Abstract
Importance of the field
Targeted liposomal drugs represent the next evolution of liposomal drug delivery in cancer treatment. In various preclinical cancer models, antibody-targeted PEGylated liposomal drugs have demonstrated superior therapeutic effects over their non-targeted counterparts. Single chain Fv (scFv) has gained popularity in recent years as the targeting agent of choice over traditional targeting agents such as monoclonal antibodies (mAb) and antibody fragments (e.g., Fab′).
Areas covered in this review
This review is focused mainly on advances in scFv-targeted liposomal drug delivery for the treatment of cancers, based on a survey of the recent literature, and on experiments done in a murine model of human B-lymphoma, using anti-CD19 targeted liposomes targeted with whole mAb, Fab′ fragments and scFv fragments.
What the reader will gain
This review examines the recent advances in PEGylated immunoliposomal drug delivery, focusing on scFv fragments as targeting agents, in comparison with Fab′ and mAb.
Take home message
For clinical development, scFv are potentially preferred targeting agents for PEGylated liposomes over mAb and Fab′, owing to factors such as decreased immunogenicity, and pharmacokinetics/biodistribution profiles that are similar to non-targeted PEGylated (Stealth®) liposomes.
Keywords: biodistribution, cancer, drug delivery, Fab′, immunoliposomes, liposomes, monoclonal antibodies, pharmacokinetics, scFv
1. Introduction
Since the first description of liposomes in the 1960s [1], liposomes have emerged as the archetypal nanoscale drug carrier, and they have entered the mainstream of drug delivery with several products approved for clinical use [2]. Several liposomal drugs, such as liposomal amphotericin B (Ambisome®, Gilead, Foster City, CA, USA;/Astellas, Deerfield, IL, USA), liposomal doxorubicin (Myocet®, Cephalon, Frazer, PA, USA), liposomal daunorubicin (DaunoXome®, Gilead) and PEGylated liposomal doxorubicin (Doxil/Caelyx®, Alza/Johnson & Johnson, New Brunswick, NJ, USA), are now on the market, and many others, including some liposomal drugs not intended for the treatment of cancers, are in various stages of clinical development (Table 1) [3–10]. Liposomes can be divided into either passively targeted or ligand-targeted (sometimes called ‘active’ targeting) when antibodies, peptides (e.g., NGR [11]), or small molecule ligands (e.g., folate [12] or transferrin [13]) are attached at the liposome surface.
Table 1.
Currently marketed liposomal drugs.
| Brand name | Drug | Formulation | Indications | Manufacturer |
|---|---|---|---|---|
| AmBisome® [3] | Amphotericin B | Liposome | Fungal infections, visceral leishmaniasis | Gilead/Astellas |
| Myocet® [4] | Doxorubicin | Liposome | Metastatic breast cancer | Cephalon |
| Doxil/Caelyx® [5] | Doxorubicin | PEGylated liposome | Kaposi’s sarcoma, refractory breast cancer, and refractory ovarian cancer | Alza/Johnson & Johnson |
| DaunoXome® [6] | Daunorubicin | Liposome | HIV-related Kaposi’s sarcoma | Gilead |
| DepoCyt® [7] | Cytosine arabioside | Liposome | Lymphomatous meningitis and neoplastic meningitis | SkyePharma (London, UK)/Enzon (Bridgewater, NJ, USA) |
| Marqibo® [8] | Vincristine | Liposome | Acute lymphoblastic leukemia and melanoma | Hana Biosciences (San Francisco, CA, USA) |
| Fluidosome™ Tobramycin [9] | Tobramycin | Liposome | Pseudomonas aeruginosa infections in cystic fibrosis | Axentis Pharma (Cleveland, OH, USA) |
| Liposomal Ciprofloxacin [10] | Ciprofloxacin | Liposome | Gram-negative infections in cystic fibrosis | Aradigm |
All nanoscale particulate carriers, including liposomes, are distributed to solid tumors, or to other sites of increased vascular permeability, by means of the enhanced permeability and retention (EPR) effect [14]. Nanoscale drug carriers concentrate in solid tumors via the EPR effect, resulting in increased local drug concentrations as they extravasate through fenestrated gaps in the tumor vasculature and localize in the tumor interstitial space [15]. Decreased lymphatic drainage in solid tumors impedes their clearance from the tumors. As the vascular endothelium in most healthy tissues, for example heart, contains tight gap junctions, limited or no extravasation of nanoparticles into these tissues occurs. Other reviews have discussed the EPR effect in greater detail [16].
The selectivity of liposomes, or other nanoparticles, for diseased cells can be enhanced further by targeting them with antibodies, antibody fragments, peptides or small ligands. Antibody-targeted liposomes, termed immunoliposomes (IL) and Stealth® (i.e., PEGylated) immunoliposomes (SIL), have been shown to increase the specific delivery of anticancer drugs to solid tumor cells by binding to tumor-associated internalizing antigens on the surface of cancer cells (reviewed in [17]). Conjugation of antibodies to the surface of liposomes has also been used to target liposomal drugs to blood-borne malignancies (e.g., lymphomas, leukemias) and to micrometastases, where the EPR effect is absent. IL and SIL have traditionally been targeted by means of whole monoclonal antibodies (mAbs), but advances in antibody engineering have allowed the use of antibody fragments such as Fab′ and single chain Fv (scFv) as targeting agents. Previous reviews have discussed the role of mAb, Fab′ and scFv in targeting liposomes [18–20]. Recently, scFv has gained popularity as targeting agents. This review focuses on a discussion of recent advances in scFv-targeted liposomal anticancer drugs, and speculation on their future utility.
1.1 Sterically stabilized (Stealth®) liposomes
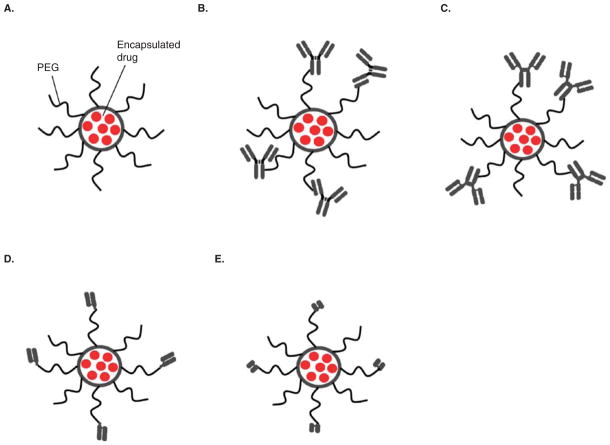
Sterically stabilized liposomes, sometimes called Stealth or PEGylated liposomes (SL), are liposomes having surface-grafted polyethylene glycol (PEG) molecules of ~ 2000 kDa (Figure 1A), but other types of hydrophilic polymer or peptide may also be used [21]. The grafting of hydrophilic PEG molecules on the surface of SL reduces their clearance by the reticuloendothelial system (RES), thereby increasing their circulation half-life to several hours, which is a considerable improvement over the circulation half-lifes of ‘classical’ liposomes that lack the PEG coating. PEGylation of the liposome surface eliminates the saturable, Michaelis–Menten clearance of classical liposomes and imparts dose-independent, zero-order pharmacokinetics (PK) for the Stealth liposomes through much of the clinical dose range [22].
Figure 1. Schematic representation of Stealth® and immunoliposomes coupled to various antibody constructs.
A. Stealth® Liposome (SL). B. Stealth® immunoliposomes (SIL) conjugated with mAb via maleimide method. C. SIL conjugated with mAb via hydrazide method. D. SIL conjugated with Fab′. E. SIL conjugated with scFv.
It can take ~ 24–48 h for circulating nanoparticles to reach peak levels in solid tumors [23], hence SL that have circulation half-lifes of several hours can recirculate continuously through the solid tumor vasculature, eventually finding their way through gaps in the tumor endothelium to accumulate in the tumor interstitial space. Once localized in tumors, therapeutics released from liposomes into the interstitial space are taken up by adjacent tumor cells to exert therapeutic effects. The rate of drug release is dependent on the method of encapsulation, and the physicochemical properties of the encapsulated drug and the membrane lipids (reviewed in [24]). The rate of drug release can, in turn, influence the therapeutic activity and the side effects of liposomal drugs. For example, doxorubicin (DXR) encapsulated in liposomes with an intermediate rate of drug release (t1/2 = 65–125 h in vivo) resulted in significant weight loss in mice, possibly owing to toxicity to the gastrointestinal tract, compared with DXR-loaded liposomes that had either a higher or a lower rate of release [25,26]. In another series of studies, maximal therapeutic activity was achieved by optimization of the rate of drug release from liposomes, and rates of drug release at either extreme resulted in decreased activity [27]. These experiments suggest that both the therapeutic activity and toxicity of liposomal drugs are related to the rate of drug release. Therefore, it is now thought to be possible to optimize the rate of drug release to achieve the maximum therapeutic activity while minimizing toxicity for individual liposomal drugs.
Interestingly, Allen et al. demonstrated that the toxicity of liposomal DXR with intermediate rates of drug release can be ameliorated through antibody-mediated targeting of the liposomal DXR in a murine model of B-cell lymphoma [26]. In these experiments, the authors showed that the toxicity of liposomal DXR having intermediate rates of drug release was reduced when those formulations were targeted to the B cells by means of an anti-CD19 mAb. The decrease in toxicity may be related to increases in the specific biodistribution of the liposomes to the CD19+ malignant B cells as a result of conjugation of anti-CD19 mAb to the surface of the liposomes.
1.2 Ligand-targeted liposomes
Although Stealth liposomes and ligand-targeted Stealth liposomes both reach tumor tissues by the same mechanism, that is, passive distribution to the tumor cells via the bloodstream, the similarities end there. Only ligand-targeted nanoparticles bind selectively to target cells, such as tumor cells. When the ligand is chosen so that it binds to an antigen or receptor that triggers receptor-mediated endocytosis, then the whole particle, including its cargo of therapeutic molecules, is internalized into the target cell [28–30]. This allows even normally impermeant molecules, for example highly charged molecules such as siRNA, to gain access to the cell interior. Ligand-targeted liposomes may also have advantages over non-targeted liposomes in other situations, for example against micro-metastases that have yet to develop a vasculature, or when directed against tumor vasculature endothelial cells [31,32] or other targets readily accessed from the vasculature. In addition to the specific delivery of anticancer drugs to antigen-expressing cells, synergistic effects may be achieved when anticancer drugs are delivered by means of IL or SIL targeted with antibodies that are capable of initiating antiproliferative or antiangiogenic signals [31,33,34]. Therefore, some clear advantages exist for the targeted delivery of liposomal anticancer drugs over non-targeted liposomes.
In various in vitro experiments, and in animal models of cancer, many (but not all) studies have demonstrated that targeted delivery of anticancer drugs with SIL results in an increased therapeutic effect over non-targeted liposomes [33,35,36]. Recently, Pastorino et al. showed, in an animal model of neuroblastoma, that targeted delivery of liposomal doxorubicin to both tumor vasculature endothelial cells (via the NGR peptide sequence) and tumor cells (via an anti-GD2 antibody) resulted in significantly enhanced therapeutic effects compared with endothelial-targeted and tumor-targeted formulations alone [31]. Li and Huang showed that targeted liposomes are able to localize to and effectively deliver siRNA to tumors in a xenograft model of human lung cancer [37]. Folate-targeted liposomes encapsulating arsenic trioxide, a toxic chemical with a low therapeutic index that has been used in the treatment of some forms of leukemia, was recently shown to be effective against malignant cells that express the folate receptor [38].
The identification of increasing numbers of tumor-specific antigens, and the development and marketing of numerous antibodies directed against these antigens, present substantial opportunities for the development of targeted drug delivery systems. Despite the availability of non-targeted liposomal anticancer drugs for more than a decade and the intense research in applications for SIL, no immunoliposomal anti-cancer drugs are being marketed at present, although at least one formulation has entered clinical trials [39].
1.3 Choice of targeting ligand
As discussed above, liposomal anticancer drugs may be targeted by means of small ligands, peptides, or antibodies and fragments of antibodies. IL/SIL can be targeted with whole monoclonal antibodies or fragments of antibodies, for example, F(ab′)2, Fab′, or scFv fragments (Figure 2). Antibodies and antibody fragments have been widely used for targeting liposomal drugs because they have the advantage of being highly specific for their target antigens, relative to other classes of targeting agents. In addition, synergistic activity may be observed when signaling antibodies are combined with combinations of anticancer drugs [40,41]. However, the production of antibodies and fragments, which require expression and purification from biological systems, is much more cost-intensive than the production of small ligands and peptides, which, although they have lower target specificity, can be chemically synthesized.
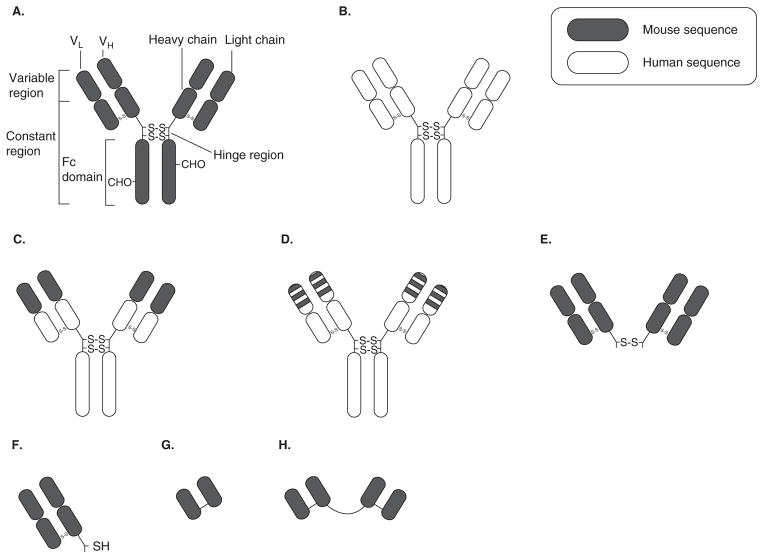
Figure 2. Schematic representation of various antibody constructs.
A. Mouse IgG. The VL and VH regions contain the antigen binding domains (i.e., complementarity-determining regions). B. Human IgG. C. Chimeric IgG with murine VL and VH regions and human constant regions. D. Humanized IgG with murine CDR sequence grafted onto human IgG backbone. E. F(ab′)2 generated by pepsin digestion of the Fc domain of IgG. F. Fab′ can be generated by reduction of the disulfide bond in the hinge region of F(ab′)2. G. ScFv, which contains recombinant VL and VH regions linked by a short peptide sequence (usually 4 Gly). Various tags (e.g., poly-His) and amino acid sequences (e.g., terminal Cys, with a sulfhydryl group for coupling to liposomes) can be engineered into the scFv construct. H. Bivalent scFv with two scFv, directed against the same epitope, joined by a polypeptide linker. Bispecific scFv can be constructed by linkage of two scFv directed against two different antigens.
1.3.1 Monoclonal antibodies and fragments of antibodies
Whole mAb is a bivalent molecule with two antigen-binding domains per mAb (Figure 2). The variable regions in the heavy (VH) and light chains (VL) contain the complementarity-determining regions (CDR) that recognize and bind to specific epitopes on antigens. The Fc region of whole mAbs bind to Fc receptors on macrophages and other cells, resulting in uptake of the mAb, which is the primary mode of clearance of mAb from circulation [42]. Fc binding can also activate secondary signals in other cells, for example, activation of mast cells, resulting in degranulation [43].
Studies have shown that some mAbs (e.g., the anti-HER2 mAb, trastuzumab) can inhibit cell signaling pathways that are essential for tumor growth, in addition to initiation of antibody-dependent cell-mediated cytotoxicity (ADCC) [44,45]. Early studies using murine mAb in humans found that murine mAb were immunogenic and resulted in the formation of human anti-mouse antibodies (HAMA) and rapid removal of the mAb from circulation [46,47]. However, it was found that the murine Fc domains were not as effective at activation of ADCC or complement-dependent cytotoxicity (CDC) in humans as are human Fc domains [48]. Despite their immunogenic nature and lower therapeutic potential, some murine mAbs such as muromonab (Orthoclone OKT3®) and ibritomomab (Zevalin®) have been approved for clinical use. Substitution of human regions for the constant regions of murine mAb is increasingly being used to decrease the immunogenicity of mAbs, resulting in chimeric mAbs [49], humanized mAbs [50] and, now the technology exists, to make fully human mAbs [51]. However, even with fully human mAbs there is the possibility that anti-idiotypic antibodies might be formed that reduce the therapeutic potential the mAb [52–54].
Small antibody fragments such as Fab′ and scFv are postulated to be less immunogenic than whole mAb as they lack the Fc domain against which most HAMA response is directed [46]. Fab′s are monovalent fragments of a mAb containing the light chain and a portion of the heavy chain (Figure 2). Single chain Fv fragments are small fragments of antibodies containing only the VH and VL domains linked by a flexible polypeptide linker (Figure 2).
In the older targeting literature, IL/SIL were targeted principally with whole mAb, but increasingly antibody fragments such as Fab′, and now scFv, are being used as targeting agents. There are several potential advantages of using scFv fragments over whole antibodies or larger fragments for liposome targeting. These include: i) slower clearance than mAb-targeted liposomes, as Fc-mediated clearance is eliminated [55]; ii) theoretically lower production cost for scFv fragments generated from bacterial cultures relative to whole antibodies generated from animal ascites or cell culture [56,57]; iii) the ability to select scFv with the desired affinity and specificity using phage display [58]; iv) the option of engineering tags into scFv constructs, which can aid in their identification and purification [59]; and v) the ability to engineer fully human fragments or fragments with low levels of non-human content, which will reduce the risk of immunogenic reactions [60,61].
Whole mAbs potentially have one advantage over Fab′ fragments or scFv fragments, which is the avidity imparted by the two binding regions on each mAb, compared with only one on Fab′ fragments or scFv fragments. However, the multivalent display of monovalent fragments on the surface of IL/SIL can restore binding avidity [62].
1.4 Antibody-coupling methods
Antibodies or ligands can be coupled to liposomes by covalent or non-covalent bonds. For preparation of SIL, whole antibodies or fragments of antibody are covalently coupled to the modified PEG termini distal to the surface of lipo somes. Various modifications to the PEG termini permit the covalent coupling of ligands, including hydrazide-PEG (Hz-PEG) [63], pyridyl-dithio propionoylamino-PEG (PDP-PEG) [64], maleimide-PEG (Mal-PEG) [65], cyanuric acid-PEG (cyanur-PEG) [66] and p-nitrophenylcarbonyl-PEG [67].
In the Mal-PEG method, antibodies are thiolated and coupled to liposomes containing Mal-PEG-polyethylene-glycol-distearoylphosphatidylethanolamine (Mal-PEG-DSPE) by means of thio-ether bonds. The advantage of the Mal-PEG method is that this method has high coupling efficiencies and results in stable covalent bonds. A drawback of the Mal-PEG method is that functionalization and thiolation of whole antibodies occur randomly and may occur in multiple locations, resulting in random orientation of antibodies relative to the surface of SIL (Figure 1B). This can lead to increased clearance of the liposomes from circulation by means of two different mechanisms: crosslinking of the immunoliposomes and rapid clearance of the resulting aggregates or the exposure of Fc groups in the case of whole mAb, and clearance of the immunoliposomes by means of Fc receptor-mediated mechanisms.
The Mal-PEG coupling method can be applied to Fab′ fragments of mAb via thiol groups in the hinge region, and to scFv fragments containing reduced sulfhydryl groups. As they lack the Fc region, Fab′-coupled and scFv-coupled SIL made by the Mal-PEG method have similar clearance to untargeted SL and circulate much longer than SIL coupled via this method to whole mAb [55,68,69]. A cysteine residue is commonly engineered at the C terminus of the scFv construct to allow for coupling to liposomes and correct orientation of the scFv on the surface of liposomes [70,71]. Site-specific coupling can be achieved in scFv through the introduction of cysteine residues in specific sites within the scFv construct. The location and the number of cysteine residues have been shown to impact the coupling efficiency to liposomes and the binding avidity of the resultant SIL [72].
The Hz-PEG method involves oxidation of the carbohydrates in the Fc region of the whole antibody into reactive aldehydes, and the formation of hydrazone bonds with the hydrazide groups on the PEG terminus. As coupling is through the Fc region of whole mAb (Figure 1C), this method interferes with the binding of the Fc region to Fc receptors; mAb-coupled SIL formed by this method show similar PK to untargeted liposomes [73].
For non-covalent coupling of antibodies to liposomes, many studies have used antibodies and PEG-lipids functionalized with proteins or small molecules that have strong affinities for each other. For example, various antibody constructs have been successfully linked to liposomes via the strong biotin and avidin interaction [74], via folate and the folate-binding protein (FBP) [75] and via poly-His tag and nickel nitrilotriacetic acid (Ni-NTA) [76]. Non-covalently coupled SIL may be less desirable than covalently coupled SIL for in vivo applications, owing to the potential for immune reactions (e.g., to avidin) or because the interaction between the linkage molecules (e.g., poly-His and Ni-NTA) may be competed away by serum proteins or cell surface receptors, resulting in loss of targeting moieties, and therefore loss of targeting [77].
1.4.1 Post-insertion approach
In addition to conventional coupling, where antibody or ligands are coupled directly to liposomes containing derivatized PEG-lipids such as Mal-PEG-DSPE, immunoliposomes can also be prepared using the post-insertion method [78]. In this method, ligands, whole antibodies, or antibody fragments, are first coupled to micelles of derivatized PEG-lipid, under conditions similar to conventional coupling. The antibody-conjugated PEG-lipids are then incubated with pre-formed liposomes, either drug-loaded or empty, under conditions that result in insertion of the conjugated PEG-lipids into the outer leaflet of the liposome membrane. Immunoliposomes prepared by the post-insertion method have been shown to have cell binding, rate of drug release and pharmacokinetics/biodistribution (PK/BD) similar to immunoliposomes prepared by conventional coupling [79,80].
The production of immunoliposomes containing different drugs is relatively simple using the post-insertion method, relative to conventional coupling, because a large batch of antibody or fragments of antibody can be coupled to PEG-lipid micelles and subsequently post-inserted into liposomes containing the drug of choice. This method is more conducive to scale-up of manufacturing of immunoliposomal drugs because an antibody-lipid conjugate can easily be inserted into an approved liposomal anticancer drug [81,82]. In addition, for antibody constructs with low storage stability (e.g., scFv), coupling to PEG-lipid micelles may increase stability of the antibody constructs and facilitate retaining activity during storage (see below).
2. Pharmacokinetics of antibody-targeted immunoliposomes
The PK/BD of SL and SIL can be affected by several factors, such as liposome size, surface modification and antibody conjugation (reviewed in [24]). In general, murine mAb-targeted SIL show rapid, biphasic clearance from circulation in mice, owing to recognition of the Fc region by macrophages in the liver and spleen [62,83], unlike the slower log-linear clearance of non-targeted liposomes [84]. In clinical practice, the same might be expected when patients are injected with liposomal drug formulations targeted by means of humanized or human mAb. SIL targeted via either Fab′ fragments or scFv fragments, both of which lack the Fc region of mAbs, have rates of clearance similar to non-targeted liposomes [29,31,55,85,86]. Repeated injection of SL and SIL, which may be required in clinical treatment protocols, may result in increased clearance of the subsequent doses of liposomal drugs and may be a result of the formation of antibodies against the PEG moiety after the initial dose [87–89]. This phenomenon has been observed with SL and mAb-targeted SIL, and because this may be a result of the formation of antibodies against the PEG moiety on the surface of liposomes, the same phenomenon may be predicted with Fab′ or scFv-targeted SIL.
As discussed above, the physical orientation of mAb on the surface of SIL can also affect the clearance of these SIL. Immunoliposomes produced by site-specific coupling of mAb methods, such as the hydrazide method, contain Fc regions that are less accessible by the RES. As a result, the PK profile of these SIL is similar to untargeted SL. The rate of clearance is also dependent on the density of mAb at the surface of liposomes. As the density of mAb on SIL increases above ~ 60–75 μg mAb/μmol phospholipid, they are cleared at increasingly rapid rates, probably by Fc-mediated mechanisms [64,90]. On the other hand, the ability of mAb-targeted liposomes to target tumor cells appears to decrease, albeit modestly, as a function of decreased antibody densities [65,91].
It has been shown in various animal models, in which SIL targeted via Fab′ versus mAb were compared, that SIL targeted via Fab′ fragments have considerably improved PK over mAb-targeted SIL; blood levels of Fab′-targeted SIL were similar to non-targeted SL [62,69,92]. SIL targeted by means of scFv fragments have PK similar to Fab′-targeted SIL and non-targeted SL owing to the lack Fc domain and fewer foreign peptide sequences (but see Section 6.1 later). Hence, Fab′ or scFv are preferred as targeting agents for IL/SIL because their clearance is not strongly dependent on antibody density, owing to the absence of the Fc region.
3. Efficacy of immunoliposomal drugs targeted by means of ScFv
Many preclinical studies have confirmed that immunoliposomal drugs targeted by means of scFv are more efficacious than either free drugs or non-targeted liposomal drugs [30,55,70,86,93,94]. In vitro studies by An et al. demonstrated that immunoliposomal topotecan targeted by means of an scFv directed against mesothelioma cells were more efficacious than non-targeted liposomal topotecan or the free drug [94]. Recently, Noble et al. demonstrated that immunoliposomal vincristine or vinblastine targeted by an anti-HER2 scFv were more efficacious than their respective non-targeted formulations or the free drug, in an animal model of breast cancer [86]. Messerschmidt et al. demonstrated a new way of delivering tumor necrosis factor (TNF) to tumor cells expressing the fibroblast activation protein (FAP), using TNF-functionalized lipidic nanoparticles that are targeted by an anti-FAP scFv [95]. Hence, data are emerging to demonstrate that targeting of liposomal drugs by scFv results in increased therapeutic effects over non-targeted liposomal drugs and suggest that scFv is a viable alternative to Fab′ or mAb.
4. Stability of ScFv constructs
Various studies have highlighted the fact that in vitro stability of scFv fragments is essential for successful targeting in vivo [96,97]. In addition, it has been shown that the stability of scFv fragments is dependent on several factors, including temperature, pH and concentration, and that substantial engineering of scFv constructs may be needed to alleviate stability issues [98,99]. Some studies have reported that low stability is an inherent issue associated with the production and the use of scFv; the lack of constant regions in scFv may play a partial role in this low stability [100].
The stability of scFv fragments can affect their therapeutic efficacy, either as the free fragment or when attached to IL/SIL. Therapeutic applications generally require large amounts of the targeting agents, and the stability of scFvs can affect their production yield [101]. In addition, scFv may be susceptible to destabilization under the conditions used during the coupling or conjugation procedures used in the production of IL/SIL and immunoconjugates. Therefore, optimization of stability is an integral part of the developmental process of any scFv-targeted therapeutic. To that end, many laboratories are working to elucidate factors that contribute to the stability of scFv, and to develop solutions that improve their stabilities.
Assessment of the stability of an scFv can be measured quantitatively by determining the free energy of folding at equilibrium as a function of increasing concentration of denaturant (e.g., urea or guanidine hydrochloride) [102]. However, interpretation of the results generated from this analysis can be problematic because it requires modeling of the data, and the presence of partially unfolded intermediates can complicate the interpretation of results [103]. The stability of scFv can also be measured by stressed incubation, where the scFv is incubated in conditions similar to its intended application (e.g., in serum at 37°C) followed by evaluation of the remaining activity or the amount of protein aggregation after the incubation [104]. In addition, dynamic light scattering has been used to assess the thermal stability of scFv [72]. In this method, the incubation temperature is gradually increased until the melting point of the scFv is reached and the intensity of the light scattering dramatically increases.
Various factors can affect the stability of scFvs. Invariably, most scFv constructs require molecular engineering to improve their stability. The Allen laboratory had experimented with several anti-CD19 scFv constructs for targeting of liposomes and has shown that most of the tested scFv constructs suffered from low stability and could benefit from some modifications [105].
Intradomain disulfide bonds at conserved positions within scFvs are associated with their intrinsic stability [102,106]. Many scFv cannot tolerate the loss of these disulfide bonds [107], and only a very few scFv constructs have been shown to fold correctly and retain their activity in the absence of intra-domain disulfide bonds [108,109]. Proba et al. demonstrated that, although the stable ABPC48 scFv derived from its wild-type parental mAb is naturally missing a disulfide bond in the VH chain, restoration of the disulfide bond by point mutation can increase the stability of the scFv above that of the unmodified ABPC48 scFv [106]. Recently, various strains of Escherichia coli with more oxidizing cytoplasm have become available for expression of disulfide-dependent proteins such as scFv (reviewed in [110]).
In addition to the intradomain disulfide bonds, other elements, such as the stability of the VL and VH domain interface [102], may affect the stability of scFv constructs. Spontaneous and transient opening of the interface between the VL and VH chains has been suggested to result in exposure of normally hidden hydrophobic patches, leading to aggregation [111]. The introduction of an interdomain disulfide bond (between the VL and VH chains) [112], and variations in the length and flexibility of the VL–VH linker [113] have been shown to increase interdomain stability by limiting the opening of the VL–VH interface. An alternative approach to increase the stability of an scFv is the CDR-grafting method, which was originally developed for humanization of murine mAb, and has also proved to be useful for scFv constructs [96,114]. In this method, the CDR sequence (i.e., the antigen-binding sequence) of an unstable scFv is grafted onto a framework of another scFv construct with proven stability. It has also been shown that the stability of scFv can be improved when some scFv are expressed as Fab fragments [115]. Other methods, such as point mutation, directed evolution and phage display of scFv constructs, have been used to improve the in vivo stability of scFv [116–118].
Cheng and Allen were able to increase the storage stability of an anti-CD19 scFv, without any modifications to its protein sequence, simply by conjugating the scFv to PEGy-lated lipid micelles (PEG-DSPE) [55]. Conjugation of the scFv to PEG-DSPE micelles resulted in preservation of its activity, compared with the unconjugated scFv, even after extended storage at sub-zero temperatures. Although this method will not increase the intrinsic stability of an scFv construct, it is postulated that aggregation of other scFv constructs could be prevented by conjugation to micelles, allowing for scale-up of their production. In fact, an anti-HER2 scFv fragment that is intended for clinical trials of immunoliposomal anticancer drugs is manufactured and coupled to PEG-DSPE micelles, and stored as the scFv-PEG-DSPE conjugate [81].
5. Production of ScFv constructs by refolding denatured ScFv
Single chain Fv are commonly expressed in bacterial cultures and extracted either from the periplasmic space or from inclusion bodies. A leader sequence is usually engineered into the scFv construct to allow transport of the scFv to the periplasmic space, which is a non-reducing environment that promotes proper protein folding. However, induced expression of scFv in bacteria often results in excessively high protein concentration in the cytoplasm, which is a reducing environment that promotes unfolding of the scFv, leading to aggregation and formation of inclusion bodies. The accumulation of recombinant proteins in inclusion bodies can account for > 20% of total cellular protein [119,120].
Native (i.e., correctly folded) scFv can be extracted from the periplasmic space, but the amount of scFv extracted is often very low. Extraction from inclusion bodies, on the other hand, involves the use of chaotropic agents to disaggregate the scFv. This procedure yields large amounts of denatured scFv that require refolding to restore activity. Many studies involving scFv-targeted liposomes have used scFv constructs that were produced and extracted from bacterial inclusion bodies [71,76,86]. Although extraction of scFv from inclusion bodies yields large quantities of scFv, various studies have suggested that the refolding of scFv is a difficult and complex process [101,103]. During the refolding process, the scFv can be trapped in a ‘thermodynamic sink’, where the protein remains in an intermediate state [121]. If the proper folding of the scFv construct can be optimized through molecular engineering, inclusion bodies can be a valuable, high-yield source of scFv.
6. Clinical applications of ScFv-targeted immunoliposomes
6.1 Effect of ScFv tags
Long circulation time is important for IL/SIL to localize and target tumor cells, hence SIL, with their long circulation half-lifes have an important advantage over IL in this regard. SIL targeted by means of scFv are expected to have circulation time similar to Fab′ and untargeted Stealth liposomes, owing to their lack of the Fc region. However, Cheng and Allen reported that scFv-targeted liposomes had rather shorter circulation times compared with non-targeted Stealth liposomes, and that this was mediated by the presence of a poly-his molecular tag engineered into the scFv fragments that increased the liver uptake of the SIL. Removal of the tag eliminated the liver uptake [55]. Further evidence can be found in the literature where recent studies have shown that a His-tagged anti-CEA scFv was also taken up rapidly by the liver (30–40% of the injected dose) [122]. In a separate study by another group, using a tag-free anti-CD45 scFv, the uptake and binding by the liver was much lower at only < 5% of the injected dose [123]. Other studies, however, have not demonstrated an increase in liver uptake of scFvs that contain a poly-his tag [124,125]. Taken together, these results further suggest that the PK/BD of scFv fragments or scFv conjugates may be influenced by molecular tags within the constructs. It can be expected that an increase in uptake of drug delivery systems (DDS) by the liver via mechanisms mediated by the poly-his tag may result in increased toxicity to the liver owing to accumulation of the DDS and the encapsulated cytotoxic drugs. In addition, molecular tags such as the c-myc tag, which may have oncogenic potentials, will be less acceptable to regulatory agencies.
Molecular tags, such as the poly-His tag, are useful in the preclinical development of scFv because they are generally used for the purification and identification of scFv. However, as discussed above, molecular tags have the potential to result in organ toxicities owing to accumulation of targeted DDS containing cytotoxic drugs. Single chain Fv fragments that do not contain molecular tags can be used to avoid unwanted accumulation in organs such as liver, and these scFv can be purified using the immunoglobulin-binding protein Protein L, which binds to the light chain of the scFv [126].
6.2 Targeting of immunoliposomes using mAb, Fab′ or scFv
With the availability of different targeting constructs, one of the questions that can be asked is which is the best antibody construct for targeting? Various studies comparing mAb and Fab′ have demonstrated that Fab′ are more suitable for clinical applications owing to improved PK/BD resulting in increased therapeutic effects [29,62]. As discussed above, several pre-clinical studies have demonstrated the therapeutic potential of scFv-targeted liposomal drugs in various solid tumor and hematological malignancy models. However, many of these studies were limited to comparison of the scFv-targeted liposomal drugs with the non-targeted formulation, and few studies have compared an scFv with the parent mAb and its Fab′.
Cheng and Allen were one of the first to compare the therapeutic potential of an scFv construct against its parent mAb and Fab′ in the same study [55]. This study demonstrated, in a mouse xenograft model of B-cell lymphoma, that SIL DXR targeted to the B-cell antigen, CD19 via anti-CD19 mAb, Fab′ or scFv, were all more efficacious than non-targeted liposomal doxorubicin or the free drug. Interestingly, of the three SIL doxorubicin formulations (i.e., anti-CD19 mAb, Fab′ or scFv), the Fab′-targeted formulation tended to be the most efficacious (although it did not reach statistical significance), whereas the scFv-targeted formulation appeared to be intermediate in efficacy between the Fab′ fragment and the mAb-targeted formulation in vivo (although, again, the differences were not significant). The scFv-targeted formulation of SIL DXR was postulated to be less therapeutically effective owing to decreased stability of the scFv fragment (see Section 4) and/or its avidity for the CD19 antigen [55].
In this model, SIL DXR targeted via the anti-CD19 mAb, although showing lower survival rates than SIL targeted via either the Fab′ or the scFv fragments, was not statistically different from the longer circulating SIL DXR targeted via the fragments. This was unexpected, as the rapid clearance of mAb-targeted liposomes was expected to impact negatively the therapeutic effects of this formulation. Perhaps the explanation lies in the fact that a hematological model was used, where the target cells were rapidly accessible to the SIL, so clearance rates did not play a significant role in the therapeutic outcome. In solid tumor models, where long circulation times are needed for substantial localization of the carrier to the tumor, one can hypothesize that the differences in the relative clearance rates between the different constructs might have greater effects on therapeutic outcome [22]. Hence, this study suggested several factors that can affect the in vivo efficacy of scFv-targeted liposomal drugs, but also demonstrated the importance of making comparisons of SIL targeted by scFv with their parent mAb and Fab′.
When all factors are taken together, the choice of targeting agent for applications involving immunoliposomal drugs depends not only on therapeutic outcome, but also on other criteria such as stability, production yields, affinity and avidity, immunogenicity and toxicity of the respective constructs. In this regard, mAb seems to have several strikes against it: it is expensive and inconvenient to produce, its clearance is accelerated by the presence of the Fc domain, and it has a higher potential to cause an immunogenic reaction. Humanized Fab′ would appear to have some advantages over the parental mAb, as it should possess similar stability, and multivalent display on IL/SILs should restore avidity, but its production from humanized mAb would not be a preferred method of manufacture. Production of recombinant humanized Fab′ in bacteria or other expression system may be an acceptable alternative. Single chain Fv are potentially valuable targeting agents, if the scFv fragments are engineered and selected for optimal affinity and stability. However, the tag-mediated clearance of scFv-targeted liposomes and the potential of the tag-containing scFv to cause toxicities need further study.
6.3 Single chain Fv in the clinic as an anticancer therapy
Several scFvs are now in different stages of clinical trials for the treatment of various cancers [127,128]. An Anti-CD22 scFv [129] and an anti-mesothelin scFv [130] have been conjugated to Pseudomonas exotoxin A (PE), whereas others, such as an anti-CEA scFv [128], are fused to an enzyme and are used in antibody-directed enzyme prodrug therapy (ADEPT) as pre-targeting agents. For the scFv-PE fusion protein, each scFv molecule carries a payload of one high potency PE molecule. For less potent therapeutics, the dose of scFv needed for therapeutic efficacy may become excessively high, which would not only become prohibitively expensive, but could also increase the frequency of adverse effects, such as the immunogenic reactions reported by Mayer [128]. Nevertheless, the success of scFv-targeted alternative therapeutics will pave the way for the clinical acceptance of scFv-targeted SIL drugs. One of the benefits of targeted immunoliposomal drugs over immunoconjugates is that a large payload of drugs can be delivered with a relatively few scFv, thereby minimizing expense, and the risk of scFv-associated adverse effects.
There are obstacles that need to be overcome for scFv-targeted SIL drugs to be successful in the clinic. The production capacity would need to be in the neighborhood of currently marketed monoclonal antibodies. Despite the reduced amount of scFv needed for targeting of DDS compared with use as a monotherapy, it can be expected that an amount in the hundred grams to kilograms range will be needed for clinical trials and clinical use. Storage stability is also a major issue that needs to be addressed, although great advances have been made [81,82].
6.4 Failure to respond and resistance to antibody-targeted immunoliposomal drugs
The response to immunoliposomal therapy is dependent on the expression of the target antigen. In most cancer cell populations there is a spectrum of antigen expression among cells, with some cells having high expression and others low or no expression of a particular antigen. Although antigen expression is usually high and stable in most B-cell populations, which are monoclonal in origin, some patient samples contain B cells that lack or have low levels of CD19 expression [131]. Obviously, B cells that do not express CD19 or that have low levels of CD19 will be less likely to respond to anti-CD19 SIL therapy. The strategy of targeting immunoliposomes to more than one antigen will increase therapeutic success in these cases [132].
For clinical applications of immunoliposomal drugs, it is crucial to consider that resistance to therapy may arise, either to the drug or to the targeting agent, or because of loss of the targeted antigen on the target cell. Recent studies have reported resistance to rituximab therapy in some patients following long-term rituximab therapy [133,134]. Several mechanisms, including downregulation of CD20 [135] and overexpression of the antiapoptotic protein Bcl-2 [136], have been proposed. In breast cancers, preclinical studies have postulated overexpression of the mucin 4 (MUC4) protein on breast cancer cells as one of the possible mechanisms to trastuzumab resistance by masking HER2/neu and induction of steric hindrance to the binding of trastuzumab to HER2/neu [137,138]. Therefore, it can be speculated that cells may acquire resistance to immunoliposomal drugs through various mechanisms. A potential approach to overcoming resistance resulting from downregulation of cell surface antigens is to target immunoliposomal drug against multiple antigens such as CD19, CD20 and CD22 expressed on B cells [62,132,139]. Other mechanisms of resistance, such as decreased expression of topoisomerase II, can potentially be combated by treatment with multiple immunoliposomal drugs with different mechanism of actions, for example, immunoliposomal DXR combined with immunoliposomal vincristine (VCR) [140].
7. Future directions
7.1 Combination of immunoliposomal drugs
Examination of different combinations of immunoliposomal drugs, containing either different drugs and/or different targeting agents, could result in enhanced efficacy. So far, only a few studies have examined the therapeutic effects of combinations of SIL drugs and/or targeting agents [31,35]. In a B-cell lymphoma model, Sapra et al. used immunoliposomal VCR, targeted by means of a combination of anti-CD19 mAb and anti-CD20 mAb [35]. In this study, additive activity was observed for the combination, compared with SIL targeted by means of either anti-CD19 or anti-CD20 alone. In a model of neuroblastoma, Pastorino et al. treated mice with liposomal DXR targeted by means of mixtures of an anti-GD2 mAb or Fab′ (tumor cell-targeted), and an NGR-peptide (tumor vasculature-targeted) [31], and also were able to demonstrate additive activity over either ligand used alone. Furthermore, Pastorino et al. demonstrated that doxorubicin and an antic-myc antisense oligodeoxynucleotides delivered via anti-GD2-targeted liposomes were more effective than either agent alone [32]. The Allen lab has also examined combinations of anti-CD19 (internalizing), anti-CD20 (non-internalizing) and anti-CD22 (internalizing) in murine models of human B lymphoma and reported that, in particular, combinations of SIL targeted via the two internalizing antibodies led to the best therapeutic outcome [139]. Combinations of vascular-targeted and tumor cell-targeted liposomes also gave improved results, compared with either therapy alone, in the treatment of murine models of metastatic breast cancer [140]. These studies showed that combining either drugs or antigens can result in increased therapeutic effects over monotherapies of immunoliposomal drugs. Co-encapsulation of two different anticancer drugs in the same liposome have recently been described [141–143], making it possible to investigate the therapeutic effects of bispecific immunoliposomes co-encapsulating a combination of drugs.
7.2 Triggered release systems
Various triggered release liposomes, including liposomes containing dioleoylphosphatidylethanolamine (DOPE) [144] and liposomes containing fusogenic peptides [145], have been developed for fusion of the liposomal and endosomal membranes after antigen-mediated endocytosis, allowing efficient release of liposomal contents into the cytoplasm. Although DOPE-containing liposomes can enhance drug delivery to cells, in vivo results were less than desired, because these liposomes had very short circulation times despite the addition of PEG onto the surface of liposomes [146]. Recently, DOPE-free liposomes targeted via transferrin and containing a fusogenic peptide (GALA) incorporated on the liposome surface have been shown to fuse efficiently with the endosomal membrane after internalization [147]; because it is known that surface PEG can interfere with liposome fusion [148], these GALA-modified liposomes were classical liposomes that lacked a stabilizing polymer such as PEG on the liposome surface. Therefore, an opportunity exists to examine the effect of long-circulating IL containing fusogenic peptides such as GALA or TAT [147,149] on the liposome surface. To make these liposomes long circulating, they can be modified with PEG molecules that are anchored on the surface of these liposomes via pH-sensitive linkages, which can be cleaved at endosomal pH [150,151]. The PEG molecules would mask the fusogenic peptides at the bilayer interface and prevent unintended membrane fusion during circulation, but on binding of the antibodies to their antigens, and antigen-mediated internalization, cleavage of the PEG-liposome linkers would expose the fusogenic peptides on the liposome surface and allow fusion to occur with the endosomal membrane.
7.3 New antibody constructs: bispecific antibodies
New antibody constructs, such as bispecific antibodies, may be useful for targeting liposomal drugs. Bispecific (bs) antibodies can be used as a pre-targeting agent for liposomes. In one example, a bsmAb with an anti-tumor domain and an anti-biotin domain was injected and allowed to localize in the tumor before initiating treatment with biotinylated liposomal drug [152]. Alternatively, immunoliposomes can be conjugated with bsmAb targeted against a tumor antigen and an antigen on effector cells, for example CD16 on NK cells [153,154]. Binding of these immunoliposomes to tumor cells would lead to specific delivery of the drug, as well as the recruitment of NK cells; this would result in direct cell-mediated cytotoxicity against the tumor cells. Bispecific antibodies can also be used to target liposomal drugs against multiple tumor antigens in order to increase binding to tumor cells; an example would be a bsmAb containing anti-CD19 and CD20 or CD22. For this application, extra advantages of using bispecific antibodies over combinations of individual mAb are that: i) two different Fab′ fragments can be generated from a single digestion; and ii) bispecific immunoliposomes can be prepared from a single coupling procedure, in comparison with previous methods that used a separate digestion and coupling step for each mAb [132]. Recently, Vallera et al. demonstrated superior toxicity to various malignant B-cell lines using a diphtheria toxin-linked bispecific scFv (CD19 and CD22), over toxin linked to either anti-CD19 or anti-CD22 scFv alone [155]. Such advances in scFv technology will pave the way for future studies using SIL targeted with bispecific scFv.
7.4 Innovative approaches to production of antibody fragments
Improving the intrinsic stability of scFv constructs and increasing production yields are crucial for successful application of scFv-targeted immunoliposomal drugs in the clinic. In addition, proper folding of the scFv during expression is essential to the activity of the scFv construct. Optimization of the expressing vectors is one of the most basic approaches to improving expression yields and has been associated with impressive yields [156]. In addition, optimization of codon use in specific expression vectors can also increase production yields [157]. Rationalized point mutations designed to improve proper folding and thermal stability should also improve production of scFv [118]. The inclusion of rare codons in the polypeptide linker between the VL and VH has also been shown to improve production yield by slowing down the transcription process and allowing for proper folding of the VL and VH regions [158]. Other approaches to facilitate proper folding of scFv during expression include the co-expression of the scFv with chaperone proteins [159]. The fusion of the scFv construct to naturally occurring E. coli proteins (e.g., maltose-binding protein and thioredoxin) also resulted in an increase in properly folded, active scFv [160,161]. The successful production of a thioredoxin-fusion scFv in a co-expression system with chaperone protein, which resulted in increased specific expression of the fusion scFv, was reported recently [162].
E. coli has traditionally been the vector of choice for expression of proteins, but induced expression of proteins such as scFv in large quantities in E. coli and the resulting formation of insoluble inclusion bodies can be toxic to the bacteria, resulting in lysis of cells and a decrease in production yield [163]. Expression of scFv in algal systems, such as Chlamydomonas reinhardtii, is relatively cost-effective in comparison with other eukaryotic systems such as mammalian cells [164]. In addition, algal systems are quite flexible, as a variety of induction methods can be used, and the culture volume can be up to 500,000 liters [164]. The main drawback for algal systems may be the relatively low yields of target proteins.
A carrier protein YebF was recently found to be secreted into the culture media of a lab strain of E. coli [165]. The investigators then went on to demonstrate that functional YebF-fusion proteins, such as YebF-α-amylase and YebF-alkaline phosphatase, both of which are larger than the typical scFv construct, can be secreted into and accumulate in the culture media. Therefore, fusion of scFv constructs to YebF may result in an increase in production yield of properly folded protein, as the large volume and oxidative environment of the culture media are less conducive to formation of inclusion bodies than the cytoplasmic environment. An extra advantage to purifying scFv from culture media, compared with purification from the periplasmic space or from inclusion bodies in the cytoplasm, is that there are fewer problems with bacterial contaminants in the culture media than in fractionated cells.
8. Conclusions
With the current advances in antibody technology and the clinical acceptance of antibodies as a treatment modality, it is time for liposomes to progress from a passive targeting platform into an active, antibody-targeted drug delivery platform. Targeted liposomes are complex systems and various factors, including the size of the liposomes, antibody coupling methods as well as the targeting agent used, can affect the PK/BD of the DDS. Single chain Fv provides some clear advantages over Fab′ and mAb, such as reduced molecular mass and improved pharmacokinetics of scFv-targeted liposomes. However, issues such as the potential for molecular tags to result in increased organ uptake need to be addressed before clinical use. Innovations in scFv engineering and expression systems will address many of the shortcomings associated with scFv, such as proper folding in oxidative environment, low in vitro stability and low production yields.
9. Expert opinion
The in vitro stability of an scFv construct is crucial to its success as a targeting agent for immunoliposomes. The refolding of scFv from denatured inclusion bodies, although it can dramatically increase the production yield, is still not optimal. Hence, this approach may not be suitable for many scFv constructs. Conjugation to PEG-lipid micelles appears to be an effective means to increase the storage stability of scFv constructs. At least for hematological malignancies, the choice of antibody constructs for the targeting of SIL appears to be somewhat independent of their circulation half-lifes, but this may not apply for solid tumors where long circulation half-lifes are needed for effective tumor localization of the IL/SIL. Other factors such as stability, production yields, cost, immunogenicity and toxicity need to be factored into the decision process when deciding which type of construct to use for targeting. ScFv is potentially a useful targeting agent for immunoliposomal drugs and hopefully this paper can help to point the direction for the future development of clinically acceptable immunoliposomal anticancer therapies.
Article highlights.
Nanomedicines containing small molecule therapeutics, peptides or gene medicines can be targeted to specific cells by means of antibodies or antibody fragments that lead to the internalization of their therapeutic contents, thereby increasing their selective toxicity.
Clearance rates of targeted nanomedicines depend on factors such as the presence of Fc domains in the targeting agents.
Antibody scFv fragments are proving to be efficacious targeting agents.
The in vitro stability of scFv fragments, which is essential for successful targeting in vivo, is dependent on several factors and substantial engineering of scFv constructs may be needed to improve their stability.
High yields of scFv can be obtained by extraction from bacterial inclusion bodies with chaotropic agents, but improper refolding of the scFv is common; low yields of native scFv can be obtained from the periplasmic space.
There are several advantages to using scFv fragments as targeting agents, but, although promising preclinical results have been reported, there are still obstacles to be overcome before a product is in the clinic.
Developments in the areas of combination targeting, triggered release systems, new antibody constructs and antibody engineering will result in further improvements in scFv-targeted nanomedicines.
Properly engineered ScFv fragments are promising targeting agents for the selective delivery of therapeutics to target cells.
Proof-of-principle for the therapeutic efficacy of scFv-targeted nanomedicines has been obtained, but engineering advances that improve the yield and stability of scFv fragments are needed before they can enter into the clinic.
This box summarizes key points contained in the article.
Acknowledgments
The authors would like to thank the Canadian Institute of Health Research (CIHR) for grant support (CIHR MOP-9127).
Footnotes
Declaration of interest
The authors do not have any conflicts of interest.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Bangham AD, Standish MM, Watkins JC. Diffusion of univalent ions across the lamellae of swollen phospholipids. J Mol Biol. 1965;13:238–52. doi: 10.1016/s0022-2836(65)80093-6. [DOI] [PubMed] [Google Scholar]
- 2.Allen TM, Cullis PR. Drug delivery systems: entering the mainstream. Science. 2004;303:1818–22. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- 3.Adler-Moore J. AmBisome targeting to fungal infections. Bone Marrow Transplant. 1994;14(Suppl 5):S3–7. [PubMed] [Google Scholar]
- 4.Batist G, Ramakrishnan G, Rao CS, et al. Reduced cardiotoxicity and preserved antitumor efficacy of liposome-encapsulated doxorubicin and cyclophosphamide compared with conventional doxorubicin and cyclophosphamide in a randomized, multicenter trial of metastatic breast cancer. J Clin Oncol. 2001;19:1444–54. doi: 10.1200/JCO.2001.19.5.1444. [DOI] [PubMed] [Google Scholar]
- 5.Northfelt DW, Dezube BJ, Thommes JA, et al. Efficacy of pegylated-liposomal doxorubicin in the treatment of AIDS-related Kaposi’s sarcoma after failure of standard chemotherapy. J Clin Oncol. 1997;15:653–9. doi: 10.1200/JCO.1997.15.2.653. [DOI] [PubMed] [Google Scholar]
- 6.Presant CA, Scolaro M, Kennedy P, et al. Liposomal daunorubicin treatment of HIV-associated Kaposi’s sarcoma. Lancet. 1993;341(8855):1242–3. doi: 10.1016/0140-6736(93)91147-e. [DOI] [PubMed] [Google Scholar]
- 7.Glantz MJ, Jaeckle KA, Chamberlain MC, et al. A randomized controlled trial comparing intrathecal sustained-release cytarabine (DepoCyt) to intrathecal methotrexate in patients with neoplastic meningitis from solid tumors. Clin Cancer Res. 1999;5:3394–402. [PubMed] [Google Scholar]
- 8.Zhigaltsev IV, Maurer N, Akhong QF, et al. Liposome-encapsulated vincristine, vinblastine and vinorelbine: a comparative study of drug loading and retention. J Control Release. 2005;104(1):103–11. doi: 10.1016/j.jconrel.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Sachetelli S, Beaulac C, Riffon R, Lagace J. Evaluation of the pulmonary and systemic immunogenicity of Fluidosomes, a fluid liposomal-tobramycin formulation for the treatment of chronic infections in lungs. Biochim Biophys Acta. 1999;1428(2–3):334–40. doi: 10.1016/s0304-4165(99)00078-1. [DOI] [PubMed] [Google Scholar]
- 10.Sweeney LG, Wang Z, Loebenberg R, et al. Spray-freeze-dried liposomal ciprofloxacin powder for inhaled aerosol drug delivery. Int J Pharm. 2005;305(1–2):180–5. doi: 10.1016/j.ijpharm.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 11.Pastorino F, Brignole C, Marimpietri D, et al. Vascular damage and anti-angiogenic effects of tumor vessel-targeted liposomal chemotherapy. Cancer Res. 2003;63(21):7400–9. [PubMed] [Google Scholar]
- 12.Lee RJ, Low PS. Folate-mediated tumor cell targeting of liposome-entrapped doxorubicin in vitro. Biochim Biophys Acta. 1995;1233:134–44. doi: 10.1016/0005-2736(94)00235-h. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki S, Inoue K, Hongoh A, et al. Modulation of doxorubicin resistance in a doxorubicin-resistant human leukaemia cell by an immunoliposome targeting transferrin receptor. Br J Cancer. 1997;76(1):83–9. doi: 10.1038/bjc.1997.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy; mechanism of tumoritropic accumulation of proteins and the antitumor agent SMANCS. Cancer Res. 1986;46(12 Pt 1):6387–92. [PubMed] [Google Scholar]
- 15.Kong G, Braun RD, Dewhirst MW. Characterization of the effect of hyperthermia on nanoparticle extravasation from tumor vasculature. Cancer Res. 2001;61(7):3027–32. [PubMed] [Google Scholar]
- 16•.Maeda H, Wu J, Sawa T, et al. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65:271–84. doi: 10.1016/s0168-3659(99)00248-5. An excellent review of the EPR effect, which formed one of the fundamental principles of drug delivery to solid tumors using liposomes and other nanoparticles. [DOI] [PubMed] [Google Scholar]
- 17•.Sapra P, Tyagi P, Allen TM. Ligand-targeted liposomes for cancer treatment. Curr Drug Deliv. 2005;2(4):369–81. doi: 10.2174/156720105774370159. A comprehensive review on the state of the art of ligand-targeted therapeutics. [DOI] [PubMed] [Google Scholar]
- 18.Allen TM. Ligand-targeted therapeutics in anticancer therapy. Nat Rev Cancer. 2002;2(10):750–63. doi: 10.1038/nrc903. [DOI] [PubMed] [Google Scholar]
- 19.Park JW, Benz CC, Martin FJ. Future directions of liposome- and immunoliposome-based cancer therapeutics. Semin Oncol. 2004;31(6 Suppl 13):196–205. doi: 10.1053/j.seminoncol.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Kontermann RE. Immunoliposomes for cancer therapy. Curr Opin Mol Ther. 2006;8(1):39–45. [PubMed] [Google Scholar]
- 21.Romberg B, Metselaar JM, Baranyi L, et al. Poly(amino acid)s: promising enzymatically degradable stealth coatings for liposomes. Int J Pharm. 2007;331(2):186–9. doi: 10.1016/j.ijpharm.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 22.Papahadjopoulos D, Allen TM, Gabizon A, et al. Sterically stabilized liposomes: improvements in pharmacokinetics and antitumor therapeutic efficacy. Proc Natl Acad Sci USA. 1991;88(24):11460–4. doi: 10.1073/pnas.88.24.11460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gabizon A, Catane R, Uziely B, et al. Prolonged circulation time and enhanced accumulation in malignant exudates of doxorubicin encapsulated in polyethylene-glycol coated liposomes. Cancer Res. 1994;54:987–92. [PubMed] [Google Scholar]
- 24•.Allen TM, Cheng WW, Hare JI, Laginha KM. Pharmacokinetics and pharmacodynamics of lipidic nano-particles in cancer. Anticancer Agents Med Chem. 2006;6(6):513–23. doi: 10.2174/187152006778699121. A comprehensive analysis of how encapsulation of therapeutics in nanoparticles affects their pharmacokinetics and pharmacodynamics. [DOI] [PubMed] [Google Scholar]
- 25.Charrois GJR, Allen TM. Drug release rate influences the pharmacokinetics, biodistribution, therapeutic activity, and toxicity of pegylated liposomal doxorubicin formulations in murine breast cancer. Biochim Biophys Acta. 2004;1663(1–2):167–77. doi: 10.1016/j.bbamem.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 26.Allen TM, Mumbengegwi DR, Charrois GJ. Anti-CD19-targeted liposomal doxorubicin improves the therapeutic efficacy in murine B-cell lymphoma and ameliorates the toxicity of liposomes with varying drug release rates. Clin Cancer Res. 2005;11:3567–73. doi: 10.1158/1078-0432.CCR-04-2517. [DOI] [PubMed] [Google Scholar]
- 27.Johnston MJ, Semple SC, Klimuk SK, et al. Therapeutically optimized rates of drug release can be achieved by varying the drug-to-lipid ratio in liposomal vincristine formulations. Biochim Biophys Acta. 2006;1758(1):55–64. doi: 10.1016/j.bbamem.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 28.Sapra P, Allen TM. Internalizing antibodies are necessary for improved therapeutic efficacy of antibody-targeted liposomal drugs. Cancer Res. 2002;62(24):7190–4. [PubMed] [Google Scholar]
- 29.Kirpotin DB, Drummond DC, Shao Y, et al. Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Cancer Res. 2006;66(13):6732–40. doi: 10.1158/0008-5472.CAN-05-4199. [DOI] [PubMed] [Google Scholar]
- 30.Roth A, Drummond DC, Conrad F, et al. Anti-CD166 single chain antibody-mediated intracellular delivery of liposomal drugs to prostate cancer cells. Mol Cancer Ther. 2007;6(10):2737–46. doi: 10.1158/1535-7163.MCT-07-0140. [DOI] [PubMed] [Google Scholar]
- 31.Pastorino F, Brignole C, Di Paolo D, et al. Targeting liposomal chemotherapy via both tumor cell-specific and tumor vasculature-specific ligands potentiates therapeutic efficacy. Cancer Res. 2006;66(20):10073–82. doi: 10.1158/0008-5472.CAN-06-2117. [DOI] [PubMed] [Google Scholar]
- 32.Pastorino F, Di Paolo D, Piccardi F, et al. Enhanced antitumor efficacy of clinical-grade vasculature-targeted liposomal doxorubicin. Clin Cancer Res. 2008;14(22):7320–9. doi: 10.1158/1078-0432.CCR-08-0804. [DOI] [PubMed] [Google Scholar]
- 33.Park JW, Hong K, Kirpotin DB, et al. Anti-HER2 immunoliposomes: Enhanced efficacy attributable to targeted delivery. Clin Cancer Res. 2002;8(4):1172–81. [PubMed] [Google Scholar]
- 34.Roth P, Hammer C, Piguet AC, et al. Effects on hepatocellular carcinoma of doxorubicin-loaded immunoliposomes designed to target the VEGFR-2. J Drug Target. 2007;15(9):623–31. doi: 10.1080/10611860701502723. [DOI] [PubMed] [Google Scholar]
- 35.Sapra P, Allen TM. Improved outcome when B-cell lymphoma is treated with combinations of immunoliposomal anticancer drugs targeted to both the CD19 and CD20 epitopes. Clin Cancer Res. 2004;10(7):2530–7. doi: 10.1158/1078-0432.ccr-03-0376. [DOI] [PubMed] [Google Scholar]
- 36.Atobe K, Ishida T, Ishida E, et al. In vitro efficacy of a sterically stabilized immunoliposomes targeted to membrane type 1 matrix metalloproteinase (MT1-MMP) Biol Pharm Bull. 2007;30(5):972–8. doi: 10.1248/bpb.30.972. [DOI] [PubMed] [Google Scholar]
- 37.Li SD, Huang L. Surface-modified LPD nanoparticles for tumor targeting. Ann NY Acad Sci. 2006;1082:1–8. doi: 10.1196/annals.1348.001. [DOI] [PubMed] [Google Scholar]
- 38.Chen H, Ahn R, Van den Bossche J, et al. Folate-mediated intracellular drug delivery increases the anticancer efficacy of nanoparticulate formulation of arsenic trioxide. Mol Cancer Ther. 2009;8(7):1955–63. doi: 10.1158/1535-7163.MCT-09-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suzuki R, Takizawa T, Kuwata Y, et al. Effective anti-tumor activity of oxaliplatin encapsulated in transferrin-PEG-liposome. Int J Pharm. 2008;346(1–2):143–50. doi: 10.1016/j.ijpharm.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 40.Emmanouilides C, Jazirehi AR, Bonavida B. Rituximab-mediated sensitization of B-non-Hodgkin’s lymphoma (NHL) to cytotoxicity induced by paclitaxel, gemcitabine, and vinorelbine. Cancer Biother Radiopharm. 2002;17(6):621–30. doi: 10.1089/108497802320970226. [DOI] [PubMed] [Google Scholar]
- 41.Jazirehi AR, Bonavida B. Cellular and molecular signal transduction pathways modulated by rituximab (rituxan, anti-CD20 mAb) in non-Hodgkin’s lymphoma: implications in chemosensitization and therapeutic intervention. Oncogene. 2005;24(13):2121–43. doi: 10.1038/sj.onc.1208349. [DOI] [PubMed] [Google Scholar]
- 42.Ternant D, Paintaud G. Pharmacokinetics and concentration-effect relationships of therapeutic monoclonal antibodies and fusion proteins. Expert Opin Biol Ther. 2005;5(Suppl 1):S37–47. doi: 10.1517/14712598.5.1.s37. [DOI] [PubMed] [Google Scholar]
- 43.Daeron M, Prouvost-Danon A, Voisin GA. Mast cell membrane antigens and Fc receptors in anaphylaxis. II. Functionally distinct receptors for IgG and for IgE on mouse mast cells. Cell Immunol. 1980;49(1):178–89. doi: 10.1016/0008-8749(80)90067-2. [DOI] [PubMed] [Google Scholar]
- 44.Sliwkowski MX, Lofgren JA, Lewis GD, et al. Nonclinical studies addressing the mechanism of action of trastuzumab (Herceptin) Semin Oncol. 1999;26(4 Suppl 12):60–70. [PubMed] [Google Scholar]
- 45.Glennie MJ, van de Winkel JG. Renaissance of cancer therapeutic antibodies. Drug Discov Today. 2003;8(11):503–10. doi: 10.1016/s1359-6446(03)02714-4. [DOI] [PubMed] [Google Scholar]
- 46.Thorpe SJ, Turner C, Heath A, et al. Clonal analysis of a human antimouse antibody (HAMA) response. Scand J Immunol. 2003;57(1):85–92. doi: 10.1046/j.1365-3083.2003.01189.x. [DOI] [PubMed] [Google Scholar]
- 47.Adams GP, Weiner LM. Monoclonal antibody therapy of cancer. Nat Biotechnol. 2005;23(9):1147–57. doi: 10.1038/nbt1137. [DOI] [PubMed] [Google Scholar]
- 48.Qu Z, Griffiths GL, Wegener WA, et al. Development of humanized antibodies as cancer therapeutics. Methods. 2005;36(1):84–95. doi: 10.1016/j.ymeth.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 49.Morrison SL, Johnson MJ, Herzenberg LA, Oi VT. Chimeric human antibody molecules: mouse antigen-binding domains with human constant region domains. Proc Natl Acad Sci USA. 1984;81(21):6851–5. doi: 10.1073/pnas.81.21.6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jones PT, Dear PH, Foote J, et al. Replacing the complementarity-determining regions in a human antibody with those from a mouse. Nature. 1986;321(6069):522–5. doi: 10.1038/321522a0. [DOI] [PubMed] [Google Scholar]
- 51••.Baskar S, Suschak JM, Samija I, et al. A human monoclonal antibody drug and target discovery platform for B-cell chronic lymphocytic leukemia based on allogeneic hematopoietic stem cell transplantation and phage display. Blood. 2009;114(20):4494–502. doi: 10.1182/blood-2009-05-222786. An interesting study to detect donor antibodies against recipient B-cell chronic lymphocytic leukemia cells after allogeneic hematopoietic stem cell transplant. A Fab library was generated from donor cells and selected using phage display. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bartelds GM, Wolbink GJ, Stapel S, et al. High levels of human anti-human antibodies to adalimumab in a patient not responding to adalimumab treatment. Ann Rheum Dis. 2006;65(9):1249–50. doi: 10.1136/ard.2005.049858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Vries MK, Wolbink GJ, Stapel SO, et al. Decreased clinical response to infliximab in ankylosing spondylitis is correlated with anti-infliximab formation. Ann Rheum Dis. 2007;66(9):1252–4. doi: 10.1136/ard.2007.072397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bartelds GM, Wijbrandts CA, Nurmohamed MT, et al. Anti-infliximab and anti-adalimumab antibodies in relation to response to adalimumab in infliximab switchers and anti-TNF naive patients: a cohort study. Ann Rheum Dis. 2009 doi: 10.1136/ard.2009.112847. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 55.Cheng WW, Allen TM. Targeted delivery of anti-CD19 liposomal doxorubicin in B-cell lymphoma: a comparison of whole monoclonal antibody, Fab′ fragments and single chain Fv. J Control Release. 2008;126(1):50–8. doi: 10.1016/j.jconrel.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 56.Kipriyanov SM, Moldenhauer G, Little M. High level production of soluble single chain antibodies in small-scale Escherichia coli cultures. J Immunol Methods. 1997;200(1–2):69–77. doi: 10.1016/s0022-1759(96)00188-3. [DOI] [PubMed] [Google Scholar]
- 57.Houdebine LM. Antibody manufacture in transgenic animals and comparisons with other systems. Curr Opin Biotechnol. 2002;13(6):625–9. doi: 10.1016/S0958-1669(02)00362-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pini A, Bracci L. Phage display of antibody fragments. Curr Protein Pept Sci. 2000;1(2):155–69. doi: 10.2174/1389203003381397. [DOI] [PubMed] [Google Scholar]
- 59.Lindner P, Bauer K, Krebber A, et al. Specific detection of his-tagged proteins with recombinant anti-His tag scFv-phosphatase or scFv-phage fusions. Biotechniques. 1997;22(1):140–9. doi: 10.2144/97221rr01. [DOI] [PubMed] [Google Scholar]
- 60.Pavlinkova G, Colcher D, Booth BJ, et al. Effects of humanization and gene shuffling on immunogenicity and antigen binding of anti-TAG-72 single-chain Fvs. Int J Cancer. 2001;94:717–26. doi: 10.1002/ijc.1523. [DOI] [PubMed] [Google Scholar]
- 61.Roque-Navarro L, Mateo C, Lombardero J, et al. Humanization of predicted T-cell epitopes reduces the immunogenicity of chimeric antibodies: new evidence supporting a simple method. Hybrid Hybridomics. 2003;22(4):245–57. doi: 10.1089/153685903322328974. [DOI] [PubMed] [Google Scholar]
- 62.Sapra P, Moase EH, Ma J, Allen TM. Improved therapeutic responses in a xenograft model of human B-lymphoma (Namalwa) for liposomal vincristine versus liposomal doxorubicin targeted via anti-CD19 IgG2a or Fab′ fragments. Clin Cancer Res. 2004;10(3):1100–11. doi: 10.1158/1078-0432.ccr-03-0041. [DOI] [PubMed] [Google Scholar]
- 63.Zalipsky S. Synthesis of end-group functionalized polyethylene glycol-lipid conjugates for preparation of polymer-grafted liposomes. Bioconjugate Chem. 1993;4:296–9. doi: 10.1021/bc00022a008. [DOI] [PubMed] [Google Scholar]
- 64.Allen TM, Brandeis E, Hansen CB, et al. A new strategy for attachment of antibodies to sterically stabilized liposomes resulting in efficient targeting to cancer cells. Biochim Biophys Acta. 1995;1237:99–108. doi: 10.1016/0005-2736(95)00085-h. [DOI] [PubMed] [Google Scholar]
- 65.Kirpotin D, Park JW, Hong K, et al. Sterically stabilized anti-HER2 immunoliposomes: design and targeting to human breast cancer cells in vitro. Biochemistry. 1997;36:66–75. doi: 10.1021/bi962148u. [DOI] [PubMed] [Google Scholar]
- 66.Bendas G, Krause A, Bakowsky U, et al. Targetability of novel immunoliposomes prepared by a new antibody conjugation technique. Int J Pharmaceutics. 1999;181(1):79–93. doi: 10.1016/s0378-5173(99)00002-2. [DOI] [PubMed] [Google Scholar]
- 67.Torchilin VP, Levchenko TS, Lukyanov AN, et al. p-Nitrophenylcarbonyl-PEG-PE-liposomes: fast and simple attachment of specific ligands, including monoclonal antibodies, to distal ends of PEG chains via p-nitrophenylcarbonyl groups. Biochim Biophys Acta. 2001;1511:397–411. doi: 10.1016/s0005-2728(01)00165-7. [DOI] [PubMed] [Google Scholar]
- 68.Mamot C, Drummond DC, Greiser U, et al. Epidermal growth factor receptor (EGFR)-targeted immunoliposomes mediate specific and efficient drug delivery to EGFR- and EGFRvIII-overexpressing tumor cells. Cancer Res. 2003;63(12):3154–61. [PubMed] [Google Scholar]
- 69.Pastorino F, Brignole C, Marimpietri D, et al. Doxorubicin-loaded Fab′ fragments of anti-disialoganglioside immunoliposomes selectively inhibit the growth and dissemination of human neuroblastoma in nude mice. Cancer Res. 2003;63(1):86–92. [PubMed] [Google Scholar]
- 70.Marty C, Langer-Machova Z, Sigrist S, et al. Isolation and characterization of a scFv antibody specific for tumor endothelial marker 1 (TEM1), a new reagent for targeted tumor therapy. Cancer Lett. 2006;235(2):298–308. doi: 10.1016/j.canlet.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 71.Cheng WW, Das D, Suresh M, Allen TM. Expression and purification of two anti-CD19 single chain Fv fragments for targeting of liposomes to CD19-expressing cells. Biochim Biophys Acta. 2007;1768(1):21–9. doi: 10.1016/j.bbamem.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 72.Messerschmidt SK, Kolbe A, Muller D, et al. Novel single-chain Fv′ formats for the generation of immunoliposomes by site-directed coupling. Bioconjug Chem. 2008;19(1):362–9. doi: 10.1021/bc700349k. [DOI] [PubMed] [Google Scholar]
- 73.Lopes de Menezes DE, Pilarski LM, Allen TM. In vitro and in vivo targeting of immunoliposomal doxorubicin to human B-cell lymphoma. Cancer Res. 1998;58(15):3320–30. [PubMed] [Google Scholar]
- 74.Hansen CB, Kao GY, Moase EH, et al. Attachment of antibodies to sterically stabilized liposomes: evaluation, comparison and optimization of coupling procedures. Biochim Biophys Acta. 1995;1239:133–44. doi: 10.1016/0005-2736(95)00138-s. [DOI] [PubMed] [Google Scholar]
- 75.Pan X, Lee RJ. Construction of anti-EGFR immunoliposomes via folate-folate binding protein affinity. Int J Pharm. 2007;336(2):276–83. doi: 10.1016/j.ijpharm.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 76.Ruger R, Muller D, Fahr A, Kontermann RE. Generation of immunoliposomes using recombinant single-chain Fv fragments bound to Ni-NTA-liposomes. J Drug Target. 2005;13(7):399–406. doi: 10.1080/10611860500353328. [DOI] [PubMed] [Google Scholar]
- 77.Ruger R, Muller D, Fahr A, Kontermann RE. In vitro characterization of binding and stability of single-chain Fv Ni-NTA-liposomes. J Drug Target. 2006;14(8):576–82. doi: 10.1080/10611860600864018. [DOI] [PubMed] [Google Scholar]
- 78.Ishida T, Iden DL, Allen TM. A combinatorial approach to producing sterically stabilized (Stealth) immunoliposomal drugs. FEBS Lett. 1999;460(1):129–33. doi: 10.1016/s0014-5793(99)01320-4. [DOI] [PubMed] [Google Scholar]
- 79.Iden DL, Allen TM. In vitro and in vivo comparison of immunoliposomes made by conventional coupling techniques with those made by a new post-insertion technique. Biochim Biophys Acta. 2001;1513(2):207–16. doi: 10.1016/s0005-2736(01)00357-1. [DOI] [PubMed] [Google Scholar]
- 80.Moreira JN, Ishida T, Gaspar R, Allen TM. Use of the post-insertion technique to insert peptide ligands into pre-formed Stealth liposomes with retention of binding activity and cytotoxicity. Pharm Res. 2002;19(3):265–9. doi: 10.1023/a:1014434732752. [DOI] [PubMed] [Google Scholar]
- 81••.Nellis DF, Ekstrom DL, Kirpotin DB, et al. Preclinical Manufacture of an Anti-HER2 scFv-PEG-DSPE, Liposome-Inserting conjugate. 1. Gram-Scale Production and Purification. Biotechnol Prog. 2005;21(1):205–20. doi: 10.1021/bp049840y. One of the first studies to describe large-scale production of an scFv for clinical trials. [DOI] [PubMed] [Google Scholar]
- 82.Nellis DF, Giardina SL, Janini GM, et al. Preclinical manufacture of anti-HER2 liposome-inserting, scFv-PEG-lipid conjugate. 2. Conjugate micelle identity, purity, stability, and potency analysis. Biotechnol Prog. 2005;21(1):221–32. doi: 10.1021/bp049839z. [DOI] [PubMed] [Google Scholar]
- 83.Lopes de Menezes DE, Pilarski LM, Belch AR, Allen TM. Selective targeting of immunoliposomal doxorubicin against human multiple myeloma in vitro and ex vivo. Biochim Biophys Acta. 2000;1466(1–2):205–20. doi: 10.1016/s0005-2736(00)00203-0. [DOI] [PubMed] [Google Scholar]
- 84.Zalipsky S, Hansen CB, Lopes de Menezes DE, Allen TM. Long-circulating polyethylene glycol-grafted immunoliposomes. J Control Release. 1996;39:153–61. [Google Scholar]
- 85.Mamot C, Drummond DC, Noble CO, et al. Epidermal growth factor receptor-targeted immunoliposomes significantly enhance the efficacy of multiple anticancer drugs in vivo. Cancer Res. 2005;65(24):11631–8. doi: 10.1158/0008-5472.CAN-05-1093. [DOI] [PubMed] [Google Scholar]
- 86.Noble CO, Guo Z, Hayes ME, et al. Characterization of highly stable liposomal and immunoliposomal formulations of vincristine and vinblastine. Cancer Chemother Pharmacol. 2009;64(4):741–51. doi: 10.1007/s00280-008-0923-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bendas G, Rothe U, Scherphof GL, Kamps JA. The influence of repeated injections on pharmacokinetics and biodistribution of different types of sterically stabilized immunoliposomes. Biochim Biophys Acta. 2003;1609(1):63–70. doi: 10.1016/s0005-2736(02)00655-7. [DOI] [PubMed] [Google Scholar]
- 88.Ishida T, Kiwada H. Accelerated blood clearance (ABC) phenomenon upon repeated injection of PEGylated liposomes. Int J Pharm. 2008;354(1–2):56–62. doi: 10.1016/j.ijpharm.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 89.Wang X, Ishida T, Kiwada H. Anti-PEG IgM elicited by injection of liposomes is involved in the enhanced blood clearance of a subsequent dose of PEGylated liposomes. J Control Release. 2007;119(2):236–44. doi: 10.1016/j.jconrel.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 90.Goren D, Horowitz AT, Zalipsky S, et al. Targeting of Stealth liposomes to erB-2 (Her/2) receptor: in vitro and in vivo studies. Br J Cancer. 1996;74:1749–56. doi: 10.1038/bjc.1996.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Houck KS, Huang L. The role of multivalency in antibody mediated liposome targeting. Biochem Biophys Res Commun. 1987;145(3):1205–10. doi: 10.1016/0006-291x(87)91565-8. [DOI] [PubMed] [Google Scholar]
- 92.Maruyama K, Takahashi N, Tagawa T, et al. Immunoliposomes bearing polyethyleneglycol-coupled Fab′ fragment show prolonged circulation time and high extravasation into targeted solid tumors in vivo. FEBS Lett. 1997;413(1):177–80. doi: 10.1016/s0014-5793(97)00905-8. [DOI] [PubMed] [Google Scholar]
- 93.Baum P, Muller D, Ruger R, Kontermann RE. Single-chain Fv immunoliposomes for the targeting of fibroblast activation protein-expressing tumor stromal cells. J Drug Target. 2007;15(6):399–406. doi: 10.1080/10611860701453034. [DOI] [PubMed] [Google Scholar]
- 94.An F, Drummond DC, Wilson S, et al. Targeted drug delivery to mesothelioma cells using functionally selected internalizing human single-chain antibodies. Mol Cancer Ther. 2008;7(3):569–78. doi: 10.1158/1535-7163.MCT-07-2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Messerschmidt SK, Musyanovych A, Altvater M, et al. Targeted lipid-coated nanoparticles: delivery of tumor necrosis factor-functionalized particles to tumor cells. J Control Release. 2009;137(1):69–77. doi: 10.1016/j.jconrel.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 96.Willuda J, Honegger A, Waibel R, et al. High thermal stability is essential for tumor targeting of antibody fragments: engineering of a humanized anti-epithelial glycoprotein-2 (epithelial cell adhesion molecule) single-chain Fv fragment. Cancer Res. 1999;59(22):5758–67. [PubMed] [Google Scholar]
- 97.Worn A, Auf der Maur A, Escher D, et al. Correlation between in vitro stability and in vivo performance of anti-GCN4 intrabodies as cytoplasmic inhibitors. J Biol Chem. 2000;275(4):2795–803. doi: 10.1074/jbc.275.4.2795. [DOI] [PubMed] [Google Scholar]
- 98.Pedroso I, Irun MP, Machicado C, Sancho J. Four-state equilibrium unfolding of an scFv antibody fragment. Biochemistry. 2002;41(31):9873–84. doi: 10.1021/bi025742e. [DOI] [PubMed] [Google Scholar]
- 99.Brockmann EC, Cooper M, Stromsten N, et al. Selecting for antibody scFv fragments with improved stability using phage display with denaturation under reducing conditions. J Immunol Methods. 2005;296(1–2):159–70. doi: 10.1016/j.jim.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 100.Shusta EV, Holler PD, Kieke MC, et al. Directed evolution of a stable scaffold for T-cell receptor engineering. Nat Biotechnol. 2000;18(7):754–9. doi: 10.1038/77325. [DOI] [PubMed] [Google Scholar]
- 101.Kipriyanov SM, Moldenhauer G, Martin AC, et al. Two amino acid mutations in an anti-human CD3 single chain Fv antibody fragment that affect the yield on bacterial secretion but not the affinity. Protein Eng. 1997;10(4):445–53. doi: 10.1093/protein/10.4.445. [DOI] [PubMed] [Google Scholar]
- 102.Worn A, Pluckthun A. Stability engineering of antibody single-chain Fv fragments. J Mol Biol. 2001;305(5):989–1010. doi: 10.1006/jmbi.2000.4265. [DOI] [PubMed] [Google Scholar]
- 103.Worn A, Pluckthun A. Different equilibrium stability behavior of ScFv fragments: identification, classification, and improvement by protein engineering. Biochemistry. 1999;38(27):8739–50. doi: 10.1021/bi9902079. [DOI] [PubMed] [Google Scholar]
- 104.Arndt MA, Krauss J, Rybak SM. Antigen binding and stability properties of non-covalently linked anti-CD22 single-chain Fv dimers. FEBS Lett. 2004;578(3):257–61. doi: 10.1016/j.febslet.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 105.Cheng WW. Targeted delivery of immunoliposomal doxorubicin to B-lymphoid cells via an anti-CD19 whole monoclonal antibody or fragments of antibody. Edmonton: University of Alberta; 2008. [Google Scholar]
- 106.Proba K, Honegger A, Pluckthun A. A natural antibody missing a cysteine in VH: consequences for thermodynamic stability and folding. J Mol Biol. 1997;265(2):161–72. doi: 10.1006/jmbi.1996.0726. [DOI] [PubMed] [Google Scholar]
- 107.Glockshuber R, Schmidt T, Pluckthun A. The disulfide bonds in antibody variable domains: effects on stability, folding in vitro, and functional expression in Escherichia coli. Biochemistry. 1992;31(5):1270–9. doi: 10.1021/bi00120a002. [DOI] [PubMed] [Google Scholar]
- 108.Proba K, Worn A, Honegger A, Pluckthun A. Antibody scFv fragments without disulfide bonds made by molecular evolution. J Mol Biol. 1998;275(2):245–53. doi: 10.1006/jmbi.1997.1457. [DOI] [PubMed] [Google Scholar]
- 109.Worn A, Pluckthun A. An intrinsically stable antibody scFv fragment can tolerate the loss of both disulfide bonds and fold correctly. FEBS Lett. 1998;427(3):357–61. doi: 10.1016/s0014-5793(98)00463-3. [DOI] [PubMed] [Google Scholar]
- 110.de Marco A. Strategies for successful recombinant expression of disulfide bond-dependent proteins in Escherichia coli. Microb Cell Fact. 2009;8:26–43. doi: 10.1186/1475-2859-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Reiter Y, Brinkmann U, Webber KO, et al. Engineering interchain disulfide bonds into conserved framework regions of Fv fragments: improved biochemical characteristics of recombinant immunotoxins containing disulfide-stabilized Fv. Protein Eng. 1994;7(5):697–704. doi: 10.1093/protein/7.5.697. [DOI] [PubMed] [Google Scholar]
- 112.Reiter Y, Brinkmann U, Jung SH, et al. Disulfide stabilization of antibody Fv: computer predictions and experimental evaluation. Protein Eng. 1995;8(12):1323–31. doi: 10.1093/protein/8.12.1323. [DOI] [PubMed] [Google Scholar]
- 113.Robinson CR, Sauer RT. Optimizing the stability of single-chain proteins by linker length and composition mutagenesis. Proc Natl Acad Sci USA. 1998;95(11):5929–34. doi: 10.1073/pnas.95.11.5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Villani ME, Morea V, Consalvi V, et al. Humanization of a highly stable single-chain antibody by structure-based antigen-binding site grafting. Mol Immunol. 2008;45(9):2474–85. doi: 10.1016/j.molimm.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 115.Quintero-Hernandez V, Juarez-Gonzalez VR, Ortiz-Leon M, et al. The change of the scFv into the Fab format improves the stability and in vivo toxin neutralization capacity of recombinant antibodies. Mol Immunol. 2007;44(6):1307–15. doi: 10.1016/j.molimm.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 116.Juarez-Gonzalez VR, Riano-Umbarila L, Quintero-Hernandez V, et al. Directed evolution, phage display and combination of evolved mutants: a strategy to recover the neutralization properties of the scFv version of BCF2 a neutralizing monoclonal antibody specific to scorpion toxin Cn2. J Mol Biol. 2005;346(5):1287–97. doi: 10.1016/j.jmb.2004.12.060. [DOI] [PubMed] [Google Scholar]
- 117.Dona MG, Giorgi C, Accardi L. Characterization of antibodies in single-chain format against the E7 oncoprotein of the human papillomavirus type 16 and their improvement by mutagenesis. BMC Cancer. 2007;7:25–38. doi: 10.1186/1471-2407-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kugler M, Stein C, Schwenkert M, et al. Stabilization and humanization of a single-chain Fv antibody fragment specific for human lymphocyte antigen CD19 by designed point mutations and CDR-grafting onto a human framework. Protein Eng Des Sel. 2009;22(3):135–47. doi: 10.1093/protein/gzn079. [DOI] [PubMed] [Google Scholar]
- 119.Guisez Y, Demolder J, Mertens N, et al. High-level expression, purification, and renaturation of recombinant murine interleukin-2 from Escherichia coli. Protein Expr Purif. 1993;4(3):240–6. doi: 10.1006/prep.1993.1031. [DOI] [PubMed] [Google Scholar]
- 120.Seddon AP, Hulmes JD, Decker MM, et al. Refolding and characterization of human recombinant heparin-binding neurite-promoting factor. Protein Expr Purif. 1994;5(1):14–21. doi: 10.1006/prep.1994.1002. [DOI] [PubMed] [Google Scholar]
- 121.Hoyer MRK, Pluckthun A. A kinetic trap is an intrinsic feature in the folding pathway of single-chain Fv fragments. Biophys Chem. 2002;96(2–3):273–84. doi: 10.1016/s0301-4622(02)00022-4. [DOI] [PubMed] [Google Scholar]
- 122.Francis RJ, Mather SJ, Chester K, et al. Radiolabelling of glycosylated MFE-23: CPG2 fusion protein (MFECP1) with 99mTc for quantitation of tumour antibody-enzyme localisation in antibody-directed enzyme pro-drug therapy (ADEPT) Eur J Nucl Med Mol Imaging. 2004;31(8):1090–6. doi: 10.1007/s00259-004-1474-4. [DOI] [PubMed] [Google Scholar]
- 123.Lin Y, Pagel JM, Axworthy D, et al. A genetically engineered anti-CD45 single-chain antibody-streptavidin fusion protein for pretargeted radioimmunotherapy of hematologic malignancies. Cancer Res. 2006;66(7):3884–92. doi: 10.1158/0008-5472.CAN-05-3443. [DOI] [PubMed] [Google Scholar]
- 124.Goel A, Beresford GW, Colcher D, et al. Divalent forms of CC49 single-chain antibody constructs in Pichia pastoris: expression, purification, and characterization. J Biochem. 2000;127(5):829–36. doi: 10.1093/oxfordjournals.jbchem.a022676. [DOI] [PubMed] [Google Scholar]
- 125.Wittel UA, Jain M, Goel A, et al. The in vivo characteristics of genetically engineered divalent and tetravalent single-chain antibody constructs. Nucl Med Biol. 2005;32(2):157–64. doi: 10.1016/j.nucmedbio.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 126.Das D, Allen TM, Suresh MR. Comparative evaluation of two purification methods of anti-CD19-c-myc-His(6)-Cys scFv. Protein Expr Purif. 2005;39(2):199–208. doi: 10.1016/j.pep.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 127.Wu AM, Senter PD. Arming antibodies: prospects and challenges for immunoconjugates. Nat Biotechnol. 2005;23(9):1137–46. doi: 10.1038/nbt1141. [DOI] [PubMed] [Google Scholar]
- 128.Mayer A, Francis RJ, Sharma SK, et al. A phase I study of single administration of antibody-directed enzyme prodrug therapy with the recombinant anti-carcinoembryonic antigen antibody-enzyme fusion protein MFECP1 and a bis-iodo phenol mustard prodrug. Clin Cancer Res. 2006;12(21):6509–16. doi: 10.1158/1078-0432.CCR-06-0769. [DOI] [PubMed] [Google Scholar]
- 129.Bang S, Nagata S, Onda M, et al. HA22 (R490A) is a recombinant immunotoxin with increased antitumor activity without an increase in animal toxicity. Clin Cancer Res. 2005;11(4):1545–50. doi: 10.1158/1078-0432.CCR-04-1939. [DOI] [PubMed] [Google Scholar]
- 130.Filpula D, Yang K, Basu A, et al. Releasable PEGylation of mesothelin targeted immunotoxin SS1P achieves single dosage complete regression of a human carcinoma in mice. Bioconjug Chem. 2007;18(3):773–84. doi: 10.1021/bc060314x. [DOI] [PubMed] [Google Scholar]
- 131.Laane E, Tani E, Bjorklund E, et al. Flow cytometric immunophenotyping including Bcl-2 detection on fine needle aspirates in the diagnosis of reactive lymphadenopathy and non-Hodgkin’s lymphoma. Cytometry B Clin Cytom. 2005;64(1):34–42. doi: 10.1002/cyto.b.20043. [DOI] [PubMed] [Google Scholar]
- 132.Laginha K, Mumbengegwi D, Allen T. Liposomes targeted via two different antibodies: assay, B-cell binding and cytotoxicity. Biochim Biophys Acta. 2005;1711(1):25–32. doi: 10.1016/j.bbamem.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 133.Maloney DG, Smith B, Rose A. Rituximab: mechanism of action and resistance. Semin Oncol. 2002;29(1 Suppl 2):2–9. doi: 10.1053/sonc.2002.30156. [DOI] [PubMed] [Google Scholar]
- 134.Smith MR. Rituximab (monoclonal anti-CD20 antibody): mechanisms of action and resistance. Oncogene. 2003;22(47):7359–73568. doi: 10.1038/sj.onc.1206939. [DOI] [PubMed] [Google Scholar]
- 135.Jilani I, O’Brien S, Manshuri T, et al. Transient down-modulation of CD20 by rituximab in patients with chronic lymphocytic leukemia. Blood. 2003;102(10):3514–20. doi: 10.1182/blood-2003-01-0055. [DOI] [PubMed] [Google Scholar]
- 136.Wobser M, Voigt H, Eggert AO, et al. Bcl-2 expression in rituximab refractory cutaneous B-cell lymphoma. Br J Cancer. 2007;96(10):1540–3. doi: 10.1038/sj.bjc.6603762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Price-Schiavi SA, Jepson S, Li P, et al. Rat Muc4 (sialomucin complex) reduces binding of anti-ErbB2 antibodies to tumor cell surfaces, a potential mechanism for herceptin resistance. Int J Cancer. 2002;99(6):783–91. doi: 10.1002/ijc.10410. [DOI] [PubMed] [Google Scholar]
- 138.Nagy P, Friedlander E, Tanner M, et al. Decreased accessibility and lack of activation of ErbB2 in JIMT-1, a herceptin-resistant, MUC4-expressing breast cancer cell line. Cancer Res. 2005;65(2):473–82. [PubMed] [Google Scholar]
- 139.Moase EA, Allen TM. Combination targeting of immunoliposomal therapeutics in a murine model of B-Cell lymphoma. 36th Annual Meeting & Exposition of the Controlled Release Society; Copenhagen, Denmark. 2009. [Google Scholar]
- 140.Hare JIM, Moase EA, Allen TM. Combined Anti-vasculature and Anti-tumor Targeting in the Treatment of Metastatic Breast Cancer. 36th Annual Meeting & Exposition of the Controlled Release Society; Copenhagen, Denmark. 2009. [Google Scholar]
- 141.Abraham SA, McKenzie C, Masin D, et al. In vitro and in vivo characterization of doxorubicin and vincristine coencapsulated within liposomes through use of transition metal ion complexation and pH gradient loading. Clin Cancer Res. 2004;10(2):728–38. doi: 10.1158/1078-0432.ccr-1131-03. [DOI] [PubMed] [Google Scholar]
- 142.Mayer LD, Harasym TO, Tardi PG, et al. Ratiometric dosing of anticancer drug combinations: controlling drug ratios after systemic administration regulates therapeutic activity in tumor-bearing mice. Mol Cancer Ther. 2006;5(7):1854–63. doi: 10.1158/1535-7163.MCT-06-0118. [DOI] [PubMed] [Google Scholar]
- 143.Tardi PG, Gallagher RC, Johnstone S, et al. Coencapsulation of irinotecan and floxuridine into low cholesterol-containing liposomes that coordinate drug release in vivo. Biochim Biophys Acta. 2007;1768(3):678–87. doi: 10.1016/j.bbamem.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 144.Caplen NJ, Kinrade E, Sorgi F, et al. In vitro liposome mediated DNA transfection of epithelial cell lines using the cationic liposome Dc Chol/Dope. Gene Ther. 1995;2(9):603–13. [PubMed] [Google Scholar]
- 145.de Lima MC, Simoes S, Pires P, et al. Gene delivery mediated by cationic liposomes: from biophysical aspects to enhancement of transfection. Mol Membr Biol. 1999;16(1):103–9. doi: 10.1080/096876899294823. [DOI] [PubMed] [Google Scholar]
- 146.Ishida T, Kirchmeier MJ, Moase EH, et al. Targeted delivery and triggered release of liposomal doxorubicin enhances cytotoxicity against human B lymphoma cells. Biochim Biophys Acta. 2001;1515:144–58. doi: 10.1016/s0005-2736(01)00409-6. [DOI] [PubMed] [Google Scholar]
- 147.Kakudo T, Chaki S, Futaki S, et al. Transferrin-modified liposomes equipped with a pH-sensitive fusogenic peptide: an artificial viral-like delivery system. Biochemistry. 2004;43(19):5618–28. doi: 10.1021/bi035802w. [DOI] [PubMed] [Google Scholar]
- 148.Kirpotin D, Hong K, Mullah N, et al. Liposomes with detachable polymer coating: destabilization and fusion of dioleoylphosphatidylethanolamine vesicles triggered by cleavage of surface-grafted poly(ethylene glycol) FEBS Lett. 1996;388:115–8. doi: 10.1016/0014-5793(96)00521-2. [DOI] [PubMed] [Google Scholar]
- 149.Torchilin VP, Rammohan R, Weissig V, Levchenko TS. TAT peptide on the surface of liposomes affords their efficient intracellular delivery even at low temperature and in the presence of metabolic inhibitors. Proc Natl Acad Sci USA. 2001;98(15):8786–91. doi: 10.1073/pnas.151247498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhang JX, Zalipsky S, Mullah N, et al. Pharmaco attributes of dioleoylphosphatidylethanolamine/cholesterylhemisuccinate liposomes containing different types of cleavable lipopolymers. Pharmacol Res. 2004;49:185–98. doi: 10.1016/j.phrs.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 151.Boomer JA, Qualls MM, Inerowicz HD, et al. Cytoplasmic delivery of liposomal contents mediated by an acid-labile cholesterol-vinyl ether-PEG conjugate. Bioconjug Chem. 2009;20(1):47–59. doi: 10.1021/bc800239b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cao Y, Suresh MR. Bispecific mAb aided liposomal drug delivery. J Drug Target. 2000;8(4):257–66. doi: 10.3109/10611860008997904. [DOI] [PubMed] [Google Scholar]
- 153.Bruenke J, Fischer B, Barbin K, et al. A recombinant bispecific single-chain Fv antibody against HLA class II and FcgammaRIII (CD16) triggers effective lysis of lymphoma cells. Br J Haematol. 2004;125(2):167–79. doi: 10.1111/j.1365-2141.2004.04893.x. [DOI] [PubMed] [Google Scholar]
- 154.Kellner C, Bruenke J, Stieglmaier J, et al. A novel CD19-directed recombinant bispecific antibody derivative with enhanced immune effector functions for human leukemic cells. J Immunother. 2008;31(9):871–84. doi: 10.1097/CJI.0b013e318186c8b4. [DOI] [PubMed] [Google Scholar]
- 155.Vallera DA, Chen H, Sicheneder AR, et al. Genetic alteration of a bispecific ligand-directed toxin targeting human CD19 and CD22 receptors resulting in improved efficacy against systemic B cell malignancy. Leuk Res. 2009;33(9):1233–42. doi: 10.1016/j.leukres.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Horn U, Strittmatter W, Krebber A, et al. High volumetric yields of functional dimeric miniantibodies in Escherichia coli, using an optimized expression vector and high-cell-density fermentation under non-limited growth conditions. Appl Microbiol Biotechnol. 1996;46(5–6):524–32. doi: 10.1007/s002530050855. [DOI] [PubMed] [Google Scholar]
- 157.Hu S, Li L, Qiao J, et al. Codon optimization, expression, and characterization of an internalizing anti-ErbB2 single-chain antibody in Pichia pastoris. Protein Expr Purif. 2006;47(1):249–57. doi: 10.1016/j.pep.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 158.Kumada Y, Sakan Y, Kajihara H, et al. Efficient production of single-chain Fv antibody possessing rare codon linkers in fed-batch fermentation. J Biosci Bioeng. 2009;107(1):73–7. doi: 10.1016/j.jbiosc.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 159.Hu X, O’Hara L, White S, et al. Optimisation of production of a domoic acid-binding scFv antibody fragment in Escherichia coli using molecular chaperones and functional immobilisation on a mesoporous silicate support. Protein Expr Purif. 2007;52(1):194–201. doi: 10.1016/j.pep.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 160.Shaki-Loewenstein S, Zfania R, Hyland S, et al. A universal strategy for stable intracellular antibodies. J Immunol Methods. 2005;303(1–2):19–39. doi: 10.1016/j.jim.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 161.Jurado P, de Lorenzo V, Fernandez LA. Thioredoxin fusions increase folding of single chain Fv antibodies in the cytoplasm of Escherichia coli: evidence that chaperone activity is the prime effect of thioredoxin. J Mol Biol. 2006;357(1):49–61. doi: 10.1016/j.jmb.2005.12.058. [DOI] [PubMed] [Google Scholar]
- 162.Sonoda H, Kumada Y, Katsuda T, Yamaji H. Functional expression of single-chain Fv antibody in the cytoplasm of Escherichia coli by thioredoxin fusion and co-expression of molecular chaperones. Protein Expr Purif. 2009 doi: 10.1016/j.pep.2009.11.003. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 163.Wall JG, Pluckthun A. The hierarchy of mutations influencing the folding of antibody domains in Escherichia coli. Protein Eng. 1999;12(7):605–11. doi: 10.1093/protein/12.7.605. [DOI] [PubMed] [Google Scholar]
- 164.Franklin SE, Mayfield SP. Prospects for molecular farming in the green alga Chlamydomonas. Curr Opin Plant Biol. 2004;7(2):159–65. doi: 10.1016/j.pbi.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 165.Zhang G, Brokx S, Weiner JH. Extracellular accumulation of recombinant proteins fused to the carrier protein YebF in Escherichia coli. Nat Biotechnol. 2006;24(1):100–4. doi: 10.1038/nbt1174. [DOI] [PubMed] [Google Scholar]