Abstract
Cisplatin is a widely used chemotherapeutic agent for the treatment of solid tumors. A serious complication of cisplatin treatment is permanent hearing loss. The aim of this study was to replicate previous genetic findings in an independent cohort of 155 pediatric patients. Associations were replicated for genetic variants in TPMT (rs12201199, P = 0.0013, odds ratio (OR) 6.1) and ABCC3 (rs1051640, P = 0.036, OR 1.8). A predictive model combining variants in TPMT, ABCC3, and COMT with clinical variables (patient age, vincristine treatment, germ-cell tumor, and cranial irradiation) significantly improved the prediction of hearing-loss development as compared with using clinical risk factors alone (area under the curve (AUC) 0.786 vs. 0.708, P = 0.00048). The novel combination of genetic and clinical factors predicted the risk of hearing loss with a sensitivity of 50.3% and a specificity of 92.7%. These findings provide evidence to support the importance of TPMT, COMT, and ABCC3 in the prediction of cisplatin-induced hearing loss in children.
Cisplatin is one of the most effective chemotherapeutic agents for children with solid tumors, including hepatoblastoma, brain tumors, and germ-cell tumors, and has contributed to a dramatic increase in the survival rate. Cisplatin has shown efficacy in standard-risk hepatoblastoma and can be used as monotherapy with a >80% 3-year event-free survival.1 A major complication that limits the use of cisplatin is the risk of drug-induced ototoxicity,2 which manifests as permanent, bilateral hearing loss in about 10–25% of adults and 26–90% of children depending on dose and treatment regimen.3–8 In children, even mild losses in hearing can significantly influence speech and language development and increase the risk of learning difficulties.9,10 In adults, the rate of hearing loss may be higher than reported due to a lack of baseline and follow-up audiometry in cisplatin protocols.
Interindividual variability of cisplatin-induced effects on hearing in patients receiving the same dose of cisplatin is considerable, from no hearing loss to high-frequency hearing loss often progressing to severe hearing impairment in the speech frequencies.10–12 Furthermore, patients show no improvement in hearing and often the progression of hearing loss continues long after the end of therapy.13 Higher cumulative cisplatin dose,3,14,15 younger age,3,14,16 cranial irradiation,15,17 and concomitant use of aminoglycosides and vincristine14,18,19 are known to influence cisplatin-induced ototoxicity.11 However, the debate over ototoxicity of vincristine continues; case reports suggest that vincristine may be ototoxic20,21 or transiently ototoxic at high doses14 whereas larger systematic clinical trials have reported that vincristine, alone, is not ototoxic.22,23 Because of the limited number of large studies, there is insufficient evidence to either support or refute the hypothesis that vincristine is an ototoxic agent. The interindividual variability in hearing loss suggests that clinical factors alone are insufficient predictors of safety.
At present, no standard methods exist to identify individuals who are at increased risk of developing hearing loss. Genetic factors involved in drug biotransformation, transport, and receptors have been recognized to influence patient drug response and susceptibility to adverse drug events, including ototoxicity.24–26 The identification of genetic markers that increase susceptibility to cisplatin-induced ototoxicity has important implications for improving patient care during cisplatin treatment.
Recently, a candidate gene study in children receiving cispl-atin identified genetic variants in thiopurine S-methyltransferase (TPMT) (rs12201199, rs1800460, rs1142345), catechol O-methyltransferase (COMT) (rs9332377, rs4646316), and several other variants, including the ATP-binding cassette transporter C3 (ABCC3) (rs1051640) as conferring increased risk of developing cisplatin-induced hearing loss.9
For clinical application, it is essential to replicate these genetic findings in independent cohorts of patients so as to reduce the number of false-positive results and to ensure that the genetic risk prediction is consistent.27 It is now well recognized that replication of genetic associations is required before any causal inferences can be drawn.27 However, several studies have reported that consistent replication is challenging and difficult to achieve. The aim of this study was to investigate replication of genetic risk factors for cisplatin-induced hearing loss in an independent cohort of patients recruited from across Canada, as well as to evaluate a predictive multi-SNP (single-nucleotide polymorphism) model.
RESULTS
Patient characteristics
Patient characteristics of the initial cohort,9 current replication cohort, and the combined cohort are listed in Table 1. Adverse drug reaction surveillance clinicians routinely update information in the Canadian Pharmacogenomics Network for Drug Safety (CPNDS) patient database as new, clinically relevant information (i.e., relapse and concomitant medication use) becomes available. For the initial cohort, we included additional demographic data, including relevant information on the clinical course of these patients in the time since the study was first reported;9 we have also included additional concomitant medication details.28 In the current replication cohort, 87 (56%) of the 155 pediatric oncology patients developed hearing loss. Concomitant vincristine treatment was significantly higher in cases than in controls in the replication cohort (66.7% vs. 27.9%, P = 2.1 × 10−6), as well as in the initial cohort (50.9% vs. 17.9%, P = 4.1 × 10−5; combined cohort P = 1.1 × 10−9). Fewer patients with osteosarcoma in the replication cohort developed hearing loss (11.5% in cases vs. 29.4% in controls, P = 0.0073), but the difference was not significant in the initial cohort (22.6% in cases vs. 28.6% in controls, P = 0.45). Furthermore, follow-up after therapy was longer in cases than in controls in the replication cohort (5.0 years vs. 2.0 years, P = 2.1 × 10−4), but the difference was not significant in the initial cohort (3.0 years vs. 2.0 years, P = 0.10).
Table 1.
Patient demographics
| Initial cohorta (n = 162) | Replication cohort (n = 155) | Combined cohort (n = 317) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases (n = 106) | Controls (n = 56) | P-value | Cases (n = 87) | Controls (n = 68) | P-value | Cases (n = 193) | Controls (n = 124) | P-value | |
| Age, years (median (min, max)) | 6.0 (0, 16) | 9.0 (0, 19) | 0.086 | 6.0 (1, 25) | 11.0 (0, 18) | 0.050 | 6.0 (0, 25) | 10.0 (0, 19) | 0.012 |
| Dose, cumulative mg/m2 (median (min, max)) | 400 (120, 720) | 400 (100, 720) | 0.66 | 400 (92, 800) | 400 (20, 768) | 0.36 | 400 (92, 800) | 400 (20, 768) | 0.71 |
| Treatment duration, months (median (min, max)) | 5.0 (1, 12) | 4.0 (1, 11) | 0.89 | 4.0 (0, 14) | 4.0 (0, 15) | 0.41 | 4.0 (0, 14) | 4.0 (0, 15) | 0.85 |
| Gender (male, n (%)) | 71 (67.0%) | 28 (50.0%) | 0.043 | 43 (49.4%) | 34 (50.0%) | 1.00 | 114 (59.1%) | 62 (50.0%) | 0.13 |
| Caucasian ethnicityb (n (%)) | 80 (75.5%) | 48 (85.7%) | 0.16 | 70 (80.5%) | 54 (79.4%) | 1.00 | 150 (77.7%) | 102 (82.3%) | 0.39 |
| Concomitant medication (n (%)) | |||||||||
| Tobramycin | 32 (30.2%) | 15 (26.8%) | 0.72 | 23 (26.4%) | 15 (22.1%) | 0.58 | 55 (28.5%) | 30 (24.2%) | 0.44 |
| Vancomycin | 25 (23.6%) | 11 (19.6%) | 0.69 | 26 (29.9%) | 14 (20.6%) | 0.20 | 51 (26.4%) | 25 (20.2%) | 0.23 |
| Vincristine | 54 (50.9%) | 10 (17.9%) | 4.1 × 10−5 | 58 (66.7%) | 19 (27.9%) | 2.1 × 10−6 | 112 (58.0%) | 29 (23.4%) | 1.1 × 10−9 |
| Gentamicin | 21 (19.8%) | 7 (12.5%) | 0.28 | 21 (24.1%) | 19 (27.9%) | 0.71 | 42 (21.8%) | 26 (21.0%) | 0.89 |
| Tumor type (n (%)) | |||||||||
| Brain tumor | 25 (23.6%) | 8 (14.3%) | 0.22 | 26 (29.9%) | 11 (16.2%) | 0.058 | 51 (26.4%) | 19 (15.3%) | 0.026 |
| Endodermal sinus tumor of thymus | 0 | 1 (1.8%) | 0.35 | 0 | 1 (1.5%) | 0.44 | 0 | 2 (1.6%) | 0.15 |
| Germ-cell tumor | 7 (6.6%) | 15 (26.8%) | 0.00063 | 7 (8.0%) | 11 (16.2%) | 0.14 | 14 (7.3%) | 26 (21.0%) | 0.00046 |
| Hepatoblastoma | 22 (20.8%) | 5 (8.9%) | 0.075 | 17 (19.5%) | 7 (10.3%) | 0.12 | 39 (20.2%) | 12 (9.7%) | 0.013 |
| Lymphoma | 0 | 1 (1.8%) | 0.35 | 1 (1.1%) | 2 (2.9%) | 0.58 | 1 (0.5%) | 3 (2.4%) | 0.30 |
| Nasopharyngeal carcinoma | 1 (0.9%) | 0 | 1.00 | 0 | 2 (2.9%) | 0.19 | 1 (0.5%) | 2 (1.6%) | 0.56 |
| Neuroblastoma | 26 (24.5%) | 9 (16.1%) | 0.23 | 24 (27.6%) | 12 (17.6%) | 0.18 | 50 (25.9%) | 21 (16.9%) | 0.073 |
| Osteosarcoma | 24 (22.6%) | 16 (28.6%) | 0.45 | 10 (11.5%) | 20 (29.4%) | 0.0073 | 34 (17.6%) | 36 (29.0%) | 0.019 |
| Other sarcoma | 1 (0.9%) | 1 (1.8%) | 1.00 | 0 | 1 (1.5%) | 0.44 | 1 (0.5%) | 2 (1.6%) | 0.56 |
| Other carcinoma | 0 | 0 | 0 | 1 (1.5%) | 0.44 | 0 | 1 (0.8%) | 0.39 | |
| Retinoblastoma | 0 | 0 | 1 (1.1%) | 0 | 1.00 | 1 (0.5%) | 0 | 1.00 | |
| Mesenchymal tumor of the liver | 0 | 0 | 1 (1.1%) | 0 | 1.00 | 1 (0.5%) | 0 | 1.00 | |
| Follow-up, years (median (min, max)) | 3 (0, 18) | 2 (0, 15) | 0.10 | 5 (0, 25) | 2 (0, 16) | 0.00021 | 4 (0, 25) | 2 (0, 16) | 9.3 × 10−5 |
| Cranial irradiation (n (%)) | 23 (21.7%) | 7 (12.5%) | 0.20 | 20 (23.0%) | 8 (11.8%) | 0.093 | 43 (22.3%) | 15 (12.1%) | 0.025 |
For age, dose, treatment duration, and follow-up, the Wilcoxon–Mann–Whitney test with normal approximation was used. For gender, ethnicity, concomitant medication, tumor type, and cranial irradiation, the Fisher’s exact test was used. Boldface numbers are statistically significant values at P < 0.05 type I error rate.
Max, maximum; min, minimum.
Results from the initial combined cohort9 compared with the current replication cohort as well as all cohorts combined.
Caucasian ethnicity assessed by principal component analysis.
Genetic results
In the replication cohort, all genetic variants assessed in TPMT (rs12201199, rs1142345, and rs1800460) showed a significant association with cisplatin-induced hearing loss (Table 2), rs12201199 being the most strongly associated variant (P = 0.0013, odds ratio (OR) 6.1). The risk allele (A) was observed in 21 (12%) cases and 3 (2%) controls. The analysis of the combined cohort of all 317 patients showed a stronger association with rs12201199 (P = 8.7 × 10−7, OR 8.9) than in the initial cohort alone.9 The other TPMT variants, rs1142345 and rs1800460, also showed a stronger association with cisplatin-induced hearing loss in the combined cohort than in the initial cohort (Table 2). Moreover, in the combined cohort, all three TPMT variants remained significantly associated with cisplatin-induced hearing loss, after correcting for clinical factors including patient age, vincristine treatment, germ-cell tumor, and cranial irradiation.
Table 2.
Genetic variants associated with cisplatin-induced hearing loss
| SNP | Allele | Initial cohorta (n = 162)
|
P-value | Replication cohort (n = 155)
|
P-value | Combined cohort (n = 317)
|
P-value | P-valueb | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 106 cases, 56 controls
|
87 cases, 68 controls
|
193 cases, 124 controls
|
||||||||||||
| Case | Ctrl | OR (95% CI) | Case | Ctrl | OR (95% CI) | Case | Ctrl | OR (95% CI) | ||||||
| TPMT | ||||||||||||||
|
| ||||||||||||||
| rs12201199 | A | 28 | 1 | 16.9 (2.3–125.9) | 0.00022 | 21 | 3 | 6.1 (1.8–20.9) | 0.0013 | 49 | 4 | 8.9 (3.2–24.9) | 8.7 × 10−7 | 4.0 × 10−5 |
|
| ||||||||||||||
| T | 184 | 111 | 153 | 133 | 337 | 244 | ||||||||
|
| ||||||||||||||
| rs1142345 | G | 19 | 1 | 10.9 (1.4–82.7) | 0.0017 | 16 | 3 | 4.5 (1.3–15.7) | 0.011 | 35 | 4 | 6.1 (2.1–17.3) | 0.00014 | 0.00039 |
|
| ||||||||||||||
| A | 193 | 111 | 158 | 133 | 351 | 244 | ||||||||
|
| ||||||||||||||
| rs1800460 | A | 16 | 0 | 18.0 (1.1–302.7) | 0.0031 | 13 | 3 | 3.6 (1.0–12.8) | 0.038 | 29 | 3 | 6.6 (2.0–21.8) | 0.00043 | 0.00073 |
|
| ||||||||||||||
| G | 196 | 110 | 161 | 133 | 357 | 243 | ||||||||
|
| ||||||||||||||
| COMT | ||||||||||||||
|
| ||||||||||||||
| rs4646316 | G | 176 | 74 | 2.5 (1.5–4.3) | 0.00055 | 141 | 104 | 1.3 (0.8–2.3) | 0.33 | 317 | 178 | 1.8 (1.2–2.6) | 0.0021 | 0.0068 |
|
| ||||||||||||||
| A | 36 | 38 | 33 | 32 | 69 | 70 | ||||||||
|
| ||||||||||||||
| rs9332377 | A | 36 | 4 | 5.5 (1.9–16.0) | 0.00018 | 38 | 23 | 1.4 (0.8–2.4) | 0.28 | 74 | 27 | 1.9 (1.2–3.1) | 0.0054 | 0.043 |
|
| ||||||||||||||
| G | 176 | 108 | 136 | 113 | 312 | 221 | ||||||||
|
| ||||||||||||||
| ABCC3 | ||||||||||||||
|
| ||||||||||||||
| rs1051640 | G | 182 | 83 | 2.1 (1.2–3.8) | 0.0092 | 146 | 101 | 1.8 (1.0–3.3) | 0.036 | 328 | 184 | 2.0 (1.3–2.9) | 0.00078 | 0.0033 |
|
| ||||||||||||||
| A | 30 | 29 | 28 | 35 | 58 | 64 | ||||||||
Boldface numbers are results more significant in current combined cohort than in previous combined cohort (ref. 9).
CI, confidence interval; Ctrl, control; OR, odds ratio; SNP, single-nucleotide polymorphism.
Results from the initial combined cohort (ref. 9) compared with the current replication cohort as well as all cohorts combined.
Adjusted for age, vincristine treatment, germ-cell tumor, and cranial irradiation.
The protective “A” allele of COMT rs4646316 was observed in 33 (19%) cases and 32 (24%) controls in the replication cohort. However, the effect of this variant in the replication cohort, although in the same direction as that of the initial cohort, was smaller (OR 1.3) and not significantly associated with hearing loss (P = 0.33). A similar effect was seen for COMT rs9332377 (P = 0.28, OR 1.4).
Next, we assessed whether other genetic variants could be replicated, thereby improving the model in predicting the risk of cisplatin-induced hearing loss. Three of the six variants showed an effect in the same direction as the initial cohort (Table 2, Supplementary Table S1 online). One synonymous variant in ABCC3 (rs1051640) (E1503E) was significantly associated with cisplatin-induced ototoxicity in the current replication cohort (P = 0.036, OR 1.8), and showed a stronger association in the combined cohort (P = 7.8 × 10−4, OR 2.0; Table 2). The protective “A” allele of ABCC3 rs1051640 was observed in 28 (16%) cases and 35 (26%) controls in the replication cohort. The association of ABCC3 rs1051640 with cisplatin-induced hearing loss remained significant in the combined cohort after adjusting for clinical factors (P = 0.0033).
By principal component analysis, a majority (80%) of patients were found to have European genetic ancestry29 (Supplementary Figure S1 online). With the aim to reduce potential bias caused by population stratification, we assessed whether associations in the replication cohort remained significant in a more homogeneous subset of patients of only European ancestry (n = 124). For this subset, the findings were similar (Supplementary Table S2 online).
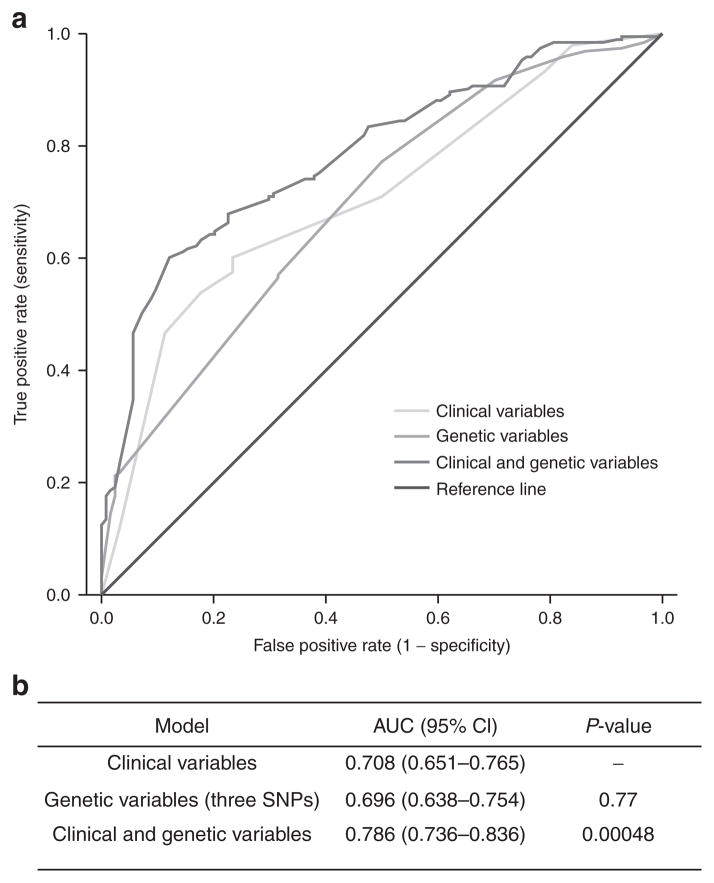
We generated a model that combines the effects of associated risk genotypes and clinical risk factors, and receiver operating characteristic analyses were performed. Three independent SNPs (TPMT rs12201199, ABCC3 rs1051640, and COMT rs4646316) were selected to construct a genetic-only model based on forward logistic regression. Although no significant association with variants in COMT in the current replication cohort was found, the combined cohort logistic regression retained COMT rs4646316 because it added significantly to the prediction model. The clinical-only model used the dichotomized variables age (median patient age cutoff: <8 years or >8 years), vincristine treatment, germ-cell tumor, and cranial irradiation. The combination of clinical and genetic variables significantly improved the prediction of cisplatin ototoxicity (Figure 1), yielding a higher area under the curve (AUC 0.786) than that of the clinical-only model (AUC 0.708, P = 0.00048).
Figure 1.
Receiver operating characteristic curves of clinical and genetic variables for the prediction of cisplatin-induced hearing loss in the combined cohort (n = 317). (a) Clinical variables are age, vincristine treatment, germ-cell tumor, and cranial irradiation, whereas genetic variables combine the effect of TPMT rs12201199, COMT rs4646316, and ABCC3 rs1051640. (b) The area under the curve for the combined cohort for each model. The P-values indicate the statistical significance between the curves for the combination of genetic and clinical variables as compared with clinical variables alone. CI, confidence interval; SNP, single-nucleotide polymorphism.
A predictive multimarker model based on these genetic variants could stratify patients by risk of ototoxicity. Using this model, we defined risk groups according to predictive values (PVs) as at lower (PV < 0.4), intermediate (0.4–0.8), and high (>0.8) risk. Accordingly, 30 (9.5%) individuals were classified as at lower risk, 245 (77.3%) at intermediate risk, and 42 (13.2%) at high risk (Table 3). In the high-risk group, 39 (92.9%) individuals developed ototoxicity (positive predictive value (PPV) 92.9%) as compared with 3 (7.1%) controls, conferring a specificity of 97.6% (Table 4). In the lower-risk group, 8 (26.7%) individuals developed ototoxicity (PPV 73.3%) as compared with 22 (73.3%) controls, conferring a sensitivity of 95.9% (Table 4).
Table 3.
Risk group comparisons and grade of hearing loss
| Patients with hearing loss (number (%a)) | Normal-hearing controls (number (%a)) | Total (number (%b)) | Grade of hearing loss (mean ± SEM) | |
|---|---|---|---|---|
| Lower risk (<0.4) | 8 (26.7%) | 22 (73.3%) | 30 (9.5%) | 0.77 ± 0.24 |
| Intermediate risk (0.4–0.8) | 146 (59.6%) | 99 (40.4%) | 245 (77.3%) | 1.69 ± 0.092 |
| High risk (>0.8) | 39 (92.7%) | 3 (7.1%) | 42 (13.2%) | 2.71 ± 0.13 |
Percentage in risk group.
Percentage of total.
Table 4.
Comparison of risk groups
| Model | Risk group | Ototox. (number, %) | Controls (number, %) | OR (95% CI)a | P-valuea | Sens | Spec | PPV | NPV |
|---|---|---|---|---|---|---|---|---|---|
| TPMT only | Risk haplotype carriersb | 43 (91.5%) | 4 (8.5%) | 9.3 (3.1, 27.4) | 5.5 × 10−5 | 22.3% | 96.8% | 91.5% | 44.4% |
| Non-carriers | 150 (55.6%) | 120 (44.4%) | |||||||
| TPMT, ABCC3, COMT | High (>0.8) | 39 (92.9%) | 3 (7.1%) | 11.0 (3.2, 37.6) | 1.3 × 10−4 | 20.2% | 97.6% | 92.9% | 44.0% |
| Low plus intermediate (<0.8) | 154 (56.0%) | 121 (44.0%) | |||||||
| TPMT, ABCC3, COMT | High plus intermediate(>0.4) | 185 (64.5%) | 102 (35.5%) | 4.8 (1.9, 11.9) | 7.1 × 10−4 | 95.9% | 17.7% | 64.5% | 73.3% |
| Low (<0.4) | 8 (26.7%) | 22 (73.3%) | |||||||
| Clinical + TPMT, ABCC3, COMT | High (>0.80) | 97 (91.5%) | 9 (8.5%) | 8.8 (4.0, 19.2) | 5.5 × 10−8 | 50.3% | 92.7% | 91.5% | 54.5% |
| Low plus intermediate (<0.8) | 96 (45.5%) | 115 (54.5%) | |||||||
| Clinical + TPMT, ABCC3, COMT | High plus intermediate(>0.45) | 161 (73.2%) | 59 (26.8%) | 3.1 (1.6, 5.9) | 4.3 × 10−4 | 83.4% | 52.4% | 73.2% | 67.0% |
| Low (<0.45) | 32 (33.0%) | 65 (67.0%) |
CI, confidence interval; NPV, negative predictive value; OR, odds ratio; Ototox, ototoxicity; PPV, positive predictive value; Sens, sensitivity; Spec, specificity.
Adjusted for age, vincristine treatment, germ-cell tumor, and cranial irradiation.
Risk haplotype carriers are individuals that are heterozygous or homozygous for TPMT rs12201199 risk variant.
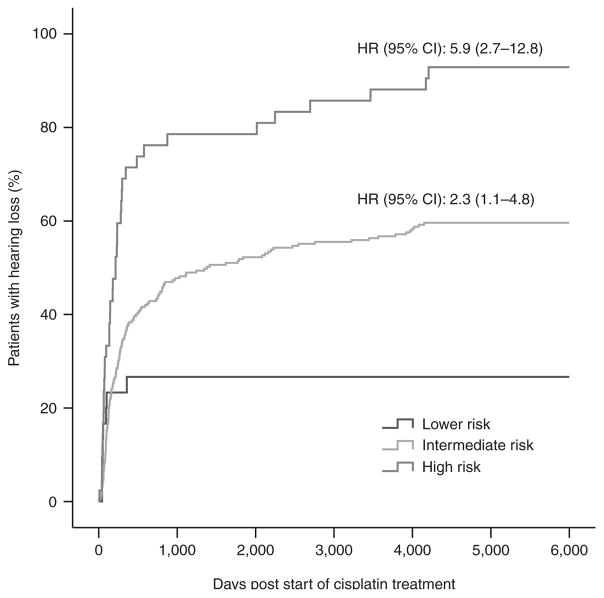
Individuals in the high-risk group also had a significantly higher risk of cisplatin-induced ototoxicity as compared with the lower- to intermediate-risk group (P = 1.3 × 10−4, OR 11.0; Table 4). Severity of hearing loss also increased with increasing risk-group status (Table 3). A Kaplan–Meier plot (Figure 2) shows that the intermediate- and high-risk groups have a significantly increased risk of hearing loss over time (Ptrend = 5.3 × 10−9).
Figure 2.
Kaplan–Meier curve of cisplatin-induced hearing loss in three different risk groups (Table 3) combining genetic factors (TPMT rs12201199, ABCC3 rs1051640, and COMT rs4646316). The curves show that the incidence of hearing loss increases with increasing risk group status. Hazard ratios (HRs) were used to compare curves with that of the lower risk group and were adjusted for clinical variables (Ptrend = 5.3 × 10−9). CI, confidence interval.
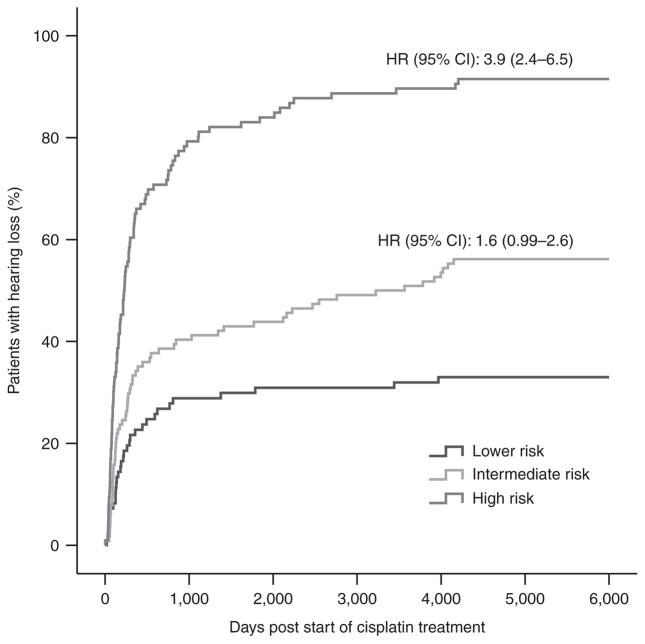
The model combining the effect of TPMT, ABCC3, and COMT was, however, able to distinguish patients as at lower or high risk better than the model based on TPMT alone (Table 4). Patients at lower risk could therefore be identified with more certainty if ABCC3 and COMT are included in the model. However, the ability to identify patients at high risk was similar between the TPMT-only model and the combined model, which includes TPMT, ABCC3, and COMT (PPV 91.5 vs. 92.9%) (Table 4). As illustrated by a Kaplan–Meier plot, we were able to identify 97 (50.3%) patients at high risk using a model combining both clinical and genetic variables, as compared with only 39 (20.2%) patients using a model based on genetics alone (Figure 3, Table 4). In the high-risk group, 97 (91.5%) individuals developed hearing loss (PPV 91.5%) as compared with 9 (8.5%) controls, conferring a specificity of 92.7% and a sensitivity of 50.3% (Table 4).
Figure 3.
Kaplan–Meier curve of cisplatin-induced hearing loss in three different risk groups (Table 3) combining clinical and genetic information. Clinical variables are age, vincristine treatment, germ-cell tumor, and cranial irradiation, whereas genetic variables combine the effect of TPMT rs12201199, COMT rs4646316, and ABCC3 rs1051640. The curves show that the incidence of hearing loss increases with increasing risk group status. Hazard ratios (HRs) were used to compare curves with that of the lower risk group and were adjusted for clinical variables (Ptrend = 3.4 × 10−19). CI, confidence interval.
DISCUSSION
The development of hearing loss after treatment with cisplatin leads to serious lifelong disability, particularly in children.2 A method to identify patients at high risk of developing serious cisplatin-induced hearing loss would significantly improve the safety of pediatric cancer therapy.
Recently, we identified genetic variants associated with cisplatin-induced hearing loss in children after cancer therapy.9 The replication of genetic associations for validation in independent populations is critical before development and clinical implementation of guidelines. In this replication study, we recruited a large independent cohort of pediatric patients from across Canada who received cisplatin therapy. We confirmed the associations of genetic variants in TPMT and ABCC3 with moderate to severe cisplatin-induced hearing loss. Genetic variants in COMT exhibited smaller effect sizes in the replication cohort (OR 2.5 vs. 1.3 and 5.5 vs. 1.4; previous vs. new cohorts). We generated a novel predictive model combining TPMT, ABCC3, and COMT genetic risk factors with clinical risk factors. The combined model was more predictive than a model based on clinical risk factors alone (AUC 0.786 vs. 0.708; P = 0.00048). The most highly associated COMT variant was included because it statistically significantly added to the predictive model with TPMT and ABCC3. The COMT and ABCC3 variants do not contribute to the prediction of patients at high risk of cisplatin-induced ototoxicity significantly; instead they stratify patients between intermediate and lower risk. The model was based on results from the combined cohort; further studies in children are needed to validate the model and assess its clinical utility.
Our study is the first to replicate the reported association between TPMT (rs12201199, rs1142345, and rs1800460) and cisplatin-induced hearing loss in a large independent cohort of pediatric patients treated for a variety of malignancies. There is evidence that patients who are heterozygous or homozygous for these TPMT variants have an increased risk of cisplatin-induced hearing loss, probably through reduced activity of the gene.9 The loss of function of TPMT is likely to increase the risk of cisplatin toxicity by inactivating the binding of the compound to purines in DNA, thereby regulating cisplatin cross-linking Another potential mechanism of cisplatin toxicity is the accumulation of S-adenosylmethionine due to reduced activity of TPMT.9,30–32
The association between the synonymous variant in ABCC3 rs1051640 (E1503E) and cisplatin-induced hearing loss was also significantly replicated. Patients who carried the G allele of rs1051640 were at increased risk of developing hearing loss after cisplatin treatment. This is the first study to describe the role of genetic variation in ABCC3 in the context of cisplatin ototoxicity. ABCC3 is a transporter that mediates the efflux of organic anions, xenobiotics, and glutathione S-conjugates.33,34 One of the mechanisms by which cancer chemotherapies, including platinum drugs, are detoxified is through conjugation of the active metabolite to glutathione, making the compound more anionic,35,36 thus enabling the compounds to be more readily exported from cells through an ATP-dependent pump. Studies in rat hepatocyte cell lines have shown that both ABCC2 and ABCC3 protein levels and mRNA expression increased after treatment with cisplatin.37,38 Studies in lung cancer cells lines have also shown that ABCC3 mRNA expression levels are significantly correlated with resistance to cisplatin and other platinum drugs.39,40 Reduction in ABCC3 activity can affect the detoxification pathway, resulting in ineffective transport of toxic compounds out of the cell, which leads to toxicity. In turn, polymorphisms may regulate ABCC3 levels or affect transporter function. Further studies are required to assess the exact mechanisms by which variants in ABCC3 are associated with cisplatin-induced hearing loss.
The allele frequencies observed in our cohort were consistent with reported population frequencies of mainly European and Asian descent. For example, the minor allele frequency of TPMT*3C ranges from 0.7 to 2.7% in Europeans and Asians, in line with our findings of 8.9% in cases and 1.6% in controls (HapMap). Furthermore, the minor allele frequency of rs1051640 ranges from 19% in the European population to 3.6% in Asian populations, in line with the observed 15% in cases and 26% in controls (HapMap).
Vincristine is a chemotherapy drug that is sometimes administered to patients receiving cisplatin. Whether vincristine itself is ototoxic is currently unclear. Case reports suggest that vincristine may be ototoxic20,21 or transiently ototoxic at high doses,14 whereas systematic clinical trials have reported that vincristine alone is not ototoxic.22,23 Bokemeyer et al. (1998) reported an increase in the prevalence of ototoxic symptoms in patients receiving vincristine together with cisplatin.14 To examine the effect of vincristine on the genetic associations with cisplatin-induced ototoxicity, binary logistic regression analyses were conducted with and without vincristine as a covariate. The regression analyses revealed that concomitant vincristine did not affect the associations of TPMT, COMT, and ABCC3 variants with cisplatin-induced ototoxicity (Supplementary Table S3 online).
Based on our a priori power calculations considering the effect sizes and genotype frequencies of the original studies, we had sufficient power for all polymorphisms to find similar effect sizes in the replication cohort. However, several SNPs previously reported to have an association could not be replicated, limiting the use of these variants in the risk prediction model. This is not surprising; many original studies have reported stronger genetic associations with larger effect sizes than in follow-up studies.41,42 There are several other potential reasons for nonreplication of initial findings. Significant interstudy heterogeneity can arise due to differences in the cohorts such as those related to population diversity, gender, and treatment protocols.41,42 This could lead to differences in the effects of variants in specific subpopulations or masking of the effects by factors that have not been sufficiently controlled for. We aimed to control for these factors by first evaluating power to ensure that the sample size is large enough to detect an association, then adjusting for clinical variables in the cohort, and finally carrying out a subgroup analysis in patients of only European ancestry. Nevertheless, a combination of these factors may explain why we could not significantly replicate the association between COMT, as well as other polymorphisms, and cisplatin-induced hearing loss. Another limitation of this study is the longer follow-up of cases than controls in the current replication cohort as compared with the initial cohort. This could introduce a potential source of bias but is likely to underestimate the positive results seen because rarely do controls have late adverse events of cisplatin therapy that were not uncovered during treatment or the 2-year period following treatment.
Combining the effect of TPMT, ABCC3, and COMT with clinical factors significantly improved the ability of the model to predict risk as compared with the model using clinical factors alone (AUC 0.786 vs. 0.708, P = 0.00048). By combining clinical risk factors and genetic markers, we could identify more patients at high risk as compared to using genetic factors alone (50.3% for combined vs. 20.2% for genetic alone). This is of particular importance in children, for whom even mild losses in hearing can cause difficulties in school performance.43,44 Several strategies have been proposed to prevent hearing loss in individuals at high risk of adverse effects from cisplatin. Patients might be placed on alternative medications that are less ototoxic (e.g., carboplatin, which is less ototoxic in older patients),16,45 receive increased monitoring for hearing loss, or be given otoprotective agents. However, the possibility of compromise of antitumor activity has raised concern.46,47 Hearing aids or cochlear implantation are also options used to manage cisplatin-induced hearing loss.
In conclusion, this study confirms previous findings and provides further evidence in an independent patient cohort for the associations of TPMT and ABCC3 with cisplatin-induced hearing loss and for the association of COMT with cisplatin-induced hearing loss in the combined cohort. The combination of TPMT, ABCC3, and COMT with clinical variables provides a novel method that promises to improve the risk prediction of hearing loss from cisplatin therapy. With the discovery of additional variants through genome-wide association studies, the current predictive model may be improved and refined further. The combination of clinical and genetic risk factors can potentially improve risk classifications for hearing loss and may allow for individualized treatment.
METHODS
Patients
Study participants were recruited through the CPNDS, a national multicenter surveillance consortium for studying adverse drug reactions.48 Between June 2008 and March 2011, a new independent replication cohort of 155 pediatric patients with cisplatin-induced hearing loss and drug-matched control patients were recruited. The previous cohort (discovery and replication) included in the combined analyses has been described previously.9
Cisplatin-induced hearing loss was diagnosed on the basis of audiometric findings using criteria described by the CTCAE v3 (Common Terminology Criteria for Adverse Events).49 To better differentiate between cases and controls, subjects with serious cisplatin-induced hearing loss were defined as with grade 2 or higher hearing impairment. Control patients were defined as those with normal audiometric data after cisplatin therapy (grade 0). Patients with grade 1 (n = 8) hearing loss were excluded from the analysis. Serious hearing impairment (grades 2–4 hearing loss) was defined as the point at which chemotherapy protocols recommend reducing or terminating cisplatin treatment. Only the most recent audiological assessment was used. Informed written consent or assent was obtained from each subject or his or her parents/legal guardians. The study was approved by the University of British Columbia/Children & Women’s Health Centre of British Columbia Research Ethics Board (H04-70358).
Genotyping
DNA of patients was extracted from blood, saliva, or buccal swabs using the QIAmp DNA purification system (Qiagen, Toronto, ON, Canada) according to the manufacturer’s protocol. We selected 11 SNPs to assess whether the prediction of risk of hearing loss could be improved. We selected genetic variants for genotyping on the basis of evidence of a significant association (P < 0.01) in the initial combined cohort9 as well as in either the initial discovery or replication cohorts.9 We first assessed whether associations with variants in TPMT (rs12201199, rs1800460, and rs1142345) and COMT (rs9332377 and rs4646316) could be replicated in the current replication cohort. We then assessed whether the associations with other variants in ABCC3 (rs1051640), MTHFR (rs3737964), VKORC1 (rs17884333 and rs8050894), and SLCO1A2 (rs4115170 and rs2306231) could be replicated. Patient DNA was genotyped using a custom Illumina GoldenGate SNP genotyping assay (Illumina, San Diego, CA). This assay included additional nonstudy SNPs for principal component analysis for the determination of patient ancestry and for quality-control purposes. All SNP genotype data were clustered manually using GenomeStudio software (Illumina, San Diego, CA). Samples with a call rate below 95% were excluded. The average genotyping call rate for included samples was 98.3%. The overall genotype call rate of the 11 study SNPs was 99.9% and all SNPs were in Hardy–Weinberg equilibrium (P > 0.05).
Statistical analysis
Clinical characteristics of patients with and without cisplatin-induced hearing loss were compared using the Wilcoxon–Mann–Whitney U-test for continuous variables and Fisher’s exact test for categorical variables. Hardy–Weinberg equilibrium was confirmed in cases and controls using the permutation version of the exact test of Hardy–Weinberg of Guo and Thompson.50
The study had a statistical power between 65 and 99% (mean 86%) based on the previous effect sizes and genotype frequencies, in order to replicate previously associated polymorphisms with cisplatin-induced hearing loss at P = 0.05. Two genetic variants in SLCO1A2 (rs11045913 and rs11045912) were excluded prior to genotyping due to inadequate power (<10%) in detecting an association. Homozygous and heterozygous odds ratios (ORs) were calculated using the homozygous genotype of the protective allele as identified in the initial cohort as a reference.9 ORs in the case of empty cells was calculated by adding 0.5 to the empty cells. The association between genetic polymorphisms and cisplatin-induced hearing loss was assessed by computing Fisher’s exact test for unadjusted (nonregression) P-values and adjusted P-values determined by logistic regression. Independent clinical factors for the prediction of cisplatin-induced hearing loss in the combined cohort were identified by forward logistic regression. The following factors were included: the first principal component, patient age, cisplatin dose, treatment duration, vincristine treatment, and germ-cell tumor. None of the variables was retained in the combined cohort at a threshold of 0.10 except concomitant vincristine treatment (P = 1.4 × 10−9). However, we also included variables that are known to increase risk of hearing loss and that were significantly different (P = 0.05) between cases and controls. Therefore, patient age, concomitant vincristine treatment, germ-cell tumor, and cranial irradiation were included as covariates. The treatment of all non-CNS germ-cell tumors is unique because these patients are treated at low individual doses of 20 mg/m2 over 5 days and are known to be at less risk of hearing loss.51 In an additional analysis, we combined the initial cohort9 and the current cisplatin replication cohort of patients to determine the overall significance of associations. Associations with variants were considered to be statistically significant if they had P < 0.05 in the new replication cohort.
Population stratification in the data set was assessed by principal component analysis using SVS/HelixTree software.29,52 Patients of African ancestry (n = 8) were excluded from the analysis to match the ancestry of the previous cohort.9,52 A graphical display of principal components was constructed using GraphPad Prism 5 software (GraphPad Software, San Diego, CA). A secondary stratified analysis of individuals of only European ancestry was based on the first two principal components.
Genetic variants were further evaluated in a multivariate logistic regression model including clinical variables using an additive model. A risk score was calculated by multiplying each variable with the estimated ß (log odds ratio) from the current combined cohort. The clinical-only model included dichotomized variables. Therefore, age (median patient age: <8 years or >8 years), vincristine treatment, germ-cell tumor, and cranial irradiation were included in the model. The genetic-only model included variants (TPMT rs12201199, ABCC3 rs1051640, and COMT rs4646316) that were selected based on statistical evidence from a forward logistic regression model in the combined cohort.
The association between genetic risk score and cisplatin-induced hearing loss was assessed using logistic regression. Three prediction models were investigated: (i) clinical variables only, (ii) genetic variables only, and (iii) clinical and genetic variables. The contribution of genetic and/or clinical risk scores to the prediction of cisplatin-induced hearing loss was investigated by comparing the area under the receiver operating characteristic curves of the prediction models. AUC estimates were obtained using the receiver operating characteristic plot function on the basis of the linear predictors obtained from the logistic regression model for clinical factors and genotypic scores. The statistical difference between the curves was calculated using DeLong’s method52 implemented in the R package pROC.53 Two-sided P-values < 0.05 were considered statistically significant.
Risk groups were defined on the basis of the PVs for each patient sample from the multi-SNP logistic regression model that included three SNPs (TPMT rs12201199, ABCC3 rs1051640, and COMT rs4646316). The threshold for genetic variables used to determine lower risk was the median PV of controls minus one standard deviation (PV < 0.4); high risk was defined as the median PV of cases plus one standard deviation (>0.8). Intermediate risk was defined as a PV between 0.4 and 0.8. The threshold for the combination of clinical and genetic variables for lower risk was defined as the median PV of controls (PV < 0.45), and high risk was defined as the median PV of cases (>0.8). Intermediate risk was defined as a PV between 0.45 and 0.8. We calculated sensitivity, specificity, PPV, and negative predictive value for each defined threshold. Kaplan–Meier curves were generated for each risk group using time from start of treatment to the time of first evidence of toxicity or last audiogram. Log-rank test was used to compare the trends of survival curves. Hazard ratios for each curve were calculated using the Cox regression model considering the lower risk group as the reference.
Statistical genetic analyses were performed using SNP and Variation Suite 7.4.5 (Golden Helix, Bozeman, MT), SPSS Statistics 19 (SPSS, Chicago, IL), and R 2.13.0 (R Development Core Team).
Supplementary Material
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Cisplatin is a widely used chemotherapeutic agent in the standard treatment of a variety of solid tumors. A serious complication of cisplatin treatment is permanent hearing loss. A previous study identified genetic variants in TPMT and COMT that were highly associated with cisplatin-induced hearing loss in children.
WHAT QUESTION DID THIS STUDY ADDRESS?
The aim of this study was to replicate previously described genetic factors and identify others that increase susceptibility to cisplatin-induced hearing loss, as well as to develop predictive models for the identification of patients at risk of toxicity.
WHAT THIS STUDY ADDS TO OUR KNOWLEDGE
This study strengthens the evidence of previous findings and provides evidence for novel genetic variants in the prediction of cisplatin-induced hearing loss in children. Furthermore, the study demonstrates that predictive models combining clinical and genetic factors can classify patients based on predicted risk of cisplatin-induced hearing loss.
HOW THIS MIGHT CHANGE CLINICAL PHARMACOLOGY AND THERAPEUTICS
The findings have the potential to influence treatment decisions toward improving safety and efficacy of cisplatin use in children.
Acknowledgments
We especially acknowledge the study participants and their families for their participation in the CPNDS project. We also acknowledge the support of the CPNDS adverse drug reaction surveillance network. We thank Michelle Higginson, Fudan Miao, and Catherine Carter for their assistance in genotyping. The CPNDS Consortium (participants are arranged geographically by institution across Canada): Vancouver—BC Children’s Hospital, Child & Family Research Institute, Centre for Molecular Medicine and Therapeutics, Pharmaceutical Outcomes and Policy Innovations: Michael Hayden, Bruce Carleton, Colin Ross, Stuart MacLeod, Beth Brooks, Anne Smith, Claudette Hildebrand, Reza Ghannadan, Rod Rassekh, Fudan Miao, Henk Visscher, Kusala Pussegoda, Michelle Higginson, Mojgan Yazdanpanah. Calgary—Alberta Children’s Hospital: Cheri Nijssen-Jordan, David Johnson, Linda Verbeek, Rick Kaczowka, Patti Stevenson, Andrea Hurton. Edmonton—Stollery Children’s Hospital: Paul Grundy, Kent Stobart, Bev Wilson, Sunil Desai, Maria Spavor, Linda Churcher, Terence Chow. Winnipeg—Winnipeg Children’s Hospital: Kevin Hall, Nick Honcharik, Sara Israels, Shanna Chan, Byron Garnham, Michelle Staub. London—London Health Sciences Centre: Michael Rieder, Becky Malkin. Hamilton—McMaster Children’s Hospital: Carol Portwine, Amy Cranston. Toronto—Hospital for Sick Children: Gideon Koren, Shinya Ito, Paul Nathan, Mark Greenberg, Facundo Garcia Bournissen, Miho Inoue, Sachi Sakaguchi, Toshihiro Tanaka, Hisaki Fujii, Mina Ogawa, Ryoko Ingram, Taro Kamiya, Smita Karande. Kingston—Kingston General Hospital: Mariana Silva, Stephanie Willing. Ottawa—Children’s Hospital of Eastern Ontario: Régis Vaillancourt, Pat Elliott-Miller, Donna Johnston, Herpreet Mankoo, Elaine Wong, Brenda Wilson, Lauren O’Connor. Ottawa—Health Canada: Maurica Maher. Montreal—Hospital Sainte-Justine: Jean-Francois Bussières, Denis Lebel, Pierre Barret, Aurélie Closon. Montreal—Montreal Heart Institute: Marie-Pierre Dubé, Yassamin Feroz Zada, Michael Phillips. Montreal—McGill University Health Centre, Montreal Children’s Hospital: Nada Jabado, Anelise Espirito Santo, Martine Nagy. Halifax—IWK Health Centre: Margaret Murray, Darlene Boliver, Marilyn Tiller, and Carol-anne Osborne. The study was supported as part of the peer-reviewed Genome Canada Applied Health Research Program, Canada Foundation for Innovation, Canadian Institutes of Health Research, Regional/National Clinical Research Initiatives, and Genome British Columbia Translational Program for Applied Health, and in part by the Child & Family Research Institute; Faculties of Pharmaceutical Sciences and Medicine, University of British Columbia; University of Western Ontario; Canada Gene Cure Foundation; Canadian Society of Clinical Pharmacology; C17 Research Network and Childhood Cancer Foundation-Candlelighters Canada; Canadian Paediatric Society, Merck Frosst; Eli Lilly; and Pfizer.
Footnotes
AUTHOR CONTRIBUTIONS
M.R.H., K.P., C.J.R., H.V., and B.C.C. wrote the manuscript. M.R.H., C.J.R., and B.C.C. designed the research. K.P., H.V., B.B., and S.R.R. performed the research. K.P., M.Y., Y.F.Z., and M.-P.D. analyzed data. M.R.H., C.J.R., and B.C.C. contributed new reagents/analytical tools.
CONFLICT OF INTEREST
The authors declared no conflict of interest.
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/cpt
References
- 1.Perilongo G, et al. Cisplatin versus cisplatin plus doxorubicin for standard-risk hepatoblastoma. N Engl J Med. 2009;361:1662–1670. doi: 10.1056/NEJMoa0810613. [DOI] [PubMed] [Google Scholar]
- 2.Kling J. US FDA contemplates collection of pharmacogenomic data. Nat Biotechnol. 2003;21:590. doi: 10.1038/nbt0603-590a. [DOI] [PubMed] [Google Scholar]
- 3.Li Y, Womer RB, Silber JH. Predicting cisplatin ototoxicity in children: the influence of age and the cumulative dose. Eur J Cancer. 2004;40:2445–2451. doi: 10.1016/j.ejca.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 4.Brock PR, Bellman SC, Yeomans EC, Pinkerton CR, Pritchard J. Cisplatin ototoxicity in children: a practical grading system. Med Pediatr Oncol. 1991;19:295–300. doi: 10.1002/mpo.2950190415. [DOI] [PubMed] [Google Scholar]
- 5.Blakley BW, Myers SF. Patterns of hearing loss resulting from cis-platinum therapy. Otolaryngol Head Neck Surg. 1993;109:385–391. doi: 10.1177/019459989310900302. [DOI] [PubMed] [Google Scholar]
- 6.Ilveskoski I, et al. Ototoxicity in children with malignant brain tumors treated with the “8 in 1” chemotherapy protocol. Med Pediatr Oncol. 1996;27:26–31. doi: 10.1002/(SICI)1096-911X(199607)27:1<26::AID-MPO6>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 7.Skinner R, Pearson AD, Amineddine HA, Mathias DB, Craft AW. Ototoxicity of cisplatinum in children and adolescents. Br J Cancer. 1990;61:927–931. doi: 10.1038/bjc.1990.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montaguti M, Brandolini C, Ferri GG, Hatzopoulos S, Prete A, Pession A. Cisplatin and carboplatin-induced ototoxicity in children: clinical aspects and perspectives for prevention. Acta Otorhinolaryngol Ital. 2002;22:14–18. [PubMed] [Google Scholar]
- 9.Ross CJ, et al. CPNDS Consortium. Genetic variants in TPMT and COMT are associated with hearing loss in children receiving cisplatin chemotherapy. Nat Genet. 2009;41:1345–1349. doi: 10.1038/ng.478. [DOI] [PubMed] [Google Scholar]
- 10.Ekborn A, Laurell G, Andersson A, Wallin I, Eksborg S, Ehrsson H. Cisplatin-induced hearing loss: influence of the mode of drug administration in the guinea pig. Hear Res. 2000;140:38–44. doi: 10.1016/s0378-5955(99)00190-2. [DOI] [PubMed] [Google Scholar]
- 11.Lewis MJ, DuBois SG, Fligor B, Li X, Goorin A, Grier HE. Ototoxicity in children treated for osteosarcoma. Pediatr Blood Cancer. 2009;52:387–391. doi: 10.1002/pbc.21875. [DOI] [PubMed] [Google Scholar]
- 12.Knight KR, Kraemer DF, Neuwelt EA. Ototoxicity in children receiving platinum chemotherapy: underestimating a commonly occurring toxicity that may influence academic and social development. J Clin Oncol. 2005;23:8588–8596. doi: 10.1200/JCO.2004.00.5355. [DOI] [PubMed] [Google Scholar]
- 13.Bertolini P, et al. Platinum compound-related ototoxicity in children: long-term follow-up reveals continuous worsening of hearing loss. J Pediatr Hematol Oncol. 2004;26:649–655. doi: 10.1097/01.mph.0000141348.62532.73. [DOI] [PubMed] [Google Scholar]
- 14.Bokemeyer C, et al. Analysis of risk factors for cisplatin-induced ototoxicity in patients with testicular cancer. Br J Cancer. 1998;77:1355–1362. doi: 10.1038/bjc.1998.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhandare N, Antonelli PJ, Morris CG, Malayapa RS, Mendenhall WM. Ototoxicity after radiotherapy for head and neck tumors. Int J Radiat Oncol Biol Phys. 2007;67:469–479. doi: 10.1016/j.ijrobp.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 16.Chang KW, Chinosornvatana N. Practical grading system for evaluating cisplatin ototoxicity in children. J Clin Oncol. 2010;28:1788–1795. doi: 10.1200/JCO.2009.24.4228. [DOI] [PubMed] [Google Scholar]
- 17.Pan CC, Eisbruch A, Lee JS, Snorrason RM, Ten Haken RK, Kileny PR. Prospective study of inner ear radiation dose and hearing loss in head-and-neck cancer patients. Int J Radiat Oncol Biol Phys. 2005;61:1393–1402. doi: 10.1016/j.ijrobp.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 18.Veenstra DL, Harris J, Gibson RL, Rosenfeld M, Burke W, Watts C. Pharmacogenomic testing to prevent aminoglycoside-induced hearing loss in cystic fibrosis patients: potential impact on clinical, patient, and economic outcomes. Genet Med. 2007;9:695–704. doi: 10.1097/gim.0b013e318156dd07. [DOI] [PubMed] [Google Scholar]
- 19.Waguespack JR, Ricci AJ. Aminoglycoside ototoxicity: permeant drugs cause permanent hair cell loss. J Physiol (Lond) 2005;567:359–360. doi: 10.1113/jphysiol.2005.094474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lugassy G, Shapira A. Sensorineural hearing loss associated with vincristine treatment. Blut. 1990;61:320–321. doi: 10.1007/BF01732887. [DOI] [PubMed] [Google Scholar]
- 21.Aydogdu I, Ozturan O, Kuku I, Kaya E, Sevinc A, Yildiz R. Bilateral transient hearing loss associated with vincristine therapy: case report. J Chemother. 2000;12:530–532. doi: 10.1179/joc.2000.12.6.530. [DOI] [PubMed] [Google Scholar]
- 22.Lugassy G, Shapira A. A prospective cohort study of the effect of vincristine on audition. Anticancer Drugs. 1996;7:525–526. doi: 10.1097/00001813-199607000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Riga M, et al. The effect of treatment with vincristine on transient evoked and distortion product otoacoustic emissions. Int J Pediatr Otorhinolaryngol. 2006;70:1003–1008. doi: 10.1016/j.ijporl.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 24.Wang L, McLeod HL, Weinshilboum RM. Genomics and drug response. N Engl J Med. 2011;364:1144–1153. doi: 10.1056/NEJMra1010600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oldenburg J, Kraggerud SM, Brydøy M, Cvancarova M, Lothe RA, Fossa SD. Association between long-term neuro-toxicities in testicular cancer survivors and polymorphisms in glutathione-s-transferase-P1 and -M1, a retrospective cross sectional study. J Transl Med. 2007;5:70. doi: 10.1186/1479-5876-5-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riedemann L, et al. Megalin genetic polymorphisms and individual sensitivity to the ototoxic effect of cisplatin. Pharmacogenomics J. 2008;8:23–28. doi: 10.1038/sj.tpj.6500455. [DOI] [PubMed] [Google Scholar]
- 27.Hirschhorn JN, Altshuler D. Once and again-issues surrounding replication in genetic association studies. J Clin Endocrinol Metab. 2002;87:4438–4441. doi: 10.1210/jc.2002-021329. [DOI] [PubMed] [Google Scholar]
- 28.Ross CJ, et al. Corrigendum: genetic variants in TPMT and COMT are associated with hearing loss in children receiving cisplatin chemotherapy. Nat Genet. 2013;45:578. doi: 10.1038/ng.478. [DOI] [PubMed] [Google Scholar]
- 29.Visscher H, et al. Application of principal component analysis to pharmacogenomic studies in Canada. Pharmacogenomics J. 2009;9:362–372. doi: 10.1038/tpj.2009.36. [DOI] [PubMed] [Google Scholar]
- 30.Weinshilboum RM. Pharmacogenomics: catechol O-methyltransferase to thiopurine S-methyltransferase. Cell Mol Neurobiol. 2006;26:539–561. doi: 10.1007/s10571-006-9095-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weinshilboum RM, Sladek SL. Mercaptopurine pharmacogenetics: monogenic inheritance of erythrocyte thiopurine methyltransferase activity. Am J Hum Genet. 1980;32:651–662. [PMC free article] [PubMed] [Google Scholar]
- 32.Ochoa B, Bobadilla N, Arrellín G, Herrera LA. S-Adenosyl-L-methionine increases serum BUN and creatinine in cisplatin-treated mice. Arch Med Res. 2009;40:54–58. doi: 10.1016/j.arcmed.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 33.Ballatori N, Hammond CL, Cunningham JB, Krance SM, Marchan R. Molecular mechanisms of reduced glutathione transport: role of the MRP/CFTR/ABCC and OATP/SLC21A families of membrane proteins. Toxicol Appl Pharmacol. 2005;204:238–255. doi: 10.1016/j.taap.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 34.Lee YM, et al. Identification and functional characterization of the natural variant MRP3-Arg1297His of human multidrug resistance protein 3 (MRP3/ABCC3) Pharmacogenetics. 2004;14:213–223. doi: 10.1097/00008571-200404000-00001. [DOI] [PubMed] [Google Scholar]
- 35.Yang P, Ebbert JO, Sun Z, Weinshilboum RM. Role of the glutathione metabolic pathway in lung cancer treatment and prognosis: a review. J Clin Oncol. 2006;24:1761–1769. doi: 10.1200/JCO.2005.02.7110. [DOI] [PubMed] [Google Scholar]
- 36.Leitner HM, Kachadourian R, Day BJ. Harnessing drug resistance: using ABC transporter proteins to target cancer cells. Biochem Pharmacol. 2007;74:1677–1685. doi: 10.1016/j.bcp.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schrenk D, Baus PR, Ermel N, Klein C, Vorderstemann B, Kauffmann HM. Up-regulation of transporters of the MRP family by drugs and toxins. Toxicol Lett. 2001;120:51–57. doi: 10.1016/s0378-4274(01)00306-x. [DOI] [PubMed] [Google Scholar]
- 38.Young LC, Campling BG, Cole SP, Deeley RG, Gerlach JH. Multidrug resistance proteins MRP3, MRP1, and MRP2 in lung cancer: correlation of protein levels with drug response and messenger RNA levels. Clin Cancer Res. 2001;7:1798–1804. [PubMed] [Google Scholar]
- 39.Young LC, Campling BG, Voskoglou-Nomikos T, Cole SP, Deeley RG, Gerlach JH. Expression of multidrug resistance protein-related genes in lung cancer: correlation with drug response. Clin Cancer Res. 1999;5:673–680. [PubMed] [Google Scholar]
- 40.Oguri T, Isobe T, Fujitaka K, Ishikawa N, Kohno N. Association between expression of the MRP3 gene and exposure to platinum drugs in lung cancer. Int J Cancer. 2001;93:584–589. doi: 10.1002/ijc.1369. [DOI] [PubMed] [Google Scholar]
- 41.Chanock SJ, et al. Replicating genotype–phenotype associations. Nature. 2007;447:655–660. doi: 10.1038/447655a. [DOI] [PubMed] [Google Scholar]
- 42.Ioannidis JP, Ntzani EE, Trikalinos TA, Contopoulos-Ioannidis DG. Replication validity of genetic association studies. Nat Genet. 2001;29:306–309. doi: 10.1038/ng749. [DOI] [PubMed] [Google Scholar]
- 43.Blair JC, Peterson ME, Viehweg SH. The effects of mild sensorineural hearing loss on academic performance of young school age children. Volta Rev. 2001;87:87–93. [Google Scholar]
- 44.Bess FH, Dodd-Murphy J, Parker RA. Children with minimal sensorineural hearing loss: prevalence, educational performance, and functional status. Ear Hear. 1998;19:339–354. doi: 10.1097/00003446-199810000-00001. [DOI] [PubMed] [Google Scholar]
- 45.Qaddoumi I, et al. Carboplatin-associated ototoxicity in children with retinoblastoma. J Clin Oncol. 2012;30:1034–1041. doi: 10.1200/JCO.2011.36.9744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Orgel E, et al. Hearing loss among survivors of childhood brain tumors treated with an irradiation-sparing approach. Pediatr Blood Cancer. 2012;58:953–958. doi: 10.1002/pbc.23275. [DOI] [PubMed] [Google Scholar]
- 47.Muldoon LL, et al. Delayed administration of sodium thiosulfate in animal models reduces platinum ototoxicity without reduction of antitumor activity. Clin Cancer Res. 2000;6:309–315. [PubMed] [Google Scholar]
- 48.Ross CJ, Carleton B, Warn DG, Stenton SB, Rassekh SR, Hayden MR. Genotypic approaches to therapy in children: a national active surveillance network (GATC) to study the pharmacogenomics of severe adverse drug reactions in children. Ann N Y Acad Sci. 2007;1110:177–192. doi: 10.1196/annals.1423.020. [DOI] [PubMed] [Google Scholar]
- 49.Cancer Therapy Evalution Program—Common Terminology Criteria for Adverse Events–Version 3. 2003 < http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf>.
- 50.Guo SW, Thompson EA. Performing the exact test of Hardy-Weinberg proportion for multiple alleles. Biometrics. 1992;48:361–372. [PubMed] [Google Scholar]
- 51.Afzal S, et al. Challenges in management of patients with intracranial germ cell tumor and diabetes insipidus treated with cisplatin and/or ifosfamide based chemotherapy. J Neurooncol. 2010;97:393–399. doi: 10.1007/s11060-009-0033-z. [DOI] [PubMed] [Google Scholar]
- 52.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 53.Robin X, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77. doi: 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.