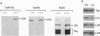Abstract
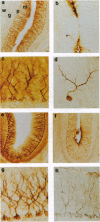
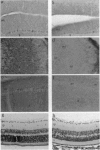
Microtubules play an important role in establishing cellular architecture. Neuronal microtubules are considered to have a role in dendrite and axon formation. Different portions of the developing and adult brain microtubules are associated with different microtubule-associated proteins (MAPs). The roles of each of the different MAPs are not well understood. One of these proteins, MAP1B, is expressed in different portions of the brain and has been postulated to have a role in neuronal plasticity and brain development. To ascertain the role of MAP1B, we generated mice which carry an insertion in the gene by gene-targeting methods. Mice which are homozygous for the modification die during embryogenesis. The heterozygotes exhibit a spectrum of phenotypes including slower growth rates, lack of visual acuity in one or both eyes, and motor system abnormalities. Histochemical analysis of the severely affected mice revealed that their Purkinje cell dendritic processes are abnormal, do not react with MAP1B antibodies, and show reduced staining with MAP1A antibodies. Similar histological and immunochemical changes were observed in the olfactory bulb, hippocampus, and retina, providing a basis for the observed phenotypes.
Full text
PDF
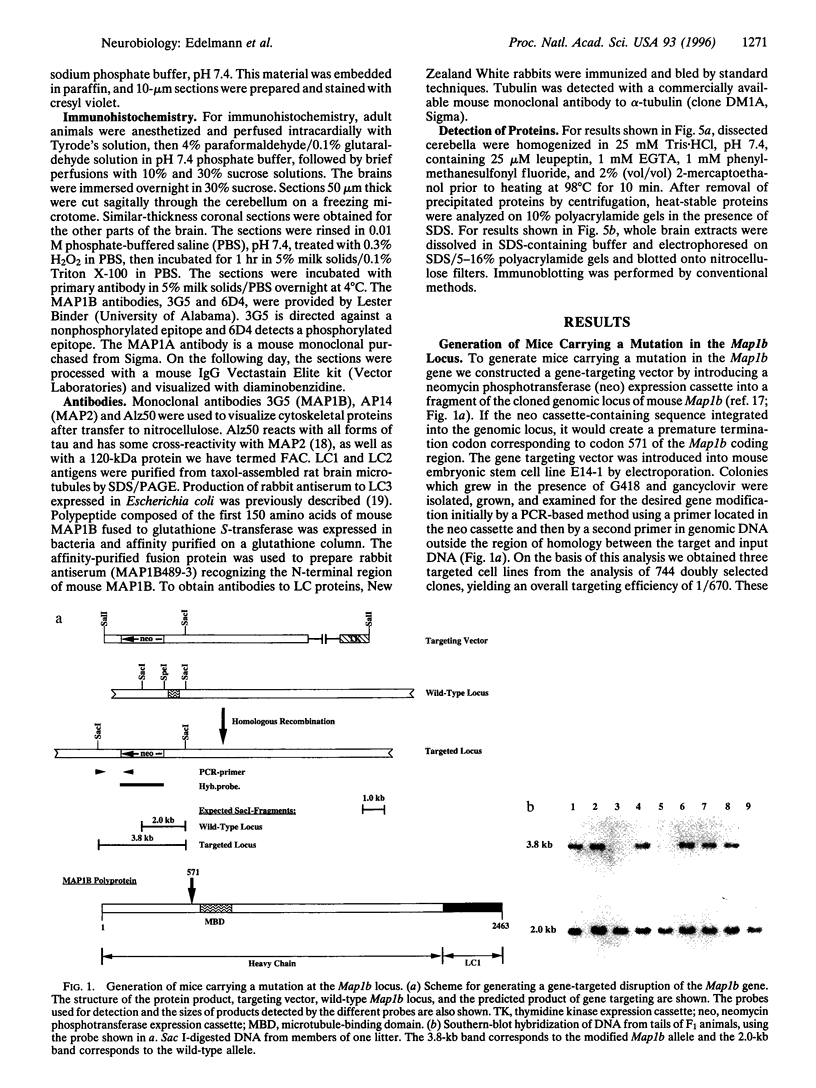
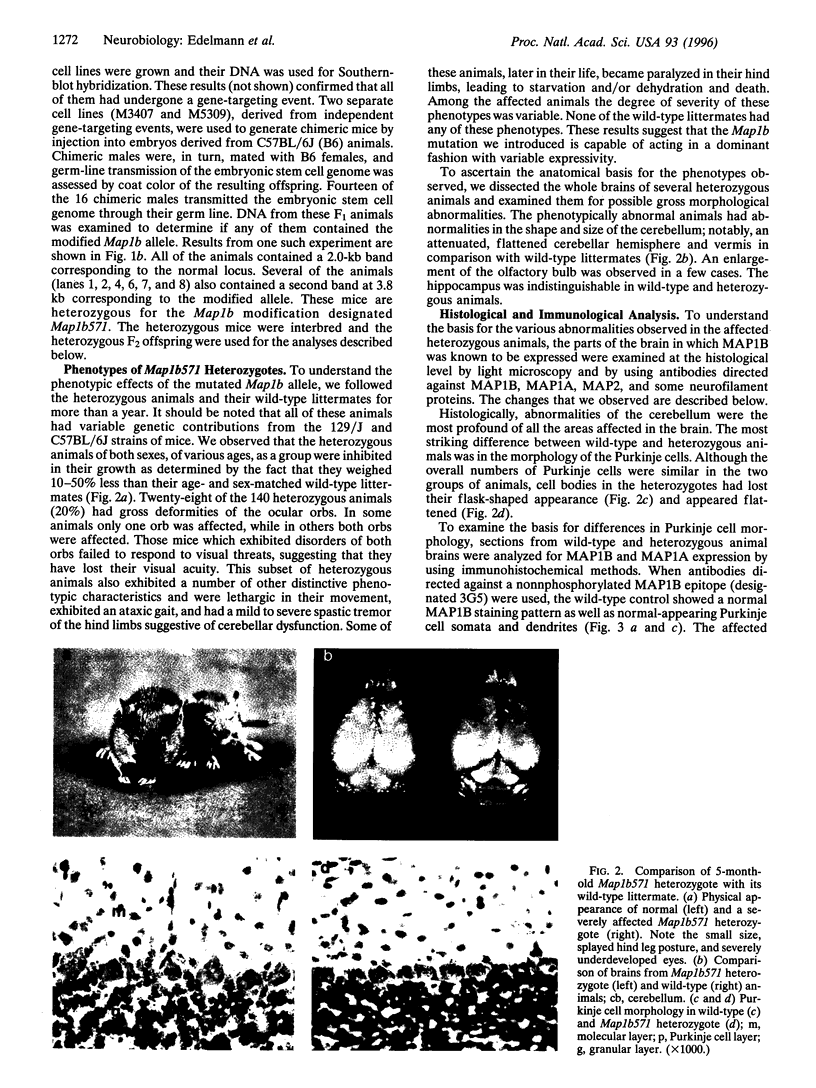
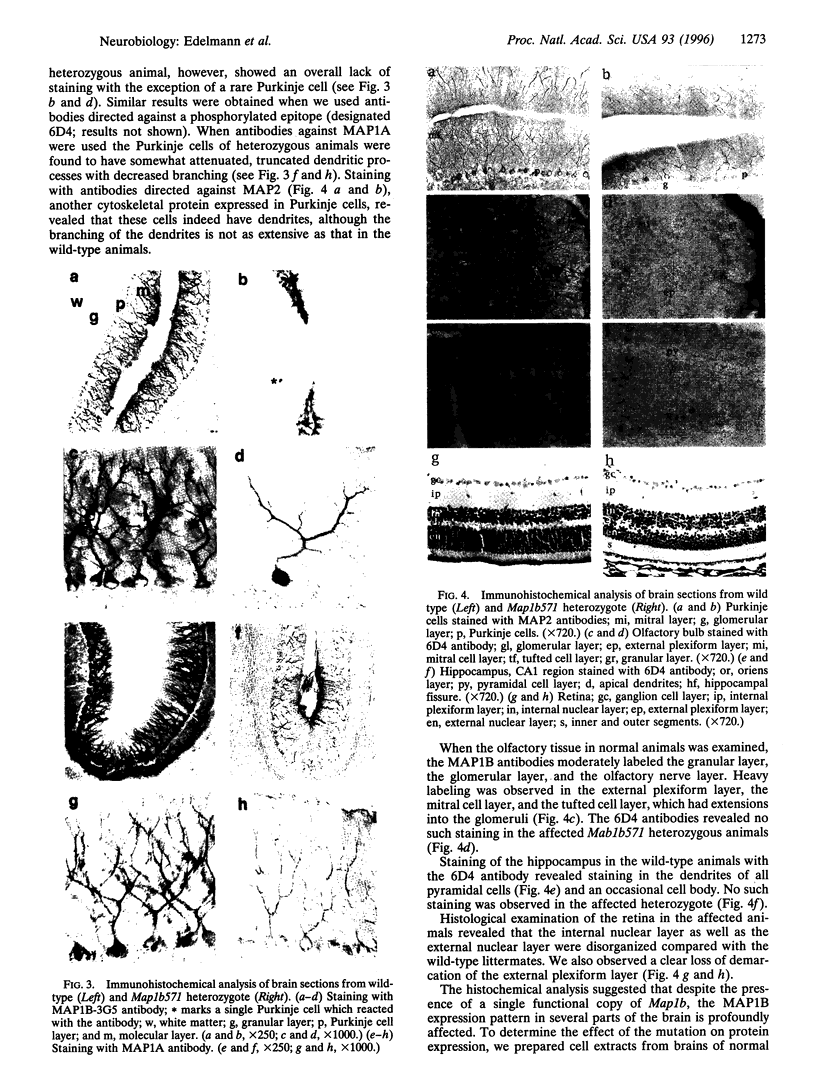


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloom G. S., Luca F. C., Vallee R. B. Microtubule-associated protein 1B: identification of a major component of the neuronal cytoskeleton. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5404–5408. doi: 10.1073/pnas.82.16.5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugg B., Matus A. PC12 cells express juvenile microtubule-associated proteins during nerve growth factor-induced neurite outgrowth. J Cell Biol. 1988 Aug;107(2):643–650. doi: 10.1083/jcb.107.2.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugg B., Reddy D., Matus A. Attenuation of microtubule-associated protein 1B expression by antisense oligodeoxynucleotides inhibits initiation of neurite outgrowth. Neuroscience. 1993 Feb;52(3):489–496. doi: 10.1016/0306-4522(93)90401-z. [DOI] [PubMed] [Google Scholar]
- Garner C. C., Garner A., Huber G., Kozak C., Matus A. Molecular cloning of microtubule-associated protein 1 (MAP1A) and microtubule-associated protein 5 (MAP1B): identification of distinct genes and their differential expression in developing brain. J Neurochem. 1990 Jul;55(1):146–154. doi: 10.1111/j.1471-4159.1990.tb08832.x. [DOI] [PubMed] [Google Scholar]
- Hammarback J. A., Obar R. A., Hughes S. M., Vallee R. B. MAP1B is encoded as a polyprotein that is processed to form a complex N-terminal microtubule-binding domain. Neuron. 1991 Jul;7(1):129–139. doi: 10.1016/0896-6273(91)90081-a. [DOI] [PubMed] [Google Scholar]
- Harada A., Oguchi K., Okabe S., Kuno J., Terada S., Ohshima T., Sato-Yoshitake R., Takei Y., Noda T., Hirokawa N. Altered microtubule organization in small-calibre axons of mice lacking tau protein. Nature. 1994 Jun 9;369(6480):488–491. doi: 10.1038/369488a0. [DOI] [PubMed] [Google Scholar]
- Ksiezak-Reding H., Davies P., Yen S. H. Alz 50, a monoclonal antibody to Alzheimer's disease antigen, cross-reacts with tau proteins from bovine and normal human brain. J Biol Chem. 1988 Jun 15;263(17):7943–7947. [PubMed] [Google Scholar]
- Kuznetsov S. A., Rodionov V. I., Nadezhdina E. S., Murphy D. B., Gelfand V. I. Identification of a 34-kD polypeptide as a light chain of microtubule-associated protein-1 (MAP-1) and its association with a MAP-1 peptide that binds to microtubules. J Cell Biol. 1986 Mar;102(3):1060–1066. doi: 10.1083/jcb.102.3.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langkopf A., Hammarback J. A., Müller R., Vallee R. B., Garner C. C. Microtubule-associated proteins 1A and LC2. Two proteins encoded in one messenger RNA. J Biol Chem. 1992 Aug 15;267(23):16561–16566. [PubMed] [Google Scholar]
- Mann S. S., Hammarback J. A. Molecular characterization of light chain 3. A microtubule binding subunit of MAP1A and MAP1B. J Biol Chem. 1994 Apr 15;269(15):11492–11497. [PubMed] [Google Scholar]
- Mansour S. L., Thomas K. R., Capecchi M. R. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature. 1988 Nov 24;336(6197):348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- Noble M., Lewis S. A., Cowan N. J. The microtubule binding domain of microtubule-associated protein MAP1B contains a repeated sequence motif unrelated to that of MAP2 and tau. J Cell Biol. 1989 Dec;109(6 Pt 2):3367–3376. doi: 10.1083/jcb.109.6.3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riederer B., Cohen R., Matus A. MAP5: a novel brain microtubule-associated protein under strong developmental regulation. J Neurocytol. 1986 Dec;15(6):763–775. doi: 10.1007/BF01625193. [DOI] [PubMed] [Google Scholar]
- Sato-Yoshitake R., Shiomura Y., Miyasaka H., Hirokawa N. Microtubule-associated protein 1B: molecular structure, localization, and phosphorylation-dependent expression in developing neurons. Neuron. 1989 Aug;3(2):229–238. doi: 10.1016/0896-6273(89)90036-6. [DOI] [PubMed] [Google Scholar]
- Schoenfeld T. A., McKerracher L., Obar R., Vallee R. B. MAP 1A and MAP 1B are structurally related microtubule associated proteins with distinct developmental patterns in the CNS. J Neurosci. 1989 May;9(5):1712–1730. doi: 10.1523/JNEUROSCI.09-05-01712.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker R. P., Binder L. I., Matus A. I. Neuronal microtubule-associated proteins in the embryonic avian spinal cord. J Comp Neurol. 1988 May 1;271(1):44–55. doi: 10.1002/cne.902710106. [DOI] [PubMed] [Google Scholar]
- Tucker R. P., Matus A. I. Developmental regulation of two microtubule-associated proteins (MAP2 and MAP5) in the embryonic avian retina. Development. 1987 Nov;101(3):535–546. doi: 10.1242/dev.101.3.535. [DOI] [PubMed] [Google Scholar]
- Vallee R. B., Davis S. E. Low molecular weight microtubule-associated proteins are light chains of microtubule-associated protein 1 (MAP 1). Proc Natl Acad Sci U S A. 1983 Mar;80(5):1342–1346. doi: 10.1073/pnas.80.5.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viereck C., Tucker R. P., Matus A. The adult rat olfactory system expresses microtubule-associated proteins found in the developing brain. J Neurosci. 1989 Oct;9(10):3547–3557. doi: 10.1523/JNEUROSCI.09-10-03547.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]