Abstract
Previous work has shown that certain β3-peptides can effectively mimic the side chain display of an α-helix and inhibit interactions between proteins, both in vitro and in cultured cells. Here we describe a β3-peptide analog of GLP-1, CC-3Act, that interacts with the GLP-1R extracellular domain (nGLP-1R) in vitro in a manner that competes with exendin-4 and induces GLP-1R-dependent cAMP signaling in cultured CHO-K1 cells expressing GLP-1R.
The primary role of pancreatic β cells is to synthesize and secrete insulin. This function is modulated in part by a set of heterotrimeric G proteins that are the immediate downstream targets of diverse G protein-coupled receptors (GPCRs). A number of GPCRs expressed by pancreatic β cells regulate insulin secretion. One, the receptor for the glucagon-like peptide-1 (GLP-1R), binds to and is activated by glucagon-like peptide-1 (GLP-1),1, 2 a 30-aa member of the incretin hormone family. In the presence of glucose, activated GLP-1R signals through the associated G protein GS to activate the adenylyl cyclase pathway and stimulate insulin secretion.3 Indeed, GLP-1R is a validated target for the treatment of type 2 diabetes mellitus.3, 4 Exendin-4, a 39-aa GLP-1 ortholog5 possessing improved serum stability, was approved for use in 2005. Additional GLP-1 mimetics with improved pharmacodynamics are in development,6, 7 and efforts have begun to identify low molecular weight compounds that may allow oral administration.8-15 Furthermore, new bi-functional peptides that activate GLP-1R and other molecular targets are being explored in pre-clinical models for enhanced anti-diabetic pharmacology.16, 17
Previous work has shown that certain β3-peptides can effectively mimic the side chain display of an α-helix and inhibit interactions between proteins, both in vitro18-20 and in cultured cells.21-23 Oligomers containing mixtures of both α- and β-amino acids have also shown success.24 Here we describe a potential β3-peptide analog of GLP-1, CC-3Act, that interacts with the GLP-1R extracellular domain (nGLP-1R) in vitro in a manner that competes with exendin-4 and induces GLP-1R-dependent cAMP signaling in cultured CHO-K1 cells expressing GLP-1R.
Our design of CC-3Act began with the sequence of exendin-4. This sequence can be divided into two regions: a 22 residue C-terminal region that associates as an α-helix (shown in purple in Figure 1) with nGLP-1R (shown in grey) and functions in isolation as a receptor antagonist (Kd = 5 nM5) and an 9 residue N-terminal region of undefined structure that is required for receptor activation.5, 25, 26 A large number side chains within the C-terminal region contribute to exendin-4/GLP-1R binding and/or receptor activation; these include E15, F22, I23, and L26; modest contributions are also made by L14, K20, A24, W25, V27 and K28. Of these side chains, five–A18, W25 F22, L26, and A/V19–are conserved between exendin–4 and GLP-1 and localize on the bound α-helix at the ligand-receptor interface (Figure 1C).27
Figure 1.
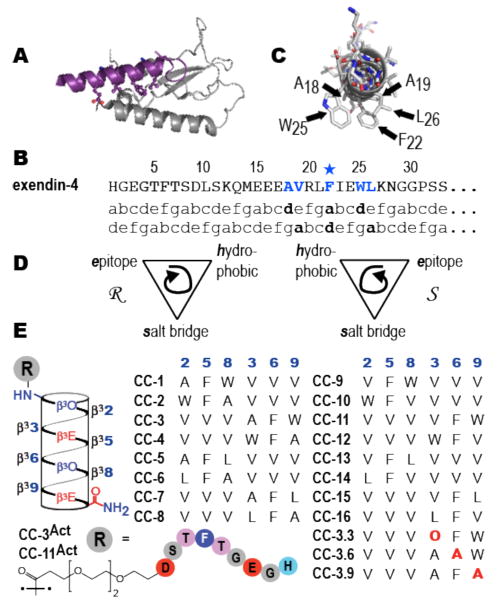
(A) View of exendin-4 (purple) in complex with nGLP-1R (grey) (PDB 3C5T) illustrating relative orientation of the incretin α-helix and the Sushi fold of the receptor extracellular domain. (B) Sequence of exendin-4 aligned with two heptad repeats. (C) View of GLP-1 looking down the helix axis to illustrate side chain arrangement at the ligand-receptor interaction face. (D) Cartoon illustrating the stereochemical relationships between the three 14-helix faces. (E) Helical net and sequences of β-peptides evaluated herein.
Our design was further refined by three principles uncovered during previous efforts to develop β3 -peptide mimics of less complex α-helical segments.18, 20-23, 28,29 These efforts revealed that homo-oligomeric β3-peptides presenting three interacting residues on one 14-helix face often perform as designed, binding their targets with affinities < 1 μM, whereas those presenting four interacting residues usually do not. These efforts also revealed that the axial orientation of the three interacting residues (N to C vs. C to N) and their stereochemical relationship (Figure 1D) could modulate Kd by more than 100-fold. The side chain that contributes most significantly to exendin-4•GLP-1R interaction is F22.30 Thus, we began by identifying a collection of side chain triads containing F22 and other components of the bound α-helix interface that could be displayed on each of two 14-helix faces and in either the N to C or C to N direction. For example, were F22 to occupy position d of a heptad repeat, the ada side chain triad would include V19, F22, and L26; were it to occupy position a, the dad triad would be A18, F22, and W25. Each of these three residue epitopes can be presented in two axial directions and on two 14-helix faces to generate a collection of 8 β3-peptides. β3-peptides presenting VFW and AFL epitopes (formally adg and gda) were also prepared to generate a collection of 16 molecules (Figure 1E).
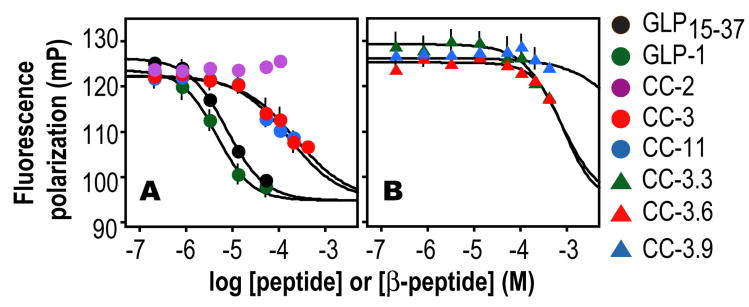
First we used an in vitro fluorescence polarization (FP) competition assay to compare the relative affinities of these 16 β3 -peptides for recombinant nGLP-1R (Figure S1). Each β3 -peptide was incubated at a concentration of 10 or 50 μM with 5 nM exendin-4Flu in the presence of 125 nM nGLP-1R, and the polarization of the mixture was monitored at equilibrium. Fourteen of the sixteen β3 -peptides evaluated had no effect on the observed polarization, even at 50 μM concentration, indicating that they had little or no effect on the fraction of exendin-4Flu bound to the nGLP-1R under these conditions. β3 -peptides CC-3 and CC-11, however, both significantly reduced the observed polarization values (22 and 33% relative to exendin-4), suggesting that they compete with exendin-4Flu for the nGLP-1R binding pocket. Incubation of 5 nM exendin-4Flu and 125 nM nGLP-1R with between 10 nM and 500 μM CC-3 or CC-11 led to a concentration-dependent decrease in the fraction of exendin-4Flu bound (Figure 2A); subsequent data analysis suggested IC50 (and Ki) values of 228 ± 35 μM (115 ± 18) and 116 ± 31 (84 ± 16) μM for CC-3 and CC-11, respectively. In comparison, the α-peptide antagonist GLP-115-37 was only 15-20 times more potent than CC-11 in this assay, competing with exendin-4Flu with IC50 and Ki values of 7.53 ± 0.54 and 3.75 ± 0.23 μM respectively. Differences between exendin-4 and GLP-1 binding to the GLP-1R ectodomain in vitro are well described.31 Even the modest affinity of GLP-1 for nGLP-1R is sufficient for subnanomolar potency in the context of the full-length receptor and peptide.
Figure 2.
Fluorescence polarization (FP) competition analysis of interactions between nGLP-1R and either peptides (GLP-1 and GLP15-37) or β3-peptides. Plots illustrate the change in the polarization of 5 nM exendin-4Flu as a function of the concentration of the ligand indicated; [nGLP-1R] = 125 nM.
Three lines of evidence suggest that CC-3 and CC-11 mimic the α-helical regions of exendin-4 and GLP-1 in their interactions with nGLP-1R. First, substitution of each component of the ‘AFW/VFW’ epitope for alanine (CC-3.6, CC-3.9) or ornithine (CC-3.3) led to (β3-peptides that compete poorly with exendin-4Flu for binding to nGLP-1R (Figure 2B). Second, CC-3 and CC-11 differ by only two methyl groups, presenting either an AFW (CC-3) or VFW (CC-11) triad in the N-to-C orientation on the same β3 -peptide face. Six other collection members carry one of these two side chain triads (CC-1, 2, and 4; CC-9, 10, 12), but differ from CC-3 and CC-11 in the relative orientation of the three side chains (N-to-C or C-to-N) or the stereochemical relationship of the epitope, hydrophobic, and salt bridge faces. Yet, only CC-3 and CC-11 competed effectively with exendin-4Flu for binding to nGLP-1R (Figure 2B).
Finally, post hoc modeling experiments provide a structural rationale for the observed differences in affinity among β3-peptides possessing alternate arrangements of the same binding epitope. We used the program pepz32 to construct models for (β3-peptides CC-1-4 and CC-9-12 in an ideal 314-helix conformation.33 After sampling of the epitope side-chains, the Cβ, Cγ, Cδ, Cɛ1 and Nζ1 atoms of the epitope residues were used to align each β3 -peptide to the corresponding side chain atoms (Cβ, Cγ, Cδ, Cε1 and Nζ1) of exendin-4 in the exendin-4•nGLP-R complex structure (PDBID 3c5t). The RMSD of these alignments varied between 1.1-1.4 Å (shown in Figure S2). While these models are too coarse to reveal detailed interactions or the precise placement of each side chain in the binding pocket, they do identify the relative orientation of side chains presented on each 314 helix face. In the case of CC-3 and CC-11, alignment of the AFW/VFW epitopes into the binding pocket orients the (β3-peptide hydrophobic face, which contains three valine side chains, towards a hydrophobic groove on the GLP-1R surface, and orients the salt-bridge face, which contains acidic and basic side chains, towards solvent. Reversing the C-to-N direction of the AFW/VFW epitope as in CC-1 and CC-9, or reversing the relative orientation of the salt-bridge and valine faces with respect to the epitope face as in CC-4 and CC-12, directs the salt-bridge face towards the hydrophobic groove on the nGLP-1R surface and points the hydrophobic valine side-chains towards solvent. These two side chain arrangements are likely to be energetically unfavorable and destabilize the interaction of these β3 -peptides with nGLP-1R. Interestingly, reversal of both the face ordering and the N-to-C directionality (as in CC-2 and CC-10) leads to side-chain placement similar to that of CC-3 and CC-11. In this case, the reduced 314 helicity of CC-2 and CC-10 may be the primary cause for their reduced binding (Figure S3).
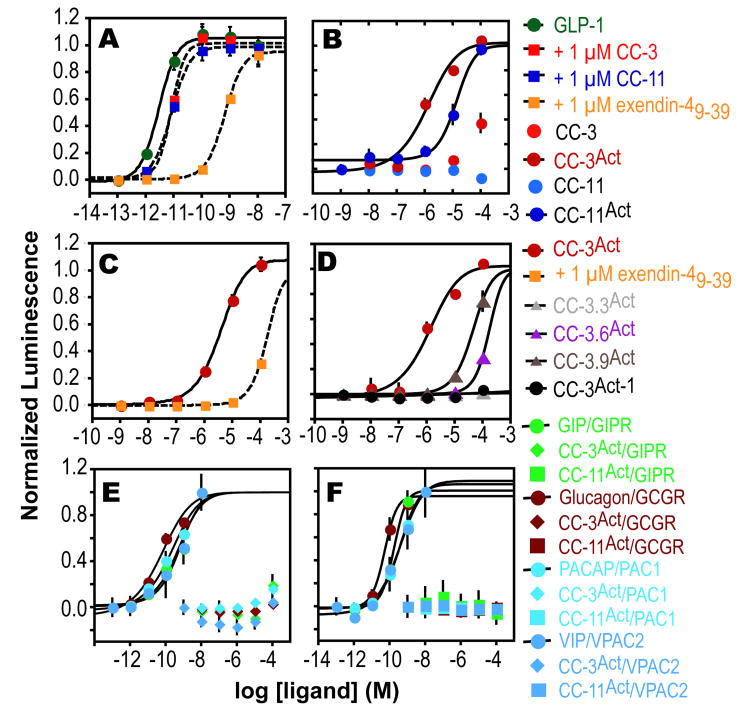
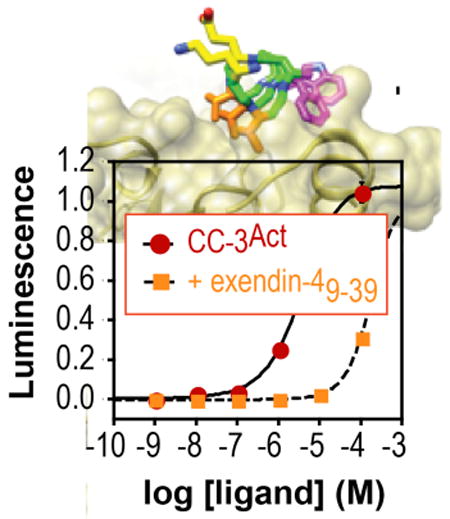
Next, CC-3 and CC-11 were converted into the potential (β3-hormones CC-3Act and CC-11Act by extending their sequence at the N-terminus with a 13-atom polyethylene glycol (PEG) chain followed by the α-peptide NH2-HGEGTFTSD, which corresponds to the nine N-terminal residues of exendin-4 and are critical for GLP-1R activation30 (Figure 1). Procedures to minimize aspartimide formation34 were employed and validated35,36 and the products were purified using pristine HPLC columns to avoid cross-contamination with GLP-1 itself. Ligand-dependent GPCR activation was monitored in GLP-1R+ CHO-K1 cells using a luciferase reporter gene under control of a cAMP response element promoter.30 As expected, GLP-1 was a potent GLP-1R agonist (EC50 = 2.86 ± 0.70 pM), and was inhibited by 1 μM exendin-49-39, CC-3 or CC-11, in accord with the relative in vitro affinities of these ligands (Figure 3A). Both CC-3Act and CC-11Act activated GLP-1R in CHO-K1 cells (EC50 = 1.2 ± 0.74 μM and 13.2 ± 2.5 μM respectively (Figure 3B). Activation of GLP-1R by CC-3Act was reduced 50-fold by 1 μM exendin-49-39 (Figure 3C), suggesting that the observed increase in cAMP resulted from a direct interaction of CC-3Act with the GLP-1R ligand-binding domain. In addition, loss of any of the three side chains that contributed to receptor affinity in vitro (Figures 1 and 2) decreased potency in the cell based assay (Figure 3D). Finally, an analog of CC-3Act lacking a PEG linker (CC-3Act-1) was inactive (Figure 3D), consistent with the need for a discrete structural relationship between the C- and N-terminal domains.
Figure 3.
Effect of β-peptides and ligands on cAMP production in cells expressing class B1 GPCRs. (A-D) Effect of various β-peptides and exendin-4 on cAMP accumulation in GLP-1R+ CHO-K1 cells. (E,F) cAMP accumulation in GIPR+, PAC1+, GCCR+, and VPAC+CHO-K1 cells upon treatment with the cognate ligand, CC-3Act or CC-11Act.
GLP-1R is one of several homologous class B1 GPCRs expressed in the pancreas. Other family members include the receptors activated by peptides known as GIP, glucagon, VIP, and PACAP.37-39 To investigate the selectivity of CC-3Act as a GLP-1R agonist, we examined its effect on the activation of these four receptors in CHO-K1 cells. Although each of these receptors were activated potently by their cognate ligands (Figure 3E), none were activated by CC-3Act (or CC-11Act), even at high concentration (Figure 3F).
Although the results reported herein suggest that CC-3Act activates GLP-1R through interactions that mimic those of GLP-1 and exendin-4, its potency was modest–a full six orders of magnitude lower than GLP-1. Even mM concentrations of CC-3Act did not inhibit forskolin-dependent activation of the adenylate cyclase pathway (Figure S4), ruling out the possibility that the lower-than-expected potency of CC-3Act was due to unexpected interference with a later step in the activation pathway. We note, however, that the high in vitro potencies of GLP-1 and orthologs are counterbalanced by the rapid elimination and short half-lives of the peptides in vivo. β3-peptides are not subject to the same degradation processes and all available studies indicate dramatically enhanced stability in vivo.40-43 Further optimization of these sequences is ongoing in our laboratory.
Supplementary Material
Acknowledgments
We are grateful to the National Institutes of Health for support of this work.
Footnotes
Supporting Information Available. Peptide synthesis and characterization, assay procedures, and supplemental figures.
References
- 1.Thorens B. Proc Natl Acad Sci USA. 1992;89:8641–8645. doi: 10.1073/pnas.89.18.8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dillon JS, Tanizawa Y, Wheeler MB, Leng XH, Ligon BB, Rabin DU, Yoo-Warren H, Permutt MA, Boyd AE. Endocrinology. 1993;133:1907–1910. doi: 10.1210/endo.133.4.8404634. [DOI] [PubMed] [Google Scholar]
- 3.Drucker DJ. Cell Metab. 2006;3:153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Lovshin JA, Drucker DJ. Nat Rev Endocrinol. 2009;5:262–269. doi: 10.1038/nrendo.2009.48. [DOI] [PubMed] [Google Scholar]
- 5.Goke R, Fehmann HC, Linn T, Schmidt H, Krause M, Eng J, Goke B. J Biol Chem. 1993;268:19650–19655. [PubMed] [Google Scholar]
- 6.Garber AJ. Expert Opin Investig Drugs. 2012;21:45–57. doi: 10.1517/13543784.2012.638282. [DOI] [PubMed] [Google Scholar]
- 7.Madsbad S, Kielgast U, Asmar M, Deacon CF, Torekov SS, Holst JJ. Diabetes Obes Metab. 2011;13:394–407. doi: 10.1111/j.1463-1326.2011.01357.x. [DOI] [PubMed] [Google Scholar]
- 8.Willard FS, Bueno AB, Sloop KW. Exp Diabetes Res. 2012;2012:709893–709893. doi: 10.1155/2012/709893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wootten D, Savage EE, Willard FS, Bueno AB, Sloop KW, Christopoulos A, Sexton PM. Mol Pharm. 2013;83:822–834. doi: 10.1124/mol.112.084525. [DOI] [PubMed] [Google Scholar]
- 10.Eng H, Sharma R, McDonald TS, Edmonds DJ, Fortin JP, Li X, Stevens BD, Griffith DA, Limberakis C, Nolte WM, Price DA, Jackson M, Kalgutkar AS. Drug Metab Dis. 2013;41:1470–1479. doi: 10.1124/dmd.113.052183. [DOI] [PubMed] [Google Scholar]
- 11.Willard FS, Wootten D, Showalter AD, Savage EE, Ficorilli J, Farb TB, Bokvist K, Alsina-Fernandez J, Furness SGB, Christopoulos A, Sexton PM, Sloop KW. Mol Pharm. 2012;82:1066–1073. doi: 10.1124/mol.112.080432. [DOI] [PubMed] [Google Scholar]
- 12.Li N, Lu J, Willars GB. Plos One. 2012;7 doi: 10.1371/journal.pone.0047936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He M, Guan N, Gao Ww, Liu Q, Wu Xy, Ma Dw, Zhong Df, Ge Gb, Li C, Chen Xy, Yang L, Liao Jy, Wang Mw. Acta Pharm Sinica. 2012;33:148–154. doi: 10.1038/aps.2011.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gong YD, Cheon HG, Lee T, Kang NS. Bull Korean Chem Soc. 2010;31:3760–3764. [Google Scholar]
- 15.Mapelli C, Natarajan SI, Meyer JP, Bastos MM, Bernatowicz MS, Lee VG, Pluscec J, Riexinger DJ, Sieber-McMaster ES, Constantine KL, Smith-Monroy CA, Golla R, Ma Z, Longhi DA, Shi D, Xin L, Taylor JR, Koplowitz B, Chi CL, Khanna A, Robinson GW, Seethala R, Anatal-Zimanyi IA, Stoffel RH, Han S, Whaley JM, Huang CS, Krupinski J, Ewing WR. J Med Chem. 2009;52:7788–7799. doi: 10.1021/jm900752a. [DOI] [PubMed] [Google Scholar]
- 16.Day JW, Ottaway N, Patterson JT, Gelfanov V, Smiley D, Gidda J, Findeisen H, Bruemmer D, Drucker DJ, Chaudhary N, Holland J, Hembree J, Abplanalp W, Grant E, Ruehl J, Wilson H, Kirchner H, Lockie SH, Hofmann S, Woods SC, Nogueiras R, Pfluger PT, Perez-Tilve D, DiMarchi R, Tschop MH. Nat Chem Biol. 2009;5:749–757. doi: 10.1038/nchembio.209. [DOI] [PubMed] [Google Scholar]
- 17.Tschoep MH, DiMarchi RD. Diabetes. 2012;61:1309–1314. doi: 10.2337/db12-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kritzer JA, Lear JD, Hodsdon ME, Schepartz A. J Am Chem Soc. 2004;126:9468–9469. doi: 10.1021/ja031625a. [DOI] [PubMed] [Google Scholar]
- 19.Michel J, Harker EA, Tirado-Rives J, Jorgensen WL, Schepartz A. J Am Chem Soc. 2009;131:6356–6357. doi: 10.1021/ja901478e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bautista AD, Appelbaum JS, Craig CJ, Michel J, Schepartz A. J Am Chem Soc. 2010;132:2904–2906. doi: 10.1021/ja910715u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stephens OM, Kim S, Welch BD, Hodsdon ME, Kay MS, Schepartz A. J Am Chem Soc. 2005;127:13126–13127. doi: 10.1021/ja053444+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bautista AD, Stephens OM, Wang L, Domaoal RA, Anderson KS, Schepartz A. Bioorg Med Chem Lett. 2009;19:3736–3738. doi: 10.1016/j.bmcl.2009.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harker EA, Daniels DS, Guarracino DA, Schepartz A. Bioorg Med Chem. 2009;17:2038–2046. doi: 10.1016/j.bmc.2009.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee EF, Smith BJ, Horne WS, Mayer KN, Evangelista M, Colman PM, Gellman SH, Fairlie WD. ChemBioChem. 2011;12:2025–2032. doi: 10.1002/cbic.201100314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mann R, Nasr N, Hadden D, Sinfield J, Abidi F, Al-Sabah S, de Maturana RL, Treece-Birch J, Willshaw A, Donnelly D. Biochem Soc Trans. 2007;35:713–716. doi: 10.1042/BST0350713. [DOI] [PubMed] [Google Scholar]
- 26.Al-Sabah S, Donnelly D. Br J Pharmacol. 2003;140:339–346. doi: 10.1038/sj.bjp.0705453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Runge S, Thogersen H, Madsen K, Lau J, Rudolph R. J Biol Chem. 2008;283:11340–11347. doi: 10.1074/jbc.M708740200. [DOI] [PubMed] [Google Scholar]
- 28.Kritzer JA, Hodsdon ME, Schepartz A. J Am Chem Soc. 2005;127:4118–4119. doi: 10.1021/ja042933r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kritzer JA, Stephens OM, Guarracino DA, Reznik SK, Schepartz A. Bioorganic & Medicinal Chemistry. 2005;13:11–6. doi: 10.1016/j.bmc.2004.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adelhorst K, Hedegaard BB, Knudsen LB, Kirk O. J Biol Chem. 1994;269:6275–6278. [PubMed] [Google Scholar]
- 31.Willard FS, Sloop KW. Exp Diabetes Res. 2012;2012:470851–470851. doi: 10.1155/2012/470851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jorgensen WL, Tirado-Rives J. J Comp Chem. 2005;26:1689–1700. doi: 10.1002/jcc.20297. [DOI] [PubMed] [Google Scholar]
- 33.DeGrado WF, Schneider JP, Hamuro Y. Chem Biol Drug Des. 1999;54:206–217. doi: 10.1034/j.1399-3011.1999.00131.x. [DOI] [PubMed] [Google Scholar]
- 34.Subiros-Funosas R, El-Faham A, Albericio F. Tetrahedron. 2011;67:8595–8606. [Google Scholar]
- 35.Kameoka D, Ueda T, Imoto T. J Biochem. 2003;134:129–135. doi: 10.1093/jb/mvg120. [DOI] [PubMed] [Google Scholar]
- 36.Ni W, Dai S, Karger BL, Zhou ZS. Anal Chem. 2010;82:7485–7491. doi: 10.1021/ac101806e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahren B. Nat Rev Drug Discov. 2009;8:369–385. doi: 10.1038/nrd2782. [DOI] [PubMed] [Google Scholar]
- 38.Regard JB, Kataoka H, Cano DA, Camerer E, Yin L, Zheng YW, Scanlan TS, Hebrok M, Coughlin SR. J Clin Inv. 2007;117:4034–4043. doi: 10.1172/JCI32994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Winzell MS, Ahren B. Peptides. 2007;28:1805–1813. doi: 10.1016/j.peptides.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 40.Hintermann T, Seebach D. CHIMIA. 1997;51:244–247. [Google Scholar]
- 41.Hook DF, Bindschadler P, Mahajan YR, Sebesta R, Kast P, Seebach D. Chem Biodiversity. 2005;2:591–632. doi: 10.1002/cbdv.200590039. [DOI] [PubMed] [Google Scholar]
- 42.Seebach D, Abele S, Schreiber JVJ, Martinoni B, Nussbaum AK, Schild H, Schulz H, Hennecke H, Woessner R, Bitsch F. CHIMIA. 1998;52:734–739. [Google Scholar]
- 43.Seebach D, Overhand M, Kühnle FNM, Martinoni B, Oberer L, Hommel U, Widmer H. Helv Chim Acta. 1996;79:913–941. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.