Abstract
Dynamic nuclear polarization (DNP) of 15N2O, known for its long-lived singlet-state order at low magnetic field, is demonstrated in organic solvent/trityl mixtures at ~1.5 K and 5 T. Both 15N polarization and intermolecular dipolar broadening are strongly affected by the sample's thermal history, indicating spontaneous formation of N2O clusters. In situ 15N NMR reveals four distinct powder-pattern spectra, attributed to the chemical-shift anisotropy (CSA) tensors of the two 15N nuclei, further split by the intramolecular dipolar coupling between their magnetic moments. 15N polarization is estimated by fitting the free-induction decay (FID) signals to the analytical model of four single-quantum transitions. This analysis implies (10:2 ± 2:2)% polarization after 37 h of DNP, and provides a direct, instantaneous probe of the absolute 15N polarization, without a need for time-consuming referencing to a thermal-equilibrium NMR signal.
Keywords: Nitrous oxide, Dynamic nuclear polarization, Singlet state, Magnetic resonance imaging, Chemical shift anisotropy, Dipolar interaction, Amorphous solid, Molecular imaging, Trityl radical
1. Introduction
The field of metabolic magnetic resonance imaging [1] has enjoyed a remarkable expansion following the success of low-temperature dynamic nuclear polarization (DNP) of 13C-labelled biomolecules, where high 13C polarization (20–75%) [2–4] is retained at room temperature after a rapid dissolution [5,6]. However, very short T1 relaxation times (~30 s) of these compounds have severely limited the applicability of 13C-DNP imaging, except for the fastest of metabolic pathways [7].
One possible way to address this problem is to utilize long-lived singlet order in homonuclear systems of spin-1/2 pairs [8–11]. A particularly attractive target system is doubly labelled 15N2O, a non-toxic anesthetic gas, which has exhibited a nuclear singlet lifetime above 25 min in a deuterated solvent [12,13] and ~7 min in human blood [14].
Here we report the first observations of DNP in the solid-state 15N2O matrix. The lineshape and the maximum achievable polarization are found to depend substantially on the thermal history of the sample, which we qualitatively explain in terms of pure-N2O clustering effects similar to those we have reported earlier in 129Xe DNP [15]. Surprisingly, cluster formation in 15N2O mixtures leads to enhanced 15N polarization, showing no signs of spin-diffusion bottle neck observed in the xenon system. Using the rich spectral structure of 15N2O NMR, we derive the detailed NMR line shape of the observed 15N2O spectra, from which one can directly and instantaneously determine the absolute 15N polarization. Our line-shape analysis provides a valuable tool for assessing and modeling DNP conditions in situ, and eliminates the need for customary referencing to thermal-equilibrium NMR signals, which can take many days of acquisition due to extremely long T1 relaxation times of 15N at these low temperatures. While the earlier studies [16] have demonstrated the effects of nuclear polarization on the overall spectral parameters such as line-shape moments, this is the first ab initio analysis of the detailed NMR spectrum observed in an isotropically oriented two-spin-1/2 solid-state molecular system, with the fit sensitivity sufficient for precise determination of the nuclear spin polarization.
2. Materials and methods
A hermetically-sealed retractable electric stirrer was used to mix Finland-acid radical [17] solution with distilled liquid 15N15NO (Cambridge Isotopes, >98% pure, >98% 15N) in an ethanol/solid CO bath (1.75 atm 15N2O pressure at 195 K) for ~1 min. Sample I contained 9.4 mg (9.4 μmol) radical, 84 mg (1.4 mmol) 1-propanol, 31 mg (0.36 mmol) CD2Cl2, 0.9 mmol 15N2O. Sample II contained 2.9 mg radical, 107 mg 1-propanol, 1.3 mmol 15N2O. After a rapid switch to the liquid nitrogen (LN2, 77 K) bath, the excess solid 15N2O at the top of the sample was sub-limated with a soldering iron. The open sample tube was transferred to the DNP probe at 120 K, which was then cooled down to 1.4 K. A home-built spectrometer recorded 15N NMR at 21.56 MHz in our 140-GHz DNP system [15]. Switching on the microwaves increased the probe temperature by 5–200 mK. Prior to the microwave sweep, the magnet's field B0 was determined by 1H2O NMR at room temperature and by solid-state 15N NMR at ~1.5 K. Free-induction decays (FID) were acquired using 1–4 μs small flip-angle pulses (7 ± 1 μs dead time). After applying a baseline correction, Gaussian broadening (50–170 Hz for plotting only), Fourier transform, and zero-order phase correction, the spectra were plotted using Igor Pro 6 (Wavemetrics).
3. Results
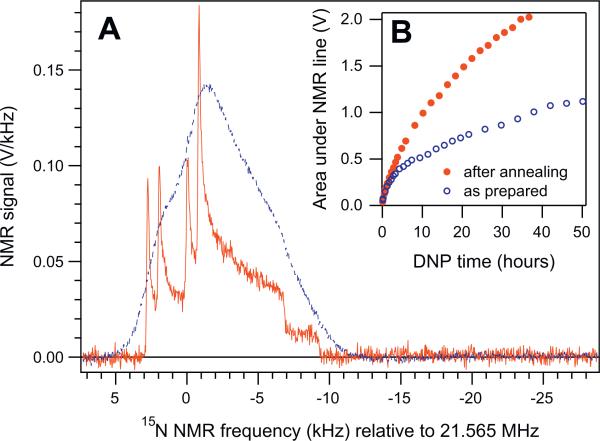
Fig. 1A shows the evolution of 15N NMR spectra during DNP in sample I. Four observed powder-pattern lineshape components are attributed to the chemical-shift anisotropy (CSA) tensors of the two 15N nuclei [18], further split by intramolecular dipolar coupling between them [19]. Fig. 1B shows the nuclear system's response to the DNP microwave frequency ν in sample II, plotted against ν/B0 (to factor out small B0 drift). The splitting between ν+ and ν–, defined as the optimal frequencies for overpopulating the ground and the excited states of the nuclear Zeeman system, was found to be 57.3 MHz, on the order of the electron-resonance linewidth or twice the 15N nuclear Larmor frequency.
Fig. 1.
(A) Evolution of 15N2O NMR spectra at T = 1:6 K and B = 4:9976 T in sample I during the first 18 h of DNP irradiation at ν+ = 140:0625 GHz, acquired using short rf pulses with a 3.7° flip angle. (B) The initial rate of increase of the area under the 15N NMR line due to DNP (dA/dt), measured in sample II (open circles) as a function of the microwave frequency-to-field ratio ν/B0 at B0 = 4:99618 T and T = 1.42 K. At each ν; 15N magnetization was destroyed by a pulse train, followed by 873 s of microwave irradiation and an 15N NMR acquisition. The dashed line is to guide the eye.
The 15N spectra shown in Fig. 1A are broadened by intermolecular interactions with the solvent atoms. This broadening strongly depends on the molecular environment of 15N2O. Fig. 2A shows two 15N NMR spectra, taken 49 min apart on the same sample I after 50 h of DNP. The N2O/solvent/trityl mixture was initially very homogeneous, as evidenced by the strong broadening of the first DNP spectrum (blue dashed line). Over several tens of seconds, this hyperpolarized sample was carefully transferred through room-temperature air and the ambient field of ~20 G to a 1.1 T holding field at 77 K. After about 3 min at 1.1 T, the sample was returned to the 5 T field of the DNP system. As can be seen from Fig. 2A, about half of the original hyperpolarization was preserved during this process. Remarkably, the strong uniform broadening of the spectrum was largely removed by this transient annealing of the sample, revealing a detailed quadruplet of powder-spectrum patterns characteristic of pure polycrystalline 15N2O. This irreversible removal of NMR line broadening persisted throughout the next DNP experiment at T = 1:6 K (red solid circles, Fig. 2B). In subsequent controlled experiments with sample II, uniformly-mixed N2O/1-propanol/trityl samples had to be annealed for tens of minutes at 160–180 K to reproduce this dramatic line narrowing and the increased DNP efficiency. At these temperatures the mixture undergoes a glass transition, whereby atomic diffusion leads to spontaneous partial segregation of the pure N2O from the mixture, leading to cluster formation. This results in a pronounced change in the 15N spin–lattice relaxation time T1 from 8 to 33 h at 1.41–1.44 K. We have also observed similar phenomena in 129Xe/1-propanol/trityl mixtures [15]. However, in contrast to 129Xe, DNP of the partially-segregated N2O/solvent/trityl mixture resulted in substantially higher average 15N polarization compared to the homogeneous mixture at the same conditions, as can be seen in Fig. 2B.
Fig. 2.
(A) Solid-state 15N NMR spectra of DNP-polarized 15N2O/solvent/trityl mixture immediately before and after (blue dashed and red solid lines, averaged over 5 and 1 scans) the annealing of sample I described in the text. (B) Evolution of the area A under the 15N NMR line during DNP at ν+ and T = 1.6 K before and after (open blue and solid red circles) the same annealing event. To avoid preamplifier saturation, the spectra with A ≥ 1.8 V were acquired with a 1.85° flip angle instead of the nominal 3.7°, and scaled up by 2.03. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Analytical model and discussion
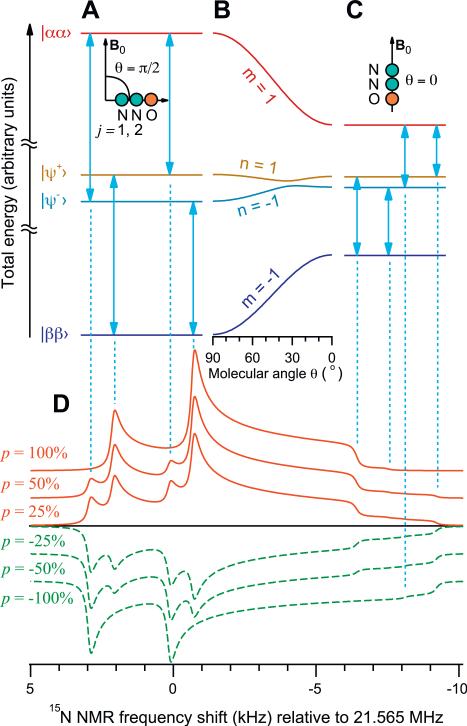
The NMR spectra of the polarized 15N2O solid may be analyzed by assuming that the 15N2O molecules are randomly oriented and equally polarized, and that all intermolecular spin interactions only produce a uniform line broadening. The linear symmetry of N2O ensures a uniaxial dependence of both the dipole–dipole and CSA tensors on the molecular angle θ (with respect to B0) via the 2nd-rank Legendre polynomial . The spin Hamiltonian in the rotating frame of the resonant radio-frequency (rf) field is:
| (1) |
where ω0 = –γB0 is the nuclear Larmor frequency, γ = –2π × 4.316 MHz/T is the 15N magnetogyric ratio, and are the isotropic and anisotropic chemical shifts for the terminal (j = 1) and central (j = 2) 15N sites, ωJ = 2πJ12 is the J-coupling, and is the nuclear dipole–dipole coupling constant for the internuclear 15N—15N distance r12. In a liquid solution, the isotropic chemical shift difference is ppm [12]. The highest- and lowest-energy eigenstates of the Hamiltonian (Eq. (1)) are the Zeeman product states and respectively [11], while the two intermediate-energy states and are coupled as shown in Fig. 3A–C. The coupling parameter is , using compact notation: and ωΔ = ω2 – ω1 for both “iso” or “ani” cases. The four single-quantum transitions (vertical arrows in Fig. 3A and C) can be parameterized by a pair of indices (Fig. 3B): m = ±1 selects the transitions connecting to either or states, whereas n = ±1 chooses a connection to one of the intermediate states . For a given molecular orientation P2(cos θ), NMR transitions of Fig. 3A–C are located relative to the rotating-frame frequency ω0 at:
| (2) |
The spin-density operator of the uniformly-polarized sample consisting of two-spin molecules is:
| (3) |
where 1 is the unit operator, and |p| ≤ 1 is the polarization level. In most NMR experiments at thermal polarization, |p| is very small, and the quadratic terms in Eq. (3) may be ignored. This is not the case in DNP experiments at |p| ~ 1. For each single-quantum coherence , a hard pulse with a flip angle ζ will generate an NMR signal amplitude , where is a rotation operator. The NMR amplitudes of the four transitions of Fig. 3A–C are thus:
| (4) |
| (5) |
whereas amn(ζ, p, P2) = 0 outside of the range of Eq. (5).
Fig. 3.
Total energy levels of the eigenstates of the Hamiltonian (Eq. (1)) for the molecules perpendicular (A: θ = π/2) and parallel (C: θ = 0) to the magnetic field B0. The detailed dependence of the energy levels on the molecular orientation θ is shown in (B). Spectral line shapes (D) for different polarization values, calculated using Eqs. (4)–(8), using the parameters of Table 1. Note the spectral features in (D) corresponding to the transitions in (A and C). The dependence of the spectral line shape on the polarization is the basis of the polarimetry technique proposed here.
For a given molecule, each transition's NMR frequency ω = Ωmn(P2) can be used to express P2(cos θ) by inverting Eq. (2) in the domain of interest:
| (6) |
where , , , , , and ϕ = 4K2 – Γ. In an orientationally-disordered 3D sample, cos θ is uniformly distributed on [–1, 1]. To account for unequal number of molecules at each ω, NMR amplitudes of Eq. (4) must be divided by the statistical weights
| (7) |
to yield the sum of four Pake-like powder patterns [20]:
| (8) |
where each element of the sum extends over its own ω range according to Eqs. (5) and (6). Although Eq. (6) is valid for 15N2O, different analytical solutions or numerical simulations are required for some other parameter ranges.
To represent homogeneous line broadening, Eq. (8) is numerically convolved with a Lorentzian of full-width-at-half-height λ. Fig. 3D shows model line shapes for different spin polarizations p, assuming a flip angle of 1.85°. Note the dependence of the spectral line shape on both the magnitude and the sign of the polarization. Computed line shapes are found to have both strongly and weakly coupled eigenstates for certain molecular orientations, making it essential not to simplify the Hamiltonian of Eq. (1) any further. Additional nonsecular dipolar terms have perturbative contributions 4 orders of magnitude below those of Eq. (1) and are therefore omitted.
Eq. (4) predicts that the relative amplitudes of the four spectral components depend on the sign and magnitude of the polarization p as well as on the molecular orientation θ (Fig. 3D): when the polarization is large and positive, the lowest energy state is overpopulated. A small flip-angle pulse (cos ζ ~ 1) excites the corresponding m = –1 single-quantum transitions, and leads to a strong positive amplitude for the two single-quantum coherences terminating at state . On the other hand, if the polarization is large and negative, the highest energy state is overpopulated, so that a small flip-angle pulse generates a large negative amplitude for the other two single-quantum transitions (m = 1). Since all four transitions have different frequencies, the shape of the spectrum, as well as its overall amplitude, thus depends on the sign and magnitude of p. The NMR spectrum of a multilevel spin system therefore provides a form of absolute polarimetry, which does not rely exclusively on the comparison of spectral amplitudes to those obtained at thermal equilibrium. This phenomenon has also been noted at millikelvin temperatures [21].
Our analytical lineshape model was fitted to the measured NMR data using a Bayesian statistical method [22–24]. 95% confidence-level intervals were computed in a Markov-chain Monte Carlo procedure [25,23,26] by minimizing the sum of square-deviations between the model (simod) and experimental FID points. The fitting weights , representing the noise due to random rf-pulse ring-down at time-points ti, were independently determined from the low-polarization spectra to fit wi = (1 + α exp(–ti/τnoise))–1, with α = 200 and τnoise = 2 μs. Including the weights wi into the fit deemphasizes the initial noisy data without discarding useful information at the rf ring-down's tail.
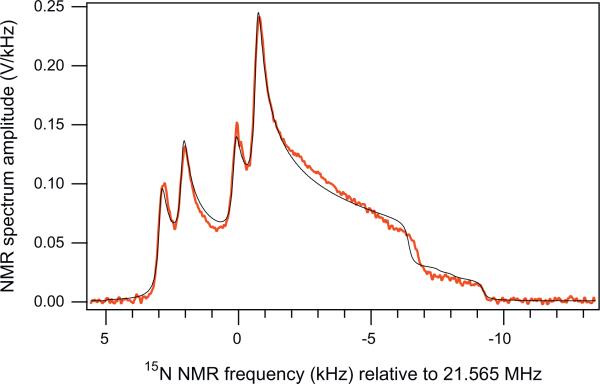
Fig. 4 shows the NMR spectrum along with the model line shape based on the best-fit parameters listed in Table 1. The J-coupling was set to the literature value –8.7 Hz [14,18]. Our isotropic chemical shift difference is in reasonable agreement with room-temperature data: 75 ppm in nematic-phase 15N2O [18] and 82.3 ppm in isotropic solution [12]. Our chemical shift anisotropies are also consistent with the nematic-phase data [18]: and (using ). The best-fit dipolar coupling b12/(2π) = –840 Hz corresponds to a N–N bond length r12 = 113.7 pm, in reasonable agreement with 112.6 pm (14N2O) and 118.6 pm (15N14NO) [27].
Fig. 4.
Comparison of the experimental spectrum (thick red curve), corresponding to the maxim-polarization data point of Fig. 2B, and the best-fit line-shape model based on Eqs. (4)–(8) and a Bayesian fitting method described in the text (thin black line). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 1.
Best-fit values and 95% confidence intervals of the essential free-fit parameters for the spectrum shown in Fig. 4.
| Parameter | Unit | Value | Parameter | Unit | Value |
|---|---|---|---|---|---|
| ppm | 74 ± 3.5 | p | % | 10.2 ± 2 | |
| ppm | –223 ± 4 | b12/(2π) | Hz | –840 ± 10 | |
| ppm | –331 ± 4 | λ | Hz | 234 ± 10 |
5. Conclusion
In summary, we have achieved 15N2O hyperpolarization by DNP in frozen mixtures with a trityl radical and an organic solvent. Annealing produces an order-of-magnitude narrowing of the NMR spectrum, suggesting the formation of pure 15N2O clusters. We have also developed analytical tools for measuring the magnitude and sign of the polarization directly from the 15N NMR spectrum. The highest observed polarization was (10.2 ± 2:2)%, still growing after 37 h of DNP. Faster polarization build-up rates may be achievable by combining DNP with 1H—15N cross-polarization [4,6]. We are now investigating how to convert the hyperpolarized solid 15N2O into a room-temperature solution, while retaining the 15N polarization.
Acknowledgments
We thank A. Popov, O. Taratula, H. Shaghaghi, and P. Manasseh for assistance with sample preparation, W. Pennie and M. Carman for machine-shop help, and J.-H. Ardenkjær-Larsen for discussions. This work is supported by NIH R01 EB010208, the Leverhulme Trust (UK) and EPSRC (UK).
References
- 1.Golman K, in t́ Zandt R, Thaning M. Real-time metabolic imaging. Proc. Natl. Acad. Sci. U. S. A. 2006;103:11270–11275. doi: 10.1073/pnas.0601319103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albers MJ, et al. Hyperpolarized 13C lactate, pyruvate, and alanine: noninvasive biomarkers for prostate cancer detection and grading. Cancer Res. 2008;68:8607–8615. doi: 10.1158/0008-5472.CAN-08-0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meyer W, et al. Dynamic polarization of 13C nuclei in solid 13C labeled pyruvic acid. Nucl. Instrum. Methods Phys. Res. A. 2011;631:1–5. [Google Scholar]
- 4.Jannin S, Bornet A, Melzi R, Bodenhausen G. High field dynamic nuclear polarization at 6.7 T: carbon-13 polarization above 70% within 20 min. Chem. Phys. Lett. 2012;549:99–102. [Google Scholar]
- 5.Golman K, et al. C-13-angiography. Acad. Radiol. 2002;9:S507–S510. doi: 10.1016/s1076-6332(03)80278-7. [DOI] [PubMed] [Google Scholar]
- 6.Bornet A, Melzi R, Jannin S, Bodenhausen G. Cross polarization for dissolution dynamic nuclear polarization experiments at readily accessible temperatures 1.2 < T < 4.2 K. Appl. Magn. Reson. 2012;43:107–117. [Google Scholar]
- 7.Rowland IJ, Peterson ET, Gordon JW, Fain SB. Hyperpolarized 13Carbon MR. Curr. Pharm. Biotechnol. 2010;11:709–719. doi: 10.2174/138920110792246636. [DOI] [PubMed] [Google Scholar]
- 8.Carravetta M, Johannessen OG, Levitt MH. Beyond the T1 limit: singlet nuclear spin states in low magnetic fields. Phys. Rev. Lett. 2004;92:153003. doi: 10.1103/PhysRevLett.92.153003. [DOI] [PubMed] [Google Scholar]
- 9.Carravetta M, Levitt MH. Long-lived nuclear spin states in high-field solution NMR. J. Am. Chem. Soc. 2004;126:6228–6229. doi: 10.1021/ja0490931. [DOI] [PubMed] [Google Scholar]
- 10.Warren WS, Jenista E, Branca RT, Chen X. Increasing hyperpolarized spin lifetimes through true singlet eigenstates. Science. 2009;323:1711–1714. doi: 10.1126/science.1167693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levitt MH. Singlet nuclear magnetic resonance. Annu. Rev. Phys. Chem. 2012;63:89–105. doi: 10.1146/annurev-physchem-032511-143724. [DOI] [PubMed] [Google Scholar]
- 12.Pileio G, Carravetta M, Hughes E, Levitt MH. The long-lived nuclear singlet state of 15N–nitrous oxide in solution. J. Am. Chem. Soc. 2008;130:12582–12583. doi: 10.1021/ja803601d. [DOI] [PubMed] [Google Scholar]
- 13.Bocan J, Pileio G, Levitt MH. Sensitivity enhancement and low-field spin relaxation in singlet NMR. Phys. Chem. Chem. Phys. 2012;14:16032–16040. doi: 10.1039/c2cp42553j. [DOI] [PubMed] [Google Scholar]
- 14.Ghosh RK, et al. Measurements of the persistent singlet state of N2O in blood and other solvents: potential as a magnetic tracer. Magn. Reson. Med. 2011;66:1177–1180. doi: 10.1002/mrm.23119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuzma NN, et al. Cluster formation restricts dynamic nuclear polarization of xenon in solid mixtures. J. Chem. Phys. 2012;137:104508. doi: 10.1063/1.4751021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abragam A, Chapellier M, Jacquinot JF, Goldman M. Absorption lineshape of highly polarized nuclear spin systems. J. Magn. Reson. 1973;10:322–346. [Google Scholar]
- 17.Macholl S, Johannesson H, Ardenkjaer-Larsen JH. Trityl biradicals and 13C dynamic nuclear polarization. Phys. Chem. Chem. Phys. 2010;12:5804–5817. doi: 10.1039/c002699a. [DOI] [PubMed] [Google Scholar]
- 18.Bhattacharyya PK, Dailey BP. 15N magnetic shielding anisotropies in 15N15NO. J. Chem. Phys. 1973;59:5820–5823. [Google Scholar]
- 19.Ouyang B, Conradi MS. NMR determination of the mechanism of molecular reorientation in solid N2O. Phys. Rev. B. 1991;44:9295–9300. doi: 10.1103/physrevb.44.9295. [DOI] [PubMed] [Google Scholar]
- 20.Pake GE. Nuclear resonance absorption in hydrated crystals – fine structure of the proton line. J. Chem. Phys. 1948;16:327–336. [Google Scholar]
- 21.Waugh JS, Gonen O, Kuhns P. Singlet nuclear magnetic resonance. J. Chem. Phys. 1987;86:3816–3818. [Google Scholar]
- 22.Bretthorst GL. Bayesian-analysis: parameter-estimation using quadrature NMR models. J. Magn. Reson. 1990;88:533–551. [Google Scholar]
- 23.von Toussaint U. Bayesian inference in physics. Rev. Mod. Phys. 2011;83:943–999. [Google Scholar]
- 24.Gregory PC. Bayesian Logical Data Analysis for the Physical Sciences. Cambridge Univ. Press; 2005. [Google Scholar]
- 25.Metropolis N, et al. Equation of state calculations by fast computing machines. J. Chem. Phys. 1953;21:1087–1092. [Google Scholar]
- 26.Håkansson P, et al. Analytical NMR powder lineshape of two spin-1/2 and polarized initial state implemented in monte carlo Baysian statistical method. Phys. Chem. Chem. Phys. 2013 (in preparation) [Google Scholar]
- 27.Douglas AE, Moller CK. The near infrared spectrum and the internuclear distances of nitrous oxide. J. Chem. Phys. 1954;22:275–279. [Google Scholar]