Abstract
The role of the complement system in oncogenesis and tumor progression remains poorly understood. We have recently demonstrated that the induction of a tumor-specific CD8+ T-cell response is improved upon transient inhibition of the complement system, which is coupled to an increased availability of natural killer cells. The complement system may therefore turn out to constitute a promising target for the development of novel anticancer therapeutics.
Keywords: cancer, complement, immunotherapy, melanoma, NK cells
More than simply “complementing” innate immune responses, complement proteins play a role in cellular turnover, growth and regeneration. Moreover, activated complement proteins have been shown to mediate antineoplastic effects by promoting complement-dependent cytotoxicity (CDC) as well as antibody-dependent cell-mediated cytotoxicity (ADCC). Yet, a growing body of evidence suggests a dichotomic role for the complement system in tumorigenesis. In fact, the complement anaphylatoxins C3a and C5a have been shown to promote the overexpression of potentially oncogenic proteins such as phosphoniositide-3-kinase (PI3K), AKT1 and mammalian target of rapamycin (mTOR).1 Moreover, overexpression of CD59, a membrane-bound regulator of the complement system found on various cancer cells including mouse B16 melanoma cells, protects malignant cells from CDC, hence promoting tumor growth in vivo and decreasing survival of tumor-bearing animals.2
Natural killer (NK) cells, granulocytic lymphocytes belonging to the innate immune system, are important effectors of cancer immunosurveillance. As they express IgG low affinity receptors (FcγRs), NK cells interact with antigen-bound immunoglobulins, hence becoming activated and producing pro-inflammatory cytokines.3 NK cells mediate cancer immunosurveillance by killing MHC class I-deficient cells, which cannot be recognized by T lymphocytes, and by limiting the metastatic dissemination of malignant cells.4 Furthermore, NK cells can crosstalk with dendritic cells (DCs) resulting not only in their own activation but also in DC maturation.5
Although a number of studies have explored the function of the complement system in various pathophysiological settings, its impact on oncogenesis and tumor progression remains unclear. We have recently set out to investigate the role of the complement system in a mouse model of melanoma, finding that transient decomplementation at the time of T-cell priming with cobra venom factor (CVF) allows for the development of a robust antitumor CD8+ T-cell response that efficiently limits disease progression. This was accompanied by the accumulation of NK cells within the tumor and spleen, and NK cells proved to be essential for the enhancement of antitumor T-cell responses observed upon decomplementation.6
Several mechanisms might explain this phenomenon. On one hand, complement proteins are known to promote the secretion of transforming growth factor β1 (TGFβ1), which facilitates angiogenesis, invasion and metastasis, as well as to limit the expression of the β chain the interleukin-2 (IL-2) receptor and the secretion of interferon γ (IFNγ) by NK cells.7 It has also been shown that the induction of complement component 5a receptor 1 (C5AR1) signaling in Toll-like receptor (TLR)-activated macrophages selectively inhibits the transcription of genes that encode IL-2 family cytokines. These cytokines play an important role in the activation and differentiation of distinct subsets of T cells as well as of NK cells. On the other hand, the clearance of apoptotic cells upon iC3b opsonization, which promotes phagocytosis upon binding to complement component 3 receptor (C3R), can be accompanied by the downregulation of co-stimulatory molecules and impaired DC maturation.8 The recruitment of large numbers of NK cells may be critical for the optimal activation of DCs and the ensuing induction of T-cell responses in these poorly inflammatory conditions. NK-cell activation can occur upon the direct recognition of target cells harboring aberrant self molecules, eventually resulting in DC maturation. In our study, we observed enhanced tumor-specific cytotoxic T lymphocyte (CTL) responses in CVF-treated vs. untreated animals in the absence of significant improvements in DC maturation.6 It is therefore likely that functional DCs have access to increased amounts of tumor-associated antigens (TAAs) in decomplemented mice owing to an improved cytotoxic activity of NK cells, eventually resulting in the elicitation of superior CTL responses.
Another important mechanism used by malignancies to suppress TAA-targeting immune responses is the stimulation of abnormal myelopoiesis and the recruitment of myeloid-derived suppressor cells (MDSCs) expressing high levels of C5AR1 to the tumor microenvironment.9,10 The binding of C5a to C5AR1 on MDSCs promotes their migration and accumulation in the tumor vasculature.10 MDSCs produce immunosuppressive molecules including reactive oxygen and nitrogen species, hence inhibiting cytotoxic CD8+ T lymphocytes as well as NK cells while stimulating the production of mitogenic and pro-angiogenic factors.10
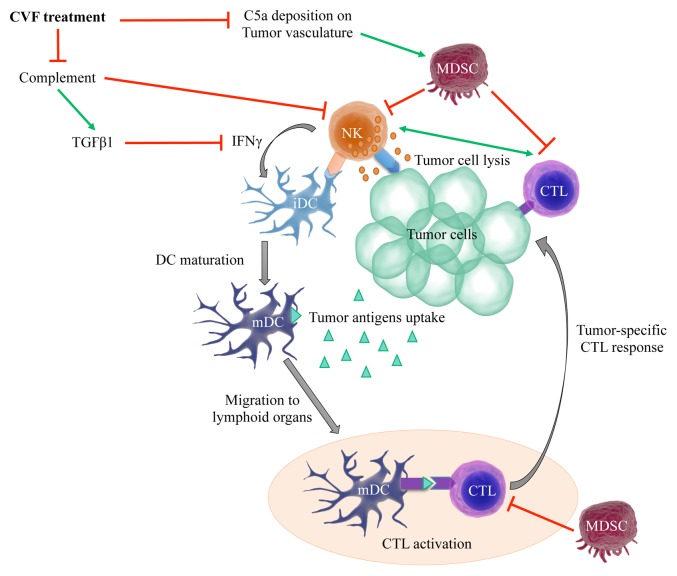
Our results were obtained in a single experimental model. They should therefore be taken with caution, as their extrapolation to other malignancies may not be possible given the properties of the complement system and other tumor types. Nevertheless, we have identified a previously unrecognized function for the complement system in oncogenesis. We demonstrated that complement proteins and NK cells interact to influence tumor growth by limiting the accumulation of tumor-specific CTLs. Furthermore, complement proteins can promote tumor infiltration by MDSCs, which suppress NK- and T-cell functions (Fig. 1). This said, the exact complement proteins involved in this process, how they functionally interact with NK cells as well as the potential mechanisms underlying the observed NK cell-CD8+ T-cell crosstalk still need to be elucidated.
Figure 1. Influence of complement proteins and NK cells on the induction of antitumor T-cell responses. Inhibition of the complement system by the cobra venom factor (CVF) increases the availability of natural killer (NK) cells within neoplastic lesions. This expanded population of NK cells may mediate superior tumoricidal activities, resulting in the release of large amounts of tumor-associated antigens, robust dendritic cell (DC) activation and improved tumor-specific cytotoxic T lymphocyte (CTL) responses. The inhibition of complement proteins can also limit the production of transforming growth factor β1 (TGFβ1) and the recruitment of myeloid-derived suppressor cells (MDSCs) to the tumor microenvironment, hence reducing its immunosuppressive potential and favoring CTL activation. IFNγ, interferon γ; iDC, immature dendritic cell; mDC, mature dendritic cell; TNFα, tumor necrosis factor α.
It will be of great interest to test other inhibitors of complement proteins in various tumor models to determine whether this novel mechanism is generally applicable. Further investigation into the interaction between complement proteins and NK cells is warranted to assist the development of novel therapeutic strategies against cancer.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed
Citation: Janelle V, Lamarre A. Role of the complement system in NK cell-mediated antitumor T-cell responses. OncoImmunology 2014; 3:e27897; 10.4161/onci.27897
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/27897
References
- 1.Venkatesha RT, Berla Thangam E, Zaidi AK, Ali H. Distinct regulation of C3a-induced MCP-1/CCL2 and RANTES/CCL5 production in human mast cells by extracellular signal regulated kinase and PI3 kinase. Mol Immunol. 2005;42:581–7. doi: 10.1016/j.molimm.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 2.Fishelson Z, Donin N, Zell S, Schultz S, Kirschfink M. Obstacles to cancer immunotherapy: expression of membrane complement regulatory proteins (mCRPs) in tumors. Mol Immunol. 2003;40:109–23. doi: 10.1016/S0161-5890(03)00112-3. [DOI] [PubMed] [Google Scholar]
- 3.Arase N, Arase H, Park SY, Ohno H, Ra C, Saito T. Association with FcRgamma is essential for activation signal through NKR-P1 (CD161) in natural killer (NK) cells and NK1.1+ T cells. J Exp Med. 1997;186:1957–63. doi: 10.1084/jem.186.12.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim S, Iizuka K, Aguila HL, Weissman IL, Yokoyama WM. In vivo natural killer cell activities revealed by natural killer cell-deficient mice. Proc Natl Acad Sci U S A. 2000;97:2731–6. doi: 10.1073/pnas.050588297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vitale M, Della Chiesa M, Carlomagno S, Pende D, Aricò M, Moretta L, Moretta A. NK-dependent DC maturation is mediated by TNFalpha and IFNgamma released upon engagement of the NKp30 triggering receptor. Blood. 2005;106:566–71. doi: 10.1182/blood-2004-10-4035. [DOI] [PubMed] [Google Scholar]
- 6.Janelle V, Langlois M-P, Tarrab E, Lapierre P, Poliquin L, Lamarre A. Transient complement inhibition promotes a tumor-specific immune response through the implication of natural killer cells. Cancer Immunology Research 2013. [DOI] [PubMed] [Google Scholar]
- 7.Penafuerte C, Galipeau J. TGF β secreted by B16 melanoma antagonizes cancer gene immunotherapy bystander effect. Cancer Immunol Immunother. 2008;57:1197–206. doi: 10.1007/s00262-008-0453-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morelli AE, Larregina AT, Shufesky WJ, Zahorchak AF, Logar AJ, Papworth GD, Wang Z, Watkins SC, Falo LD, Jr., Thomson AW. Internalization of circulating apoptotic cells by splenic marginal zone dendritic cells: dependence on complement receptors and effect on cytokine production. Blood. 2003;101:611–20. doi: 10.1182/blood-2002-06-1769. [DOI] [PubMed] [Google Scholar]
- 9.Gallina G, Dolcetti L, Serafini P, De Santo C, Marigo I, Colombo MP, Basso G, Brombacher F, Borrello I, Zanovello P, et al. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest. 2006;116:2777–90. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Markiewski MM, DeAngelis RA, Benencia F, Ricklin-Lichtsteiner SK, Koutoulaki A, Gerard C, Coukos G, Lambris JD. Modulation of the antitumor immune response by complement. Nat Immunol. 2008;9:1225–35. doi: 10.1038/ni.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]