Abstract
Background
Pain is a prominent feature of acute dengue as well as a clinical criterion in World Health Organization guidelines in diagnosing dengue. We conducted a prospective cohort study to compare levels of pain during acute dengue between different ethnicities and dengue severity.
Methods
Demographic, clinical and laboratory data were collected. Data on self-reported pain was collected using the 11-point Numerical Rating Scale. Generalized structural equation models were built to predict progression to severe disease.
Results
A total of 499 laboratory confirmed dengue patients were recruited in the Prospective Adult Dengue Study at Tan Tock Seng Hospital, Singapore. We found no statistically significant differences between pain score with age, gender, ethnicity or the presence of co-morbidity. Pain score was not predictive of dengue severity but highly correlated to patients’ day of illness. Prevalence of abdominal pain in our cohort was 19%. There was no difference in abdominal pain score between grades of dengue severity.
Conclusion
Dengue is a painful disease. Patients suffer more pain at the earlier phase of illness. However, pain score cannot be used to predict a patient’s progression to severe disease.
Introduction
The World Health Organization (WHO) reported that over 40% of the global population is at risk of dengue and an estimated 390 million dengue infections happen worldwide every year.
Dengue incidence has increased and its geographic range expanded.[1] Many studies on the clinical features of dengue were pediatric cohorts.[2], [3] In Singapore, dengue infections have shifted from a primarily childhood to an adult disease. [4] Today, more than 90% of dengue patients are adults. We previously identified the occurrence of bleeding, lymphopenia, hypoproteinemia and elevated serum urea as independent predictors for dengue hemorrhagic fever (DHF) [5].
Pain is an important component of dengue diagnostic criteria. In WHO 1997 guideline, probable dengue requires the presence of fever with any two of headache, retro-orbital pain, mylagia, arthralgia, rash, positive tourniquet test or or hemarroghic manifestation. [6] In WHO 2009 guideline, the diagnostic criteria were revised to comprise fever and two of nausea/vomiting, rash, aches and pains, positive tourniquet test, leucopenia and any warning sign. Warning signs include abdominal pain or tenderness, persistent vomiting, clinical fluid accumulation, mucosal bleeding, lethargy, hepatomegaly and hematocrit rise with rapid platelet count drop [7].
Higher prevalence of abdominal pain, backache, headache and myalgia was noted in adult dengue patients.[3], [8], [9] Abdominal pain and myalgia were associated with dengue severity and mortality.[10]–[12] While these studies identified aches and pains as an important factor for dengue diagnosis and prognosis, we have not found any study assessing the degree of pain reported by dengue patients in predicting progression to severe disease to date. To fill the lacuna in literature, we conducted a prospective cohort study to determine the relationship between pain intensity and disease severity.
Methods
Ethics Statement
Ethical approval was provided by the Domain Specific Review Board of the National Healthcare Group, Singapore (DSRB/E/2009/432). Written informed consent was obtained from all subjects.
Patient Cohort
Acutely febrile patients above the age of 18 were recruited prospectively from January 2010 to September 2012 at the Communicable Disease Center, Tan Tock Seng Hospital, Singapore, a tertiary referral infectious disease centre. All dengue diagnostic testing was performed by the Enviromental Health Institute, a WHO Collaborating Center for Reference and Research on Arbovirus and its Associated Vectors in Singapore. Patients were classified as having laboratory-confirmed dengue according to WHO standards if they were RT-PCR or NS1 positive using the dengue NS1 antigen (Ag) strip (Bio-Rad Laboratories, Marnes-la-Coquette, France).[13] Only laboratory-confirmed dengue patients were included. Detailed daily clinical and laboratory data, including pain score, were collected prospectively until discharge or at the recovery phase of illness. Pain score for all patients, including those who did not return for subsequent follow-ups, were included to increase the sample size.
The Numeric Rating Scale (NRS) is a widely used tool to assess patients’ pain level in chronic and acute illnesses with good responsiveness and sensitivity.[14]–[16]. The NRS was the preferred pain rating scale owing to its ease of use and accurate assessment.[17] The scale ranges from 0 to 10 with 0 being no pain and 10 being the worst possible pain; this is aided with the use of the face pain scale of facial expressions at pain scale of 0,2,4,8 and 10. [18] Separate information on abdominal pain score was captured as a warning sign in the WHO 2009 guideline. On enrolment, patients were asked to rate their pain intensity from day of illness to day of enrolment. Pain scores were then recorded prospectively on subsequent follow-ups.
Outcome Variable
The primary outcome was the severity of dengue: 1: Dengue fever, 2: Dengue hemorrhagic fever (DHF; Grades I–II) [6] and 3: Severe disease, defined as fulfilling dengue shock syndrome (DSS) and severe dengue (SD) [7].
Statistical Analysis
Ordinal logistic regression was used to determine how pain score was associated with age, gender, co-morbidity and ethnicity (1: Chinese 2: Malay 3: Indian 4: Others). Generalized structural equation model (GSEM) was applied as a more confirmatory analysis to analyze the data accounting for temporal data structure and the complex dependence among pain score, fever and the outcome.[19] As the latest development in structural equation modeling which unifies regression models with latent class models, GSEM is deemed to be the most appropriate in the proposed data analysis. It could estimate the direct and indirect effects of pain score and fever on the outcome with a single model set-up. In conventional regression analysis one may have to construct multiple models for dealing with studies involving a final outcome, a set of covariates and some variables that are both predictors for the final outcome and intermediate outcomes.
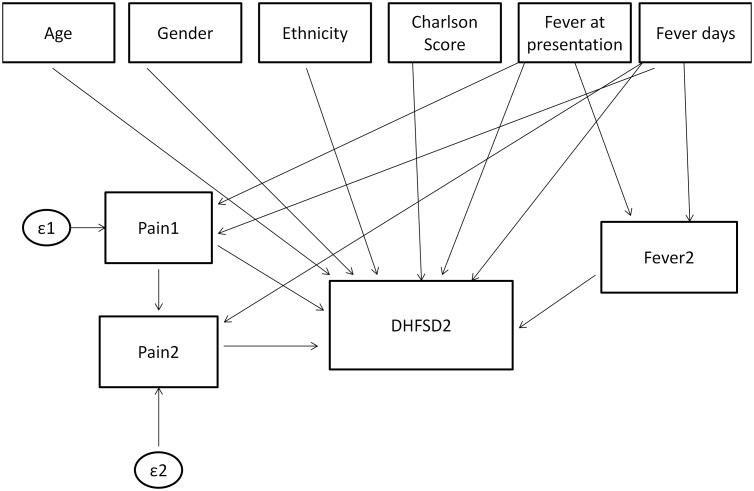
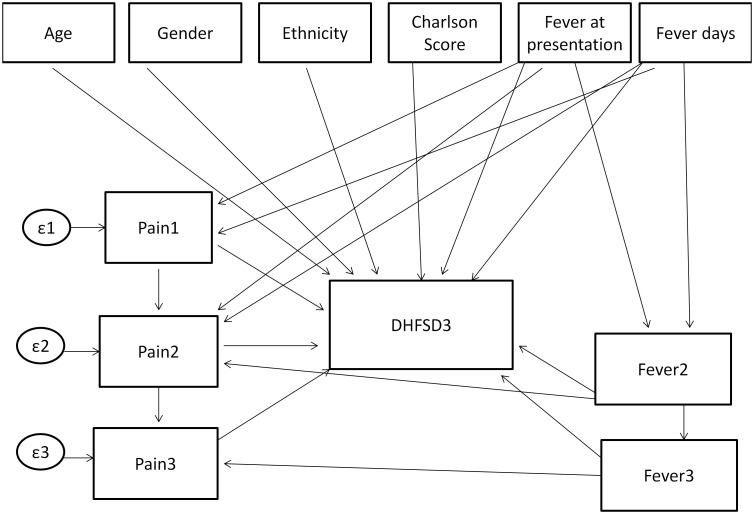
Two sets of analyses were carried out, with the outcome ascertained on day 2 and day 3 of enrolment. In both analyses, the number of fever days reported by patients preceded fever at presentation which in turn preceded days 2 and 3 of enrolment (see Figures 1 and 2).
Figure 1. Model specification for study day 2.
Feveratpresentation = Fever present at enrolment (Yes/No), Feverdays = Number of fever days up to enrolment, Fever2 = Fever on study day 2, Pain1 = Pain Score at enrolment/presentation, Pain2 = Pain Score on study day 2, DHFSD2 = Outcome on study day 2.
Figure 2. Model specification for study day 3.
The first GSEM model to predict dengue severity on the second day of enrolment (day 2) involved baseline covariates such as age, gender, ethnicity, Charlson’s co-morbidity score[20], fever at presentation and number of fever days before day 2 as the direct effects to the outcome. The intermediate variables involved whether patient had fever on day 2, pain score on study days 1 and 2. Fever at presentation and the number of fever days were hypothesized to be associated with pain score on day 1 and fever on day 2. Pain score on day 1 was assumed to be associated with pain score on day 2 (Figure 1). To predict dengue severity on the third day of enrolment (day 3), the model was extended to accommodate the pain score and fever on day 3 (Figure 2). The GSEM is most appropriate when such data structures with irreversible temporal effects are required in analysis.
Estimated with maximum likelihood, the models were analysed with the multinomial distribution and logit link. Analyzed with Stata MP 13 (Stata Corp, Texas, USA), all statistical tests were conducted with 95% confidence intervals.
Results
Demographic Description of Cohort
Of the 850 patients recruited, 499 tested positive for RT-PCR or NS1 were included in the analysis. There were 111 DHF I–II and 13 DHF III–IV(DSS) cases according to WHO 1997 classification. Twenty were severe dengue cases in accordance with the WHO 2009 classification. The median duration of fever was 6 (3–9) days. One patient died owing to myocarditis. One patient was diagnosed with both DSS and SD. In total, 32 patients fulfilled the severe disease classification.
The median age of the cohort was 34 years (5th–95th percentile, 21–51 years). Male patients comprised 79% and 77.6% of the cohort were Chinese. Medical co-morbidity, mainly myocardial infarction, diabetes and cerebrovascular dieseases was present in 1.8%. The majority (76.3%) of patients had fever on the first visit. The median pain score was similar in DF, DHF I–II and severe disease patients (5 [0–8], p = 0.7). (Table 1) There were no significant statistical association between pain score at first visit and age (p = 0.09), gender (p = 0.32), co-morbidity (p = 0.49) or race (p = 0.19).
Table 1. Demographic description of study cohort.
| DF (n = 356) | DHF I–II (n = 111) | Severe disease (n = 32) | Overall (n = 499) | |
| Age in years | 32 (21–46) | 40 (22–58) | 36 (23–57) | 34 (21–51) |
| Male gender | 289 (81.1%) | 89 (80.2%) | 18 (56.2%) | 396 (79.4%) |
| Chinese ethnicity | 258 (72.5%) | 103 (92.8%) | 26 (81.2%) | 387 (77.6%) |
| Any co-morbidity | 5 (1.4%) | 3 (2.7%) | 1 (3.1%) | 9 (1.8%) |
| Fever at 1st visit | 250 (70.2%) | 100 (90.1%) | 31 (96.9%) | 381 (76.3%) |
| Fever days at 1st visit | 6 (3–9) | 6 (3–8) | 5 (3–9) | 6 (3–8) |
| Pain score at 1st visit | 5 (0–8) | 5 (0–8) | 5 (0–8) | 5 (0–8) |
For categorical variables, absolute numbers (and the relative percentage) are being indicated. For continuous variables, medians (and the relative 5th–95th percentile) are being indicated.
Temporal Trend of Overall Pain Score
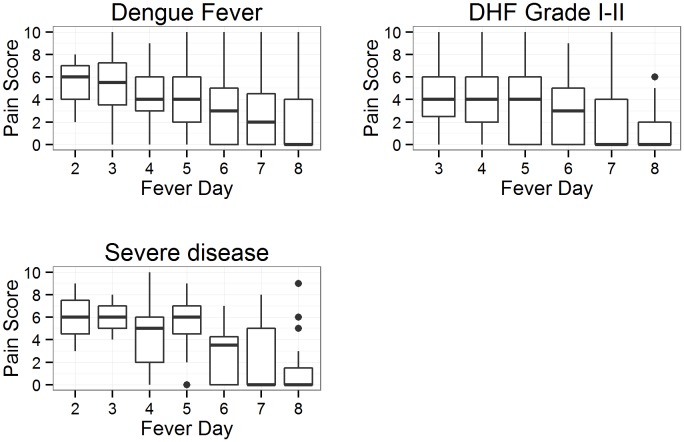
Figure 3 shows that DF patients generally experienced reduction in pain as they were recovering from dengue infection. Median pain score for patients with DHF I–II or severe disease overlapped. However, pain score diminished to the range of 0–2 on fever day 8. It is visually difficult to draw conclusions on differences between dengue severity from the plots due to the large overlap. (For detailed sample sizes for each day, refer to Table S1).
Figure 3. Boxplot of pain scores over fever days by dengue severity.
The line inside the box represent the median, box edges represent 25th and 75th percentile and vertical lines represent 1.5 times the interquartile range. Points outside these limits are considered outliers.
Predictive Modeling of Overall Pain Score and Co-variates
Study day 2
Pain score on study days 1 and 2, number of fever days, ethnicity and co-morbidity were not significantly associated with dengue severity (Table 2). The GSEM results showed that age and fever at presentation were predictive of severe disease on study day 2. On average patient’s risk of severe disease increased by 3% with every year of increase in age. In addition, fever at presentation increased the risk of developing severe disease (Table 2). Patients experienced less pain as they recovered (odds ratio [OR]: 1.52 [1.33–74]).
Table 2. GSEM results for likelihood of developing severe dengue disease on days 2 and 3 of enrolment.
| Baseline co-variates | Study Day 2 (n = 438) aOR (95% CI) | Study Day 3 (n = 399) aOR (95% CI) |
| Age | 1.03 (1–1.06)* | 1.05 (1.00–1.10)* |
| Gender | ||
| Male | Reference | |
| Female | 1.48 (0.68–3.23) | 1.08 (0.32–3.65) |
| Ethnicity | ||
| Chinese | Reference | |
| Malay | 0.59 (0.07–4.96) | 2.14 (0.15–30.07) |
| Indian | 0.14 (0.02–1.06) | 0.24 (0.03–1.95) |
| Others | 0.59 (0.07–4.90) | 2.70 (0.24–30.73) |
| Charlson’s co-morbidity score | ||
| 0 | Reference | |
| ≥1 | 1.51 (0.25–8.91) | 1.56 (0.12–21.20) |
| Fever at presentation | ||
| No | Reference | |
| Yes | 6.76 (1.41–32.4)* | 0.77(0.17–3.60) |
| Fever duration in days | 1.29 (0.96–1.73) | 1.27 (0.82–1.97) |
| Fever on Study day 2 | ||
| No | Reference | |
| Yes | 1.93 (0.85–4.37) | 0.54 (0.18–1.62) |
| Fever on Study day 3 | ||
| No | NA | Reference |
| Yes | NA | 3.97 (1.18–13.34)* |
| Pain score at presentation | 0.96 (0.83–1.10) | 1.09 (0.90–1.32) |
| Study Day 2 pain score | 0.96 (0.82–1.13) | 1.15 (0.92–1.42) |
| Study Day 3 pain score | NA | 3.97 (1.18–13.34)* |
*Statistically significant; equivalent to p<0.05.
Study day 3
Similarly, pain score on study day 2 was not predictive but age and fever on day 3 of study were predictive of dengue severity. The risk of age on severe disease largely remained the same as the day before. In addition, fever on day 3 increased a patient’s risk of severe disease. Notably, patients who developed severe disease on day 3 were more likely to report higher pain scores (Table 2).
Abdominal Pain
Ninety five patients (19%) experienced abdominal pain. Four of them had SD while the other 3 had DSS. Of these 7 cases, 3 of them experienced the worst abdominal pain on the same day as they were diagnosed with severe disease or after progressing on to severe disease. The median abdominal pain score for patients with severe disease was 5 (5th–95th percentile, 2–9) and 4 (5th–95th percentile, 1–10) for non-severe disease (p = 0.38).
Discussion
Abdominal pain was identified as one of four warning signs in a retrospective Puerto Rican study on dengue deaths.[21] In Cuba, headache was present in all twelve fatalities.[22] In Singapore abdominal pain was present in half of adult dengue deaths.[23] In Lucknow, India, severe abdominal pain was noted in 18% of children and adults with dengue shock syndrome[24]. In the Philippines, abdominal pain was significantly more common in children with DHF.[25] Abdominal pain was significantly associated with the need for major interventions in the DENCO study [26] while in Brazilian children, abdominal pain was independently associated with severe dengue.[27] Notably, be it dengue shock syndrome or uncomplicated dengue, headache and abdominal pain were significantly more common in adults compared with children [3].
To our knowledge, this is the first study to analyze the utility of a numeric pain score in predicting the progression of dengue severity. We explored the utility of pain score in dengue prognosis accounting for baseline covariates which might affect pain. The results from the GSEM model should not be interpreted as a predictive model for dengue disease severity. We found that pain intensity measured by numeric rating scale experienced by adult dengue patients did not differ by ethnicity, co-morbidity and dengue severity. Additionally abdominal pain intensity did not differ by dengue severity. Age and fever at presentation appeared to correlate with dengue severity. The impact on age on dengue severity was previously studied [28].
There were several limitations in our study. We were unable to monitor patients’ parameters before enrolment. Our patient profile at a tertiary center may not reflect those in primary care. Patients were recruited at a median of 6 days of fever. The daily level of pain by recall before enrolment can be unreliable and may be associated with recall bias. Patients who visited the clinic only on enrolment day were included in the analysis for increasing the sample size; we assumed that patients were most painful on enrolment day and subsequent follow-up were missed as they recovered. Our study was conducted in an adult cohort which may not generalize to children with dengue. Further work needs to be done to monitor level of pain in the early course of illness to better understand the relationship between pain and disease progression in dengue.
Conclusion
This study provides evidence for clinicians against using pain score alone in clinical triage or in predicting progression to severe disease.
Supporting Information
Sample size at each fever day stratified by outcome (DHF I–II = Dengue hemarroghic fever grades 1–2).
(DOC)
Acknowledgments
We are grateful to all our research assistants and patients who have participated in our study. We are thankful to Dr Tun Linn Thein and Dr Hao Ying for their valuable suggestions.
Funding Statement
This study was funded by a National Medical Research Council (http://www.nmrc.gov.sg) Translational and Clinical Research Flagship Programme Grant NMRC/TCR/005 for Scientific exploration, Translational research, Operational evaluation of disease prevention and Preventative measures through new treatment strategies against Dengue (STOP Dengue). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, et al. (2013) The global distribution and burden of dengue. Nature 496: 504–507 10.1038/nature12060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Thein S, Aung MM, Shwe TN, Aye M, Zaw A, et al. (1997) Risk factors in dengue shock syndrome. Am J Trop Med Hyg 56: 566–572. [DOI] [PubMed] [Google Scholar]
- 3.Dinh The T, Le Thi Thu T, Nguyen Minh D, Tran Van N, Tran Tinh H, et al. (2012) Clinical Features of Dengue in a Large Vietnamese Cohort: Intrinsically Lower Platelet Counts and Greater Risk for Bleeding in Adults than Children. PLoS Negl Trop Dis 6. Available: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3383761/. Accessed 12 March 2014. [DOI] [PMC free article] [PubMed]
- 4. Ooi E-E, Goh K-T, Gubler DJ (2006) Dengue Prevention and 35 Years of Vector Control in Singapore. Emerg Infect Dis 12: 887–893 10.3201/eid1206.051210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee VJ, Lye DCB, Sun Y, Fernandez G, Ong A, et al. (2008) Predictive value of simple clinical and laboratory variables for dengue hemorrhagic fever in adults. J Clin Virol Off Publ Pan Am Soc Clin Virol 42: 34–39 10.1016/j.jcv.2007.12.017 [DOI] [PubMed] [Google Scholar]
- 6.WHO | Dengue guidelines for diagnosis, treatment, prevention and control: new edition (n.d.). WHO. Available: http://www.who.int/rpc/guidelines/9789241547871/en/. Accessed 10 March 2014.
- 7.WHO | Dengue haemorrhagic fever: diagnosis, treatment, prevention and control. 2nd edition. Geneva: World Health Organization. (n.d.). WHO. Available: http://www.who.int/csr/resources/publications/dengue/Denguepublication/en/. Accessed 10 March 2014.
- 8. Khan MIH, Anwar E, Agha A, Hassanien NSM, Ullah E, et al. (2013) Factors predicting severe dengue in patients with dengue Fever. Mediterr J Hematol Infect Dis 5: e2013014 10.4084/MJHID.2013.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wichmann O, Hongsiriwon S, Bowonwatanuwong C, Chotivanich K, Sukthana Y, et al. (2004) Risk factors and clinical features associated with severe dengue infection in adults and children during the 2001 epidemic in Chonburi, Thailand. Trop Med Int Health 9: 1022–1029 10.1111/j.1365-3156.2004.01295.x [DOI] [PubMed] [Google Scholar]
- 10. Thein T-L, Leo Y-S, Fisher DA, Low JG, Oh HML, et al. (2013) Risk Factors for Fatality among Confirmed Adult Dengue Inpatients in Singapore: A Matched Case-Control Study. PLoS ONE 8: e81060 10.1371/journal.pone.0081060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Junia J, Garna H, Setiabudi D (2007) Clinical risk factors for dengue shock syndrome in children. Paediatr Indones 47: 7–11. [Google Scholar]
- 12.Pongpan S, Wisitwong A, Tawichsari C, Patumanond J (2013) Prognostic Indicators for Dengue Infection Severity. Int J Clin Pediatr. Available: http://ijcp.elmerpress.com/index.php/ijcp/article/view/73. Accessed 10 March 2014.
- 13. Lai Y-L, Chung Y-K, Tan H-C, Yap H-F, Yap G, et al. (2007) Cost-Effective Real-Time Reverse Transcriptase PCR (RT-PCR) To Screen for Dengue Virus followed by Rapid Single-Tube Multiplex RT-PCR for Serotyping of the Virus. J Clin Microbiol 45: 935–941 10.1128/JCM.01258-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bijur PE, Latimer CT, Gallagher EJ (2003) Validation of a Verbally Administered Numerical Rating Scale of Acute Pain for Use in the Emergency Department. Acad Emerg Med 10: 390–392 10.1111/j.1553-2712.2003.tb01355.x [DOI] [PubMed] [Google Scholar]
- 15. Williamson A, Hoggart B (2005) Pain: a review of three commonly used pain rating scales. J Clin Nurs 14: 798–804 10.1111/j.1365-2702.2005.01121.x [DOI] [PubMed] [Google Scholar]
- 16. Farrar JT, Young Jr JP, LaMoreaux L, Werth JL, Poole RM (2001) Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. PAIN 94: 149–158 10.1016/S0304-3959(01)00349-9 [DOI] [PubMed] [Google Scholar]
- 17. Chanques G, Viel E, Constantin J-M, Jung B, de Lattre S, et al. (2010) The measurement of pain in intensive care unit: comparison of 5 self-report intensity scales. Pain 151: 711–721 10.1016/j.pain.2010.08.039 [DOI] [PubMed] [Google Scholar]
- 18. Downie WW, Leatham PA, Rhind VM, Wright V, Branco JA, et al. (1978) Studies with pain rating scales. Ann Rheum Dis 37: 378–381 10.1136/ard.37.4.378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rabe-Hesketh S, Skrondal A, Pickles A (2004) Generalized multilevel structural equation modeling. Psychometrika 69: 167–190 10.1007/BF02295939 [DOI] [Google Scholar]
- 20. Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40: 373–383. [DOI] [PubMed] [Google Scholar]
- 21. Rigau-Pérez JG, Laufer MK (2006) Dengue-Related Deaths in Puerto Rico, 1992–1996: Diagnosis and Clinical Alarm Signals. Clin Infect Dis 42: 1241–1246 10.1086/501355 [DOI] [PubMed] [Google Scholar]
- 22. Guzmán MG, Alvarez M, Rodríguez R, Rosario D, Vázquez S, et al. (1999) Fatal dengue hemorrhagic fever in Cuba, 1997. Int J Infect Dis IJID Off Publ Int Soc Infect Dis 3: 130–135. [DOI] [PubMed] [Google Scholar]
- 23. Ong A, Sandar M, Chen MI, Sin LY (2007) Fatal dengue hemorrhagic fever in adults during a dengue epidemic in Singapore. Int J Infect Dis 11: 263–267 10.1016/j.ijid.2006.02.012 [DOI] [PubMed] [Google Scholar]
- 24. Agarwal R, Kapoor S, Nagar R, Misra A, Tandon R, et al. (1999) A clinical study of the patients with dengue hemorrhagic fever during the epidemic of 1996 at Lucknow, India. Southeast Asian J Trop Med Public Health 30: 735–740. [PubMed] [Google Scholar]
- 25. Carlos CC, Oishi K, Cinco MTDD, Mapua CA, Inoue S, et al. (2005) Comparison of Clinical Features and Hematologic Abnormalities Between Dengue Fever and Dengue Hemorrhagic Fever Among Children in the Philippines. Am J Trop Med Hyg 73: 435–440. [PubMed] [Google Scholar]
- 26. Alexander N, Balmaseda A, Coelho ICB, Dimaano E, Hien TT, et al. (2011) Multicentre prospective study on dengue classification in four Southeast Asian and three Latin American countries. Trop Med Int Health 16: 936–948 10.1111/j.1365-3156.2011.02793.x [DOI] [PubMed] [Google Scholar]
- 27. Giraldo D, Sant’Anna C, Périssé ARS, March M de FP, Souza AP, et al. (2011) Characteristics of children hospitalized with dengue fever in an outbreak in Rio de Janeiro, Brazil. Trans R Soc Trop Med Hyg 105: 601–603 10.1016/j.trstmh.2011.07.007 [DOI] [PubMed] [Google Scholar]
- 28. García-Rivera EJ, Rigau-Pérez JG (2003) Dengue severity in the elderly in Puerto Rico. Rev Panam Salud Pública Pan Am J Public Health 13: 362–368. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sample size at each fever day stratified by outcome (DHF I–II = Dengue hemarroghic fever grades 1–2).
(DOC)