Abstract
Depression is a group of brain disorders with varied origins, complex genetics and obscure neurobiology. Definitions of clinical phenotypes are not rooted in their neurobiology, and animal models of behavioural despair have considerable limitations. Nevertheless, investigation of subtle alterations in gene expression, of correlations between genotype and brain activity, and of environmental variables interacting with genetic variants have advanced research into the genetics of depression. Although the postgenomic era is still in its infancy, several milestones have already been reached: variation in gene expression has been confirmed to play a predominant role in individual differences; gene–environment interactions have been established in humans and in a nonhuman primate model; gene–phenotype correlations have been substantiated by functional neuroimaging; and the notion of gene networks that control brain development is increasingly recognized. Given the etiologic and psychobiologic complexity of mood disorders, it is not surprising that the identification of specific genetic factors is extremely difficult and continues to be among the last frontiers of gene hunting.
Medical subject headings: bipolar disorder; depression; environment; gene expression; genetics; models, animal; serotonin
Abstract
La dépression est un ensemble de troubles cérébraux d'origines variées, dont les caractéristiques génétiques sont complexes et la neurobiologie est mal connue. Les définitions des phénotypes cliniques ne sont pas enracinées dans leur neurobiologie et les modèles animaux de désespoir comportemental ont des limites importantes. Les analyses d'altérations subtiles de l'expression génique, de corrélations entre le génotype et l'activité cérébrale, et de l'interaction entre des variables environnementales et des variantes génétiques ont néanmoins fait progresser la recherche sur les caractéristiques génétiques de la dépression. Même si l'ère postgénomique commence à peine, on a déjà franchi plusieurs étapes marquantes : on a confirmé que la variation de l'expression génique joue un rôle prédominant dans les différences individuelles; on a établi des liens entre les gènes et l'environnement chez les êtres humains et dans un modèle de primate non humain; la neuro-imagerie fonctionnelle a confirmé l'existence de liens entre les gènes et le phénotype et l'on reconnaÎt de plus en plus le concept de réseaux géniques qui contrôlent le développement du cerveau. Compte tenu de la complexité étiologique et psychobiologique des troubles de l'humeur, il n'est pas étonnant que des facteurs génétiques spécifiques soient extrêmement difficiles à identifier et qu'ils demeurent aux limites extrêmes de la recherche génique.
Introduction
Depression is an etiologically heterogeneous group of brain disorders characterized by a wide range of symptoms that reflect alterations in cognitive, psychomotor and emotional processes. Affected individuals differ remarkably regarding the profile of clinical features, severity and course of illness as well as their response to drug treatment and reintegration efforts. Genetic epidemiology has assembled convincing evidence that mood disorders including depression are substantially influenced by genetic factors and that the genetic component is highly complex, polygenic and epistatic. Because the mode of inheritance of depression is complex, it has been concluded that multiple genes of modest effect, in interaction with each other and in conjunction with environmental events, produce vulnerability to the disorder. Investigation of gene–environment interactions in humans and nonhuman primates, as well as gene inactivation studies in mice, have further advanced the identification of genes that are essential for the development and plasticity of brain systems related to depression.
Family studies and gene–environment interaction
Epidemiologic studies of unipolar major depression have revealed a population prevalence of 2%–19% and an age-adjusted risk for first-degree relatives of patients with unipolar major depression of 5%–25%.1,2 In a meta-analysis of 5 large and rigorously selected family studies of major depression, familiality in this disease was demonstrated by a relative risk of 2.8 for affected subject versus first-degree relative status.3 Early age of onset and multiple episodes of depression seem to increase the familial aggregation, and different affective disorders are often present in the same family.4 Relatives of patients with bipolar disorder also have an increased risk of unipolar depression, and affective disorders tend to coexist with anxiety in many families.5,6,7
Twin and family-based studies have accrued considerable evidence that a complex genetic mechanism is involved in vulnerability to depressive disorders.8,9 Compared with the general population, first-degree relatives of depressed individuals have a nearly 3-fold increase in their risk of developing a major depressive disorder. In general, twin studies of depressive adults suggest that genes and specific environmental factors are critical and that shared environmental factors, although important in less severe subtypes of depression, are possibly of less significance.10,11,12,13 The heritability of unipolar depression appears to be remarkable, with estimates between 40% and 70%. Depression- associated genetic factors are largely shared with generalized anxiety disorder, whereas environmental determinants seem to be distinct.14,15,16 This notion is consistent with recent models of emotional disorders, which view depression and anxiety as sharing common vulnerabilities but differing in dimensions including, for instance, focus of attention or psychosocial liability. Although life events may precipitate depression, examination of familial liability along with social adversity reveals that environmental effects tend to be contaminated by genetic influences.16,17 The predisposition to suffer life events is likely to be influenced by shared family environment, and some events may be associated with genetic factors.
Whereas genetic research has typically focused either on depression-related traits or on major depressive disorders, with few investigations evaluating the genetic and environmental relation between the two, it is crucial to identify whether a certain quantitative trait pathogenetically influences the disorder or whether the trait is a syndromal dimension of the disorder. The concept of traits influencing disease liability also supports the hypothesis that a genetic predisposition, coupled with early life stress in critical stages of development, may result in a phenotype that is neurobiologically vulnerable to stress and may lower an individual's threshold for developing depression on additional exposure to stress.
Molecular genetics
Linkage analysis of extended pedigrees is a practical approach to identifying disease genes for monogenic diseases that display a mendelian mode of inheritance. In these studies, highly polymorphic genetic markers that are present throughout the genome allow the identification of the chromosomal region containing the disease-relevant genetic variation. Despite meiotic recombination events, marker alleles that remain close to the disease-causing variant tend to cosegregate with the disease within a family. Because mood disorders, including depression, are believed to be heterogeneous in origin, different susceptibility genes may be operative in different families. As large families with monogenic inheritance of depressive disorders are rare, they are unlikely to be representative of the majority of cases. Although a rare disease-causing mutation in a single gene would not explain most cases, identification of such a major gene effect may facilitate the investigation of the poorly understood pathophysiology of mood disorders.
Differential psychopathologic ascertainment aimed at the delineation of distinct clinical subtype (e.g., early age of onset, stress reactivity, suicidality) may increase the probability of identifying a truly monogenic family. In addition to the mode of inheritance, the analysis requires assumptions to be made regarding the penetrance of the variant and the frequency of the susceptibility allele in the general population. For affective disorders, as well as for many other complex diseases, these parameters are not known. Therefore, nonparametric methods, such as the affected pedigree member method, which investigates pairs of affected relatives, in most cases siblings, are preferred. Because siblings share on average 50% of their alleles, pairs of siblings affected by the same disorder will have increased allele sharing for markers close to the disease gene. This method is independent of whether the disease is dominant, recessive or nonmendelian.
Bipolar disorder, a serious affective disorder with a lifetime risk of about 1%, in which individuals suffer from episodes of extreme depression and mania, is by far the most frequently studied mood disorder using linkage analysis. In most families, bipolar disorder is now thought to be influenced by multiple genes, as well as environmental influences.18,19 Gene–gene and gene–environment interactions therefore complicate attempts to understand the cause of this complex disorder. Locus heterogeneity is also thought to be present, in which several distinct genes contribute to the disorder, perhaps even within the same family.
Past linkage studies in unipolar depression suffered from poor design and included only a small number of families,20,21 whereas 2 state-of-the-art genome-wide linkage scans have recently been published and several others are presently in progress. Zubenko et al22 have reported several chromosomal loci that may influence the development of recurrent major depressive disorder in 81 families. The highest maximum logarithm of the odds ratio (LOD) score observed occurred at marker D2S2321 (205 cM), located 121 kb proximal to the cyclic adenosine monophosphate (AMP) response element binding protein 1 (CREB1), a ubiquitous nuclear transcription factor.23 Nineteen chromosomal regions contained linkage peaks that reached genome-wide statistical significance. Six linkage peaks were revealed after an analysis of covariance controlled for the effects of gender and epistatic interaction with the CREB1 locus. Based on a systematic analysis of candidate genes located in the linked chromosomal regions, it is concluded that gene products derived from these genes participate in cellular signalling pathways that converge on CREB and that allelic variants of downstream target genes of CREB may affect the susceptibility of mood disorders. Because CREB is a pivotal regulatory protein in many cell types, additional spatiotemporally specific (protein) factors are likely to contribute to the genetic risk, in order to specify the depression-associated endophenotypes that constitute a clinically relevant depressive syndrome. The next level of complexity will be reached with the identification of those genetic factors that modify the disease mechanism (as well as treatment response and long-term course of illness) in individual patients with a disorder of the depressive spectrum.
Abkevich et al24 conducted a genome-wide scan in 1890 individuals from 110 pedigrees with a strong family history of major depression and provided strong evidence for the existence of a sex-specific disposition locus for major depression on chromosome 12q22–12q23.2. Interestingly, a previous linkage analysis for quantitative trait loci (QTLs) influencing variation in the neuroticism personality trait also identified a gender-specific locus on chromosome 12q23.1.25 Although the findings of these 3 linkage analyses require rigorous replication, they confirm previous evidence that 1 or more genes involved in psychiatric diseases are present on chromosome 12q.
A considerable number of linkage studies have been published, suggesting a number of candidate regions on different chromosomes, including 1q, 4p, 10p, 12q, 13q, 18p, 18q, 21q, 22q and Xq, but no bipolar disorder susceptibility gene has been identified yet.26,27 A recent meta-analysis of all reported genome scans found the strongest evidence for bipolar susceptibility loci on 13q and 22q.28 The same regions were also implicated in schizophrenic spectrum disorders, suggesting sharing of susceptibility genes. Additional loci, including regions on chromosomes 2p, 4q, 6q, 7q, 8q, 9q, 10q, 14q, 16p and 17q,29,30,31,32 have also been linked to bipolar disorder but have yet to be replicated by independent studies. However, although some of these chromosomal regions meet strict criteria for significant evidence of linkage, narrowing the linkage regions and identifying candidate genes have proved difficult, and no genes have yet been conclusively demonstrated to affect risk for bipolar disorder. The inconsistencies appear to result from still widely underestimated genetic heterogeneity and insufficient statistical power to detect loci with smaller effects.
On the other hand, the clinical (categorial) diagnosis, no matter how carefully assessed, does not reflect neurobiologic (dimensional) processes and may, therefore, not be the appropriate phenotype for genetic analysis. The definition of more homogeneous disease phenotypes or clinical subtypes, such as the presence of catatonic features or response to antibipolar treatment, or functional neuroimaging-derived endophenotypes with less genetic complexity, preferably biologically measurable but not necessarily exclusive for the disease, is a prerequisite, if not an absolute requirement, for future study designs.33 Chromosomal rearrangements, such as the balanced translocation of chromosomes 1 and 11 that is associated with schizophrenic and affective disorders or other chromosomal aberrations, such as the 22q11 microdeletion, may also have the potential to identify the chromosomal location of a candidate gene.34 Last, population isolates may be used to increase the probability of all affected individuals having the same disease alleles.
Because the power of linkage analysis to detect small gene effects is quite limited, at least with realistic cohort sizes, molecular genetic research in depression has primarily relied on association analysis using DNA variants in or near candidate genes with etiologic or pathophysiologic relevance. Gene variants with a significant impact on the functionality of components of brain neurotransmission, such as the serotonin (5-HT) system, are a rational starting point. Based on converging lines of evidence that 5-HT and serotonergic gene expression are involved in a myriad of processes during brain development, as well as synaptic plasticity in adulthood, depression-related temperamental predispositions and behaviour are likely to be influenced by genetically driven variability of 5-HT function. Consequently, the contribution of genetic variants of the 5-HT transporter (5-HTT), a protein critically involved in the control of 5-HT function, to the risk of mood disorders, including depression and bipolar disorder, has been explored in several independent population-based and family-based studies.35,36 Moreover, evidence is accumulating that a repeat polymorphism in the 5'-flanking transcriptional control region of the 5-HTT gene (5HTTLPR), resulting in allelic variation of 5-HTT expression and function, is associated with personality traits of negative emotionality including anxiety, depression and aggressiveness (neuroticism and agreeableness).37,38 The short and long 5HTTLPR variants differentially modulate transcriptional activity of the 5-HTT gene promoter, 5-HTT protein concentration, 5-HT uptake activity in lymphoblastoid cells, mRNA concentrations in the raphe complex of the human brain post mortem, platelet 5-HT uptake and content, 5-HT system responsivity elicited by pharmacologic challenge tests, mood changes following tryptophan depletion and in-vivo single-photon emission computed tomography (SPECT) imaging of human brain 5-HTT, with the short variant being associated with lower 5-HTT expression and function.39
Depression-related traits
A growing body of evidence implicates personality traits, such as neuroticism or the anxiety-related cluster, in the comorbidity of mood disorders.6,14,40 The dimensional structure of neuroticism comprising fearfulness, depression, negative emotionality and stress reactivity has been delineated by systematic research. As indexed by the personality scale of neuroticism, general vulnerability is likely to overlap genetically with both anxiety and depression. Separation of depression from depression-related personality disorders in current consensual diagnostic systems has therefore enhanced interest in the link between temperament, personality and mood disorders as well as the impact of this interrelation on the heterogeneity within diagnostic entities, prediction of long-term course and treatment response. This concept may predict that when a QTL, such as 5HTTLPR, is found for neuroticism, the same QTL should be associated with symptoms of anxiety and depression.40 Anxiety and mood disorders are, therefore, likely to represent the extreme end of variation in negative emotionality. The genetic factor contributing to the extreme ends of dimensions of variation commonly recognized as a disorder may be quantitatively, not qualitatively, different from the rest of the distribution. This vista has important implications for identifying genes for complex traits related to distinct disorders.
However, the effect sizes for 5HTTLPR–personality associations indicate that this polymorphism has only a moderate influence on these behavioural predispositions that corresponds to less than 5% of the total variance, based on estimates from twin studies using these and related measures that have consistently demonstrated that genetic factors contribute 40%–60% of the variance in neuroticism and other related personality traits.38 The associations represent only a modest share of the genetic contribution to depression-related and anxiety-related traits. An additive contribution of comparable size or epistatic interaction has, in fact, been found in studies of other quantitative traits. Thus, the results are consistent with the view that the influence of a single, common polymorphism on continuously distributed traits is likely to be modest, if not minimal.
A modulatory effect of allelic variation of 5-HTT function on cortical activity provided the first evidence that genotype–phenotype correlations may be accessible by functional imaging of the brain. Recently, Hariri et al41 reported that individuals with 1 or 2 copies of the low-activity short 5HTTLPR variant exhibit greater amygdala neuronal activity, as assessed by functional magnetic resonance imaging (fMRI), in response to fearful stimuli compared with individuals homozygous for the high-activity long allele. These findings confirm that genetically driven variation of serotonergic function contributes to the response of brain regions underlying human emotional behaviour and indicate that differential excitability of the amygdala to emotional stimuli may contribute to increased fear and anxiety-related responses.
Variants that change the structure of the 5-HTT protein are rare and their potential to alter 5-HT uptake activity remains to be determined.42,43,44,45 Most of these variants have yet to be explored with respect to a functional effect on transport activity or association with a behavioural phenotype or disorder. Nevertheless, 2 nonsynonymous single nucleotide polymorphisms (SNPs) that change the coding sequence of the 5-HTT gene were found to segregate with complex serotonergic dysfunction-related phenotypes including obsessive–compulsive disorder (OCD) and other 5-HT spectrum disorders or were associated with severe depression. OCD is characterized by either obsessions or compulsions that cause marked distress and are time consuming or significantly interfere with an individual's normal routine or functioning. Obsessions are recurrent and persistent ideas, thoughts, impulses or images that an individual attempts to ignore or suppress, whereas compulsions are repetitive, purposeful and intentional behaviours performed in response to an obsession to neutralize or prevent discomfort, worry and anxiety or some dreaded event. OCD often co-occurs with other disorders, including depression.
A missense mutation resulting in a conserved Ile-425-Val substitution in the 5-HTT gene was detected in 2 affected individuals and their family members with OCD and related disorders.46 Six of 7 family members with the variant had OCD or OC personality disorder. In addition, the affected individuals and their immediate family members carrying the Ile-425-Val variant met diagnostic criteria for other disorders including autism, social phobia, anorexia nervosa, tic disorder, depression and alcohol abuse or dependence. The evolutionary conserved Ile-425-Val substitution is located in transmembrane domain 8 (TMD8) and may modify the α-helical secondary structure of the 5-HTT protein and, consequently, transport function. Studies of the expression of the mutant 5-HTT gene cDNA in human cells demonstrated a gain of function through constitutive activation of 5-HT transport in a nitric oxide-stimulated pathway resulting in a 2-fold increase in 5-HT uptake.47 Taken together, these findings strongly indicate that gain-of-function mutations associated with coding sequence may contribute to the expression of psychopathology related to serotonergic dysfunction in some families.
Interestingly, 2 brothers from one of the families with OCD and autism who carried the Ile-425-Val variant also had the long/long 5HTTLPR genotype that was previously found to be associated with or preferentially transmitted in both OCD48,49 and autism.50,51 Moreover, a conservative Leu-255-Met substitution located in TMD4 was detected in a patient with delusional depression, who was also found to carry a short/short 5HTTLPR genotype, which is implicated in anxiety- related and depression-related traits.43 Possibly, low 5-HTT gene expression in interaction with the Leu-255-Met variant, which is highly conserved among various species, could additively perturb 5-HTT function or regulation. These 2 examples of co-occurrence and possible cooperativity of allelic variation in gene expression and protein structure might represent a “double hit” with functional consequences in the same gain-of-function and loss-of-function direction for both of these 5-HTT gene variations.52
Analogous to the 5-HTT gene and its allelic variation of expression, a functional SNP (C-1019G) in the transcriptional control region of the gene for the 5-HT1A receptor (HTR1A) is associated with anxiety-related and depression-related personality traits,53 as well as depression and suicidality. In-vitro experiments demonstrated that the G variant displays differential binding efficiency of transcriptional regulators (repressors/enhancers) that may lead to allelic variation of 5-HT1A receptor expression and function.54 The agoraphobic subtype of panic disorder also appears to be associated with HTR1A.55 These findings further support multiple lines of evidence that implicate the 5-HT1A receptor in the pathophysiology of anxiety and depression, as well as in the mode of action of anxiolytic and antidepressant drugs. Patients with panic disorder and depression exhibit an attenuation of 5-HT1A receptor-mediated hypothermic and neuroendocrine responses, reflecting dysfunction of both presynaptic and postsynaptic 5-HT1A receptors.56,57 Likewise, a decrease in ligand binding to 5-HT1A receptors, as assessed by positron emission tomography, has been shown in forebrain areas and in the raphe of depressed patients.58,59 Downregulation and hyporesponsivity of 5HT1A receptors in patients with major depression are not reversed by antidepressant drug treatment,59,60,61 thus further supporting the notion that low receptor function is a trait and, therefore, a pathogenetic mechanism of the disease.
Gene-expression profiling
Large-scale, high-throughput, cDNA microarray technology now allows the identification of altered gene expression patterns in the human postmortem brain of patients with neuropsychiatric disorders including depression, bipolar disorder and schizophrenia, which is an important step in understanding gene function in these complex disorders. Changes in expression of a number of genes have been reported in bipolar disorder. A meta-analysis of studies using microarrays and other techniques has identified modifications in the expression of genes related to neurotransmission, survival of neuronal and glial cells, and signal transduction.62,63 Depression, bipolar disorder and schizophrenia seem to share a number of changes in gene expression. Whereas bipolar disorder is associated with low expression of several genes and more closely resembles schizophrenia in the overall pattern of changes, depression shows the smallest number of modifications. Intriguingly, decreased glial fibrillary acidic protein levels and numbers of oligodendrocytes were observed in depression, bipolar disorder and schizophrenia, which suggests that glial dysfunction may be a common substrate for all of these disorders. These observations match the increasing number of reports that describe glial abnormalities in neuropsychiatric disorders. Because glial cells are central to homeostatic and regulatory processes that affect neuronal development, plasticity and survival, this concept suggests that glial regulation of nitric oxide, glutamate, dopamine or serotonin neurotransmission, or calcium homeostasis may have special importance for these disorders. Finally, profiling of gene-expression patterns associated with clinically effective drugs in cell systems and in the animal model represents a complementary approach and may provide new insights into understanding drug mechanisms.64,65,66
Animal models
Quantitative genetic research on animal models consists primarily of inbred strain and selection studies. Whereas comparisons between different inbred strains of mice expose remarkable differences in measures of depression-related and anxiety-related behaviour, differences within strains can be attributed to environmental influences. Inbred and recombinant inbred strain studies are highly efficient in dissecting genetic influences, for investigating interactions between genotype and environment, and for testing the disposition–stress model.
Mice strains that have been selectively bred to display a phenotype of interest are currently being used to identify genetic loci that contribute to behavioural traits, including fearfulness, emotionality and behavioural despair. However, linkage analyses provide only a rough chromosomal localization, whereas the next step, identifying the relevant genes by positional cloning, remains a challenging task. Because mice and humans share many orthologous genes mapped to syntenic chromosomal regions, it is conceivable that individual genes identified for one or more types of murine fear-related behaviour may be developed as animal models for human anxiety. Following chromosomal mapping of polymorphic genes and evaluation of gene function using genetically engineered mice, behavioural parameters are investigated. As an example, findings in mice with a targeted inactivation of the 5-HTT gene emphasize the relevance of adaptive 5-HT uptake function and 5-HT homeostasis in the developing human brain as well as molecular processes underlying anxiety-related traits,67,68,69,70,71,72 in addition to 5-HT spectrum disorders including depression and bipolar disorder.36 Despite growing evidence for a potential role of 5-HTT in the integration of synaptic connections in the rodent, nonhuman primate and human brain during critical periods of development, adult life and old age, knowledge of the molecular mechanisms involved in these fine-tuning processes remains fragmentary.66,72 Thus, the combination of elaborate genetic and behavioural analyses may lead to the identification of many genes with effects on the variation and development of murine depression-related and anxiety-related behaviour.
Finally, recent advances in conditional knockout and knockin (and overexpression) techniques in mice increasingly affect our understanding of the neurobiologic basis and the neurodevelopmental processes of depression-related and anxiety-related behaviour.72 However, the majority of neural substrates and circuitries that regulate emotional processes or cause mood disorders remain remarkably elusive. Among the reasons for the lack of progress are several conceptional deficiencies regarding the psychobiology of emotionality and behavioural despair, which make it difficult to develop and validate reliable models of depression. The clinical presentation of mood disorders and the lack of consensus on clinical categories further complicates the development of mouse models for depressive disorders. The dilemma that no single paradigm mimics the diagnostic entities or treatment response of mood disorders may reflect the inadequacy of classification rather than failure to develop valid mouse models.
Gene–environment interaction in humans and in the nonhuman primate model
Because the genetic basis of present-day temperamental and behavioural traits may reflect selective forces among our remote ancestors, research efforts have recently been focused on nonhuman primates, especially rhesus macaques. In this primate model, environmental influences are probably less complex, can be more easily controlled for and are thus less likely to confound associations between behaviour and genes. All forms of emotionality in rhesus monkeys (major categories are anxiety and aggression) appear to be modulated by environmental factors, and marked disruptions to the mother–infant relationship probably confer increased risk.
One of the most replicated findings in psychobiology is the observation of lower 5-HIAA, the major metabolite of 5-HT, in the brain and cerebrospinal fluid (CSF) in impulsive aggression and suicidal behaviour. In rhesus monkeys, brain 5-HT turnover, as measured by cisternal CSF 5-HIAA concentrations, shows a strong heritable component and is trait-like, with demonstrated stability over an individual's lifetime.73,74,75 Early experiences have long-term consequences for the function of the central 5-HT system, as indicated by robustly altered CSF 5-HIAA levels, as well as anxiety, depression and aggression-related behaviour, in rhesus monkeys deprived of their mother at birth and raised only with peers. This model of maternal separation was therefore used to study gene–environment interaction by testing for associations between central 5-HT turnover and the polymorphism in the transcriptional control region of the 5-HTT gene (rh5HTTLPR).76 The findings suggest that the rh5HTTLPR genotype is predictive of CSF 5-HIAA concentrations in rhesus monkeys, but that early experiences make unique contributions to variation in later 5-HT system function, which provides evidence of an environment-dependent association between the 5-HTT gene and a direct measure of brain 5-HT turnover and thus, ultimately, function. The consequences of deleterious early experiences of maternal separation seem consistent with the notion that 5HTTLPR may influence the risk for mood disorders.
The interactive effect of the rh5HTTLPR genotype and early rearing environment on social play and aggression was also explored.77 Infant rhesus monkeys homozygous for the long variant were more likely to engage in rough play than were individuals with the long/short variant, with a significant interaction between the 5-HTT genotype and rearing condition. Peer-reared infants carrying the short variant were less likely to play with peers than those homozygous for the long allele, whereas the rh5HTTLPR genotype had no effect on the incidence of social play among mother-reared monkeys. Socially dominant mother-reared monkeys were more likely than their peer-reared counterparts to engage in aggression. In contrast, peer-reared but not mother-reared monkeys with the low-activity short allele exhibited more aggressive behaviours than their long/long counterparts. This genotype by rearing interaction for aggressive behaviour indicates that peer-reared subjects with the short allele, while unlikely to win in a competitive encounter, are more inclined to persist in aggression once it begins. Moreover, high composite scores for alcohol intake and alcohol-elicited aggression are associated with the low-activity short rh5HTTLPR variant in male rhesus monkeys, which provides a potential model for type II alcoholism.78
Taken together, these findings provide evidence of an environment-dependent association between allelic variation of 5-HTT expression and central 5-HT function, and they illustrate the possibility that specific genetic factors play a role in 5-HT-mediated behaviour in primates. Because rhesus monkeys exhibit temperamental and behavioural traits that parallel anxiety, depression and aggression-related personality dimensions observed in humans with the low-activity 5HTTLPR variant, it may be possible to search for evolutionary continuity in the genetic mechanism for individual differences. Nonhuman primate studies may also be useful in helping to identify environmental factors that either compound the vulnerability conferred by a particular genetic makeup or, conversely, act to improve the behavioural outcome associated with a distinct genetic makeup.
Consequently, it is increasingly accepted that much of the impact of genes on emotionality, including anxiety and depression, depends on interactions between genes and the environment. Such interactions would lead to the expression of environmental effects only in the presence of a permissive genetic background. Not unexpectedly, a recent study by Caspi et al79 robustly confirmed that individuals with 1 or 2 short versions of 5HTTLPR are up to 2 times more likely to get depressed after stressful events such as bereavement, romantic disasters, illnesses or losing their job. Moreover, childhood maltreatment significantly increased the probability of developing depressive syndromes in later life in individuals with the low-activity short allele of 5HTTLPR. These results further support the notion that a combination of genetic disposition and specific life events may interact to facilitate the development of mental illness. What went largely unconsidered, though, were its implications for the relevance of studying the genetics of personality. Depression is strongly associated with anxiety-related and depression-related traits, the personality dimensions that have been linked to allelic variation of 5-HTT function. Given the high comorbidity between anxiety and depression and the evidence for their modulation by common genetic factors,80 it is likely that predisposition to mood disorders will also be determined by environmental influences whose impact on the brain is under genetic control.
Another finding by Caspi et al,79 that early trauma inflicted by childhood maltreatment interacts with allelic variation of 5-HTT function increases vulnerability to development of mood disorders, is particularly interesting. A remarkable body of evidence suggests that emotionality and stress reactivity can be influenced by experiences early in life, and it has long been supposed that severe early life trauma may increase the risk for anxiety and affective disorders.81 For example, adults who experience 4 of a list of 7 severe early traumatic events showed a more than 4-fold increased risk of depressive symptoms and about a 12-fold increased risk of attempted suicide.82 No direct correlation between any specific childhood trauma and a specific adult anxiety or mood disorder could be made, however, suggesting that other, possibly genetic, factors determine the precise pathology that is precipitated by the traumatic event. The observation that individuals are particularly susceptible to adverse environmental influences during early developmental stages is confirmed by animal studies that have demonstrated the influential effects of the quality of maternal care on lifelong emotional behaviour and brain functioning.
Conclusion
Although monoaminergic dysfunction is likely to occur in depression, pathogenetic mechanisms continue to be inadequately understood at the neuronal and molecular level. A complementary approach to genetic studies of depression and related disorders involves investigation of genes (i.e., construction of transcriptome maps) and their protein products (i.e., application of proteomics) implicated in the brain neurocircuitries of emotionality and behavioural despair in animal models. Based on converging lines of evidence that genetically driven variability of expression and function of proteins that regulate the function of brain neurotransmitter systems (e.g., receptors, ion channels, transporters, enzymes) are associated with complex behavioural traits, research is now giving emphasis to the molecular basis of depression-related and anxiety-related behaviours in rodents and, increasingly, nonhuman primates.
More functionally relevant polymorphisms in genes within a single neurotransmitter system, or in genes that constitute a functional unit in their concerted actions, need to be identified and assessed in both large population-based and family-based association studies to avoid stratification artifacts and to elucidate complex interactions of multiple loci. Even pivotal regulatory proteins of neurotransmission, such as receptors, transporters and modifying enzymes, will have only a modest impact, whereas noise from nongenetic mechanisms may seriously obstruct identification of relevant genes. Although current methods for the detection of gene–environment interaction in behavioural genetics are largely indirect, the most relevant consequence of gene identification for behavioural traits related to depression may be that it will provide the tools required to systematically clarify the effects of gene–environment interaction.
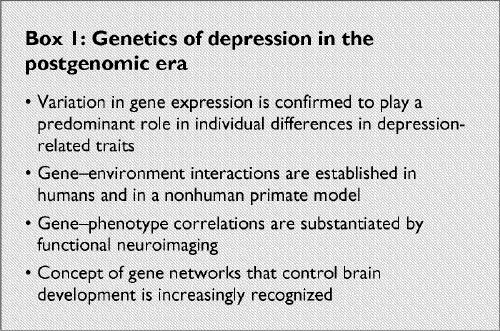
Although depression confronts geneticists with similar challenges to other complex medical diseases, such as hypertension or diabetes, it is unique in that it is a consequence of the dysfunctional master control organ, the brain. Although the postgenomic era is still in its infancy, several milestones have already been reached (Box 1): variation in gene expression has been confirmed to play a predominant role in individual differences; gene–environment interactions have been established in humans and in a nonhuman primate model; gene–phenotype correlations have been substantiated by functional neuroimaging; and the notion of gene networks that control brain development is increasingly recognized. Given the psychobiologic complexity of depressive disorders, it is not surprising that the identification of specific genetic factors is extremely difficult and continues to be among the last frontiers of gene hunting. Future research will take advantage of the completion of the sequencing of the human and mouse genome coinciding with the revolution in bioinformatics. Integration of these emerging technologies for genetic analysis will provide the basis for gene identification and functional studies in depression.
Box 1.
Acknowledgments
The author's work is supported by a grant from the European Commission entitled “New molecules in mood disorders: a genomic, neurobiological and systems approach in animal models and human disorder.”
Footnotes
Competing interests: None declared.
Correspondence to: Dr. Klaus Peter Lesch, Molecular and Clinical Psychobiology, Department of Psychiatry and Psychotherapy, University of Würzburg, Füchsleinstrasse 15, 97080 Würzburg, Germany; fax 49-931-20177620; kplesch@mail.uni-wuerzburg.de
Submitted July 4, 2003; Revised Feb. 20, 2004; Accepted Mar. 1, 2004
References
- 1.Farmer A, Harris T, Redman K, Sadler S, Mahmood A, McGuffin P. Cardiff depression study. A sib-pair study of life events and familiality in major depression. Br J Psychiatry 2000; 176:150-5. [DOI] [PubMed]
- 2.Harrington RC, Rutter M, Weissman M. Psychiatric disorders in the relatives of depressed probands I: comparison of pre- pubertal, adolescent and early adult onset cases. J Affect Disord 1997; 42:9-22. [DOI] [PubMed]
- 3.Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry 2000; 157:1552-62. [DOI] [PubMed]
- 4.Kovacs M, Devlin B, Pollock M, Richards C, Mukerji P. A controlled family history study of childhood-onset depressive disorder. Arch Gen Psychiatry 1997;54:613-23. [DOI] [PubMed]
- 5.Merikangas KR, Chakravarti A, Moldin SO, Araj H, Blangero JC, Burmeister M, et al. Future of genetics of mood disorders research. Biol Psychiatry 2002;52:457-77. [DOI] [PubMed]
- 6.Thapar A, McGuffin P. Anxiety and depressive symptoms in childhood—a genetic study of comorbidity. J Child Psychol Psychiatry 1997;38:651-6. [DOI] [PubMed]
- 7.Jones I, Craddock N. Candidate gene studies of bipolar disorder. Ann Med 2001;33:248-56. [DOI] [PubMed]
- 8.Kendler KS. Major depression and the environment: a psychiatric genetic perspective. Pharmacopsychiatry 1998;31:5-9. [DOI] [PubMed]
- 9.Malhi GS, Moore J, McGuffin P. The genetics of major depressive disorder. Curr Psychiatry Rep 2000;2:165-9. [DOI] [PubMed]
- 10.Kendler KS, Neale MC, Sullivan P, Corey LA, Gardner CO, Prescott CA. A population-based twin study in women of smoking initiation and nicotine dependence. Psychol Med 1999;29:299-308. [DOI] [PubMed]
- 11.Lyons MJ, Eisen SA, Goldberg J, True W, Lin N, Meyer JM, et al. A registry-based twin study of depression in men. Arch Gen Psychiatry 1998;55:468-72. [DOI] [PubMed]
- 12.McGuffin P, Katz R, Watkins S, Rutherford J. A hospital-based twin register of the heritability of DSM-IV unipolar depression. Arch Gen Psychiatry 1996;53:129-36. [DOI] [PubMed]
- 13.Silberg J, Pickles A, Rutter M, Hewitt J, Simonoff E, Maes H, et al. The influence of genetic factors and life stress on depression among adolescent girls. Arch Gen Psychiatry 1999;56:225-32. [DOI] [PubMed]
- 14.Kendler KS, Walters EE, Neale MC, Kessler RC, Heath AC, Eaves LJ. The structure of the genetic and environmental risk factors for six major psychiatric disorders in women. Phobia, generalized anxiety disorder, panic disorder, bulimia, major depression, and alcoholism. Arch Gen Psychiatry 1995;52:374-83. [DOI] [PubMed]
- 15.Kendler KS, Kessler RC, Walters EE, MacLean C, Neale MC, Heath AC, et al. Stressful life events, genetic liability, and onset of an episode of major depression in women. Am J Psychiatry 1995;152:833-42. [DOI] [PubMed]
- 16.Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry 1999;156:837-41. [DOI] [PubMed]
- 17.Thapar A, Harold G, McGuffin P. Life events and depressive symptoms in childhood—shared genes or shared adversity? A research note. J Child Psychol Psychiatry 1998;39:1153-8. [PubMed]
- 18.Taylor L, Faraone SV, Tsuang MT. Family, twin, and adoption studies of bipolar disease. Curr Psychiatry Rep 2002;4:130-3. [DOI] [PubMed]
- 19.Craddock N, Khodel V, Van Eerdewegh P, Reich T. Mathematical limits of multilocus models: the genetic transmission of bipolar disorder. Am J Hum Genet 1995;57:690-702. [PMC free article] [PubMed]
- 20.Neiswanger K, Zubenko GS, Giles DE, Frank E, Kupfer DJ, Kaplan BB. Linkage and association analysis of chromosomal regions containing genes related to neuroendocrine or serotonin function in families with early-onset, recurrent major depression. Am J Med Genet 1998;81:443-9. [PubMed]
- 21.Balciuniene J, Yuan QP, Engstrom C, Lindblad K, Nylander PO, Sundvall M, et al. Linkage analysis of candidate loci in families with recurrent major depression. Mol Psychiatry 1998;3:162-8. [DOI] [PubMed]
- 22.Zubenko GS, Maher B, Hughes HB 3rd, Zubenko WN, Stiffler JS, Kaplan BB, et al. Genome-wide linkage survey for genetic loci that influence the development of depressive disorders in families with recurrent, early-onset, major depression. Am J Med Genet 2003;123B:1-18. [DOI] [PubMed]
- 23.Zubenko GS, Hughes HB 3rd, Stiffler JS, Brechbiel A, Zubenko WN, Maher BS, et al. Sequence variations in CREB1 cosegregate with depressive disorders in women. Mol Psychiatry 2003;8:611-8. [DOI] [PubMed]
- 24.Abkevich V, Camp NJ, Hensel CH, Neff CD, Russell DL, Hughes DC, et al. Predisposition locus for major depression at chromosome 12q22-12q23.2. Am J Hum Genet 2003;73:1271-81. [DOI] [PMC free article] [PubMed]
- 25.Fullerton J, Cubin M, Tiwari H, Wang C, Bomhra A, Davidson S, et al. Linkage analysis of extremely discordant and concordant sibling pairs identifies quantitative-trait loci that influence variation in the human personality trait neuroticism. Am J Hum Genet 2003;72:879-90. [DOI] [PMC free article] [PubMed]
- 26.Baron M. Manic-depression genes and the new millennium: poised for discovery. Mol Psychiatry 2002;7:342-58. [DOI] [PubMed]
- 27.Sklar P. Linkage analysis in psychiatric disorders: the emerging picture. Annu Rev Genomics Hum Genet 2002;3:371-413. [DOI] [PubMed]
- 28.Badner JA, Gershon ES. Meta-analysis of whole-genome linkage scans of bipolar disorder and schizophrenia. Mol Psychiatry 2002;7:342-58. [DOI] [PubMed]
- 29.Dick DM, Foroud T, Flury L, Bowman ES, Miller MJ, Rau NL, et al. Genomewide linkage analyses of bipolar disorder: a new sample of 250 people from the National Institute of Mental Health Genetics Initiative [published erratum appears in Am J Hum Genet 2003;73:979]. Am J Hum Genet 2003;73:104-14. [DOI] [PMC free article] [PubMed]
- 30.Nurnberger JI Jr, Foroud T. Genetics of bipolar affective disorder. Curr Psychiatry Rep 2000;2:147-57. [DOI] [PubMed]
- 31.Liu J, Juo SH, Dewan A, Grunn A, Tong X, Brito M, et al. Evidence for a putative bipolar disorder locus on 2p13–16 and other potential loci on 4q31, 7q34, 8q13, 9q31, 10q21–24, 13q32, 14q21 and 17q11–12. Mol Psychiatry 2003;8:333-42. [DOI] [PubMed]
- 32.Bennett P, Segurado R, Jones I, Bort S, McCandless F, Lambert D, et al. The Wellcome trust UK-Irish bipolar affective disorder sibling-pair genome screen: first stage report. Mol Psychiatry 2002;7:189-200. [DOI] [PubMed]
- 33.Leboyer M, Bellivier F, Nosten-Bertrand M, Jouvent R, Pauls D, Mallet J. Psychiatric genetics: search for phenotypes. Trends Neurosci 1998;21:102-5. [DOI] [PubMed]
- 34.MacIntyre DJ, Blackwood DH, Porteous DJ, Pickard BS, Muir WJ. Chromosomal abnormalities and mental illness. Mol Psychiatry 2003;8:275-87. [DOI] [PubMed]
- 35.Lesch KP. Neuroticism and serotonin: a developmental genetic perspective. In: Plomin R, DeFries J, Craig I, McGuffin P, editors. Behavioral genetics in the postgenomic era. Washington: American Psychiatric Press; 2002. p. 389-423.
- 36.Lesch KP. Serotonin transporter: from genomics and knockouts to behavioral traits and psychiatric disorders. In: Briley M, Sulser F, editors. Molecular genetics of mental disorders. London: Martin Dunitz Publishers; 2001. p. 221-67.
- 37.Greenberg BD, Li Q, Lucas FR, Hu S, Sirota LA, Benjamin J, et al. Association between the serotonin transporter promoter polymorphism and personality traits in a primarily female population sample. Am J Med Genet 2000;96:202-16. [DOI] [PubMed]
- 38.Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 1996;274:1527-31. [DOI] [PubMed]
- 39.Lesch KP, Greenberg BD, Higley JD, Murphy DL. Serotonin transporter, personality, and behavior: toward dissection of gene–gene and gene–environment interaction. In: Benjamin J, Ebstein R, Belmaker RH, editors. Molecular genetics and the human personality. Washington: American Psychiatric Press; 2002. p. 109-35.
- 40.Lesch KP. Genetic dissection of anxiety and related disorders. In: Nutt D, Ballenger T, editors. Anxiety disorders. Oxford: Blackwell; 2003. p. 229-50.
- 41.Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science 2002;297:400-3. [DOI] [PubMed]
- 42.Glatt CE, DeYoung JA, Delgado S, Service SK, Giacomini KM, Edwards RH, et al. Screening a large reference sample to identify very low frequency sequence variants: comparisons between two genes. Nat Genet 2001;27:435-8. [DOI] [PubMed]
- 43.Di Bella D, Catalano M, Balling U, Smeraldi E, Lesch KP. Systematic screening for mutations in the coding region of the 5-HTT gene using PCR and DGGE. Am J Med Genet 1996;67:541-5. [DOI] [PubMed]
- 44.Lesch KP, Gross J, Wolozin BL, Murphy DL, Riederer P. Primary structure of the serotonin transporter in unipolar depression and bipolar disorder. Biol Psychiatry 1995;37:215-23. [DOI] [PubMed]
- 45.Altemus M, Murphy DL, Greenberg B, Lesch KP. Intact coding region of the serotonin transporter in obsessive–compulsive disorder. Am J Med Genet 1996;67:104-9. [DOI] [PubMed]
- 46.Ozaki N, Goldman D, Kaye WH, Plotnicov K, Greenberg BD, Lappalainen J, et al. Serotonin transporter missense mutation associated with a complex neuropsychiatric phenotype. Mol Psychiatry 2003;8:895. [DOI] [PubMed]
- 47.Kilic F, Murphy DL, Rudnick G. A human serotonin transporter mutation causes constitutive activation of transport activity. Mol Pharmacol 2003;64:440-6. [DOI] [PubMed]
- 48.Bengel D, Greenberg BD, Cora-Locatelli G, Altemus M, Heils A, Li Q, et al. Association of the serotonin transporter promoter regulatory region polymorphism and obsessive–compulsive disorder. Mol Psychiatry 1999;4:463-6. [DOI] [PubMed]
- 49.McDougle CJ, Epperson CN, Price LH, Gelernter J. Evidence for linkage disequilibrium between serotonin transporter protein gene (SLC6A4) and obsessive compulsive disorder. Mol Psychiatry 1998;3:270-3. [DOI] [PubMed]
- 50.Klauck SM, Poustka F, Benner A, Lesch KP, Poustka A. Serotonin transporter (5-HTT) gene variants associated with autism? Hum Mol Genet 1997;6:2233-8. [DOI] [PubMed]
- 51.Yirmiya N, Pilowsky T, Nemanov L, Arbelle S, Feinsilver T, Fried I, et al. Evidence for an association with the serotonin transporter promoter region polymorphism and autism. Am J Med Genet 2001;105:381-6. [DOI] [PubMed]
- 52.Lesch KP, Murphy DL. Molecular genetics of transporters for norepinephrine, dopamine, and serotonin in behavioral traits and complex diseases. In: Bröer S, Wagner CA, editors. Membrane transporter diseases. New York: Kluwer Academic/ Plenum; 2003. p. 349-64.
- 53.Strobel A, Gutknecht L, Rothe C, Reif A, Mössner R, Zeng Y, et al. Allelic variation in 5-HT1A receptor expression is associated with anxiety- and depression-related personality traits. J Neural Transm 2004;110:1445-53. [DOI] [PubMed]
- 54.Lemonde S, Turecki G, Bakish D, Du L, Hrdina PD, Brown CD, et al. Impaired repression at a 5-hydroxytryptamine 1A receptor gene polymorphism associated with major depression and suicide. J Neurosci 2003;23:8788-99. [DOI] [PMC free article] [PubMed]
- 55.Rothe C, Gutknecht L, Freitag C, Tauber R, Mossner R, Franke P, et al. Association of a functional -1019C>G 5-HT1A receptor gene polymorphism with panic disorder with agoraphobia [electronic publication ahead of print]. Int J Neuropsychopharmacol 2004;1-4. [DOI] [PubMed]
- 56.Lesch KP, Mayer S, Disselkamp-Tietze J, Hoh A, Wiesmann M, Osterheider M, et al. 5-HT1A receptor responsivity in unipolar depression. Evaluation of ipsapirone-induced ACTH and cortisol secretion in patients and controls. Biol Psychiatry 1990;28:620-8. [DOI] [PubMed]
- 57.Lesch KP, Wiesmann M, Hoh A, Muller T, Disselkamp-Tietze J, Osterheider M, et al. 5-HT1A receptor-effector system responsivity in panic disorder. Psychopharmacology (Berl) 1992;106:111-7. [DOI] [PubMed]
- 58.Drevets WC, Frank E, Price JC, Kupfer DJ, Holt D, Greer PJ, et al. PET imaging of serotonin 1A receptor binding in depression. Biol Psychiatry 1999;46:1375-87. [DOI] [PubMed]
- 59.Sargent PA, Kjaer KH, Bench CJ, Rabiner EA, Messa C, Meyer J, et al. Brain serotonin1A receptor binding measured by positron emission tomography with [11C]WAY-100635: effects of depression and antidepressant treatment. Arch Gen Psychiatry 2000;57:174-80. [DOI] [PubMed]
- 60.Lesch KP, Disselkamp-Tietze J, Schmidtke A. 5-HT1A receptor function in depression: effect of chronic amitriptyline treatment. J Neural Transm Gen Sect 1990;80:157-61. [DOI] [PubMed]
- 61.Lesch KP, Hoh A, Schulte HM, Osterheider M, Muller T. Long-term fluoxetine treatment decreases 5-HT1A receptor responsivity in obsessive–compulsive disorder. Psychopharmacology (Berl) 1991;105:415-20. [DOI] [PubMed]
- 62.Knable MB, Torrey EF, Webster MJ, Bartko JJ. Multivariate analysis of prefrontal cortical data from the Stanley Foundation Neuropathology Consortium. Brain Res Bull 2001;55:651-9. [DOI] [PubMed]
- 63.Knable MB, Barci BM, Bartko JJ, Webster MJ, Torrey EF. Molecular abnormalities in the major psychiatric illnesses: classification and regression tree (CRT) analysis of post-mortem prefrontal markers. Mol Psychiatry 2002;7:392-404. [DOI] [PubMed]
- 64.Bosetti F, Seemann R, Bell JM, Zahorchak R, Friedman E, Rapoport SI, et al. Analysis of gene expression with cDNA microarrays in rat brain after 7 and 42 days of oral lithium administration. Brain Res Bull 2002;57:205-9. [DOI] [PubMed]
- 65.Lesch KP, Schmitt A. Antidepressants and gene expression profiling: How to SNARE novel drug targets. Pharmacogenomics J 2002;2:346-8. [DOI] [PubMed]
- 66.Lesch KP. Serotonergic gene expression and depression: implications for developing novel antidepressants. J Affect Disord 2001;62:57-76. [DOI] [PubMed]
- 67.Murphy DL, Li Q, Engel S, Wichems C, Andrews A, Lesch KP, et al. Genetic perspectives on the serotonin transporter. Brain Res Bull 2001;56:487-94. [DOI] [PubMed]
- 68.Holmes A, Yang RJ, Lesch KP, Crawley JN, Murphy DL. Mice lacking the serotonin transporter exhibit abnormalities in exploratory locomotion and anxiety-like behavior. Neuropsychopharmacology 2003;28:2077-88. [DOI] [PubMed]
- 69.Bengel D, Murphy DL, Andrews AM, Wichems CH, Feltner D, Heils A, et al. Altered brain serotonin homeostasis and locomotor insensitivity to 3,4-methylenedioxymethamphetamine (“ecstasy”) in serotonin transporter-deficient mice. Mol Pharmacol 1998;53:649-55. [DOI] [PubMed]
- 70.Persico AM, Revay RS, Mössner R, Hall FS, Revay RS, Sora I, et al. Barrel pattern formation in somatosensory cortical layer IV requires serotonin uptake by thalamocortical endings, while vesicular monoamine release is necessary for development of supragranular layers. J Neurosci 2001;21:6862-73. [DOI] [PMC free article] [PubMed]
- 71.Salichon N, Gaspar P, Upton AL, Picaud S, Hanoun N, Hamon M, et al. Excessive activation of serotonin (5-HT) 1B receptors disrupts the formation of sensory maps in monoamine oxidase a and 5-HT transporter knock-out mice. J Neurosci 2001;21: 884-96. [DOI] [PMC free article] [PubMed]
- 72.Lesch KP, Zeng Y, Reif A, Gutknecht L. Anxiety-related traits in mice with modified genes of the serotonergic pathway. Eur J Pharmacol 2003;480:185-204. [DOI] [PubMed]
- 73.Higley JD, Suomi SJ, Linnoila M. CSF monoamine metabolite concentrations vary according to age, rearing, and sex, and are influenced by the stressor of social separation in rhesus monkeys. Psychopharmacology 1991;103:551-6. [DOI] [PubMed]
- 74.Higley JD, Thompson WW, Champoux M, Goldman D, Hasert MF, Kraemer GW, et al. Paternal and maternal genetic and environmental contributions to cerebrospinal fluid monoamine metabolites in rhesus monkeys (Macaca mulatta). Arch Gen Psychiatry 1993;50:615-23. [DOI] [PubMed]
- 75.Kraemer GW, Ebert MH, Schmidt DE, McKinney WT. A longitudinal study of the effect of different social rearing conditions on cerebrospinal fluid norepinephrine and biogenic amine metabolites in rhesus monkeys. Neuropsychopharmacology 1989;2:175-89. [DOI] [PubMed]
- 76.Bennett AJ, Lesch KP, Heils A, Long JC, Lorenz JG, Shoaf SE, et al. Early experience and serotonin transporter gene variation interact to influence primate CNS function. Mol Psychiatry 2002;7:118-22. [DOI] [PubMed]
- 77.Barr CS, Newman TK, Becker ML, Parker CC, Champoux M, Lesch KP, et al. The utility of the non-human primate; model for studying gene by environment interactions in behavioral research. Genes Brain Behav 2003;2:336-40. [DOI] [PubMed]
- 78.Barr CS, Newman TK, Becker ML, Champoux M, Lesch KP, Suomi SJ, et al. Serotonin transporter gene variation is associated with alcohol sensitivity in rhesus macaques exposed to early-life stress. Alcohol Clin Exp Res 2003;27:812-7. [DOI] [PubMed]
- 79.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 2003;301:386-9. [DOI] [PubMed]
- 80.Kendler KS. Major depression and generalised anxiety disorder. Same genes, (partly) different environments—revisited. Br J Psychiatry Suppl 1996;30:68-75. [PubMed]
- 81.Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry 2001;49:1023-39. [DOI] [PubMed]
- 82.Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med 1998;14:245-58. [DOI] [PubMed]


