicotine addiction in rats. Methods Mol Biol. 2012;829:243–256. doi: 10.1007/978-1-61779-458-2_15. [DOI] [PubMed] [Google Scholar]
- Chang GQ, Gaysinskaya V, Karatayev O, Leibowitz SF. Maternal high-fat diet and fetal programming: increased proliferation of hypothalamic peptide-producing neurons that increase risk for overeating and obesity. J Neurosci. 2008;28:12107–12119. doi: 10.1523/JNEUROSCI.2642-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens KJ, Caille S, Cador M. The effects of response operandum and prior food training on intravenous nicotine self-administration in rats. Psychopharmacology (Berl) 2010;211:43–54. doi: 10.1007/s00213-010-1866-z. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology (Berl) 1989;99:473–478. doi: 10.1007/BF00589894. [DOI] [PubMed] [Google Scholar]
- Dallongeville J, Marecaux N, Fruchart JC, Amouyel P. Cigarette smoking is associated with unhealthy patterns of nutrient intake: a meta-analysis. The Journal of nutrition. 1998;128:1450–1457. doi: 10.1093/jn/128.9.1450. [DOI] [PubMed] [Google Scholar]
- Di Matteo V, Pierucci M, Benigno A, Esposito E, Crescimanno G, Di Giovanni G. Critical role of nitric oxide on nicotine-induced hyperactivation of dopaminergic nigrostriatal system: Electrophysiological and neurochemical evidence in rats. CNS neuroscience & therapeutics. 2010;16:127–136. doi: 10.1111/j.1755-5949.2010.00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz WH. Health consequences of obesity in youth: childhood predictors of adult disease. Pediatrics. 1998;101:518–525. [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Knopf S, Brown C. Nicotine self-administration in rats. Psychopharmacology (Berl) 1995;122:390–394. doi: 10.1007/BF02246272. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Mielke MM, Booth S, Gharib MA, Hoffman A, Maldovan V, Shupenko C, McCallum SE. Nicotine self-administration in rats on a progressive ratio schedule of reinforcement. Psychopharmacology (Berl) 1999;147:135–142. doi: 10.1007/s002130051153. [DOI] [PubMed] [Google Scholar]
- Dourmashkin JT, Chang GQ, Hill JO, Gayles EC, Fried SK, Leibowitz SF. Model for predicting and phenotyping at normal weight the long-term propensity for obesity in Sprague-Dawley rats. Physiol Behav. 2006;87:666–678. doi: 10.1016/j.physbeh.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Ellman GL, Courtney KD, Andres V, Jr., Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Feduccia AA, Chatterjee S, Bartlett SE. Neuronal nicotinic acetylcholine receptors: neuroplastic changes underlying alcohol and nicotine addictions. Front Mol Neurosci. 2012;5:83. doi: 10.3389/fnmol.2012.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, Ghee SM, See RE. Nicotine self-administration and reinstatement of nicotine-seeking in male and female rats. Drug and alcohol dependence. 2012;121:240–246. doi: 10.1016/j.drugalcdep.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JO, Birch LL. Fat preferences and fat consumption of 3- to 5-year-old children are related to parental adiposity. J Am Diet Assoc. 1995;95:759–764. doi: 10.1016/S0002-8223(95)00212-X. [DOI] [PubMed] [Google Scholar]
- Flores CM, Davila-Garcia MI, Ulrich YM, Kellar KJ. Differential regulation of neuronal nicotinic receptor binding sites following chronic nicotine administration. Journal of neurochemistry. 1997;69:2216–2219. doi: 10.1046/j.1471-4159.1997.69052216.x. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Kenny PJ. Intravenous nicotine self-administration and cue-induced reinstatement in mice: effects of nicotine dose, rate of drug infusion and prior instrumental training. Neuropharmacology. 2011;61:687–698. doi: 10.1016/j.neuropharm.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotti C, Guiducci S, Tedesco V, Corbioli S, Zanetti L, Moretti M, Zanardi A, Rimondini R, Mugnaini M, Clementi F, Chiamulera C, Zoli M. Nicotinic acetylcholine receptors in the mesolimbic pathway: primary role of ventral tegmental area alpha6beta2* receptors in mediating systemic nicotine effects on dopamine release, locomotion, and reinforcement. J Neurosci. 2010;30:5311–5325. doi: 10.1523/JNEUROSCI.5095-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon TS, Rao G, Arslanian SA. Childhood obesity and type 2 diabetes mellitus. Pediatrics. 2005;116:473–480. doi: 10.1542/peds.2004-2536. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Hartmann J, Kiewert C, Duysen EG, Lockridge O, Greig NH, Klein J. Excessive hippocampal acetylcholine levels in acetylcholinesterase-deficient mice are moderated by butyrylcholinesterase activity. Journal of neurochemistry. 2007;100:1421–1429. doi: 10.1111/j.1471-4159.2006.04347.x. [DOI] [PubMed] [Google Scholar]
- Hussaini AE, Nicholson LM, Shera D, Stettler N, Kinsman S. Adolescent obesity as a risk factor for high-level nicotine addiction in young women. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2011;49:511–517. doi: 10.1016/j.jadohealth.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Imaki T, Xiao-Quan W, Shibasaki T, Yamada K, Harada S, Chikada N, Naruse M, Demura H. Stress-induced activation of neuronal activity and corticotropin-releasing factor gene expression in the paraventricular nucleus is modulated by glucocorticoids in rats. J Clin Invest. 1995;96:231–238. doi: 10.1172/JCI118026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabbaj M. Individual differences in vulnerability to drug abuse: the high responders/low responders model. CNS Neurol Disord Drug Targets. 2006;5:513–520. doi: 10.2174/187152706778559318. [DOI] [PubMed] [Google Scholar]
- Kaizer RR, da Silva AC, Morsch VM, Correa MC, Schetinger MR. Diet-induced changes in AChE activity after long-term exposure. Neurochemical research. 2004;29:2251–2255. doi: 10.1007/s11064-004-7033-3. [DOI] [PubMed] [Google Scholar]
- Kane JK, Parker SL, Matta SG, Fu Y, Sharp BM, Li MD. Nicotine up-regulates expression of orexin and its receptors in rat brain. Endocrinology. 2000;141:3623–3629. doi: 10.1210/endo.141.10.7707. [DOI] [PubMed] [Google Scholar]
- Keath JR, Iacoviello MP, Barrett LE, Mansvelder HD, McGehee DS. Differential modulation by nicotine of substantia nigra versus ventral tegmental area dopamine neurons. J Neurophysiol. 2007;98:3388–3396. doi: 10.1152/jn.00760.2007. [DOI] [PubMed] [Google Scholar]
- Kellar KJ, Giblin BA, Lumpkin MD. Regulation of brain nicotinic cholinergic recognition sites and prolactin release by nicotine. Prog Brain Res. 1989;79:209–216. doi: 10.1016/s0079-6123(08)62480-2. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Chartoff E, Roberto M, Carlezon WA, Jr., Markou A. NMDA receptors regulate nicotine-enhanced brain reward function and intravenous nicotine self-administration: role of the ventral tegmental area and central nucleus of the amygdala. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2009;34:266–281. doi: 10.1038/npp.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmin A, Jerlhag E, Liljequist S, Engel J. Effects of subunit selective nACh receptors on operant ethanol self-administration and relapse-like ethanol-drinking behavior. Psychopharmacology (Berl) 2009;203:99–108. doi: 10.1007/s00213-008-1375-5. [DOI] [PubMed] [Google Scholar]
- Le AD, Li Z, Funk D, Shram M, Li TK, Shaham Y. Increased vulnerability to nicotine self-administration and relapse in alcohol-naive offspring of rats selectively bred for high alcohol intake. J Neurosci. 2006;26:1872–1879. doi: 10.1523/JNEUROSCI.4895-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Chakraborty-Chatterjee M, Lev-Ran S, Barnes C, Pushparaj A, Gamaleddin I, Yan Y, Khaled M, Goldberg SR. Varenicline decreases nicotine self-administration and cue-induced reinstatement of nicotine-seeking behaviour in rats when a long pretreatment time is used. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2012;15:1265–1274. doi: 10.1017/S1461145711001398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz SF, Dourmashkin JT, Chang GQ, Hill JO, Gayles EC, Fried SK, Wang J. Acute high-fat diet paradigms link galanin to triglycerides and their transport and metabolism in muscle. Brain research. 2004;1008:168–178. doi: 10.1016/j.brainres.2004.02.030. [DOI] [PubMed] [Google Scholar]
- Livingstone PD, Wonnacott S. Nicotinic acetylcholine receptors and the ascending dopamine pathways. Biochem Pharmacol. 2009;78:744–755. doi: 10.1016/j.bcp.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Lof E, Olausson P, deBejczy A, Stomberg R, McIntosh JM, Taylor JR, Soderpalm B. Nicotinic acetylcholine receptors in the ventral tegmental area mediate the dopamine activating and reinforcing properties of ethanol cues. Psychopharmacology (Berl) 2007;195:333–343. doi: 10.1007/s00213-007-0899-4. [DOI] [PubMed] [Google Scholar]
- Mameli-Engvall M, Evrard A, Pons S, Maskos U, Svensson TH, Changeux JP, Faure P. Hierarchical control of dopamine neuron-firing patterns by nicotinic receptors. Neuron. 2006;50:911–921. doi: 10.1016/j.neuron.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Markou A, Paterson NE. The nicotinic antagonist methyllycaconitine has differential effects on nicotine self-administration and nicotine withdrawal in the rat. Nicotine Tob Res. 2001;3:361–373. doi: 10.1080/14622200110073380. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Pauly JR, Grun EU, Collins AC. ST/b and DBA/2 mice differ in brain alpha-bungarotoxin binding and alpha 7 nicotinic receptor subunit mRNA levels: a quantitative autoradiographic analysis. Brain Res Mol Brain Res. 1996;39:207–222. doi: 10.1016/0169-328x(96)00027-7. [DOI] [PubMed] [Google Scholar]
- Maskos U, Molles BE, Pons S, Besson M, Guiard BP, Guilloux JP, Evrard A, Cazala P, Cormier A, Mameli-Engvall M, Dufour N, Cloez-Tayarani I, Bemelmans AP, Mallet J, Gardier AM, David V, Faure P, Granon S, Changeux JP. Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature. 2005;436:103–107. doi: 10.1038/nature03694. [DOI] [PubMed] [Google Scholar]
- Miao H, Liu C, Bishop K, Gong ZH, Nordberg A, Zhang X. Nicotine exposure during a critical period of development leads to persistent changes in nicotinic acetylcholine receptors of adult rat brain. Journal of neurochemistry. 1998;70:752–762. doi: 10.1046/j.1471-4159.1998.70020752.x. [DOI] [PubMed] [Google Scholar]
- Miyata G, Meguid MM, Fetissov SO, Torelli GF, Kim HJ. Nicotine's effect on hypothalamic neurotransmitters and appetite regulation. Surgery. 1999;126:255–263. [PubMed] [Google Scholar]
- Moretti M, Mugnaini M, Tessari M, Zoli M, Gaimarri A, Manfredi I, Pistillo F, Clementi F, Gotti C. A comparative study of the effects of the intravenous self-administration or subcutaneous minipump infusion of nicotine on the expression of brain neuronal nicotinic receptor subtypes. Mol Pharmacol. 2010;78:287–296. doi: 10.1124/mol.110.064071. [DOI] [PubMed] [Google Scholar]
- Morganstern I, Ye Z, Liang S, Fagan S, Leibowitz SF. Involvement of cholinergic mechanisms in the behavioral effects of dietary fat consumption. Brain research. 2012;1470:24–34. doi: 10.1016/j.brainres.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugnaini M, Tessari M, Tarter G, Merlo Pich E, Chiamulera C, Bunnemann B. Upregulation of [3H]methyllycaconitine binding sites following continuous infusion of nicotine, without changes of alpha7 or alpha6 subunit mRNA: an autoradiography and in situ hybridization study in rat brain. Eur J Neurosci. 2002;16:1633–1646. doi: 10.1046/j.1460-9568.2002.02220.x. [DOI] [PubMed] [Google Scholar]
- Naef L, Moquin L, Dal Bo G, Giros B, Gratton A, Walker CD. Maternal high-fat intake alters presynaptic regulation of dopamine in the nucleus accumbens and increases motivation for fat rewards in the offspring. Neuroscience. 2011;176:225–236. doi: 10.1016/j.neuroscience.2010.12.037. [DOI] [PubMed] [Google Scholar]
- Naef L, Srivastava L, Gratton A, Hendrickson H, Owens SM, Walker CD. Maternal high fat diet during the perinatal period alters mesocorticolimbic dopamine in the adult rat offspring: reduction in the behavioral responses to repeated amphetamine administration. Psychopharmacology (Berl) 2008;197:83–94. doi: 10.1007/s00213-007-1008-4. [DOI] [PubMed] [Google Scholar]
- NIDA. Tobacco Addiction (Nicotine) 2012. [Google Scholar]
- Nishizaki T, Ikeuchi Y, Matsuoka T, Sumikawa K. Short-term depression and long-term enhancement of ACh-gated channel currents induced by linoleic and linolenic acid. Brain research. 1997;751:253–258. doi: 10.1016/s0006-8993(96)01405-9. [DOI] [PubMed] [Google Scholar]
- Nishizaki T, Nomura T, Matsuoka T, Enikolopov G, Sumikawa K. Arachidonic acid induces a long-lasting facilitation of hippocampal synaptic transmission by modulating PKC activity and nicotinic ACh receptors. Brain Res Mol Brain Res. 1999;69:263–272. doi: 10.1016/s0169-328x(99)00117-5. [DOI] [PubMed] [Google Scholar]
- Ohmori N, Itoi K, Tozawa F, Sakai Y, Sakai K, Horiba N, Demura H, Suda T. Effect of acetylcholine on corticotropin-releasing factor gene expression in the hypothalamic paraventricular nucleus of conscious rats. Endocrinology. 1995;136:4858–4863. doi: 10.1210/endo.136.11.7588217. [DOI] [PubMed] [Google Scholar]
- Ong ZY, Muhlhausler BS. Maternal "junk-food" feeding of rat dams alters food choices and development of the mesolimbic reward pathway in the offspring. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2011;25:2167–2179. doi: 10.1096/fj.10-178392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orosco M, Rouch C, Dauge V. Behavioral responses to ingestion of different sources of fat. Involvement of serotonin? Behav Brain Res. 2002;132:103–109. doi: 10.1016/s0166-4328(01)00397-7. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Justinova Z, Mascia P, Pistis M, Luchicchi A, Lecca S, Barnes C, Redhi GH, Adair J, Heishman SJ, Yasar S, Aliczki M, Haller J, Goldberg SR. Novel use of a lipid-lowering fibrate medication to prevent nicotine reward and relapse: preclinical findings. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37:1838–1847. doi: 10.1038/npp.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker SL, Fu Y, McAllen K, Luo J, McIntosh JM, Lindstrom JM, Sharp BM. Up-regulation of brain nicotinic acetylcholine receptors in the rat during long-term self-administration of nicotine: disproportionate increase of the alpha6 subunit. Mol Pharmacol. 2004;65:611–622. doi: 10.1124/mol.65.3.611. [DOI] [PubMed] [Google Scholar]
- Pasumarthi RK, Fadel J. Stimulation of lateral hypothalamic glutamate and acetylcholine efflux by nicotine: implications for mechanisms of nicotine-induced activation of orexin neurons. Journal of neurochemistry. 2010;113:1023–1035. doi: 10.1111/j.1471-4159.2010.06666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson NE, Markou A. Prolonged nicotine dependence associated with extended access to nicotine self-administration in rats. Psychopharmacology (Berl) 2004;173:64–72. doi: 10.1007/s00213-003-1692-7. [DOI] [PubMed] [Google Scholar]
- Pauly JR, Marks MJ, Gross SD, Collins AC. An autoradiographic analysis of cholinergic receptors in mouse brain after chronic nicotine treatment. J Pharmacol Exp Ther. 1991;258:1127–1136. [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain, in Stereotaxic Coordinates, Compact. Third Edition. San Diego, C.A.: Academic Press, Inc.; 1997. edn. [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Addy NA, Mineur YS, Brunzell DH. It is not "either/or": activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Progress in neurobiology. 2008;84:329–342. doi: 10.1016/j.pneurobio.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Kenny PJ. Molecular Mechanisms Underlying Behaviors Related to Nicotine Addiction. Cold Spring Harb Perspect Med. 2012 doi: 10.1101/cshperspect.a012112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, Fuxe K, Changeux JP. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Pons S, Fattore L, Cossu G, Tolu S, Porcu E, McIntosh JM, Changeux JP, Maskos U, Fratta W. Crucial role of alpha4 and alpha6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration. J Neurosci. 2008;28:12318–12327. doi: 10.1523/JNEUROSCI.3918-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puhl MD, Cason AM, Wojnicki FH, Corwin RL, Grigson PS. A history of bingeing on fat enhances cocaine seeking and taking. Behavioral neuroscience. 2011;125:930–942. doi: 10.1037/a0025759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos EJ, Meguid MM, Zhang L, Miyata G, Fetissov SO, Chen C, Suzuki S, Laviano A. Nicotine infusion into rat ventromedial nuclei and effects on monoaminergic system. Neuroreport. 2004;15:2293–2297. doi: 10.1097/00001756-200410050-00030. [DOI] [PubMed] [Google Scholar]
- Rasmussen BA, Perry DC. An autoradiographic analysis of [125I]alpha-bungarotoxin binding in rat brain after chronic nicotine exposure. Neurosci Lett. 2006;404:9–14. doi: 10.1016/j.neulet.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Raybuck JD, Gould TJ. The role of nicotinic acetylcholine receptors in the medial prefrontal cortex and hippocampus in trace fear conditioning. Neurobiol Learn Mem. 2010;94:353–363. doi: 10.1016/j.nlm.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raygada M, Cho E, Hilakivi-Clarke L. High maternal intake of polyunsaturated fatty acids during pregnancy in mice alters offsprings' aggressive behavior, immobility in the swim test, locomotor activity and brain protein kinase C activity. The Journal of nutrition. 1998;128:2505–2511. doi: 10.1093/jn/128.12.2505. [DOI] [PubMed] [Google Scholar]
- Rezvani K, Teng Y, Shim D, De Biasi M. Nicotine regulates multiple synaptic proteins by inhibiting proteasomal activity. J Neurosci. 2007;27:10508–10519. doi: 10.1523/JNEUROSCI.3353-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro AC, LeSauter J, Dupre C, Pfaff DW. Relationship of arousal to circadian anticipatory behavior: ventromedial hypothalamus: one node in a hunger-arousal network. Eur J Neurosci. 2009;30:1730–1738. doi: 10.1111/j.1460-9568.2009.06969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro AC, Sawa E, Carren-LeSauter I, LeSauter J, Silver R, Pfaff DW. Two forces for arousal: Pitting hunger versus circadian influences and identifying neurons responsible for changes in behavioral arousal. Proc Natl Acad Sci U S A. 2007;104:20078–20083. doi: 10.1073/pnas.0710096104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Rizzo TA, Silverman BL, Metzger BE, Cho NH. Behavioral adjustment in children of diabetic mothers. Acta paediatrica. 1997;86:969–974. doi: 10.1111/j.1651-2227.1997.tb15181.x. [DOI] [PubMed] [Google Scholar]
- Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24:726–731. doi: 10.1016/s0166-2236(00)02002-6. [DOI] [PubMed] [Google Scholar]
- Saules KK, Levine MD, Marcus MD, Pomerleau CS. Differences in smoking patterns among women smokers with childhood versus later onset of weight problems. Eating behaviors. 2007;8:418–422. doi: 10.1016/j.eatbeh.2006.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz RD, Kellar KJ. Nicotinic cholinergic receptor binding sites in the brain: regulation in vivo. Science. 1983;220:214–216. doi: 10.1126/science.6828889. [DOI] [PubMed] [Google Scholar]
- Shacka JJ, Robinson SE. Exposure to prenatal nicotine transiently increases neuronal nicotinic receptor subunit alpha7, alpha4 and beta2 messenger RNAs in the postnatal rat brain. Neuroscience. 1998;84:1151–1161. doi: 10.1016/s0306-4522(97)00564-2. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Adamson LK, Grocki S, Corrigall WA. Reinstatement and spontaneous recovery of nicotine seeking in rats. Psychopharmacology (Berl) 1997;130:396–403. doi: 10.1007/s002130050256. [DOI] [PubMed] [Google Scholar]
- Shram MJ, Funk D, Li Z, Le AD. Nicotine self-administration, extinction responding and reinstatement in adolescent and adult male rats: evidence against a biological vulnerability to nicotine addiction during adolescence. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33:739–748. doi: 10.1038/sj.npp.1301454. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. Nicotine and the adolescent brain: insights from an animal model. Neurotoxicol Teratol. 2002;24:369–384. doi: 10.1016/s0892-0362(02)00199-x. [DOI] [PubMed] [Google Scholar]
- Sorge RE, Clarke PB. Rats self-administer intravenous nicotine delivered in a novel smoking-relevant procedure: effects of dopamine antagonists. J Pharmacol Exp Ther. 2009;330:633–640. doi: 10.1124/jpet.109.154641. [DOI] [PubMed] [Google Scholar]
- Soulis G, Papalexi E, Kittas C, Kitraki E. Early impact of a fat-enriched diet on behavioral responses of male and female rats. Behavioral neuroscience. 2007;121:483–490. doi: 10.1037/0735-7044.121.3.483. [DOI] [PubMed] [Google Scholar]
- Sparks JA, Pauly JR. Effects of continuous oral nicotine administration on brain nicotinic receptors and responsiveness to nicotine in C57Bl/6 mice. Psychopharmacology (Berl) 1999;141:145–153. doi: 10.1007/s002130050818. [DOI] [PubMed] [Google Scholar]
- Sullivan EL, Grove KL. Metabolic imprinting in obesity. Forum of nutrition. 2010;63:186–194. doi: 10.1159/000264406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto N, Austin JD, Vezina P. Locomotor response to novelty predicts a rat's propensity to self-administer nicotine. Psychopharmacology (Berl) 2001;158:175–180. doi: 10.1007/s002130100867. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Jayasena CN, Bloom SR. Obesity and appetite control. Experimental diabetes research. 2012;2012:824305. doi: 10.1155/2012/824305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi A, Ishimaru H, Ikarashi Y, Maruyama Y. Decrease of norepinephrine and preservation of acetylcholine in the hypothalamus of VMH obese rats. Brain Res Bull. 1995;36:97–99. doi: 10.1016/0361-9230(94)00173-x. [DOI] [PubMed] [Google Scholar]
- Tel G, Ozturk M, Duru ME, Harmandar M, Topcu G. Chemical composition of the essential oil and hexane extract of Salvia chionantha and their antioxidant and anticholinesterase activities. Food Chem Toxicol. 2010;48:3189–3193. doi: 10.1016/j.fct.2010.08.020. [DOI] [PubMed] [Google Scholar]
- Teo MY, van Wyk M, Lin J, Lipski J. Differential effects of nicotine on the activity of substantia nigra and ventral tegmental area dopaminergic neurons in vitro. Acta Neurobiol Exp (Wars) 2004;64:119–130. doi: 10.55782/ane-2004-1498. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Perry DC. Prenatal nicotine exposure is associated with an increase in [125I]epibatidine binding in discrete cortical regions in rats. Pharmacology, biochemistry, and behavior. 2000;67:319–323. doi: 10.1016/s0091-3057(00)00379-8. [DOI] [PubMed] [Google Scholar]
- Vajreswari A, Rupalatha M, Rao PS. Effect of altered dietary n-6-to-n-3 fatty acid ratio on erythrocyte lipid composition and membrane-bound enzymes. J Nutr Sci Vitaminol (Tokyo) 2002;48:365–370. doi: 10.3177/jnsv.48.365. [DOI] [PubMed] [Google Scholar]
- van de Kamp JL, Collins AC. Prenatal nicotine alters nicotinic receptor development in the mouse brain. Pharmacology, biochemistry, and behavior. 1994;47:889–900. doi: 10.1016/0091-3057(94)90293-3. [DOI] [PubMed] [Google Scholar]
- Vucetic Z, Kimmel J, Totoki K, Hollenbeck E, Reyes TM. Maternal high-fat diet alters methylation and gene expression of dopamine and opioid-related genes. Endocrinology. 2010;151:4756–4764. doi: 10.1210/en.2010-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters CL, Brown S, Changeux JP, Martin B, Damaj MI. The beta2 but not alpha7 subunit of the nicotinic acetylcholine receptor is required for nicotine-conditioned place preference in mice. Psychopharmacology (Berl) 2006;184:339–344. doi: 10.1007/s00213-005-0295-x. [DOI] [PubMed] [Google Scholar]
- WHO. Diet, nutrition and the prevention of chronic diseases. WHO Technical Report Series. 2003 [PubMed]
- Wise RA. Roles for nigrostriatal--not just mesocorticolimbic--dopamine in reward and addiction. Trends Neurosci. 2009;32:517–524. doi: 10.1016/j.tins.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Nashmi R, McKinney S, Cai H, McIntosh JM, Lester HA. Chronic nicotine selectively enhances alpha4beta2* nicotinic acetylcholine receptors in the nigrostriatal dopamine pathway. J Neurosci. 2009;29:12428–12439. doi: 10.1523/JNEUROSCI.2939-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Pushparaj A, Le Strat Y, Gamaleddin I, Barnes C, Justinova Z, Goldberg SR, Le Foll B. Blockade of dopamine d4 receptors attenuates reinstatement of extinguished nicotine-seeking behavior in rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37:685–696. doi: 10.1038/npp.2011.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZJ, Blaha V, Meguid MM, Oler A, Miyata G. Infusion of nicotine into the LHA enhances dopamine and 5-HT release and suppresses food intake. Pharmacology, biochemistry, and behavior. 1999;64:155–159. doi: 10.1016/s0091-3057(99)00111-2. [DOI] [PubMed] [Google Scholar]
- Yoburn BC, Glusman M. The effects of intrahypothalamic hemicholinium-3 on muricide, irritability and feeding. Pharmacology, biochemistry, and behavior. 1984;20:829–833. doi: 10.1016/0091-3057(84)90001-7. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Solati J, Oryan S, Parivar K. Effect of intra-amygdala injection of nicotine and GABA receptor agents on anxiety-like behaviour in rats. Pharmacology. 2008;82:276–284. doi: 10.1159/000161129. [DOI] [PubMed] [Google Scholar]
- Zhang JF, Zheng F. The role of paraventricular nucleus of hypothalamus in stress-ulcer formation in rats. Brain research. 1997;761:203–209. doi: 10.1016/s0006-8993(97)00257-6. [DOI] [PubMed] [Google Scholar]
- Zhou FM, Liang Y, Dani JA. Endogenous nicotinic cholinergic activity regulates dopamine release in the striatum. Nature neuroscience. 2001;4:1224–1229. doi: 10.1038/nn769. [DOI] [PubMed] [Google Scholar]


