Abstract
Interleukin-1β (IL-1β) can limit tumor growth by promoting T cell-mediated antitumor immune responses. Several chemotherapeutic agents can stimulate the production of IL-1β by tumor-infiltrating leukocytes via the NLRP3 inflammasome. We have recently demonstrated that some chemotherapeutics can also trigger the secretion of IL-1β by driving the assembly of the caspase-8- and FADD-containing platform known as the ripoptosome.
Keywords: IL-1β, caspase-1, caspase-8, chemotherapy, inflammasome, ripoptosome
Innate immunological signals delivered by tumor-resident dendritic cells (DCs) and macrophages (MΦ) can contribute to the efficacy of chemotherapeutic agents by engaging T cell-mediated adaptive immune responses that limit the expansion of chemotherapy-resistant tumor cells.1 Interleukin-1β (IL-1β) has emerged as a particularly relevant MΦ/DC-derived cytokine that—depending on tumor type and microenvironmental features—can either inhibit tumor growth by T cell-dependent mechanisms or support tumor progression. The latter activity of IL-1β may involve1 the release of paracrine growth factors from stromal cells of the tumor environment;2 enhanced tumor angiogenesis; and/or3 the accumulation of myeloid-derived suppressor cells (MDSC), which suppress anticancer immunosurveillance (reviewed in Refs.1,2). Given such contrasting roles for IL-1β in tumor growth and anticancer therapy, it is important to define the signaling pathways that underlie the production of IL-1β by MΦs and DCs in the context of diverse anticancer chemotherapeutic regimens.
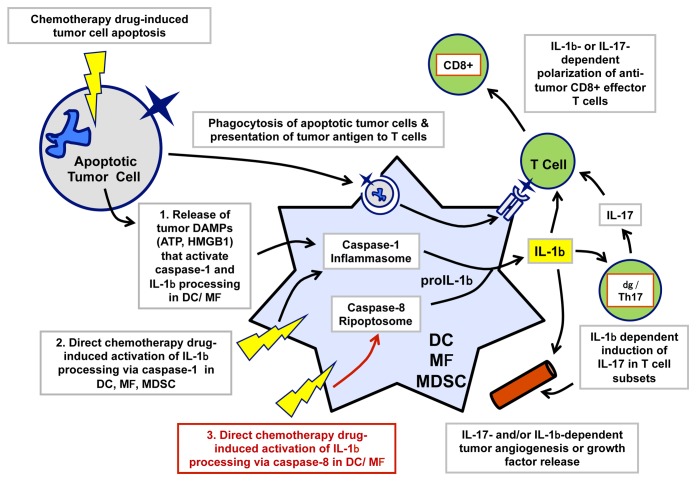
Previous studies have identified 2 distinct, but not mutually exclusive, molecular cascades that lead to the secretion of IL-1β (Fig. 1). The aim of most chemotherapeutic agents is to directly promote apoptosis or necrosis among rapidly dividing neoplastic cells, which generally results in the accumulation of tumor-derived macromolecules (e.g., high mobility group box 1, HMGB1) and small metabolites (e.g., ATP) in the tumor microenvironment. These molecules act as danger-associated molecular patterns (DAMPs) and engage the canonical cascade involving the assembly of a multiprotein caspase-1-activating platform that includes NLR family, pyrin domain containing 3 (NLRP3) as well as the PYD and CARD domain containing adapter (PYCARD), best known as ASC. This complex, commonly known as the NLRP3 inflammasome, promotes the secretion of bioactive IL-1β from tumor-resident MΦs and DCs. This indirect pathway of IL-1β secretion involving a paracrine cancer cell/myeloid cell signaling axis has been documented in neoplastic lesions exposed to multiple chemotherapeutic agents, including (but not limited to) oxaliplatin, doxorubicin, and mitoxantrone.3 A second mechanism underlying the secretion of IL-1β within neoplastic lesions involves the ability of specific chemotherapeutics to directly stimulate the NLRP3 inflammasome in MΦs and DCs. This is consistent with the ability of NLRP3 to sense an extraordinarily diverse range of stressful conditions, involving perturbations of the homeostasitic functions of the plasma membrane as well as of the lysosomal or mitochondrial compartments.4 The production of IL-1β via a cell-autonomous signaling axis has been observed in various myeloid leukocytes exposed to a subset of chemotherapeutic agents that can trigger mitochondrial (e.g., doxorubicin, staurosporine) or lysosomal dysfunction (gemcitabine, 5-fluorouracil)5-7.
Figure 1. Production and functions of interleukin-1β within the tumor microenvironment. Macrophages, dendritic cells, and myeloid-derived suppressor cells (MDSCs) represent the major cellular source of interleukin (IL)-1β within the tumor microenvironment. In the context of anticancer chemotherapy, local IL-1β can support antitumor immune responses by directly (or indirectly, via IL-17) polarizing tumor-reactive CD8+ T lymphocytes into effector cells that kill or suppress chemotherapy-resistant cancer cells. However, IL-1β may also directly (or indirectly, via IL-17) support tumor progression by stimulating the release of growth factors from stromal cells or by promoting angiogenesis. The secretion of biologically active IL-1β involves the convergence of two signaling cascades, commonly known as “signal 1” and “signal 2.” The former involves the NF-κB-dependent upregulation of pro-IL-1β, while the latter involves the assembly of a caspase-containing complex that catalyzes the proteolytic processing of pro-IL-1β into mature IL-1β. Caspase-1 is the canonical IL-1β-converting enzyme (ICE) and is itself activated by multiple stimuli that promote the assembly of the so-called inflammasome. Previous studies have identified 2 pathways by which chemotherapeutic agents can activate the inflammasome and hence promote the secretion of IL-1β by tumor-resident myeloid cells. The first one is an indirect axis whereby mediators released from dying cancer cells (e.g., ATP) act as paracrine agonists on myeloid cell receptors (e.g., the ATP-gated ion channel P2RX7), hence triggering the assembly of the inflammasome. The second one involves the ability of some chemotherapeutic agents to direct stimulate apoptotic or necrotic signaling cascades in myeloid cells, ultimately converging on caspase-1 activation. Recent data indicate that some chemotherapeutic agents can synergize with Toll-like receptor 4 (TLR4) signaling to promote assembly of caspase-8-containing ripoptosomes, acting as alternative, non-canonical platforms for the proteolytic maturation of IL-1β (in red).
We have recently described a third, mechanistically distinct molecules cascade by which some chemotherapeutics can promote IL-1β production.8 Although this pathway also involves a direct activity of chemotherapy on MΦs and DCs, it is independent of the classical NLRP3 inflammasome but rather reflects the engagement of a signaling cascade involving receptor-interacting protein kinase 1 (RIPK1) and resulting in the assembly of a Fas (TNFRSF6)-associated via death domain protein (FADD)- and caspase-8-containing supramolecular complex known as the ripoptosome (Fig. 1). These findings add to a growing literature on the important role for caspase-8 as both an alternative IL-1β-converting enzyme and a regulator of canonical inflammasomes.4 Notably, the ripoptosome-dependent production of IL-1β was triggered by some common chemotherapeutic agents (e.g., doxorubicin, staurosporine) but not by others (e.g., oxaliplatin, cisplatin). The ability of the various drugs to activate caspase-8 in myeloid cells and hence drive IL-1β secretion correlated with their efficacy to inhibit the expression of members of the inhibitor of apoptosis protein (IAP) family. The release of DIABLO (an IAP-binding protein best known as Smac) from mitochondria suppresses the E3 ubiquitin ligase activity of IAPs, hence inhibiting the ubiquitin-dependent degradation of ripoptosomes.9 The downregulation of baculoviral IAP repeat containing 2 (BIRC2, a member of the IAP family also known as cIAP1) in doxorubicin-treated DCs appeared to be critical for the preservation of the FADD/caspase-8 ripoptosome, corroborating previous results that implicated the downregulation of IAPs in the processing of IL-1β as triggered by Smac mimetics.10
The identification of a caspase-8-dependent pathway for IL-1β production has several implications in the context of anticancer chemotherapy. First, Toll-like receptor 4 (TLR4) signaling in the tumor-resident MΦs or DCs of cancer patients undergoing chemotherapy may be activated by commensal bacteria-derived endotoxins that leak across compromised gut epithelial barriers or by tumor-derived DAMPs, such as HMGB1. Such an activation of TLR4 may synergize with chemotherapeutics, such as doxorubicin, to trigger the FADD/caspase-8 ripoptosome in vivo. Second, in contrast to caspase-1 (which is predominantly expressed in myeloid cells), caspase-8 is ubiquitously expressed. The caspase-8-dependent processing of IL-1β may thus occur in non-myeloid stromal compartments of the tumor microenvironment, including epithelial cells, endothelial cells and fibroblasts, as well as in some neoplastic cells, which do not express high levels of caspase-1 but may express pro-IL-1β in particular inflammatory contexts.
Ongoing studies indicate that the ability of particular chemotherapeutic agents to drive the caspase-8-dependent processing of IL-1β correlates with their ability to elicit pro-apoptotic signaling cascades in MΦs or DCs, even in the context of the NF-κB signals that are required for production of pro-IL-1β. This is likely to be relevant given the prominent role of NF-κB in transactivation of anti-apoptotic/pro-survival genes. Malignant cells proliferate rapidly in comparison to MΦs and DCs, and most chemotherapeutic agents act on cancer cells by inducing molecular injuries (e.g., DNA damage) that activate networks resulting in the transcriptional or post-transcriptional activation of pro-apoptotic proteins (e.g., pro-apoptotic Bcl-2 family members). A key issue is therefore to discriminate how a particular drug may differently integrate NF-κB-driven pro-inflammatory vs. anti-apoptotic gene expression in malignant cells vs. tumor-resident immune cells. Moreover, given the key role of organellar dysfunction in the activation of NLR3 inflammasomes and ripoptosomes,4,9 the analysis of the effects of chemotherapeutic agents on mitochondrial, lysosomal, and plasma membrane integrity will likely provide novel insights into the multiple pathways underlying IL-1β production in different tumor models.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
Grant support: NIH-R01-GM36387 (GRD)
Citation: Antonopoulos C, Dubyak GR. Chemotherapy engages multiple pathways leading to IL-1β production by myeloid leukocytes. OncoImmunology 2013; 2:e27499; 10.4161/onci.27499
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/27499
References
- 1.Zitvogel L, Kepp O, Galluzzi L, Kroemer G. Inflammasomes in carcinogenesis and anticancer immune responses. Nat Immunol. 2012;13:343–51. doi: 10.1038/ni.2224. [DOI] [PubMed] [Google Scholar]
- 2.Janowski AM, Kolb R, Zhang W, Sutterwala FS. Beneficial and Detrimental Roles of NLRs in Carcinogenesis. Front Immunol. 2013;4:370. doi: 10.3389/fimmu.2013.00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, Vermaelen K, Panaretakis T, Mignot G, Ullrich E, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15:1170–8. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 4.Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13:397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sauter KA, Wood LJ, Wong J, Iordanov M, Magun BE. Doxorubicin and daunorubicin induce processing and release of interleukin-1β through activation of the NLRP3 inflammasome. Cancer Biol Ther. 2011;11:1008–16. doi: 10.4161/cbt.11.12.15540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimada K, Crother TR, Karlin J, Dagvadorj J, Chiba N, Chen S, Ramanujan VK, Wolf AJ, Vergnes L, Ojcius DM, et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36:401–14. doi: 10.1016/j.immuni.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruchard M, Mignot G, Derangère V, Chalmin F, Chevriaux A, Végran F, Boireau W, Simon B, Ryffel B, Connat JL, et al. Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat Med. 2013;19:57–64. doi: 10.1038/nm.2999. [DOI] [PubMed] [Google Scholar]
- 8.Antonopoulos C, El Sanadi C, Kaiser WJ, Mocarski ES, Dubyak GR. Proapoptotic chemotherapeutic drugs induce noncanonical processing and release of IL-1β via caspase-8 in dendritic cells. J Immunol. 2013;191:4789–803. doi: 10.4049/jimmunol.1300645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mocarski ES, Upton JW, Kaiser WJ. Viral infection and the evolution of caspase 8-regulated apoptotic and necrotic death pathways. Nat Rev Immunol. 2012;12:79–88. doi: 10.1038/nri3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vince JE, Wong WW, Gentle I, Lawlor KE, Allam R, O’Reilly L, Mason K, Gross O, Ma S, Guarda G, et al. Inhibitor of apoptosis proteins limit RIP3 kinase-dependent interleukin-1 activation. Immunity. 2012;36:215–27. doi: 10.1016/j.immuni.2012.01.012. [DOI] [PubMed] [Google Scholar]