Abstract
A novel cable-driven robotic gait training system has been tested to improve the locomotor function in individuals post stroke. Seven subjects with chronic stroke were recruited to participate in this 6 weeks robot-assisted treadmill training paradigm. A controlled assistance force was applied to the paretic leg at the ankle through a cable-driven robotic system. The force was applied from late stance to mid-swing during treadmill training. Body weight support was provided as necessary to prevent knee buckling or toe drag. Subjects were trained 3 times a week for 6 weeks. Overground gait speed, 6 minute walking distance, and balance were evaluated at pre, post 6 weeks robotic training, and at 8 weeks follow up. Significant improvements in gait speed and 6 minute walking distance were obtained following robotic treadmill training through a cable-driven robotic system. Results from this study indicate that it is feasible to improve the locomotor function in individuals post stroke through a flexible cable-driven robot.
I. Introduction
Stroke is currently the leading cause of disability in the U.S. with approximately 1.1 million individuals currently living with stroke-related disabilities. Impaired mobility is an important factor in determining the degree of physical disability after stroke [1]. While up to 80% of individuals with stroke may ultimately recover the ability to walk a short distance [2], most of them do not achieve the locomotor capacity necessary for community ambulation. Limited community walking reduces the probability of successful return to work and decreases participation in community activities [3].
Body weight supported treadmill training (BWSTT) has been used to improve walking capability in individuals post-stroke and is becoming increasingly popular. By providing partial body weight support over a treadmill and manual facilitation from therapists, previous research has demonstrated improvements in temporal-spatial gait patterns, including gait velocity [4–7], endurance [8], balance [7], and symmetry [9]. In particular, changes in impairments and functional limitations observed with intensive BWSTT are often greater than that achieved during conventional or lower intensity physical therapy [5]. However, BWSTT requires greater involvement of the physical therapist, especially for those patients who need substantial assistance [4].
Several robotic systems have been developed for automating locomotor training of individuals post stroke, such as the Lokomat [10] and Gait Trainer (GT) [11]. The Lokomat is a motorized exoskeleton that drives hip and knee motion in the sagittal plane using four DC motors [10]. The GT drives the patient’s feet through a stepping motion using a crank-and-rocker mechanism attached to foot platforms [11]. These robotic systems had at their onset the basic design goal of firmly assisting patients in producing correctly shaped and timed locomotor movements.
While current robotic gait training relieves the strenuous effort of the therapists and increases the total duration of training, the functional gains are limited for some patient [12, 13]. In particular, results from a study with chronic ambulatory stroke survivors indicated that robotic-assisted BWSTT using the Lokomat is even less effective in improving walking ability in individuals post-stroke than physical therapist-assisted locomotor training [12]. Such results suggest that currently available robotic-assisted BWSTT does not have an advantage in terms of regaining gait function in patients post-stroke except for reducing the labor effort of the physical therapist. As a consequence, there is a need to improve the techniques of robotic BWSTT in order to produce greater functional improvements in individuals post stroke.
Recently, a novel cable-driven robotic gait training system (CaLT) has been developed [14]. The new robotic trainer uses a light-weight cable driven with controlled forces applied to the legs. The CaLT is highly backdrivable, complaint, and gives patients the freedom to voluntarily move their legs in a natural gait pattern during BWSTT. In this study, we tested the feasibility of using this cable-driven robotic system to improve the locomotor function in individuals post stroke.
II. Methods
A. Subjects
Seven individuals with chronic hemiparetic stroke were recruited to participate in this pilot study. Mean age at the time of study enrollment was 57.1 ± 7.7 years old. The average interval between stroke and the onset of robotic BWSTT was 9.1 ± 7.0 years (range 2–21 ys). Five out of 7 are male. Specific inclusion criteria for the participation in the study included: a) age between 21 and 75 years old; b) > 6 months duration after unilateral, supratentorial, ischemic or hemorrhage stroke with lesion location confirmed by radiographic findings; c) no prior stroke; d) demonstration of impaired walking function (self-selected walking speed ≤ 0.99 m/s); f) able to stand and walk (>10 meters) without physical assistance, with the use of assistive devices or orthoses (below knee) as needed.
Exclusion criteria included significant cardiorespiratory/metabolic disease, or other neurological or orthopedic injury that may limit exercise participation or impair locomotion; scores on the Mini Mental Status examination (MMSE) < 24 [15]; stroke of the brainstem or cerebellar lesions; uncontrolled hypertension (systolic > 200 mm Hg, diastolic > 110 mm Hg). All subjects required medical clearance prior to participation. Subjects were excluded if they were unable to tolerate 30 minutes of standing or undergoing concurrent physical therapy. All procedures were approved by the Institutional Review Board of the Northwestern University Medical School. Written informed consent was obtained from all subjects.
B. Apparatus
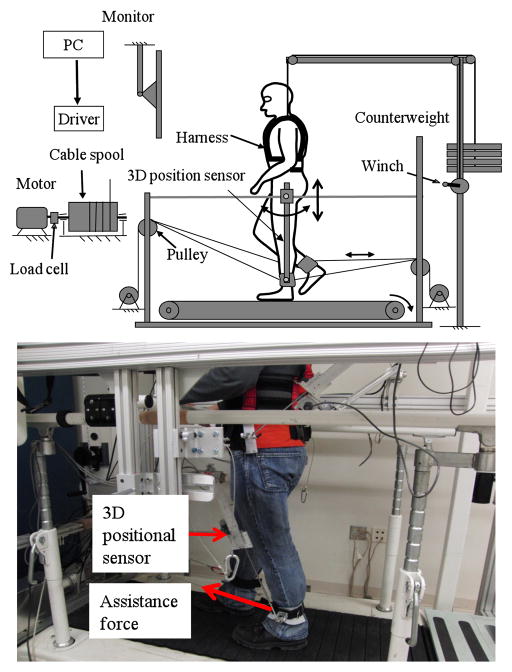
A detailed description of the system has been reported previously [14]. In brief, four nylon-coated stainless-steel cables, driven by four motors through 4 cable spools and pulleys, are affixed to custom cuffs that are strapped to the legs (around the ankles) to produce an assistance force up to 45N (see Figure 1). The frontal pulleys are located at 42 cm above the moving belt. Four, one-degree of freedom reaction torque load cells are integrated between the output shafts of the motors and the cable spools to record the applied torques. Ankle kinematics of both legs are measured using two custom, 3 dimensional position sensors. The ankle position signals were used by the operator to control the timing and magnitude of applied forces, at targeted phases of gait.
Figure 1.
Cable-driven robotic gait training system.
Control is implemented through a custom LabVIEW program, which sends control signals to the motor drives through an analog output to set the applied forces. The controller automatically adjusts the load provided by the cables based on the kinematic performance of the subject. The load is applied starting at pre-swing (10% gait cycle prior to toe off) through mid-swing of gait. The force applied to the legs was determined in real time using the following equation:
| (1) |
where t is time; kP and kD are the position and velocity gains (which are adjustable depending the tolerance of the subject); x(t), ẋ(t), xd(t) and ẋd(t)are the measured and desired ankle horizontal position and velocity during the swing phase. The desired positions were determined from the mean recorded ankle trajectory using the position sensor for two healthy subjects walking on the treadmill.
C. Protocol
For each training session, subjects were fitted with an overhead harness attached to a counterweight support system, with the counterweight providing as much support as necessary to prohibit knee buckling or toe drag during stepping. The treadmill speed was consistent with their maximum comfortable walking speed, determined on the treadmill at the start of each training session. Blood pressure and heart rate were monitored during treadmill training. Short rest breaks were provided as necessary.
At the initiation of locomotor training, the load was applied to the ankle of the paretic leg through the cable robot. At the beginning of each training session, a physical therapist determined the position and velocity gains based on the tolerance of subject. Then, the amount of the load was real-time controlled by the controller, based on the kinematic performance of the subject in accordance with the control algorithm described above.
D. Outcome measures
Outcome measures were evaluated for each participant prior to training, after 6 weeks of training, and at 8 weeks after training was completed. Primary measures were self-selected and fast overground walking velocity collected on a 10 m instrumented walkway (GaitMat II, E.Q. Inc, Chalfont, PA), and walking distance assessed through the 6-minute walk test [16]. Balance was assessed using the Berg Balance Scale [17].
E. Data Analysis
Data was analyzed using scores at pre- vs. post 6 weeks training, and pre vs. 8 weeks follow up assessment. Overgound gait speed and 6-minute walk distance were analyzed using repeated measures ANOVAs for the effect of training (pre vs. post training, pre training vs. follow up), with significance noted at p < 0.05. In addition, balance (Berg Balance Scale) was also analyzed using repeated measures ANOVAs, with significance noted at p < 0.05.
III. Results
All 7 subjects finished 18 sessions of robotic treadmill training. Partial body weight support was provided for one subject (starting at 32% and decreased to 16% at the last training session).
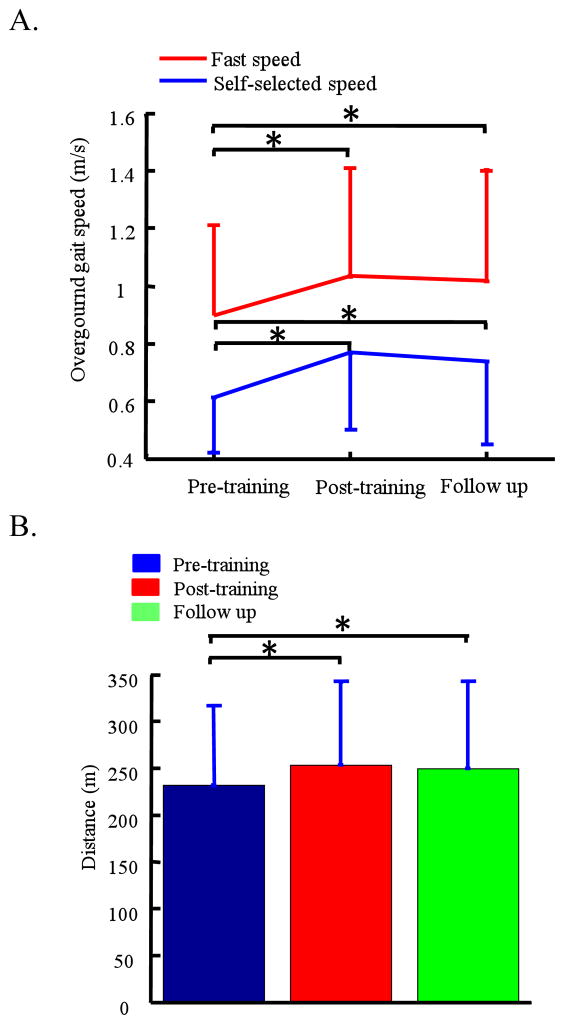
A significant improvement of walking function in individuals post stroke was obtained following 6 weeks of robotic BWSTT using the CaLT. Specifically, self-selected overground walking speed significantly increased from 0.61 ± 0.20 m/s at the baseline to 0.77 ± 0.27 m/s post training (n = 7, one-way repeated measures ANOVA, p = 0.01). Fast walking speed significantly increased from 0.90 ± 0.31 m/s at the baseline to 1.03 ± 0.38 m/s post training (p = 0.02), see Figure 2A. Further, the improved walking speeds were partially retained at 8 weeks follow up. For instance, the self-selected and fast walking speed at the follow up were significantly greater than that at the baseline (0.74 ± 0.29 m/s vs. 0.61 ± 0.20 m/s, p = 0.03, for self-selected speed, and 1.02 ± 0.38 m/s vs. 0.90 ± 0.31 m/s, p = 0.03 for fast walking speed). In addition, the 6-minute walk distance significantly increased after training (232 ± 86 m vs. 254 ± 88 m, for pre and post training, p = 0.01), and was significantly greater at 8 weeks follow up than that at the baseline (250 ± 94 vs. 232 ± 86, p = 0.01), see Figure 2B. Balance had no significant change following robotic treadmill training. Specifically, the Berg Balance Scale Score increased from 49 ± 5 at the baseline to 50 ± 5 post training, although not significant (p = 0.3), and declined to 49 ± 5 at 8 weeks follow up (see Figure 3).
Figure 2.
Overground gait speed, A, and 6-minute walk distance, B, of 7 subjects at pre, post 6 weeks robotic-assisted treadmill training, and 8 weeks after the end of training. An instrumented walkway (GaitMat II, E.Q., Inc) was used to measure the overground gait speed. Three trials were tested and averaged for each test condition.
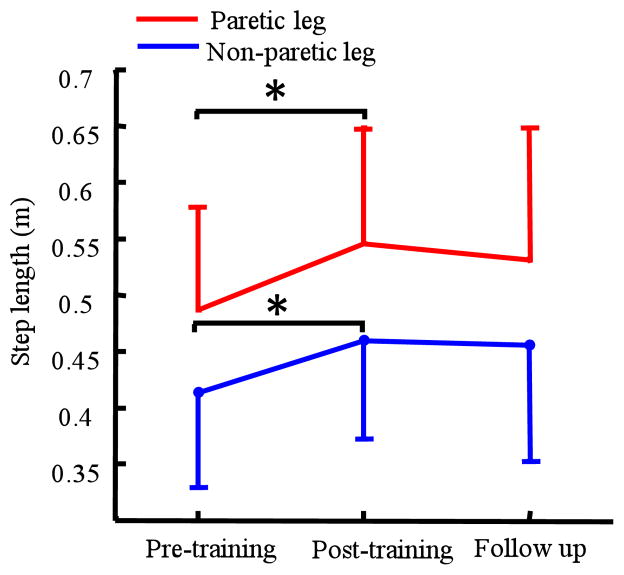
Figure 3.
Step length of 7 subjects at pre, post 6 weeks robotic treadmill training, and 8 weeks after the end of training.
Both step length and cadence significantly improved following robotic gait training. Specifically, the step length of the non-paretic and paretic leg significantly increased from 0.41 ± 0.09 m and 0.49 ± 0.09 m at the baseline to 0.46 ± 0.09 m (p = 0.02) and 0.55 ± 0.1m (p = 0.002), respectively, post training, although no significant changes were observed at follow up (0.46 ± 0.10 m, p = 0.05 and 0.53 ± 0.12m, p = 0.06 for non-paretic and paretic leg, respectively), Figure 3A. Cadence significantly increased from 80 ± 18 steps/min at baseline to 88 ± 24 steps/min post training (p = 0.04), although no significant changes were noted at follow up, 86 ± 24 steps/min (p = 0.06).
IV. Discussion
Improvements in overgound walking were obtained following gait training using a flexible cable-driven robotic system, i.e., CaLT, in individuals post stroke. Specifically, self-selected and fast walking speed, as well as 6-minute walk distance were improved following robotic gait training. Further, the improvements in walking function were partially retained at 8 weeks post training, indicating a clinical significance of such intervention.
The functional gains obtained in the current study with the cable driven robotic gait training is comparable to outcomes following physical therapist assisted BWSTT, i.e., 0.16 ± 0.10 m/s vs. 0.13 ± 0.11 m/s for the self-selected walking speed, and 0.14 ± 0.12 m/s vs. 0.13 ± 0.12 m/s for the fast walking speed [12], but larger than the outcomes following robotic gait training with a fixed trajectory control strategy, i.e., 0.16 ± 0.10 m/s vs. 0.07 ± 0.07 m/s for the self-selected walking speed, and 0.14 ± 0.12 m/s vs. 0.06 ± 0.08 m/s for the fast walking speed [12]. These functional improvements may be due to the features of the cable-driven robotic system, which is designed to mimic the way in which a physical therapist would provide an assistance force to the paretic leg during treadmill training in individuals post stroke.
Maintaining variation in kinematics during BWSTT is considered to be critical in improving the locomotor function in individuals post stroke. For instance, results from animal experiments show that motor learning is more effective with a robotic algorithm that allows variability in the stepping pattern than with a fixed trajectory paradigm [18]. In addition, results from human study have shown that intralimb coordination after stroke was improved by physical therapist assisted BWSTT, which allowed for kinematic variability, but not robotic gait training with a fixed trajectory, which reduces kinematic variability [19]. In the current study, the cable driven robotic system, which is highly backdrivable, has limited constraint of leg kinematics during treadmill training. This type of training seems more effective in improving locomotor function in individuals post stroke than with fixed trajectory training. In particular, both the step lengths of the paretic and non-paretic legs improved, suggesting an improvement in motor control of the paretic leg following robotic training.
The subjects who participated in the current study were all ambulatory patients with self-selected walking speeds ranging from 0.23 to 0.83 m/s. Six out 7 subjects were community walkers (i.e., self-selected walking speed > 0.5 m/s). For these patients, cable-driven robotic gait training appeared to be effective to improve locomotor function. However, it remains unclear whether cable-driven robotic gait training will be effective in improving the locomotor function of individuals who are more severely affected.
V. Conclusion
The cable driven locomotor training system proposed in this study provides a promising adjunct for treatment of patients post-stroke through robotic-assisted treadmill training. The cable-driven robotic gait training system is highly backdrivable, complaint, and allows freedom for patients to voluntarily move their legs during BWSTT. Results from this study indicate that it is feasible to improve the locomotor function in individuals post-stroke using the cable-driven robotic gait training system.
Acknowledgments
This work was supported by the NIH under Grant 1R21HD058267 (Wu), and in partial the Falk Medical Research Trust.
The authors thank Dr. James Stinear for lab space support.
Contributor Information
Ming Wu, Email: w-ming@northwestern.edu, Sensor Motor Performance Program, Rehabilitation Institute of Chicago; Department of Physical Medicine and Rehabilitation, Northwestern University Medical School, Chicago, IL, 60611, USA (phone: 312-238-0700; fax: 312-238-2208;).
Jill M. Landry, Email: jlandry@ric.org, Sensor Motor Performance Program, Rehabilitation Institute of Chicago, Chicago, IL, 60611, USA
Sheng-Che Yen, Email: syen@ric.org, Sensor Motor Performance Program, Rehabilitation Institute of Chicago, Chicago, IL, 60611 USA.
Brian D. Schmit, Email: brain.schmit@marquette.edu, Department of Biomedical Engineering, Marquette University, Milwaukee, WI, USA
T. George Hornby, Email: g-hornby@northwestern.edu, Department of Physical Therapy, University of Illinois at Chicago, Chicago, IL, USA.
Miriam Rafferty, Sensor Motor Performance Program, Rehabilitation Institute of Chicago, Chicago, IL, 60611, USA.
References
- 1.Perry J, et al. Classification of walking handicap in the stroke population. Stroke. 1995 Jun;26:982–9. doi: 10.1161/01.str.26.6.982. [DOI] [PubMed] [Google Scholar]
- 2.Jorgensen HS, et al. Recovery of walking function in stroke patients: the Copenhagen Stroke Study. Arch Phys Med Rehabil. 1995 Jan;76:27–32. doi: 10.1016/s0003-9993(95)80038-7. [DOI] [PubMed] [Google Scholar]
- 3.Treger I, et al. Return to work in stroke patients. Disabil Rehabil. 2007 Sep 15;29:1397–403. doi: 10.1080/09638280701314923. [DOI] [PubMed] [Google Scholar]
- 4.Hesse S, et al. Treadmill training with partial body weight support compared with physiotherapy in nonambulatory hemiparetic patients. Stroke. 1995 Jun;26:976–81. doi: 10.1161/01.str.26.6.976. [DOI] [PubMed] [Google Scholar]
- 5.Pohl M, et al. Speed-dependent treadmill training in ambulatory hemiparetic stroke patients: a randomized controlled trial. Stroke. 2002 Feb;33:553–8. doi: 10.1161/hs0202.102365. [DOI] [PubMed] [Google Scholar]
- 6.Sullivan KJ, et al. Step training with body weight support: effect of treadmill speed and practice paradigms on poststroke locomotor recovery. Arch Phys Med Rehabil. 2002 May;83:683–91. doi: 10.1053/apmr.2002.32488. [DOI] [PubMed] [Google Scholar]
- 7.Visintin M, et al. A new approach to retrain gait in stroke patients through body weight support and treadmill stimulation. Stroke. 1998 Jun;29:1122–8. doi: 10.1161/01.str.29.6.1122. [DOI] [PubMed] [Google Scholar]
- 8.Macko RF, et al. Treadmill exercise rehabilitation improves ambulatory function and cardiovascular fitness in patients with chronic stroke: a randomized, controlled trial. Stroke. 2005 Oct;36:2206–11. doi: 10.1161/01.STR.0000181076.91805.89. [DOI] [PubMed] [Google Scholar]
- 9.Trueblood PR. Partial body weight treadmill training in persons with chronic stroke. Neuro Rehabilitation. 2001;16:141–53. [PubMed] [Google Scholar]
- 10.Colombo G, et al. Treadmill training of paraplegic patients using a robotic orthosis. J Rehabil Res Dev. 2000 Nov-Dec;37:693–700. [PubMed] [Google Scholar]
- 11.Hesse S, Uhlenbrock D. A mechanized gait trainer for restoration of gait. J Rehabil Res Dev. 2000 Nov-Dec;37:701–8. [PubMed] [Google Scholar]
- 12.Hornby TG, et al. Enhanced gait-related improvements after therapist- versus robotic-assisted locomotor training in subjects with chronic stroke: a randomized controlled study. Stroke. 2008 Jun;39:1786–92. doi: 10.1161/STROKEAHA.107.504779. [DOI] [PubMed] [Google Scholar]
- 13.Husemann B, et al. Effects of locomotion training with assistance of a robot-driven gait orthosis in hemiparetic patients after stroke: a randomized controlled pilot study. Stroke. 2007 Feb;38:349–54. doi: 10.1161/01.STR.0000254607.48765.cb. [DOI] [PubMed] [Google Scholar]
- 14.Wu M, et al. A cable-driven locomotor training system for restoration of gait in human SCI. Gait Posture. 2011 Feb;33:256–60. doi: 10.1016/j.gaitpost.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 15.Folstein MF, et al. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975 Nov;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 16.Harada ND, et al. Mobility-related function in older adults: assessment with a 6-minute walk test. Arch Phys Med Rehabil. 1999 Jul;80:837–41. doi: 10.1016/s0003-9993(99)90236-8. [DOI] [PubMed] [Google Scholar]
- 17.Berg K, et al. The Balance Scale: reliability assessment with elderly residents and patients with an acute stroke. Scand J Rehabil Med. 1995 Mar;27:27–36. [PubMed] [Google Scholar]
- 18.Cai LL, et al. Implications of assist-as-needed robotic step training after a complete spinal cord injury on intrinsic strategies of motor learning. J Neurosci. 2006 Oct 11;26:10564–8. doi: 10.1523/JNEUROSCI.2266-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewek MD, et al. Allowing intralimb kinematic variability during locomotor training poststroke improves kinematic consistency: a subgroup analysis from a randomized clinical trial. Phys Ther. 2009 Aug;89:829–39. doi: 10.2522/ptj.20080180. [DOI] [PMC free article] [PubMed] [Google Scholar]