Abstract
In a quantitative meta-analysis, using the activation likelihood estimation method, we examined the neural regions involved in bilingual cognitive control, particularly when engaging in switching between languages. The purpose of this study was to evaluate the bilingual cognitive control model based on a qualitative analysis [Abutalebi, J., & Green, D. W. (2008). Control mechanisms in bilingual language production: Neural evidence from language switching studies. Language and Cognitive Processes, 23, 557–582.]. After reviewing 128 peer-reviewed articles, ten neuroimaging studies met our inclusion criteria and in each study, bilinguals switched between languages in response to cues. We isolated regions involved in voluntary language switching, by including reported contrasts between the switching conditions and high level baseline conditions involving similar tasks but requiring the use of only one language. Eight brain regions showed significant and reliable activation: left inferior frontal gyrus, left middle temporal gyrus, left middle frontal gyrus, right precentral gyrus, right superior temporal gyrus, midline pre-SMA and bilateral caudate nuclei. This quantitative result is consistent with bilingual aphasia studies that report switching deficits associated with lesions to the caudate nuclei or prefrontal cortex. It also extends the previously reported qualitative model. We discuss the implications of the findings for accounts of bilingual cognitive control.
Keywords: bilingualism, meta-analysis, functional neuroimaging, cognitive control, language switching
Introduction
Bilingual language processing has been studied primarily at the behavioral level. Recent neuroimaging techniques allow researchers to investigate the neural correlates of bilingual language processing underlying these behavioral findings. Most of the neuroimaging literature on bilingual language processing has focused on identifying the common and unique brain regions responsible for processing the first (L1) and second language (L2), with a smaller number of studies specifically examining brain regions responsible for language switching, a key aspect of language control in bilingual speakers. The sample sizes in these latter studies were relatively small and participants typically spoke specific pairs of languages (e.g., Spanish-French or German-English), limiting any generalization regarding the regions involved in language switching. Meta-analysis of neuroimaging studies provides a method for overcoming this limitation by capturing the commonality across studies while minimizing sampling bias within individual studies.
Abutalebi and Green (2008) proposed a neurocognitive model of bilingual language switching based on a qualitative review of published neuroimaging studies involving either switching between two languages or translation. The model consisted of five brain regions considered to be crucial for bilingual language switching: left dorsolateral prefrontal cortex (DLPFC), anterior cingulate cortex (ACC), caudate nucleus and bilateral supramarginal gyri (SMG). They proposed that this subcortical-cortical circuit sustains the intensive cognitive demand of managing two languages, such as facilitating the selection of the appropriate language while suppressing the irrelevant language and monitoring language use.
Abutalebi and Green (2008) proposed that the regions involved in language switching are also involved in cognitive control or executive functions more generally. Of particular relevance, left DLPFC and bilateral SMG (part of the inferior parietal lobule) are part of a proposed fronto-parietal network of attention (Toro, Fox & Paus, 2008). The other two regions in the bilingual network, namely the anterior cingulate cortex (ACC) and caudate nucleus, have also been implicated in conditions that require cognitive control (van Schouwenburg, den Ouden & Cools, 2010; Westlye, Grydeland, Walhovd & Fjell, 2010; Kerns et al., 2004). For example, the ACC is typically associated with error detection (e.g., Ide & Li, 2010) and was included as part of a “salience network” that is thought to allocate neural resources to internal processing or external stimulation in order to guide behavior (Seeley, Menon, Schatzberg, et al., 2007). The caudate nucleus has been implicated in studies examining motor response control (Boehler, Appelbaum, Krebs, Hopf & Woldorff, 2010) and goal-directed behavior (Grahn, Parkinson & Owen, 2008). It also plays a role in mediating cortical activation in the ACC and prefrontal regions to enhance switching the focus of attention between stimulus representations (Hedden & Gabrieli, 2010). Thus, the brain areas in the bilingual language control network proposed by Abutalebi and Green (2008) are similar to those implicated in other forms of higher cognitive function, supporting the notion that bilingual language control, especially language switching, is a demanding task sharing features with other types of cognitive control.
Studies of bilingual patients with lesions to one of the regions in the proposed bilingual control network provide further support for the model. For instance, a recent review of the effects of lesion size and location on the recovery patterns of bilingual patients with aphasia (Green and Abutalebi, 2008) suggested that lesions in the left caudate nucleus lead to an impairment in language control, resulting in problems with language switching or language mixing. Accordingly, activation of the caudate nucleus would be most evident in situations that require manipulating two languages.
The purpose of the present meta-analysis was to evaluate the bilingual cognitive control network proposed by Abutalebi and Green (2008) using a quantitative approach. We aimed to identify brain regions that showed common functional activity in response to the cognitive control demand involved in bilingual language switching. Ten neuroimaging studies examining bilingual language processing were included, all of which had experimental conditions that required language switching (Table 1A). Moreover, all had high level baseline conditions similar to the experimental condition, but without language switching or translation (Table 1B). Coordinates of brain regions showing significant activation in the contrast between the experimental and high level baseline conditions were analyzed using the Activation Likelihood Estimation (ALE) method (Eickhoff et al., 2009; Turkeltaub et al., 2002).
Table 1A.
List of studies contributing data to the meta-analysis
| Expt. | First Author | Year | Sample size | Age | L2A onset age | L1 | L2 | Number of Foci |
|---|---|---|---|---|---|---|---|---|
| 1 | Abutalebi | 2008 | 12 | 25.4 | 11.6 | German | French | 16 |
| 2 | Abutalebi | 2007 | 12 | 30.2 | 3 | Italian | French | 16 |
| 3 | Hernandez | 2001 | 6 | 21.7 | 5 | Spanish | English | 1 |
| 4 | Lehtonen | 2005 | 11 | 31.8 | 26.7 | Finnish | Norwegian | 2 |
| 5 | Price | 1999 | 6 | 30.5 | 8.8 | German | English | 20 |
| 6 | Wang | 2007 | 12 | 19.5 | 12.7 | Mandarin | English | 5 |
| 7 | Hernandez | 2009 | 12 | 21.4 | 5 | Spanish | English | 4 |
| 8 | van Heuven | 2008 | 12 | 24.1 | 11.2 | Dutch | English | 27 |
| 9 | Rinne | 2000 | 8 | 32–56 | N/A | Finnish | English | 6 |
| 10 | Wang | 2009 | 15 | 19–23 | 12.1 | Mandarin | English | 7 |
Table 1B.
Tasks used in the studies.
| Expt. | Experimental task | Contrast baseline task |
|---|---|---|
| 1 | Overt picture naming in bilingual context based on cues | Overt L1 naming |
| 2 | Passively listening to narratives alternating in two languages | Passive listening to one language |
| 3 | Picture naming in alternating languages | Picture naming in one language |
| 4 | Decide whether probes were translated correctly | Decide whether same sentences were presented |
| 5 | Covert translation of words | Covert word reading |
| 6 | Covert picture naming according to language switching cues | Covert picture naming in single language trials |
| 7 | Covert picture naming according to language switching cues | Covert picture naming in single language trials |
| 8 | Decide whether stimuli were English words (English-Dutch cognates) | Same task with English control words |
| 9 | Simultaneous translation | Simultaneous repeat of Finnish or English text |
| 10 | Digit naming based on language cues | Digit naming in one language |
Method
Neuroimaging studies using fMRI or PET involving bilinguals were selected using a systematic search on PubMed. Only peer-reviewed journal articles published in English were included. The search terms included were “humans” <AND> “bilingual” <OR> “bilinguals” <OR> “bilingualism” <AND> “neuroimaging” <OR> “MRI” <OR> “magnetic resonance imaging” <OR> “PET” <OR> “positron emission tomography” <NOT> “aphasia” <OR> “aphasic”. This systematic search resulted in 128 studies. Excluding review articles, studies involving event-related potentials (ERP), bimodal bilinguals (sign language and English) or only patients (i.e., no control group), a total of 63 studies remained. Of these studies, 18 of them involved an experimental condition in which bilinguals had to use both languages to engage in language switching. The tasks involved in these conditions were picture naming, passive listening, silent translation, semantic decision and digit naming. In order to obtain coordinates for activated brain regions specifically responsible during language switching, we chose those studies that included high-level baseline tasks that were similar to those in the experimental condition, but only required processing in one language. Three studies did not include a high-level baseline and were excluded (Nelson, et al., 2009; Rodriguez-Fornells, et al., 2002, 2005). Furthermore, four studies that only reported region of interest (ROI) analysis and one study that did not report the full coordinates were also excluded (Chee et al., 2003; Crinion et al., 2006; Hernandez et al., 2000; Illes et al., 1999 and Klein et al., 2006). The final sample included 10 studies with a total of 104 foci.
Participants and task descriptions
Demographic information of the bilingual participants reported in these studies is presented in Table 1A and a description of the experimental and high-level baseline conditions in Table 1B.
The combined sample of 106 bilinguals from the ten studies was composed of mostly young adults with the mean age of 25.6 years (SD = 4.7, excluding the two studies reporting only the age ranges of the bilinguals, and that of Rinne (2000) who recruited adults aged 32 to 56). Although the sample involved both early and late bilinguals, with an average age of second language acquisition of around 10 years (SD = 6.9), and varying levels of second language proficiency, all reported active and regular use of both languages since the acquisition of their second language. Luk, De Sa and Bialystok (in press) have shown that onset age of active bilingualism, but not age of second language acquisition, negatively correlated with cognitive control performance. Therefore, we combined the early and late bilinguals here as defined by their ages of second language acquisition in this analysis as none of the studies reported participants’ onset age of active bilingualism. All studies, other than those of Abutalebi et al. (2007) and Rinne et al. (2000), required the processing of visual stimuli.
Data analyses
The data entered into the analysis consisted of reported coordinates for the contrast between a language switching experimental condition and a high-level baseline condition involving single language processing. Coordinates reported in Montreal Neurological Institute (MNI) space and Talairach space were recorded separately. Then, the MNI coordinates were converted to Talairach space for subsequent analysis by the built-in transformation algorithm in BrainMap GingerALE 2.0.4 (Lancaster, Tordesillas-Gutierrez, Martinez, Salinas, Evans, Zilles, Mazziotta, & Fox, 2007). BrainMap GingerALE 2.0.4, initially developed by Turkeltaub et al. (2002), was later modified and improved as reported in Laird et al. (2005) and Eickhoff et al. (2009). The ALE random-effect method treats the coordinates for each reported cluster maximum as the central focal point of a spatial probability distribution. Convergence of activated foci is determined by computing ALE values constructed to reveal the activation probabilities of each voxel. For our analysis, we used the more conservative non-additive ALE method. This method limits the bias of resulting ALE values as a result of studies reporting multiple foci within close proximity. Furthermore, this non-additive algorithm results in cluster extents that are smaller than those from the original algorithm, allowing more precise localization of clusters (Turkeltaub, Eickhoff, Laird, Fox, Wiener & Fox, in press). Significance of convergence across studies was determined by a permutation test comparing the ALE maps against a null distribution determined empirically to model spatial uncertainty (Eickhoff et al., 2009). The resulting products were p-values for each voxel, which were then thresholded at P < .01, corrected for false discovery rate (FDR) to control for multiple comparison (Genovese, Lazar & Nichols, 2002). Furthermore, a minimum cluster size of 100 mm3 was applied to the thresholded ALE map to create the final output. Anatomical labels were assigned to significant clusters identified in the final ALE map according to the Talairach Daemon data labels included in GingerALE 2.0.4 (Lancaster et al., 2000). All the results are reported in Talairach space (Talairach & Tournoux, 1988). The thresholded ALE maps were overlaid onto the anatomical image in Talairach space distributed by the BrainMap GingerALE 2.0.4.
Results
In total, ten distinct clusters were identified using a false discovery rate (FDR) of P = .01. The anatomical labels of the clusters and their corresponding Talairach coordinates and volumes are presented in Table 2.
Table 2.
Clusters resulting from the ALE analysis listed in the order of ALE values for FDR = .01.
| Cluster | Talairach Coordinates | ALE value | Volume (mm3) | Contributing studiesa | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| a. Left middle frontal gyrus (BA 46) | −46 | 18 | 26 | 0.01358 | 528 | 1, 2, 9 |
| b. Midline pre-SMA (BA 6) | 2 | 8 | 58 | 0.01131 | 488 | 1, 8, 10 |
| c. Left inferior frontal gyrus (BA 47) | −32 | 20 | −8 | 0.01173 | 304 | 4, 5 |
| d. Right precentral gyrus (BA 6) | 44 | −4 | 30 | 0.01220 | 272 | 1, 7 |
| e. Right caudate | 16 | 8 | 12 | 0.01177 | 224 | 1, 5 |
| f. Left middle temporal gyrus (BA 37) | −50 | −44 | −6 | 0.01078 | 160 | 8, 10 |
| g. Left inferior frontal gyrus (BA 44) | −50 | 18 | 6 | 0.00996 | 216 | 1, 2 |
Number corresponds to the Experiment ID in Table 1A.
The clusters showing reliable activation with a volume greater than 100 mm3 were: left middle frontal gyrus (BA 9, 46), midline pre-supplementary motor area (BA 6), left inferior frontal gyrus (BA 44 and 47), left middle temporal gyrus (BA 37), right superior temporal gyrus (BA 22), right precentral gyrus (BA 6) and bilateral caudate (Figure 1).
Figure 1.
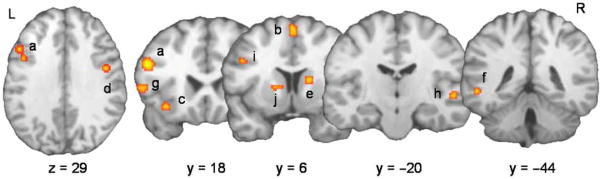
Clusters reliably activated at FDR with p = 0.01. The letters correspond to the clusters identified in Table 2.
The clusters were mostly left lateralized and concentrated in the frontal area. The only regions identified by the meta-analysis in common with the previous model (Abutalebi and Green, 2008) were the caudate and left prefrontal cortex. The ACC and bilateral SMG proposed in the theoretical model were not identified in the meta-analysis.
Discussion
In the present quantitative meta-analysis, we aimed to identify the neural correlates of bilingual cognitive control in language switching using the ALE method. The regions identified in the meta-analysis partially overlapped with the qualitative model reported by Abutalebi and Green (2008). Notably, the brain regions active across studies during bilingual language switching were largely left lateralized, and six of the ten were in the frontal regions. In addition, subcortical regions, namely the bilateral caudate, survived the stringent FDR correction. This finding is consistent with the argument that the frontal-subcortical circuit involving the caudate is critical for language control (Green & Abutalebi, 2008), suggesting there is no single brain region specific to bilingual language switching. Instead, activation in multiple brain regions, both at the cortical and subcortical level, is responsible for bilingual language switching. It is also in line with research showing that bilingual patients with aphasia who had lesions in subcortical brain regions exhibit deficits in language switching.
In contrast to the earlier model, the meta-analysis results showed significant activation likelihood in the midline pre-SMA rather than the ACC. Additional clusters of activation were identified in the left middle temporal gyrus and in the right precentral gyrus. No bilateral SMG activation was identified. We consider these differences between our results and the previous bilingual cognitive control model in turn.
The lack of a significant likelihood of activation in bilateral SMG may reflect the nature of the baseline tasks. The majority of the experimental conditions in the studies required phonological processing (such as translation and picture naming), and these processes were also involved in the high-level baseline tasks. Therefore, bilateral SMG may participate in general language processing in bilinguals, but not be differentially activated during switching.
The lack of a significant likelihood of activation in ACC is more surprising and may also reflect response to the high-level baseline tasks. ACC plays a role in error monitoring and detection, showing less activity during correct trials and more activity during error trials (Velanova, Wheeler & Luna, 2008). Since all the coordinates included in the analysis were derived from contrasts between language switching and high-level baseline conditions, recruitment of the error monitoring processes of the ACC might have occurred in both conditions. In addition, variations across studies in how participants adjust to performance challenges may explain the absence of any overall effect. Indeed, when ACC foci were reported in some of the studies included in our analysis (Abutalebi et al., 2008; van Heuven et al., 2008) these were widely distributed along the extension of the ACC. This variability in location would result in the absence of any common detectable activation focus in the ACC using ALE.
Although our analysis detected no ACC activation, it did detect significant activation in midline pre-SMA, which has been increasingly recognized, along with the dorsal ACC, in the performance of demanding tasks in terms of response control, performance monitoring, error detection, feedback, and related processes (Bush et al., 2000; Hester et al., 2005; Nachev et al., 2008). The pre-SMA is sometimes combined with dorsal ACC to form a region known as the Rostral Cingulate Zone (Ridderinkhof, et al., 2004a, 2004b). Our results suggest that it is this more superior part of the Rostral Cingulate Zone (i.e., falling in the pre-SMA area) that is more consistently found in studies investigating language switching. This argues for a role of the pre-SMA in initiating and executing speech production (for a review, see Price, 2010) especially under conditions of language conflict (Liu, Hu, Guo & Peng, 2010).
Alternatively, and more generally, the pre-SMA may participate in a control trade-off with the ACC. Recently, Hikosaka and Isoda (2010) proposed that the ACC operates retroactively to control switching performance while the pre-SMA acts proactively. Although, there is no direct evidence for this distinction in language production, Kuipers and Thierry (2010) have shown that bilinguals detect language change (English vs. Welsh) as early as 200ms after the onset of word presentation (compared to around 400ms for monolinguals who perceived the Welsh words as meaningless). Early activation in response to a cue may then be sufficient to trigger proactive control and so elicit pre-SMA rather than ACC, activation. Further research could examine whether cognitive control involved in bilingual language switching involves proactive or retroactive control by means of neuroimaging methodologies that allow high temporal and spatial resolution.
The meta-analysis identified three regions, right precentral gyrus and bilateral temporal gyri, that were not in the previous model. A recent study by Nakamura et al. (2010) suggests the temporal region is subject to top-down control by the left inferior frontal cortex during language switching. Activation in precentral gyrus may relate to switching between two sets of motor preparatory acts for picture naming. While the left temporal activation relates to general language processing, activation in the right temporal gyrus may relate to attentional demand required in language processing (Sabri, Binder, Deasi, Medler, Leitl & Liebenthal, 2008). However, the contribution of these regions’ activation in bilingual language switching is yet to be determined. Future functional neuroimaging studies adopting a network analysis approach may reveal the functional connectivity between these regions and other frontal regions identified in this analysis.
In conclusion, our selection criteria admitted a small set of studies that unanimously reported contrasts between experimental conditions involving switching between L1 and L2 and high-level baseline conditions, allowing the examination of brain areas engaged in bilingual language switching. These regions overlap with those identified in other studies of cognitive control and executive functions (see Introduction). We acknowledge the small number of studies and the heterogeneity of tasks involved in these studies. Despite these factors, the meta-analysis of these data yielded robust results even at a conservative level of FDR and provided crucial evidence that the cognitive control of language switching involves a distributed set of brain regions. The regions identified overlapped with those specified in the bilingual control model (Abutalebi & Green, 2008). Importantly, bilateral caudate and the left prefrontal regions were robustly observed, supporting the frontal-subcortical circuit for bilingual language control. The meta-analysis extended this model to other brain regions shown to be crucial to cognitive control and to other regions implicated in lexical and semantic processing that are the likely targets of language control. Interestingly, the brain regions reported in the present meta-analysis have also been reported in other studies examining neural correlates of non-language cognitive control, implying that bilingual language switching involves high level cognitive processes that are not specific to language processing. Future studies investigating bilingual processing should adopt a network approach (Abutalebi, Rosa, Tettamanti, Green & Cappa, 2009; Luk, Anderson, Craik, Grady & Bialystok, 2010; Xiang, Fonteijn, Norris & Hagoort, 2010) to identify functional connectivity among regions showing activation to further explore the networks underlying language control. In addition, examination of white matter connectivity between brain regions found in this meta-analysis would shed light on how structural connections support the control function of these brain regions.
Acknowledgments
This work was supported by Canadian Institutes of Health Research grant to C.L.G. (MOP14036) and by the Canada Research Chairs program, the Ontario Research Fund, and the Canadian Foundation for Innovation. D.G. was supported by the Wellcome Trust.
References
- Abutalebi J, Annoni JM, Zimine I, Pegna AJ, Seghier ML, et al. Language control and lexical competition in bilinguals: An event-related fMRI study. Cerebral Cortex. 2008;18:1496–1505. doi: 10.1093/cercor/bhm182. [DOI] [PubMed] [Google Scholar]
- Abutalebi J, Brambati SM, Annoni JM, Moro A, Cappa SF, Perani D. The neural cost of the auditory perception of language switches: An event-related functional magnetic resonance imaging study in bilinguals. The Journal of Neuroscience. 2007;27:13762–13769. doi: 10.1523/JNEUROSCI.3294-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abutalebi J, Green DW. Control mechanisms in bilingual language production: Neural evidence from language switching studies. Language and Cognitive Processes. 2008;23:557–582. [Google Scholar]
- Abutalebi J, Rosa PA, Tettamanti M, Green DW, Cappa SF. Bilingual aphasia and language control: A follow-up fMRI and intrinsic connectivity study. Brain and Language. 2009;109:141–156. doi: 10.1016/j.bandl.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Boehler CN, Appelbaum LG, Krebs RM, Hopf JM, Woldorff MG. Pinning down response inhibition in the brain – conjunction analyses of the Stop-signal task. NeuroImage. 2010;52:1621–1632. doi: 10.1016/j.neuroimage.2010.04.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Chee MWL, Soon CS, Lee HL. Common and segregated neuronal networks for different languages revealed using functional magnetic resonance adaptation. Journal of Cognitive Neuroscience. 2003;15:85–97. doi: 10.1162/089892903321107846. [DOI] [PubMed] [Google Scholar]
- Crinion J, Turner R, Grogan A, Hanakawa T, Noppeney U, et al. Language control in the bilingual brain. Science. 2006;312:1537–1540. doi: 10.1126/science.1127761. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: A random-effects approach based on empirical estimates of spatial uncertainty. Human Brain Mapping. 2009;30:2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols TE. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Grahn JA, Parkinson JA, Owen AM. The cognitive functions of the caudate nucleus. Progress in Neurobiology. 2008;86:141–155. doi: 10.1016/j.pneurobio.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JD. Shared and selective neural correlates of inhibition, facilitation, and shifting processes during executive control. Neuroimage. 2010;51:421–431. doi: 10.1016/j.neuroimage.2010.01.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez AE. Language switching in the bilingual brain: What’s next? Brain and Language. 2009;109:133–140. doi: 10.1016/j.bandl.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Hernandez AE, Dapretto M, Mazziotta J, Bookheimer S. Language switching and language representation in Spanish-English bilinguals: An fMRI study. NeuroImage. 2001;14:510–520. doi: 10.1006/nimg.2001.0810. [DOI] [PubMed] [Google Scholar]
- Hernandez AE, Martinez A, Kohnert K. In search of the language switch: An fMRI study of picture naming in Spanish-English bilinguals. Brain and Language. 2000;73:421–431. doi: 10.1006/brln.1999.2278. [DOI] [PubMed] [Google Scholar]
- Hester R, Foxe JJ, Molholm S, Shpaner M, Garavan H. Neural mechanisms involved in error processing: a comparison of errors made with and without awareness. Neuroimage. 2005;27:602–608. doi: 10.1016/j.neuroimage.2005.04.035. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Isoda M. Switching from automatic to controlled behavior: cortico-basal ganglia mechanisms. Trends in Cognitive Science. 2010;14:154–61. doi: 10.1016/j.tics.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide JS, Li CS. A cerebellar thalamic cortical circuit for error-related cognitive control. NeuroImage. doi: 10.1016/j.neuroimage.2010.07.042. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illes J, Francis WS, Desmond JE, Gabrieli JDE, Glover GH, Poldrack R, et al. Convergent cortical representation of semantic processing in bilinguals. Brain and Language. 1999;70:347–363. doi: 10.1006/brln.1999.2186. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, III, Cho RY, Stenger VA, Aizenstein H, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Klein D, Zatorre RJ, Chen JK, Milner B, Crane J, Belin P, Bouffard M. Bilingual brain organization: A functional magnetic resonance adaptation study. NeuroImage. 2006;31:366–375. doi: 10.1016/j.neuroimage.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Kuipers JR, Thierry G. Event-related brain potentials reveal the time-course of language change detection in early bilinguals. NeuroImage. 2010;50:1633–1638. doi: 10.1016/j.neuroimage.2010.01.076. [DOI] [PubMed] [Google Scholar]
- Laird AR, Fox M, Price CJ, Glahn DC, Uecker AM, et al. ALE meta-analysis: Controlling the false discovery rate and performing statistical contrasts. Human Brain Mapping. 2005;25:155–164. doi: 10.1002/hbm.20136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas-Gutierrez D, Martinez M, Salinas F, Evans A, Zilles K, Mazziotta JC, Fox PT. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Human Brain Mapping. 2007;28:1194–1205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parson LM, Liotti CS, Freitas CS, et al. Automated Talairach atlas labels for functional brain mapping. Human Brain Mapping. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtonen MH, Laine M, Niemi J, Thomsen T, Vorobyev VA, Hugdahl K. Brain correlates of sentence translation in Finnish-Norwegian bilinguals. Neuroreport. 2005;16:607–610. doi: 10.1097/00001756-200504250-00018. [DOI] [PubMed] [Google Scholar]
- Liu H, Hu Z, Guo T, Peng D. Speaking words in two languages with one brain: Neural overlap and dissociation. Brain Research. 2010;1316:75–82. doi: 10.1016/j.brainres.2009.12.030. [DOI] [PubMed] [Google Scholar]
- Luk G, Anderson JAE, Craik FIM, Grady C, Bialystok E. Distinct neural correlates for two types of inhibition in bilinguals: Response inhibition versus interference suppression. Brain and Cognition. 2010;74:347–357. doi: 10.1016/j.bandc.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Luk G, De Sa E, Bialystok E. Bilingualism: Language and Cognition. Is there a relation between onset age of bilingualism and enhancement of cognitive control? (in press) [Google Scholar]
- Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre- supplementary motor areas. Nature Reviews Neuroscience. 2008;9:856–869. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Kouider S, Makuuchi M, Kuroki C, Hanajima R, et al. Neural control of cross-language asymmetry in the bilingual brain. Cerebral Cortex. 2010;20:2244–2251. doi: 10.1093/cercor/bhp290. [DOI] [PubMed] [Google Scholar]
- Nelson JR, Liu Y, Fiez J, Perfetti CA. Assimilation and accommodation patterns in ventral occipitotemporal cortex in learning a second writing system. Human Brain Mapping. 2009;30:810–820. doi: 10.1002/hbm.20551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: A review of 100 fMRI studies published in 2009. Annals of the New York Academy of Sciences. 2010;1191:62–88. doi: 10.1111/j.1749-6632.2010.05444.x. [DOI] [PubMed] [Google Scholar]
- Price CJ, Green DW, von Studnitz R. A functional imaging study of translation and language switching. Brain. 1999;122:2221–2235. doi: 10.1093/brain/122.12.2221. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004a;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, van den Wildenberg WPS, Segalowitz J, Carter CS. Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain and Cognition. 2004b;56:129–140. doi: 10.1016/j.bandc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Rinne JO, Tommola J, Laine M, Krause BJ, Schmidt D, et al. The translating brain: cerebral activation patterns during simultaneous interpreting. Neuroscience Letters. 2000;294:85–88. doi: 10.1016/s0304-3940(00)01540-8. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Fornells A, Rotte M, Heinze HJ, Nösselt T, Münte TF. Brain potential and functional MRI evidence for how to handle two languages with one brain. Nature. 2002;415:1026–1029. doi: 10.1038/4151026a. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Fornells A, van der Lugt A, Rotte M, Britti B, Heinze HJ, Nösselt T, Münte TF. Second language interferes with word production in fluent bilinguals: brain potential and functional imaging evidence. Journal of Cognitive Neuroscience. 2005;17:422–433. doi: 10.1162/0898929053279559. [DOI] [PubMed] [Google Scholar]
- Sabri M, Binder JR, Desai R, Medler DA, Leitl MD, Liebenthal E. Attentional and linguistic interactions in speech perception. NeuroImage. 2008;39:1444–1456. doi: 10.1016/j.neuroimage.2007.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York, NY: Thieme; 1998. [Google Scholar]
- Toro R, Fox PT, Paus T. Functional coactivation map of the human brain. Cerebral Cortex. 2008;18:2553–2559. doi: 10.1093/cercor/bhn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: Method and validation. NeuroImage. 2002;16:765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eickhoff SB, Laird AR, Fox M, Wiener M, Fox P. Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Human Brain Mapping. 2011 doi: 10.1002/hbm.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velanova K, Wheeler ME, Luna B. Maturational changes in anterior cingulate and frontoparietal recruitment support the development of error processing and inhibitory control. Cerebral Cortex. 2008;18:2505–2522. doi: 10.1093/cercor/bhn012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heuven WJB, Schriefers H, Dijkstra T, Hagoort P. Language conflict in the bilingual brain. Cerebral Cortex. 2008;18:2706–2716. doi: 10.1093/cercor/bhn030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schouwenburg MR, den Ouden HE, Cools R. The human basal ganglia modulate frontal-posterior connectivity during attention shifting. Journal of Neuroscience. 2010;30:9910–9918. doi: 10.1523/JNEUROSCI.1111-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Xue G, Chen C, Xue F, Dong Q. Neural bases of asymmetric language switching in second-language learners: An ER-fMRI study. NeuroImage. 2007;35:862–870. doi: 10.1016/j.neuroimage.2006.09.054. [DOI] [PubMed] [Google Scholar]
- Wang Y, Kuhl PK, Chen C, Dong Q. Sustained and transient language control in the bilingual brain. NeuroImage. 2009;47:414–422. doi: 10.1016/j.neuroimage.2008.12.055. [DOI] [PubMed] [Google Scholar]
- Westlye LT, Grydeland H, Walhovd KB, Fjell AM. Associations between regional cortical thickness and attentional networks as measured by the Attention Network Test. Cerebral Cortex. doi: 10.1093/cercor/bhq101. (in press) [DOI] [PubMed] [Google Scholar]
- Xiang HD, Fonteijn HM, Norris DG, Hagoort P. Topographical functional connectivity pattern in the perisylvian language networks. Cerebral Cortex. 2010;20:549–560. doi: 10.1093/cercor/bhp119. [DOI] [PubMed] [Google Scholar]


