Abstract
Background
In Korea, cancer is the third leading cause of death among adolescents and young adults (AYAs). However, cancer incidence and survival trends among AYAs (15–29 years) have never been studied in Korea. Therefore, this study aimed to investigate the incidence and relative survival rates and their trends among AYAs in Korea.
Materials and Methods
Cancer incidence data from 1999–2010 were obtained from the Korea Central Cancer Registry (KCCR). Each cancer was classified into subgroups according to the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) AYA site recode. Percent distributions, age-specific incidence rates, age-standardized incidence rates per million, and annual percent changes (APCs) were calculated for AYAs according to sex. Five-year relative survival rates were estimated for cases diagnosed between 1993 and 2010 and followed up to 2011.
Results
The age-standardized incidence rates of all cancers combined were 196.4 and 367.8 per million for males and females, respectively (male-to-female (M/F) ratio: 0.5). The age-standardized incidence rates increased from 208.7 per million in 1999 to 396.4 per million in 2010, and the APC was 6.3% (P<0.001). The five most common cancers among AYAs were thyroid carcinoma, non-Hodgkin lymphoma, stomach carcinoma, breast carcinoma, and acute myeloid leukemia. In males, the 5-year relative survival rate improved, from 46.5% in 1993–1995 to 75.9% in 2006–2010. In females, the 5-year relative survival rate also improved, from 66.7% in 1993–1995 to 89.1% in 2006–2010.
Conclusions
Our study showed increases in cancer incidence and improvements in the 5-year relative survival rate among Korean AYAs. This study also provides additional data regarding temporal and geographic trends in cancer that may enhance future efforts to identify factors affecting cancer incidence and responses to treatment among AYAs.
Introduction
Cancers in adolescents and young adults (AYAs; 15–29 years) have distinctive characteristics compared with cancers in children and older cohorts.
The incidence pattern of specific cancer types differ between AYAs and younger and older patients. In addition, the genetic and histologic patterns of cancers among AYAs differ from those of older patients [1]. Because cancer is uncommon among AYAs, this patient population has not drawn public attention compared with the pediatric and adult populations. However, an increase in cancer incidence among AYAs has been reported in Europe [2]–[4] and the United States [5].
Over 200,000 new cancer patients are diagnosed annually in Korea [6], and approximately 3,200 (1.6%) AYAs were diagnosed with cancer in Korea in 2010. According to the U.S. National Cancer Institute Surveillance Epidemiology and End Results (SEER), 2% of all invasive cancers are diagnosed in individuals aged 15–29 years [5]. Although only a small proportion of all malignancies are diagnosed in AYAs, high-grade and later-stage tumors of certain cancers are more likely to be diagnosed in this patient population [1]. Furthermore, cancer diagnosis in AYAs can greatly influence future quality of life and life expectancy [7]. In fact, in Korea, cancer is the leading cause of death among AYAs, after suicide and traffic accidents [8]. However, to the best of our knowledge, cancer incidence and survival among AYAs in Korea have never been studied. Therefore, this study aimed to investigate cancer incidence and survival among AYAs in Korea. We examined the trends in cancer incidence rates from 1999 to 2010 and the trends in relative survival rates from 1993 to 2010 among Korean AYAs.
Materials and Methods
Data Sources
In 1980, the Korean Ministry of Health and Welfare started the Korea Central Cancer Registry (KCCR), a nationwide, hospital-based cancer registry [9]. Until 1998, the registry collected cancer cases from more than 180 hospitals in Korea annually, and these data represent 80–90% of all cancer incidence in Korea [10]. Since 1999, the KCCR has covered the entire population under the population-based cancer registry program [6]. The Korea National Cancer Incidence Database (KNCIDB) KCCR data from 1999 to 2002 and from 2003 to 2007 have been published in Cancer Incidence in Five Continents, which reflects the completeness and validity of the incidence data [11].
Incidence data were collected for Korean AYAs aged 15–29 years who were newly diagnosed with cancer between 1999 and 2010. The incidence data were collected from the KNCIDB of the KCCR and included age, sex, diagnosis date, primary tumor site, morphology, the diagnostic method, and stage at diagnosis.
Survival data for individuals aged 15–29 years who were newly diagnosed with cancer from 1993–2010 were obtained from the KNCIDB, and the patients’ vital status was followed until December 31, 2011. The survival analysis was based on the KNCIDB data and mortality data obtained from Statistics Korea.
Case Definition
In accordance with the guidelines of the National Cancer Institute SEER Program [5] and the Canadian Cancer Society [12] in this study, AYAs were defined as adolescents and young adults aged 15–29 years.
Cancer sites were coded by primary site and morphology using the International Classification of Diseases for Oncology (third edition) [13]. Cancers were classified according to the SEER AYA scheme, which is based on a modified version of the International Classification of Childhood Cancer [5]. In particular, the SEER AYA scheme is based on an updated classification proposed by Barr et al. and is composed of ten major groups and second- and third-level subgroups according to the site of origin [14].
Incidence
Age-specific incidence rates per million were analyzed in each diagnostic subgroup according to the age at diagnosis (15–19 years, 20–24 years, and 25–29 years), and age-standardized rates (ASRs) according to sex were calculated using the world standard population defined by the World Health Organization [15]. Trends in annual ASRs were calculated using the annual percent change (APC), which was estimated using the following formula: 100×(eβ-1), where β is the slope calculated from a linear regression of log age-standardized incidence rates in a calendar year [16]. The male-to-female (M/F) ratio was the ratio of the ASR among males to the ASR among females. Comparisons of age-standardized incidence rates in males and females were calculated by the direct method [17].
Survival
Relative survival rates were estimated according to the time period: 1993–1995, 1996–2000, 2001–2005 and 2006–2010. Relative survival rates according to the diagnostic group were calculated by dividing observed survival by expected survival among comparable groups in the general population [18] using the Ederer II method [19]. These survival rates were estimated using “complete analysis”, which included “right-censored” patients. Due to this inclusion of the early survival experience of more recently recruited patients, the analysis provided more up-to-date and precise survival rates in long-term survival [20]. Trends in 5-year relative survival rates were also calculated. Additionally, the effects of sex, diagnostic group, and time period on survival were assessed using a relative excess risk model. All analyses were performed using SAS version 9.2.
Results
Incidence
From 1999 to 2010, 39,639 cancer cases (2.3% of all cancer patients) were newly diagnosed in AYAs. Of these cases, 14,016 (35.4%) and 25,623 (64.6%) cases were diagnosed in males and females, respectively. According to the age at diagnosis, 6,396 (16.1%) cases were diagnosed at 15–19 years of age, 10,433 (26.3%) cases were diagnosed at 20–24 years, and 22,810 (26.3%) cases were diagnosed at 25–29 years.
The number of cases per age group (15–19 years, 20–24 years and 25–29 years) by sex, age-specific incidence rates, and age-standardized incidence rates among both males and females and M/F ratios according to the diagnostic group are shown in Table 1.
Table 1. Number of cases, age-specific incidence rates, and age-standardized incidence rates from 1999 to 2010 according to sex and age.
| Diagnostic group (SEER) | Males | Females | Total | M/F | ||||||||||||||
| 15–19 years | 20–24 years | 25–29 years | 15–29 years | 15–19 years | 20–24 years | 25–29 years | 15–29 years | Ratio* | ||||||||||
| Cases | CR | Cases | CR | Cases | CR | Cases | ASR | Cases | CR | Cases | CR | Cases | CR | Cases s | ASR | ASR | ||
| All Cancers | 3,249 | 150.9 | 3,891 | 170.7 | 6,876 | 273.4 | 14,016 | 196.4 | 3,147 | 160.4 | 6,542 | 305.6 | 15,934 | 663.3 | 25,623 | 367.8 | 279.9 | 0.5 † |
| All Cancers (excluding thyroid carcinoma) ‡ | 3,065 | 142.3 | 3,445 | 151.1 | 5,665 | 225.2 | 12,175 | 171.7 | 2,275 | 115.9 | 3,660 | 171.0 | 8,793 | 366.0 | 14,728 | 213.6 | 192.2 | 0.8 † |
| 1. Leukemias | 737 | 17.1 | 600 | 13.2 | 694 | 13.8 | 2,031 | 14.8 | 482 | 12.3 | 430 | 10.0 | 549 | 11.4 | 1,461 | 11.3 | 13.1 | 1.3 † |
| 1.1 Acute lymphoid leukemia | 307 | 7.1 | 177 | 3.9 | 130 | 2.6 | 614 | 4.6 | 170 | 4.3 | 110 | 2.6 | 95 | 2.0 | 375 | 3.0 | 3.9 | 1.5† |
| 1.2 Acute myeloid leukemia | 265 | 12.3 | 230 | 10.1 | 287 | 11.4 | 782 | 11.3 | 226 | 11.5 | 190 | 8.9 | 283 | 11.8 | 699 | 10.8 | 11.0 | 1.1† |
| 1.3 Chronic myeloid leukemia | 96 | 4.5 | 131 | 5.7 | 187 | 7.4 | 414 | 5.8 | 37 | 1.9 | 75 | 3.5 | 92 | 3.8 | 204 | 3.0 | 4.5 | 1.9† |
| 1.4 Other and unspecified leukemia | 69 | 3.2 | 62 | 2.7 | 90 | 3.6 | 221 | 3.2 | 49 | 2.5 | 55 | 2.6 | 79 | 3.3 | 183 | 2.8 | 3.0 | 1.1† |
| 2. Lymphomas | 516 | 24.0 | 533 | 23.4 | 612 | 24.3 | 1,661 | 23.9 | 269 | 13.7 | 414 | 19.3 | 568 | 23.6 | 1,251 | 18.7 | 21.4 | 1.3 † |
| 2.1 Non-Hodgkin lymphoma | 406 | 18.9 | 437 | 19.2 | 524 | 20.8 | 1,367 | 19.6 | 199 | 10.1 | 314 | 14.7 | 461 | 19.2 | 974 | 14.5 | 17.1 | 1.4† |
| 2.2 Hodgkin lymphoma | 110 | 5.1 | 96 | 4.2 | 88 | 3.5 | 294 | 4.3 | 70 | 3.6 | 100 | 4.7 | 107 | 4.5 | 277 | 4.2 | 4.3 | 1.0 |
| 3. CNS and Other Intracranial and Intraspinal Neoplasms | 332 | 15.4 | 320 | 14.0 | 406 | 16.1 | 1,058 | 15.2 | 228 | 11.6 | 214 | 10.0 | 361 | 15.0 | 803 | 12.2 | 13.8 | 1.2 † |
| 3.1 Astrocytoma | 88 | 4.1 | 110 | 4.8 | 171 | 6.8 | 369 | 5.2 | 82 | 4.2 | 74 | 3.5 | 155 | 6.5 | 311 | 4.7 | 4.9 | 1.1† |
| 3.2 Other glioma | 44 | 2.0 | 50 | 2.2 | 101 | 4.0 | 195 | 2.7 | 42 | 2.1 | 33 | 1.5 | 88 | 3.7 | 163 | 2.4 | 2.6 | 1.1† |
| 3.3 Ependymoma | 23 | 1.1 | 24 | 1.1 | 33 | 1.3 | 80 | 1.1 | 15 | 0.8 | 21 | 1.0 | 22 | 0.9 | 58 | 0.9 | 1.0 | 1.3† |
| 3.4. Medulloblastoma and other PNET | 66 | 3.1 | 35 | 1.5 | 24 | 1.0 | 125 | 1.9 | 47 | 2.4 | 30 | 1.4 | 24 | 1.0 | 101 | 1.6 | 1.8 | 1.2† |
| 3.5 Other specified intracranial and intraspinal neoplasms | 11 | 0.5 | 9 | 0.4 | 10 | 0.4 | 30 | 0.4 | 3 | 0.2 | 7 | 0.3 | 11 | 0.5 | 21 | 0.3 | 0.4 | 1.4† |
| 3.6 Unspecified intracranial and intraspinal neoplasms | 100 | 4.6 | 92 | 4.0 | 67 | 2.7 | 259 | 3.8 | 39 | 2.0 | 49 | 2.3 | 61 | 2.5 | 149 | 2.3 | 3.1 | 1.7† |
| 4. Osseous and Chondromatous Neoplasms | 379 | 17.6 | 219 | 9.6 | 146 | 5.8 | 744 | 11.3 | 189 | 9.6 | 124 | 5.8 | 120 | 5.0 | 433 | 6.9 | 9.2 | 1.6 † |
| 4.1 Osteosarcoma | 257 | 11.9 | 104 | 4.6 | 53 | 2.1 | 414 | 6.4 | 126 | 6.4 | 49 | 2.3 | 47 | 2.0 | 222 | 3.7 | 5.1 | 1.8† |
| 4.2 Chondrosarcoma | 28 | 1.3 | 39 | 1.7 | 45 | 1.8 | 112 | 1.6 | 10 | 0.5 | 33 | 1.5 | 34 | 1.4 | 77 | 1.1 | 1.4 | 1.4† |
| 4.3 Ewing tumor | 53 | 2.5 | 44 | 1.9 | 18 | 0.7 | 115 | 1.7 | 30 | 1.5 | 14 | 0.7 | 21 | 0.9 | 65 | 1.0 | 1.4 | 1.7† |
| 4.4 Other specified and unspecified bone tumors | 41 | 1.9 | 32 | 1.4 | 30 | 1.2 | 103 | 1.5 | 23 | 1.2 | 28 | 1.3 | 18 | 0.7 | 69 | 1.1 | 1.3 | 1.4† |
| 5. Soft Tissue Sarcomas | 220 | 10.2 | 248 | 10.9 | 345 | 13.7 | 813 | 11.5 | 174 | 8.9 | 223 | 10.4 | 302 | 12.6 | 699 | 10.5 | 11.1 | 1.1 † |
| 5.1 Fibromatous neoplasms | 44 | 2.0 | 63 | 2.8 | 92 | 3.7 | 199 | 2.8 | 35 | 1.8 | 59 | 2.8 | 88 | 3.7 | 182 | 2.7 | 2.7 | 1.0 |
| 5.2 Rhabdomyosarcoma | 58 | 2.7 | 26 | 1.1 | 25 | 1.0 | 109 | 1.7 | 36 | 1.8 | 16 | 0.7 | 19 | 0.8 | 71 | 1.2 | 1.4 | 1.4† |
| 5.3 Other soft tissue sarcoma | 118 | 5.5 | 159 | 7.0 | 228 | 9.1 | 505 | 7.1 | 103 | 5.2 | 148 | 6.9 | 195 | 8.1 | 446 | 6.7 | 6.9 | 1.1† |
| 6. Germ Cell and Trophoblastic Neoplasms | 340 | 15.8 | 417 | 18.3 | 473 | 18.8 | 1,230 | 17.6 | 341 | 17.4 | 300 | 14.0 | 295 | 12.3 | 936 | 14.7 | 16.1 | 1.2 † |
| 6.1 Germ cell and trophoblastic neoplasms of gonads | 84 | 3.9 | 231 | 10.1 | 364 | 14.5 | 679 | 9.3 | 283 | 14.4 | 243 | 11.4 | 195 | 8.1 | 721 | 11.4 | 10.3 | 0.8† |
| 6.2 Germ cell and trophoblastic neoplasms of nongonadal sites | 256 | 11.9 | 186 | 8.2 | 109 | 4.3 | 551 | 8.3 | 58 | 3.0 | 57 | 2.7 | 100 | 4.2 | 215 | 3.2 | 5.9 | 2.5† |
| 7. Melanoma and Skin Carcinomas | 21 | 1.0 | 40 | 1.8 | 107 | 4.3 | 168 | 2.3 | 19 | 1.0 | 42 | 2.0 | 98 | 4.1 | 159 | 2.3 | 2.3 | 1.0 |
| 7.1 Melanoma | 12 | 0.6 | 20 | 0.9 | 50 | 2.0 | 82 | 1.1 | 11 | 0.6 | 26 | 1.2 | 46 | 1.9 | 83 | 1.2 | 1.2 | 0.9 |
| 7.2 Skin carcinomas | 9 | 0.4 | 20 | 0.9 | 57 | 2.3 | 86 | 1.2 | 8 | 0.4 | 16 | 0.7 | 52 | 2.2 | 76 | 1.1 | 1.1 | 1.1 |
| 8. Carcinomas | 516 | 24.0 | 1,272 | 55.8 | 3,653 | 145.2 | 5,441 | 73.0 | 1,257 | 64.1 | 4,451 | 207.9 | 12,959 | 539.4 | 18,667 | 262.2 | 165.0 | 0.3 † |
| 8.1 Thyroid carcinoma | 184 | 8.5 | 446 | 19.6 | 1,211 | 48.1 | 1,841 | 24.7 | 872 | 44.4 | 2,882 | 134.6 | 7,141 | 297.3 | 10,895 | 154.2 | 87.7 | 0.2† |
| 8.2 Other carcinoma of head and neck | 81 | 3.8 | 133 | 5.8 | 213 | 8.5 | 427 | 5.9 | 72 | 3.7 | 133 | 6.2 | 199 | 8.3 | 404 | 6.0 | 5.9 | 1.0 |
| 8.2.1 Nasopharyngeal carcinoma | 40 | 1.9 | 43 | 1.9 | 47 | 1.9 | 130 | 1.9 | 17 | 0.9 | 19 | 0.9 | 28 | 1.2 | 64 | 1.0 | 1.4 | 1.9† |
| 8.2.2 Other sites in lip, oral cavity, and pharynx | 36 | 1.7 | 81 | 3.6 | 143 | 5.7 | 260 | 3.6 | 47 | 2.4 | 104 | 4.9 | 152 | 6.3 | 303 | 4.4 | 4.0 | 0.8 |
| 8.2.3 Nasal cavity, middle ear, sinuses, larynx, and other ill-defined sites in head/neck | 5 | 0.2 | 9 | 0.4 | 23 | 0.9 | 37 | 0.5 | 8 | 0.4 | 10 | 0.5 | 19 | 0.8 | 37 | 0.5 | 0.5 | 1.0 |
| 8.3 Carcinoma of trachea, bronchus, and lung | 25 | 1.2 | 50 | 2.2 | 104 | 4.1 | 179 | 2.4 | 16 | 0.8 | 52 | 2.4 | 121 | 5.0 | 189 | 2.7 | 2.6 | 0.9† |
| 8.4 Carcinoma of breast | 2 | 0.1 | 3 | 0.1 | 5 | 0.1 | 15 | 0.8 | 265 | 12.4 | 1,672 | 69.6 | 1,952 | 26.5 | 13.0 | 0.003† | ||
| 8.5 Carcinoma of genitourinary tract | 30 | 1.4 | 93 | 4.1 | 290 | 11.5 | 413 | 5.5 | 154 | 7.8 | 524 | 24.5 | 1,986 | 82.7 | 2,664 | 37.1 | 20.9 | 0.1† |
| 8.5.1 Carcinoma of kidney | 17 | 0.8 | 52 | 2.3 | 173 | 6.9 | 242 | 3.2 | 18 | 0.9 | 34 | 1.6 | 108 | 4.5 | 160 | 2.3 | 2.8 | 1.4† |
| 8.5.2 Carcinoma of bladder | 12 | 0.6 | 30 | 1.3 | 103 | 4.1 | 145 | 1.9 | 2 | 0.1 | 16 | 0.7 | 31 | 1.3 | 49 | 0.7 | 1.3 | 2.8† |
| 8.5.3 Carcinoma of gonads | - | - | 3 | 0.1 | 2 | 0.1 | 5 | 0.1 | 123 | 6.3 | 242 | 11.3 | 420 | 17.5 | 785 | 11.5 | 5.6 | 0.01† |
| 8.5.4 Carcinoma of cervix and uterus | - | - | - | - | - | - | - | - | 8 | 0.4 | 229 | 10.7 | 1,399 | 58.2 | 1,636 | 22.2 | 10.8 | - |
| 8.5.5 Carcinoma of other and ill-defined sites in genitourinary tract | 1 | 0.0 | 8 | 0.4 | 12 | 0.5 | 21 | 0.3 | 3 | 0.2 | 3 | 0.1 | 28 | 1.2 | 34 | 0.5 | 0.4 | 0.6† |
| 8.6 Carcinoma of gastrointestinal tract | 161 | 7.5 | 500 | 21.9 | 1,715 | 68.2 | 2,376 | 31.5 | 113 | 5.8 | 553 | 25.8 | 1,740 | 72.4 | 2,406 | 33.5 | 32.5 | 0.9† |
| 8.6.1 Carcinoma of colon and rectum | 67 | 3.1 | 176 | 7.7 | 530 | 21.1 | 773 | 10.3 | 46 | 2.3 | 165 | 7.7 | 402 | 16.7 | 613 | 8.7 | 9.5 | 1.2† |
| 8.6.2 Carcinoma of stomach | 41 | 1.9 | 179 | 7.9 | 764 | 30.4 | 984 | 12.9 | 33 | 1.7 | 298 | 13.9 | 1,116 | 46.5 | 1,447 | 19.9 | 16.3 | 0.6† |
| 8.6.3 Carcinoma of liver and intrahepatic bile ducts | 51 | 2.4 | 121 | 5.3 | 373 | 14.8 | 545 | 7.3 | 18 | 0.9 | 66 | 3.1 | 151 | 6.3 | 235 | 3.3 | 5.4 | 2.2† |
| 8.6.4 Carcinoma of pancreas | 1 | 0.0 | 16 | 0.7 | 21 | 0.8 | 38 | 0.5 | 12 | 0.6 | 14 | 0.7 | 32 | 1.3 | 58 | 0.9 | 0.7 | 0.6† |
| 8.6.5 Carcinoma of other and ill-defined sites in gastrointestinal tract | 1 | 0.0 | 8 | 0.4 | 27 | 1.1 | 36 | 0.5 | 4 | 0.2 | 10 | 0.5 | 39 | 1.6 | 53 | 0.7 | 0.6 | 0.6† |
| 8.7 Carcinoma of other and ill-defined sites | 35 | 1.6 | 48 | 2.1 | 117 | 4.7 | 200 | 2.7 | 15 | 0.8 | 42 | 2.0 | 100 | 4.2 | 157 | 2.2 | 2.5 | 1.2† |
| 8.7.1 Adrenocortical carcinoma | 1 | 0.0 | 5 | 0.2 | 6 | 0.2 | 12 | 0.2 | 3 | 0.2 | 7 | 0.3 | 8 | 0.3 | 18 | 0.3 | 0.2 | 0.6† |
| 8.7.2 Carcinoma of other and ill-defined sites, NOS | 34 | 1.6 | 43 | 1.9 | 111 | 4.4 | 188 | 2.6 | 12 | 0.6 | 35 | 1.6 | 92 | 3.8 | 139 | 2.0 | 2.3 | 1.3† |
| 9. Miscellaneous Specified Neoplasms, NOS | 120 | 5.6 | 117 | 5.1 | 181 | 7.2 | 418 | 6.0 | 100 | 5.1 | 141 | 6.6 | 254 | 10.6 | 495 | 7.3 | 6.6 | 0.8 † |
| 9.1 Other pediatric and embryonal tumors, NOS | 30 | 1.4 | 20 | 0.9 | 24 | 1.0 | 74 | 1.1 | 20 | 1.0 | 17 | 0.8 | 22 | 0.9 | 59 | 0.9 | 1.0 | 1.2† |
| 9.2 Other specified and embryonal tumors, NOS | 90 | 4.2 | 97 | 4.3 | 157 | 6.2 | 344 | 4.9 | 80 | 4.1 | 124 | 5.8 | 232 | 9.7 | 436 | 6.4 | 5.6 | 0.8† |
| 10. Unspecified Malignant Neoplasms | 67 | 3.1 | 125 | 5.5 | 259 | 10.3 | 451 | 6.2 | 88 | 4.5 | 203 | 9.5 | 428 | 17.8 | 719 | 10.4 | 8.2 | 0.6 † |
*M/F Ratio = Male ASR/Female ASR.
P-values <0.05.
Thyroid carcinoma was excluded from the calculation of the incidence rate of all cancers combined because of its unusually high incidence rate.
CR, crude incidence rate; CNS, central nervous system; PNET, primitive neuroectodermal tumor; NOS, not otherwise specified.
Between 1999 and 2010, the overall age-standardized incidence rate of cancers among AYAs in Korea was 279.9 per million. Cancer incidence was higher in females (367.8 per million) than in males (196.4 per million), and for all cancers combined, the male/female ratio was 0.5 (P<0.05). The higher rate among females was largely due to a much higher incidence rate of thyroid carcinomas (24.7 per million among males vs. 154.2 per million among females). Because the incidence rate of thyroid carcinoma was unusually high, the age-standardized incidence rates of all cancers combined were recalculated, excluding thyroid carcinoma (group 8.1). Removing thyroid carcinomas, the overall ASR of cancers was 192.2 per million (171.7 per million for males and 213.6 per million for females) (Table 1).
The incidence increased with age, from 150.9 in males and 160.4 in females per million at 15–19 years of age to 170.7 in males and 305.6 in females per million, respectively, at 20–24 years of age. The incidence further increased at 25–59 years of age, to 273.4 in males and 663.3 in females per million, respectively (Table 1). The incidence rates were correlated with age group for most subtypes, with the notable exceptions of leukemia and osseous/chondromatous neoplasms, which were more common among younger AYAs (Table 1).
Table 2 shows the secular trends in cancer incidence among AYAs from 1999 to 2010 according to the diagnostic group. The incidence rate of all cancers among AYAs significantly increased, from 208.7 per million in 1999 to 396.4 per million in 2010 (APC = 6.3%; P<0.05). Over the studied time period, there was also a steady increase in the incidence of cancer among AYAs for both males (APC = 3.9%) and females (APC = 7.8%) (Figure 1).
Table 2. Trends in age-standardized incidence rates among Korean AYAs and estimated annual percent changes (APCs).
| 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | APC | |
| All cancers | 208.7 | 204.7 | 230.3 | 226.4 | 256.3 | 266.2 | 275.6 | 290.8 | 315.8 | 355.6 | 370.4 | 396.4 | 6.3* |
| All cancers (excluding thyroid carcinoma) † | 174.8 | 170.9 | 188.2 | 178.9 | 193.6 | 188.0 | 193.5 | 197.1 | 203.8 | 210.7 | 208.2 | 207.5 | 1.8* |
| 1 Leukemias | 23.4 | 24.1 | 27.8 | 26.0 | 24.5 | 24.5 | 25.6 | 26.6 | 28.2 | 29.6 | 28.9 | 26.5 | 1.5* |
| 1.1 Acute lymphoid leukemia | 7.0 | 7.6 | 7.4 | 7.2 | 6.7 | 6.9 | 7.7 | 8.0 | 8.4 | 8.3 | 8.9 | 8.8 | 2.2* |
| 1.2 Acute myeloid leukemia | 10.4 | 9.2 | 12.3 | 12.2 | 10.5 | 10.5 | 10.8 | 12.5 | 11.3 | 12.7 | 11.1 | 9.3 | 0.3 |
| 1.3 Chronic myeloid leukemia | 3.5 | 4.3 | 4.7 | 3.7 | 4.7 | 4.4 | 4.5 | 3.6 | 4.9 | 5.0 | 5.3 | 5.5 | 2.9* |
| 1.4 Other and unspecified leukemia | 2.5 | 3.0 | 3.4 | 2.8 | 2.7 | 2.6 | 2.7 | 2.5 | 3.6 | 3.5 | 3.6 | 2.8 | 1.5 |
| 2 Lymphomas | 16.6 | 15.0 | 17.7 | 17.2 | 21.8 | 23.1 | 21.5 | 23.9 | 23.9 | 26.7 | 26.3 | 26.0 | 5.3* |
| 2.1 Non-Hodgkin lymphoma | 14.5 | 12.5 | 14.4 | 13.6 | 17.2 | 18.2 | 17.7 | 19.6 | 18.6 | 20.7 | 20.8 | 19.9 | 4.5* |
| 2.2 Hodgkin lymphoma | 2.1 | 2.4 | 3.3 | 3.6 | 4.6 | 4.9 | 3.8 | 4.3 | 5.3 | 6.0 | 5.5 | 6.1 | 9.1* |
| 3 CNS and Other Intracranial and Intraspinal Neoplasms (all behaviors) | 12.7 | 12.9 | 12.0 | 13.4 | 15.0 | 14.5 | 12.9 | 14.6 | 14.5 | 15.7 | 14.1 | 13.3 | 1.2 |
| 3.1. Astrocytoma | 4.8 | 3.8 | 4.7 | 4.5 | 4.7 | 5.4 | 4.6 | 5.0 | 6.3 | 5.6 | 4.8 | 5.3 | 2.3* |
| 3.2 Other glioma | 1.4 | 1.8 | 1.5 | 2.8 | 2.8 | 2.5 | 2.5 | 2.6 | 2.7 | 4.2 | 3.3 | 3.4 | 8.0* |
| 3.3 Ependymoma | 0.5 | 1.0 | 1.1 | 0.9 | 1.1 | 0.9 | 0.7 | 1.2 | 1.0 | 1.4 | 1.2 | 1.3 | 4.9* |
| 3.4. Medulloblastoma and other PNET | 1.6 | 1.5 | 1.6 | 2.0 | 2.3 | 2.3 | 1.2 | 2.4 | 1.3 | 2.0 | 2.0 | 1.1 | -0.8 |
| 3.5 Other specified intracranial and intraspinal neoplasms | 0.6 | 0.4 | 0.4 | 0.2 | 0.5 | 0.4 | 0.3 | 0.2 | 0.5 | 0.5 | 0.2 | 0.3 | -3.9 |
| 3.6 Unspecified intracranial and intraspinal neoplasms | 3.7 | 4.5 | 2.8 | 3.0 | 3.7 | 2.9 | 3.6 | 3.1 | 2.8 | 2.0 | 2.5 | 2.1 | -5.0* |
| 4 Osseous & Chondromatous Neoplasms | 8.4 | 8.7 | 11.4 | 7.9 | 10.6 | 8.5 | 8.2 | 7.6 | 9.7 | 10.2 | 8.8 | 10.5 | 0.6 |
| 4.1 Osteosarcoma | 5.0 | 4.9 | 6.8 | 4.9 | 5.6 | 5.3 | 4.9 | 3.8 | 4.4 | 6.1 | 4.4 | 5.2 | -1.1 |
| 4.2 Chondrosarcoma | 1.4 | 1.4 | 1.1 | 0.9 | 1.9 | 1.0 | 1.3 | 1.1 | 1.5 | 1.6 | 1.2 | 2.0 | 2.3 |
| 4.3 Ewing tumor | 0.7 | 1.3 | 1.7 | 1.2 | 1.4 | 1.0 | 1.2 | 1.3 | 1.9 | 1.3 | 1.9 | 2.1 | 5.8* |
| 4.4 Other specified and unspecified bone tumors | 1.3 | 1.1 | 1.8 | 0.9 | 1.7 | 1.1 | 0.8 | 1.5 | 1.9 | 1.2 | 1.3 | 1.2 | 0.4 |
| 5 Soft Tissue Sarcomas | 8.9 | 8.5 | 10.6 | 9.5 | 11.0 | 10.9 | 9.7 | 12.5 | 13.0 | 12.9 | 13.6 | 12.8 | 4.1* |
| 5.1 Fibromatous neoplasms | 1.4 | 1.8 | 1.7 | 2.0 | 3.2 | 2.7 | 2.5 | 3.3 | 3.2 | 4.5 | 3.1 | 4.2 | 9.6* |
| 5.2 Rhabdomyosarcoma | 1.2 | 1.6 | 1.2 | 1.6 | 0.6 | 2.5 | 1.3 | 2.4 | 1.5 | 0.7 | 1.0 | 1.3 | -1.6 |
| 5.3 Other soft tissue sarcoma | 6.3 | 5.0 | 7.7 | 5.9 | 7.2 | 5.7 | 5.9 | 6.8 | 8.3 | 7.7 | 9.4 | 7.3 | 3.2* |
| 6 Germ Cell and Trophoblastic Neoplasms | 13.5 | 14.0 | 14.8 | 14.3 | 14.5 | 14.7 | 17.0 | 16.4 | 19.1 | 17.8 | 18.3 | 20.2 | 3.6* |
| 6.1 Germ cell and trophoblastic neoplasms of gonads | 9.1 | 8.6 | 9.9 | 9.0 | 9.0 | 10.2 | 10.3 | 10.3 | 11.2 | 12.0 | 11.4 | 12.9 | 3.3* |
| 6.2 Germ cell and trophoblastic neoplasms of nongonadalsites | 4.4 | 5.5 | 4.9 | 5.3 | 5.6 | 4.4 | 6.7 | 6.1 | 8.0 | 5.9 | 7.0 | 7.3 | 4.2* |
| 7 Melanoma and Skin Carcinomas | 1.7 | 2.1 | 2.6 | 1.8 | 1.0 | 1.5 | 3.7 | 2.5 | 2.3 | 2.7 | 3.2 | 2.5 | 4.8 |
| 7.1 Melanoma | 1.1 | 1.2 | 1.2 | 0.9 | 0.5 | 0.9 | 2.0 | 0.9 | 0.9 | 1.4 | 1.9 | 1.1 | 2.6 |
| 7.2 Skin carcinomas | 0.6 | 0.8 | 1.5 | 0.9 | 0.6 | 0.5 | 1.7 | 1.6 | 1.4 | 1.3 | 1.4 | 1.5 | 7.2* |
| 8 Carcinomas | 105.3 | 102.0 | 116.4 | 123.2 | 141.5 | 155.2 | 163.7 | 173.1 | 191.8 | 225.1 | 244.4 | 270.7 | 9.4* |
| 8.1 Thyroid carcinoma | 33.9 | 33.8 | 42.1 | 47.4 | 62.6 | 78.2 | 82.1 | 93.6 | 112.0 | 144.9 | 162.2 | 188.9 | 17.9* |
| 8.2 Other carcinoma of head and neck | 4.9 | 5.1 | 5.0 | 5.9 | 6.7 | 5.9 | 6.1 | 5.3 | 6.8 | 6.7 | 8.1 | 5.2 | 2.5 |
| 8.2.1 Nasopharyngeal carcinoma | 1.1 | 1.8 | 1.2 | 1.2 | 1.9 | 1.6 | 1.3 | 1.1 | 1.4 | 1.7 | 1.8 | 1.4 | 1.2 |
| 8.2.2 Other sites in lip, oral cavity and pharynx | 3.2 | 3.0 | 3.3 | 4.2 | 3.9 | 3.8 | 4.4 | 3.9 | 5.0 | 4.4 | 5.5 | 3.6 | 3.4* |
| 8.2.3 Nasal cav,mid ear,sinuses,larynx,oth ill-defhead/neck | 0.6 | 0.4 | 0.4 | 0.5 | 0.9 | 0.6 | 0.4 | 0.3 | 0.5 | 0.7 | 0.7 | 0.3 | -0.6 |
| 8.3 Carcinoma of trachea,bronchus, and lung | 2.4 | 1.5 | 2.9 | 2.2 | 2.5 | 3.0 | 3.1 | 3.0 | 1.9 | 2.2 | 2.9 | 3.4 | 2.6 |
| 8.4 Carcinoma of breast | 8.5 | 11.4 | 12.3 | 13.6 | 12.2 | 12.6 | 15.1 | 13.8 | 13.9 | 14.1 | 13.8 | 15.3 | 3.5* |
| 8.5 Carcinoma of genitourinary tract | 18.4 | 14.7 | 17.2 | 18.7 | 21.5 | 21.1 | 20.9 | 23.8 | 22.2 | 24.5 | 24.0 | 25.7 | 4.2* |
| 8.5.1 Carcinoma of kidney | 2.2 | 0.9 | 2.3 | 2.1 | 2.1 | 2.8 | 2.5 | 4.2 | 2.4 | 4.3 | 4.4 | 3.3 | 9.1* |
| 8.5.2 Carcinoma of bladder | 1.4 | 0.8 | 1.4 | 1.1 | 1.7 | 1.5 | 1.8 | 1.1 | 1.6 | 1.2 | 0.9 | 1.4 | 0.7 |
| 8.5.3 Carcinoma of gonads | 5.9 | 5.9 | 4.9 | 5.5 | 6.8 | 5.2 | 5.8 | 5.2 | 6.4 | 4.9 | 4.4 | 6.1 | -0.8 |
| 8.5.4 Carcinoma of cervix and uterus | 8.6 | 6.7 | 8.4 | 9.5 | 10.3 | 11.1 | 10.3 | 13.0 | 11.3 | 13.9 | 13.7 | 14.6 | 6.2* |
| 8.5.5 Carc of other and ill-def sites, geniourinary tract | 0.2 | 0.3 | 0.2 | 0.5 | 0.5 | 0.4 | 0.5 | 0.3 | 0.5 | 0.2 | 0.5 | 0.3 | 2.8 |
| 8.6 Carcinoma of gastrointestinal tract | 34.2 | 32.3 | 34.2 | 33.1 | 34.3 | 31.6 | 34.0 | 31.2 | 31.7 | 31.1 | 31.1 | 30.4 | -1.0* |
| 8.6.1 Carcinoma of colon and rectum | 9.1 | 8.1 | 8.4 | 7.7 | 9.1 | 8.3 | 9.2 | 9.4 | 9.8 | 12.9 | 11.8 | 11.3 | 3.7* |
| 8.6.2 Carcinoma of stomach | 18.8 | 17.3 | 18.4 | 18.7 | 17.4 | 15.8 | 17.3 | 15.4 | 15.3 | 13.2 | 13.7 | 13.2 | -3.3* |
| 8.6.3 Carcinoma of liver and intrahepatic bile ducts | 5.2 | 5.5 | 6.8 | 5.8 | 6.3 | 5.4 | 6.0 | 5.2 | 5.3 | 3.6 | 4.3 | 4.5 | -3.2* |
| 8.6.4 Carcinoma of pancreas | 0.4 | 0.8 | 0.3 | 0.6 | 0.6 | 1.0 | 0.8 | 0.8 | 0.8 | 0.6 | 0.4 | 1.1 | 4.8 |
| 8.6.5 Carc other and ill-def sites, gastrointestinal tract | 0.7 | 0.6 | 0.4 | 0.2 | 0.8 | 1.0 | 0.6 | 0.4 | 0.6 | 0.8 | 1.0 | 0.3 | 0.1 |
| 8.7 Carcinoma of other and ill-def sites | 3.0 | 3.3 | 2.7 | 2.3 | 1.8 | 2.8 | 2.4 | 2.5 | 3.1 | 1.7 | 2.4 | 2.0 | -3.0 |
| 8.7.1 Adrenocortical carcinoma | 0.2 | 0.2 | 0.1 | 0.2 | 0.2 | 0.1 | 0.1 | 0.4 | 0.5 | 0.1 | 0.4 | - | 6.7 |
| 8.7.2 Carcinoma of other and ill-defined sites, NOS | 2.8 | 3.0 | 2.6 | 2.1 | 1.6 | 2.7 | 2.3 | 2.1 | 2.6 | 1.6 | 1.9 | 2.0 | -3.4* |
| 9 Miscellaneous specified neoplasms, NOS | 3.1 | 4.4 | 4.3 | 4.3 | 7.5 | 6.4 | 7.5 | 8.2 | 8.2 | 9.7 | 8.1 | 9.1 | 9.8* |
| 9.1 Other pediatric and embryonal tumors, NOS | 0.7 | 1.3 | 0.7 | 0.8 | 1.4 | 0.6 | 1.3 | 0.9 | 0.9 | 1.5 | 0.7 | 1.4 | 3.0 |
| 9.2 Other specified and embryonal tumors, NOS | 2.5 | 3.1 | 3.6 | 3.5 | 6.0 | 5.8 | 6.3 | 7.4 | 7.3 | 8.3 | 7.4 | 7.7 | 11.3 |
| 10 Unspecified Malignant Neoplasms | 15.1 | 13.1 | 12.7 | 8.8 | 8.8 | 7.1 | 5.6 | 5.3 | 5.0 | 5.1 | 4.6 | 4.7 | -10.9* |
*P-values <0.05.
Thyroid carcinoma was excluded from the calculation of the incidence rate of all cancers combined because of its unusually high incidence rate.
AYAs, adolescents and young adults (aged 15–29 years); CNS, central nervous system; PNET, primitive neuroectodermal tumor; NOS, not otherwise specified.
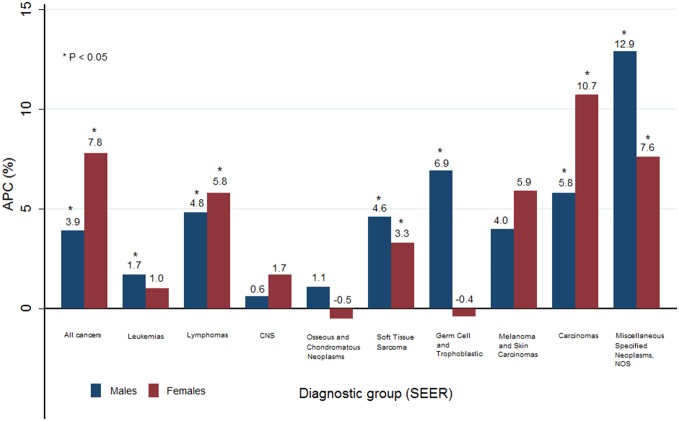
Figure 1. Annual percent change (APC) from 1999–2010 according to the diagnostic group (SEER).
Most cancer sites showed a trend of increasing incidence, with the exception of unspecified malignant neoplasms (group 10) among AYAs (APC = −10.9%). For miscellaneous specified neoplasms, NOS (group 9), a rapid increase in the incidence rate was observed for both sexes (APC = 9.8%), but the number of cases was small. For carcinomas (group 10), a large increase in the incidence rate was also observed among AYAs (APC = 9.4%). In particular, among carcinomas, the incidence of thyroid carcinoma showed the most rapid increase (APC = 17.9%; P<0.05). The annual percent change in all cancer combined, excluding thyroid carcinoma, was 1.8% (P<0.05) among AYAs (Table 2), with 1.9% (P<0.05) for males and 1.8% (P<0.05) for females (data not shown). Although the incidence of kidney carcinoma also exhibited a notable increase (APC = 9.1%), the number of cases was small.
The incidence of lymphomas (group 2) exhibited a large increase among AYAs (APC = 5.3%, P<0.05). In particular, Hodgkin lymphoma rapidly increased in incidence (APC = 9.1%, P<0.05) (Table 2).
Cancer incidence trends within diagnostic groups were observed to differ by gender. Among males, the incidence of most cancer sites was increased. Miscellaneous specified neoplasms, NOS, showed the largest increase in incidence (APC = 12.9%, P<0.05), followed by germ cell and trophoblastic neoplasms (APC = 6.9%, P<0.05). Among females, the incidence of most cancer sites was also increased. Carcinomas (APC = 10.7%, P<0.05) and miscellaneous specified neoplasms, NOS (APC = 7.6%, P<0.05), showed the greatest increases. However, osseous/chondromatous neoplasms (APC = −0.5%) and germ cell and trophoblastic neoplasms (APC = −0.4%) slightly decreased in incidence (Figure 1). Additionally, a notable increase in the incidence of carcinomas among females was observed in the cervix (APC = 6.2%, P<0.05) and breast (APC = 3.5%, P<0.05) (data not shown).
Survival
A total of 52,077 cancer cases diagnosed from 1993 to 2010 were used for the survival analysis. Table 3 shows the 5-year relative survival rates and numbers of cases in the four time periods (1993–1995, 1996–2000, 2001–2005 and 2006–2010). For all cancers combined, the 5-year relative survival rate of AYAs significantly improved, from 58.9% in 1993–1995 to 84.8% in 2006–2010 (P<0.05). AYAs with leukemia and lymphoma showed the most marked improvement in survival from 25.8% (95% CI: 22.9–28.7) and 55.4% (95% CI: 50.6–59.9) in 1993–1995 to 58.8% (95% CI: 55.5–61.8) and 83.6% (95% CI: 80.9–85.9) in 2006–2010, respectively. Conversely, decreases in survival were observed from 1993–1995 to 2006–2010 for other glioma (group 3.2), carcinoma of gonads (group 8.5.3), and carcinoma of pancreas (group 8.6.4) (Table 3).
Table 3. Five-year RSRs for Korean AYAs according to the time period of cancer diagnosis.
| Diagnostic group (SEER) | Both sexes | Change† | P | |||||||
| 1993–1995 | 1996–2000 | 2001–2005 | 2006–2010 | |||||||
| Cases | RSR | Cases | RSR | Cases | RSR | Cases | RSR | |||
| All Cancers | 6,387 | 58.9 | 12,453 | 66.4 | 14,310 | 76.5 | 18,927 | 84.8 | 25.9 | * |
| All Cancers (excluding thyroid carcinoma) ‡ | 5,525 | 52.6 | 10,474 | 60.2 | 10,555 | 68.2 | 10,884 | 74.4 | 21.8 | * |
| 1. Leukemias | 866 | 25.8 | 1,440 | 35.9 | 1,397 | 47.7 | 1,435 | 58.8 | 33.0 | * |
| 1.1 Acute lymphoid leukemia | 237 | 19.9 | 433 | 29 | 379 | 33.9 | 425 | 48.6 | 28.7 | * |
| 1.2 Acute myeloid leukemia | 375 | 26.0 | 602 | 37.5 | 624 | 47.2 | 588 | 52.7 | 26.7 | * |
| 1.3 Chronic myeloid leukemia | 141 | 41.4 | 251 | 51.2 | 252 | 76 | 262 | 90.5 | 49.1 | * |
| 1.4 Other and unspecified leukemia | 113 | 17.8 | 154 | 24.1 | 142 | 36 | 160 | 58.8 | 41.0 | * |
| 2. Lymphomas | 439 | 55.4 | 937 | 65 | 1,133 | 75.8 | 1,315 | 83.6 | 28.2 | * |
| 2.1 Non-Hodgkin lymphoma | 372 | 51.3 | 790 | 60.6 | 906 | 72.5 | 1,037 | 82.0 | 30.7 | * |
| 2.2 Hodgkin lymphoma | 67 | 78.0 | 147 | 88.8 | 227 | 88.8 | 278 | 89.4 | 11.4 | * |
| 3. CNS and Other Intracranial and Intraspinal Neoplasms | 371 | 53.4 | 689 | 54.5 | 730 | 60 | 750 | 65.6 | 12.2 | * |
| 3.1. Astrocytoma | 179 | 43.2 | 305 | 45.1 | 275 | 45.2 | 291 | 54.3 | 11.1 | * |
| 3.2 Other glioma | 65 | 72.7 | 83 | 61.7 | 141 | 66.1 | 177 | 71.2 | -1.5 | |
| 3.3 Ependymoma | 14 | 86.1 | 49 | 79.9 | 57 | 88.0 | 62 | 87.0 | 0.9 | |
| 3.4 Medulloblastoma and other PNET | 18 | 44.6 | 84 | 49 | 92 | 47.9 | 84 | 52.3 | 7.7 | |
| 3.5 Other specified intracranial and intraspinal neoplasms | 13 | 61.9 | 27 | 74.4 | 20 | 80.2 | 18 | 72.6 | 10.7 | |
| 3.6 Unspecified intracranial and intraspinal neoplasms | 82 | 55.2 | 141 | 61.2 | 145 | 76.1 | 118 | 80.6 | 25.4 | * |
| 4. Osseous and Chondromatous Neoplasms | 262 | 48.3 | 516 | 66.1 | 479 | 68.7 | 447 | 74.8 | 26.5 | * |
| 4.1 Osteosarcoma | 160 | 43.3 | 305 | 65.5 | 281 | 65.3 | 231 | 72.2 | 28.9 | * |
| 4.2 Chondrosarcoma | 28 | 64.6 | 75 | 87.0 | 69 | 87.2 | 77 | 94.9 | 30.3 | * |
| 4.3 Ewing tumor | 30 | 40.2 | 66 | 39.5 | 67 | 52.4 | 72 | 51.0 | 10.8 | |
| 4.4 Other specified and unspecified bone tumors | 44 | 61.7 | 70 | 71.7 | 62 | 80.9 | 67 | 85.5 | 23.8 | * |
| 5. Soft Tissue Sarcomas | 243 | 58.7 | 481 | 58.8 | 554 | 67.5 | 617 | 73.4 | 14.7 | * |
| 5.1 Fibromatous neoplasms | 51 | 78.8 | 103 | 78 | 135 | 93.6 | 193 | 95.1 | 16.3 | * |
| 5.2 Rhabdomyosarcoma | 32 | 28.3 | 73 | 37.1 | 66 | 34.9 | 55 | 37.5 | 9.2 | |
| 5.3 Other soft tissue sarcoma | 160 | 58.4 | 305 | 57.6 | 353 | 63.6 | 369 | 67.8 | 9.4 | * |
| 6. Germ Cell and Trophoblastic Neoplasms | 278 | 81.6 | 745 | 87 | 842 | 89.9 | 936 | 91.7 | 10.1 | * |
| 6.1 Germ cell and trophoblastic neoplasms of gonads | 174 | 89.4 | 465 | 92.3 | 558 | 94.3 | 613 | 96.2 | 6.8 | * |
| 6.2 Germ cell and trophoblastic neoplasms of nongonadal sites | 104 | 68.6 | 280 | 78.1 | 284 | 81.2 | 323 | 83.2 | 14.6 | |
| 7. Melanoma and Skin Carcinomas | 56 | 64.6 | 111 | 59.7 | 122 | 77.3 | 138 | 86.3 | 21.7 | * |
| 7.1 Melanoma | 25 | 32.2 | 64 | 43.9 | 63 | 60.5 | 59 | 65.0 | 32.8 | * |
| 7.2 Skin carcinomas | 31 | 90.8 | 47 | 81.2 | 59 | 95.2 | 79 | 99.0 | 8.2 | * |
| 8. Carcinomas | 3,205 | 67.5 | 6,607 | 72.2 | 8,366 | 82.4 | 12,601 | 89.9 | 22.4 | * |
| 8.1 Thyroid carcinoma | 862 | 99.3 | 1,979 | 99.6 | 3,755 | 99.9 | 8,043 | 99.9 | 0.6 | * |
| 8.2 Other carcinoma of head and neck | 140 | 69.6 | 289 | 77.8 | 339 | 83.1 | 333 | 85.5 | 15.9 | * |
| 8.2.1 Nasopharyngeal carcinoma | 40 | 70.4 | 104 | 65.7 | 79 | 80.0 | 75 | 85.1 | 14.7 | * |
| 8.2.2 Other sites in lip, oral cavity, and pharynx | 73 | 79.8 | 159 | 87.7 | 233 | 85.6 | 235 | 88.6 | 8.8 | |
| 8.2.3 Nasal cavity, middle ear, sinuses,larynx, and other ill-defined sites in head/neck | 27 | 41.0 | 26 | 65.6 | 27 | 70.6 | 23 | 60.5 | 19.5 | |
| 8.3 Carcinoma of trachea, bronchus, and lung | 73 | 24.8 | 136 | 36.2 | 144 | 47.4 | 132 | 43.1 | 18.3 | * |
| 8.4 Carcinoma of breast | 310 | 68.0 | 691 | 77.2 | 825 | 82.0 | 841 | 86.5 | 18.5 | * |
| 8.5 Carcinoma of genitourinary tract | 602 | 85.3 | 1,191 | 85.5 | 1,194 | 88.9 | 1,371 | 86.5 | 1.2 | |
| 8.5.1 Carcinoma of kidney | 35 | 68.9 | 94 | 76.9 | 142 | 87.6 | 209 | 87.0 | 18.1 | * |
| 8.5.2 Carcinoma of bladder | 39 | 85.1 | 77 | 94.0 | 90 | 93.6 | 73 | 95.9 | 10.8 | |
| 8.5.3 Carcinoma of gonads | 234 | 86.6 | 396 | 84.5 | 326 | 88.2 | 291 | 82.7 | -3.9 | |
| 8.5.4 Carcinoma of cervix and uterus | 285 | 86.6 | 605 | 86.7 | 611 | 89.1 | 781 | 87.0 | 0.4 | |
| 8.5.5 Carcinoma of other and ill-defined sites,genitourinary tract | 9 | 78.3 | 19 | 79.2 | 25 | 84.2 | 17 | 88.1 | 9.8 | |
| 8.6 Carcinoma of gastrointestinal tract | 1,123 | 38.0 | 2,127 | 42.3 | 1,974 | 50.2 | 1,766 | 59.2 | 21.2 | * |
| 8.6.1 Carcinoma of colon and rectum | 246 | 47.0 | 527 | 52.8 | 500 | 63.2 | 603 | 73.9 | 26.9 | * |
| 8.6.2 Carcinoma of stomach | 723 | 36.3 | 1,239 | 41.4 | 1,091 | 49.0 | 831 | 58.2 | 21.9 | * |
| 8.6.3 Carcinoma of liver andintrahepatic bile ducts | 90 | 23.5 | 277 | 23.6 | 308 | 33.6 | 256 | 31.6 | 8.1 | * |
| 8.6.4 Carcinoma of pancreas | 30 | 56.9 | 35 | 57.3 | 39 | 59.1 | 42 | 43.8 | -13.1 | |
| 8.6.5 Carcinoma of other and ill-defined sites ingastrointestinal tract | 34 | 29.6 | 49 | 45.1 | 36 | 39.0 | 34 | 59.1 | 29.5 | * |
| 8.7 Carcinoma of other and ill-defined sites | 95 | 41.3 | 194 | 39.3 | 135 | 44.6 | 115 | 54.0 | 12.7 | * |
| 8.7.1 Adrenocortical carcinoma | N/S | |||||||||
| 8.7.2 Carcinoma of other and ill-defined sites, NOS | 88 | 40.0 | 181 | 39.4 | 128 | 46.2 | 99 | 56.2 | 16.2 | * |
| 9. Miscellaneous Specified Neoplasms, NOS | 121 | 54.0 | 213 | 72.5 | 348 | 79.2 | 453 | 79.7 | 25.7 | * |
| 9.1 Other pediatric and embryonaltumors, NOS | 33 | 30.5 | 43 | 46.7 | 51 | 57.0 | 51 | 63.1 | 32.6 | * |
| 9.2 Other specified and embryonal tumors, NOS | 88 | 62.8 | 170 | 79.1 | 297 | 83.0 | 402 | 81.7 | 18.9 | * |
| 10. Unspecified Malignant Neoplasms | 546 | 62.0 | 714 | 70.8 | 339 | 79.0 | 235 | 77.8 | 15.8 | * |
*P-values <0.05 for trend.
Change (%) in the 5-year RSR from 1993–1995 to 2006–2010.
Thyroid carcinoma was excluded from the calculation of the incidence rate of all cancers combined because of its unusually high incidence rate.
N/S: not shown because <20 cases were reported in each period.
AYAs, adolescents and young adults (aged 15–29 years); CNS, central nervous system; PNET, primitive neuroectodermal tumor; NOS, not otherwise specified.
Survival rates for thyroid carcinoma (group 8.1) and skin carcinoma (group 7.2) were very high across all time periods. The five-year relative survival rate for thyroid carcinoma among males increased slightly, from 95.3% in 1993–1995 to 99.7% in 2006–2010, whereas the rate was unchanged among females from 1993–1995 (99.9%) to 2006–2010 (100.0%). The survival rates for germ cell and trophoblastic neoplasms (group 6), skin carcinoma (group 7.2), and carcinoma of the genitourinary tract (group 8.5) consistently exceeded 80–90% in all time periods. Conversely, the lowest survival rates were observed for rhabdomyosarcoma (group 5.2); carcinoma of the trachea, bronchus, and lung (group 8.3); and carcinoma of liver and intrahepatic bile ducts (group 8.6.3) (Table 3).
The survival rates for all cancers combined significantly increased from 1993 to 2010 in both males and females. In particular, the 5-year relative survival rate increased from 46.5% to 75.9% in males (P<0.05) and from 66.7% to 89.1% in females (P<0.05). However, the 5-year relative survival rate for all cancers combined was slightly lower in males than in females, regardless of whether thyroid carcinoma was excluded (Table 4).
Table 4. Five-year RSRs for Korean AYAs according to the time period of cancer diagnosis and sex.
| Diagnostic group (SEER) | Males | Females | ||||||||||||||||||
| 1993–1995 | 1996–2000 | 2001–2005 | 2006–2010 | Change† | P | 1993–1995 | 1996–2000 | 2001–2005 | 2006–2010 | Change† | P | |||||||||
| Cases | RSR | Cases | RSR | Cases | RSR | Cases | RSR | Cases | RSR | Cases | RSR | Cases | RSR | Cases | RSR | |||||
| All Cancers | 2,478 | 46.5 | 4,801 | 55.1 | 5,166 | 65.8 | 6,149 | 75.9 | 29.4 | * | 3,909 | 66.7 | 7,652 | 73.6 | 9,144 | 82.6 | 12,778 | 89.1 | 22.4 | * |
| All Cancers (excluding thyroid carcinoma) ‡ | 2,366 | 44.2 | 4,516 | 52.4 | 4,696 | 62.3 | 4,924 | 70.5 | 26.3 | * | 3,159 | 58.9 | 5,958 | 66.0 | 5,859 | 72.9 | 5,960 | 77.6 | 18.7 | * |
| 1. Leukemias | 492 | 25.4 | 791 | 33.0 | 826 | 46.2 | 841 | 57.9 | 32.5 | * | 374 | 26.3 | 649 | 39.4 | 571 | 49.8 | 594 | 60.0 | 33.7 | * |
| 1.1 Acute lymphoid leukemia | 140 | 22.3 | 253 | 27.4 | 236 | 30.2 | 272 | 49.2 | 26.9 | * | 97 | 16.5 | 180 | 31.2 | 143 | 39.9 | 153 | 47.4 | 30.9 | * |
| 1.2 Acute myeloid leukemia | 194 | 26.5 | 307 | 30.4 | 318 | 44.5 | 313 | 51.1 | 24.6 | * | 181 | 25.5 | 295 | 44.8 | 306 | 50.1 | 275 | 54.7 | 29.2 | * |
| 1.3 Chronic myeloid leukemia | 87 | 40.5 | 152 | 51.6 | 183 | 74.0 | 173 | 89.1 | 48.6 | * | 54 | 42.7 | 99 | 50.6 | 69 | 81.3 | 89 | 93.2 | 50.5 | * |
| 1.4 Other and unspecified leukemia | 71 | 9.9 | 79 | 25.5 | 89 | 37.2 | 83 | 51.4 | 41.5 | * | 42 | 31.0 | 75 | 22.7 | 53 | 34.0 | 77 | 67.3 | 36.3 | * |
| 2. Lymphomas | 262 | 50.7 | 558 | 60.9 | 637 | 72.8 | 748 | 80.9 | 30.2 | * | 177 | 62.3 | 379 | 71.1 | 496 | 79.6 | 567 | 87.0 | 24.7 | * |
| 2.1 Non-Hodgkin lymphoma | 230 | 47.3 | 480 | 56.7 | 522 | 70.2 | 601 | 79.2 | 31.9 | * | 142 | 57.9 | 310 | 66.6 | 384 | 75.7 | 436 | 85.9 | 28.0 | * |
| 2.2 Hodgkin lymphoma | 32 | 75.5 | 78 | 86.3 | 115 | 84.6 | 147 | 87.3 | 11.8 | * | 35 | 80.2 | 69 | 91.5 | 112 | 93.0 | 131 | 91.3 | 11.1 | * |
| 3. CNS and Other Intracranial and Intraspinal Neoplasms | 220 | 49.9 | 393 | 52.2 | 417 | 59.4 | 423 | 63.0 | 13.1 | * | 151 | 58.4 | 296 | 57.6 | 313 | 60.8 | 327 | 68.9 | 10.5 | * |
| 3.1. Astrocytoma | 104 | 36.8 | 169 | 39.8 | 156 | 43.1 | 155 | 48.4 | 11.6 | 75 | 52.2 | 136 | 51.6 | 119 | 48.0 | 136 | 60.8 | 8.6 | ||
| 3.2 Other glioma | 41 | 76.1 | 53 | 56.9 | 68 | 70.8 | 102 | 66.9 | -9.2 | 24 | 66.9 | 30 | 70.2 | 73 | 61.8 | 75 | 77.0 | 10.1 | ||
| 3.3 Ependymoma | 5 | 100.6 | 32 | 84.8 | 28 | 86.0 | 39 | 94.0 | -6.6 | 9 | 78.0 | 17 | 70.7 | 29 | 89.8 | 23 | 74.6 | -3.4 | ||
| 3.4 Medulloblastoma and other PNET | 8 | 37.8 | 43 | 44.4 | 51 | 37.4 | 46 | 41.5 | 3.7 | 10 | 50.1 | 41 | 53.8 | 41 | 61.1 | 38 | 63.8 | 13.7 | ||
| 3.5 Other specified intracranial and intraspinal neoplasms | N/S | N/S | - | |||||||||||||||||
| 3.6 Unspecified intracranial and intraspinal neoplasms | 54 | 54.1 | 80 | 61.5 | 102 | 77.7 | 72 | 78.3 | 24.2 | * | 28 | 57.3 | 61 | 60.8 | 43 | 72.2 | 46 | 84.3 | 27.0 | * |
| 4. Osseous and Chondromatous Neoplasms | 163 | 48.8 | 317 | 65.0 | 290 | 66.1 | 296 | 74.7 | 25.9 | * | 99 | 47.6 | 199 | 68.0 | 189 | 72.6 | 151 | 75.4 | 27.8 | * |
| 4.1 Osteosarcoma | 106 | 42.7 | 195 | 64.4 | 174 | 60.5 | 160 | 71.4 | 28.7 | * | 54 | 44.6 | 110 | 67.4 | 107 | 73.0 | 71 | 74.6 | 30.0 | * |
| 4.2 Chondrosarcoma | 17 | 71.1 | 43 | 86.5 | 44 | 93.5 | 44 | 93.5 | 22.4 | 11 | 54.7 | 32 | 87.7 | 25 | 76.2 | 33 | 96.4 | 41.7 | ||
| 4.3 Ewing tumor | 15 | 47.0 | 43 | 37.4 | 38 | 44.9 | 46 | 49.7 | 2.7 | 15 | 33.4 | 23 | 43.6 | 29 | 62.2 | 26 | 53.3 | 19.9 | ||
| 4.4 Other specified and unspecified bone tumors | 25 | 60.4 | 36 | 75.4 | 34 | 82.6 | 46 | 89.4 | 29.0 | * | 19 | 63.3 | 34 | 67.8 | 28 | 78.7 | 21 | 77.1 | 13.8 | |
| 5. Soft Tissue Sarcomas | 126 | 52.7 | 244 | 57.7 | 287 | 63.3 | 342 | 68.8 | 16.1 | * | 117 | 65.1 | 237 | 60.1 | 267 | 72.1 | 275 | 78.6 | 13.5 | * |
| 5.1 Fibromatous neoplasms | 23 | 78.8 | 61 | 75.8 | 64 | 90.9 | 103 | 93.4 | 14.6 | * | 28 | 78.8 | 42 | 81.1 | 71 | 96.0 | 90 | 96.7 | 17.9 | * |
| 5.2 Rhabdomyosarcoma | 20 | 25.2 | 43 | 35.0 | 41 | 34.2 | 35 | 34.1 | 8.9 | 12 | 33.4 | 30 | 40.1 | 25 | 36.1 | 20 | 44.9 | 11.5 | ||
| 5.3 Other soft tissue sarcoma | 83 | 52.2 | 140 | 56.7 | 182 | 60.1 | 204 | 62.8 | 10.6 | * | 77 | 65.1 | 165 | 58.3 | 171 | 67.4 | 165 | 73.4 | 8.3 | |
| 6. Germ Cell and Trophoblastic Neoplasms | 98 | 72.9 | 323 | 79.3 | 457 | 85.8 | 574 | 88.8 | 15.9 | * | 180 | 86.4 | 422 | 92.9 | 385 | 94.7 | 362 | 96.3 | 9.9 | * |
| 6.1 Germ cell and trophoblastic neoplasms of gonads | 47 | 90.0 | 157 | 85.8 | 255 | 90.5 | 331 | 95.4 | 5.4 | * | 127 | 89.2 | 308 | 95.7 | 303 | 97.5 | 282 | 97.0 | 7.8 | * |
| 6.2 Germ cell and trophoblastic neoplasms of nongonadal sites | 51 | 57.2 | 166 | 73.2 | 202 | 79.9 | 243 | 79.9 | 22.7 | 53 | 79.5 | 114 | 85.3 | 82 | 84.3 | 80 | 93.6 | 14.1 | ||
| 7. Melanoma and Skin Carcinomas | 24 | 71.3 | 66 | 57.9 | 66 | 73.0 | 67 | 83.9 | 12.6 | * | 32 | 59.6 | 45 | 62.4 | 56 | 82.3 | 71 | 89.1 | 29.5 | * |
| 7.1 Melanoma | 11 | 45.8 | 38 | 45.0 | 33 | 51.7 | 26 | 45.0 | -0.8 | 14 | 21.5 | 26 | 42.4 | 30 | 70.1 | 33 | 76.5 | 55.0 | * | |
| 7.2 Skin carcinomas | 13 | 93.0 | 28 | 75.4 | 33 | 94.3 | 41 | 97.9 | 4.9 | 18 | 89.2 | 19 | 89.7 | 26 | 96.4 | 38 | 100.2 | 11.0 | ||
| 8. Carcinomas | 872 | 52.3 | 1,787 | 57.0 | 1,935 | 68.2 | 2,561 | 80.5 | 28.2 | * | 2,333 | 73.1 | 4,820 | 77.9 | 6,431 | 86.6 | 10,040 | 92.4 | 19.3 | * |
| 8.1 Thyroid carcinoma | 112 | 95.3 | 285 | 97.3 | 470 | 100.2 | 1,225 | 99.7 | 4.4 | * | 750 | 99.9 | 1,694 | 99.9 | 3,285 | 99.8 | 6,818 | 100.0 | 0.1 | |
| 8.2 Other carcinoma of head and neck | 76 | 62.3 | 147 | 71.1 | 177 | 79.4 | 170 | 86.8 | 24.5 | * | 64 | 78.4 | 142 | 84.7 | 162 | 87.2 | 163 | 84.5 | 6.1 | |
| 8.2.1 Nasopharyngeal carcinoma | 28 | 71.9 | 70 | 60.3 | 56 | 80.6 | 48 | 91.6 | 19.7 | * | 12 | 66.9 | 34 | 76.7 | 23 | 78.4 | 27 | 77.6 | 10.7 | |
| 8.2.2 Other sites in lip, oral cavity, and pharynx | 32 | 72.4 | 65 | 86.6 | 108 | 79.9 | 112 | 86.6 | 14.2 | 41 | 85.6 | 94 | 88.5 | 125 | 90.5 | 123 | 90.3 | 4.7 | ||
| 8.2.3 Nasal cavity, middle ear, sinuses, larynx, and other ill-defined sites in head/neck | N/S | N/S | ||||||||||||||||||
| 8.3 Carcinoma of trachea, bronchus, and lung | 41 | 24.6 | 68 | 37.0 | 65 | 52.5 | 64 | 39.6 | 15.0 | 32 | 25.1 | 68 | 35.4 | 79 | 43.1 | 68 | 46.7 | 21.6 | * | |
| 8.4 Carcinoma of breast | N/S | 309 | 67.9 | 686 | 77.3 | 822 | 81.9 | 838 | 86.6 | 18.7 | * | |||||||||
| 8.5 Carcinoma of genitourinary tract | 52 | 83.3 | 116 | 91.0 | 158 | 91.5 | 197 | 92.2 | 8.9 | 550 | 85.5 | 1,075 | 85.0 | 1,036 | 88.5 | 1,174 | 85.5 | 0 | ||
| 8.5.1 Carcinoma of kidney | 14 | 79.2 | 43 | 86.5 | 85 | 89.8 | 129 | 91.9 | 12.7 | 21 | 62.1 | 51 | 68.8 | 57 | 84.4 | 80 | 78.8 | 16.7 | * | |
| 8.5.2 Carcinoma of bladder | 30 | 87.3 | 61 | 95.6 | 61 | 95.4 | 60 | 95.0 | 7.7 | N/S | ||||||||||
| 8.5.3 Carcinoma of gonads | N/S | 231 | 86.8 | 392 | 84.4 | 324 | 88.1 | 290 | 82.6 | -4.2 | ||||||||||
| 8.5.4 Carcinoma of cervix and uterus | N/S | 285 | 86.6 | 605 | 86.7 | 611 | 89.1 | 781 | 87.0 | 0.4 | ||||||||||
| 8.5.5 Carcinoma of other and ill-defined sites in genitourinary tract | N/S | N/S | ||||||||||||||||||
| 8.6 Carcinoma of gastrointestinal tract | 536 | 43.1 | 1,064 | 43.0 | 984 | 49.4 | 842 | 56.5 | 13.4 | * | 587 | 33.3 | 1,063 | 41.5 | 990 | 51.0 | 924 | 61.7 | 28.4 | * |
| 8.6.1 Carcinoma of colon and rectum | 140 | 51.1 | 299 | 50.1 | 287 | 62.6 | 330 | 73.6 | 22.5 | * | 106 | 41.6 | 228 | 56.3 | 213 | 64.0 | 273 | 74.6 | 33.0 | * |
| 8.6.2 Carcinoma of stomach | 307 | 44.0 | 530 | 48.0 | 445 | 51.9 | 310 | 56.5 | 12.5 | * | 416 | 30.6 | 709 | 36.5 | 646 | 47.0 | 521 | 59.3 | 28.7 | * |
| 8.6.3 Carcinoma of liver and intrahepatic bile ducts | 62 | 26.0 | 193 | 19.3 | 226 | 28.4 | 170 | 31.4 | 5.4 | * | 28 | 17.9 | 84 | 33.4 | 82 | 47.7 | 86 | 31.4 | 13.5 | |
| 8.6.4 Carcinoma of pancreas | N/S | 21 | 66.9 | 18 | 83.5 | 24 | 71.0 | 28 | 69.3 | 2.4 | ||||||||||
| 8.6.5 Carcinoma other and ill-defined sites in gastrointestinal tract | 18 | 28.0 | 25 | 44.3 | 11 | 45.6 | 18 | 45.3 | 17.3 | 16 | 31.3 | 24 | 46.0 | 25 | 36.1 | 16 | 75.2 | 43.9 | * | |
| 8.7 Carcinoma of other and ill-defined sites | 54 | 31.7 | 102 | 44.3 | 78 | 51.5 | 60 | 48.1 | 16.4 | * | 41 | 53.8 | 92 | 33.8 | 57 | 35.2 | 55 | 60.2 | 6.4 | |
| 8.7.1 Adrenocortical carcinoma | N/S | N/S | ||||||||||||||||||
| 8.7.2 Carcinoma of other and ill-defined sites, NOS | 51 | 33.6 | 94 | 44.9 | 76 | 51.5 | 53 | 48.7 | 15.1 | * | 37 | 48.8 | 87 | 33.4 | 52 | 38.5 | 46 | 66.1 | 17.3 | |
| 9. Miscellaneous Specified Neoplasms, NOS | 51 | 49.4 | 80 | 52.8 | 154 | 71.0 | 221 | 81.0 | 31.6 | * | 70 | 57.3 | 133 | 84.4 | 194 | 85.7 | 232 | 78.6 | 21.3 | |
| 9.1 Other pediatric and embryonal tumors, NOS | 21 | 33.6 | 24 | 33.5 | 32 | 53.3 | 27 | 62.6 | 29.0 | * | 12 | 25.1 | 19 | 63.3 | 19 | 63.3 | 24 | 63.4 | 38.3 | |
| 9.2 Other specified and embryonal tumors, NOS | 30 | 60.4 | 56 | 61.0 | 122 | 75.6 | 194 | 83.4 | 23.0 | * | 58 | 64.0 | 114 | 87.9 | 175 | 88.2 | 208 | 80.3 | 16.3 | |
| 10. Unspecified Malignant Neoplasms | 170 | 40.9 | 242 | 56.1 | 97 | 64.2 | 76 | 70.7 | 29.4 | * | 376 | 71.5 | 472 | 78.4 | 242 | 84.9 | 159 | 81.3 | 9.8 | * |
*P-values <0.05 for trend.
Change (%) in the 5-year RSRs from 1993–1995 to 2006–2010.
Thyroid carcinoma was excluded from the incidence rate of all cancers combined because of its unusually high incidence rate.
N/S: not shown because <20 cases were reported in each period.
AYAs, adolescents and young adults (aged 15–29 years); CNS, central nervous system; PNET, primitive neuroectodermal tumor; NOS, not otherwise specified.
Leukemia (group 1) showed the greatest increase in survival in both males (32.5%) and females (33.7%). In particular, chronic myeloid leukemia had the largest and second-largest increases in survival in males (48.6%; from 40.5% to 89.1%) and females (50.5%; from 42.7% to 93.2%), respectively.
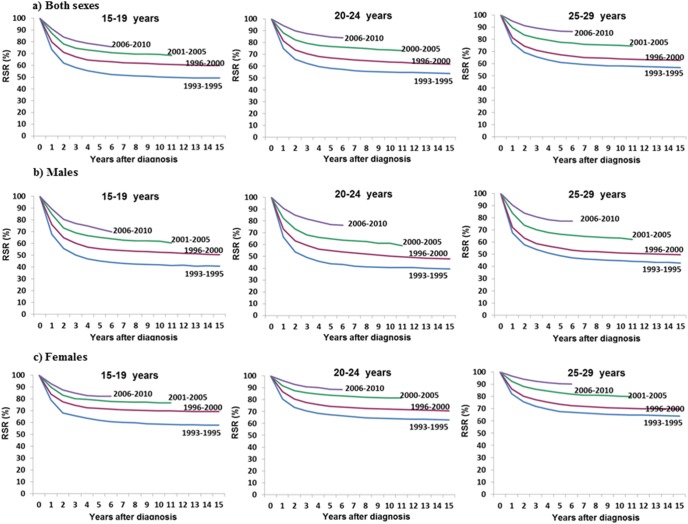
Figure 2 depicts the 5-year relative survival rates of all cancer patients in each of the four time periods according to age (15–19 years, 20–24 years and 25–29 years) and sex. Both gender, the 5-year relative survival rates increased in all age groups. For males aged 15–19 years, the 5-year relative survival rates in 1993–1995, 1996–2000, 2001–2005, and 2006–2010 for all cancers combined were 45.3% (95.% CI: 41.2–49.2), 55.4% (95% CI: 52.5–58.1), 65.3% (95% CI: 62.5–67.9), and 72.2% (95% CI: 69.2–75.0), respectively. The survival rates of males aged 20–24 years were 43.9% (95% CI: 40.2–47.5), 55.0% (95% CI: 52.2–57.7), 65.0% (95% CI: 62.5–67.3), and 77.0% (95% CI: 74.3–79.5) in 1993–1995, 1996–2000, 2001–2005, and 2006–2010, respectively. The survival rates of males aged 25–29 years were 48.8% (95% CI: 45.9–51.7), 55.0% (95% CI: 52.9–57.0), 66.5% (95% CI: 64.6–68.3), and 77.2% (95% CI: 75.3–79.0) in 1993–1995, 1996–2000, 2001–2005, and 2006–2010, respectively. For females aged 15–19 years, the 5-year relative survival rates for all cancers combined were 62.0% (95% CI: 58.0–65.8), 72.0% (95% CI: 69.4–74.5), 78.8% (95% CI: 76.3–81.0), and 82.2% (95% CI: 79.7–84.5) in 1993–1995, 1996–2000, 2001–2005, and 2006–2010, respectively. The survival rates of females aged 20–24 years were 67.4% (95% CI: 64.6–70.1), 74.4% (95% CI: 72.4–76.3), 83.7% (95% CI: 82.2–85.1), and 88.9% (95% CI: 87.4–90.2) in 1993–1995, 1996–2000, 2001–2005, and 2006–2010, respectively. The survival rates of females aged 25–29 years were 67.7% (95% CI: 65.7–69.6), 73.6% (95% CI: 72.2–74.9), 82.9% (95% CI: 81.9–83.9), and 90.4% (95% CI: 89.5–91.2) in 1993–1995, 1996–2000, 2001–2005, and 2006–2010, respectively.
Figure 2. Trends in relative survival after cancer in Korea according to age and the time period.
Discussion
This is the first study to investigate cancer incidence, survival and their trends among AYAs using the population-based national cancer registry in Korea. The major findings of this study were that cancer in AYAs showed a trend of increasing incidence, with an increase of 6.3% per year (P<0.05), from 1999 to 2010 and that age- and gender-related cancer incidence patterns differed according to the primary site. Moreover, five-year relative survival rates for most cancers improved from 1993–1995 (58.9%) to 2006–2010 (84.8%) among AYAs.
When comparing our study with studies from other countries, cancer incidence rates among AYAs in our study were lower than incidence rates in the U.S. [5], France [21], Portugal [22], and Netherlands [3] and among males in Canada [12], even though the time period and age group differs. In other studies, cancer incidence in AYA males was generally similar to or higher than cancer incidence in AYA females. Conversely, we reported much lower incidence rates in males than in females. The reason for this difference in the incidence rate by gender was that thyroid carcinoma has the highest incidence and rapidly increased in incidence among AYA females in Korea.
Consistent with other studies, we found a rising incidence of cancer among AYAs during the study period.
The data on AYAs in Korea reported here exhibited several important differences from site-specific cancer incidence rates among AYAs in other regions of the world. Since the 2000s, an annual increase in incidence of 0.6–2.0% has been reported in several countries [16], [23]–[25]. However, our results showed an annual increase in incidence of 6.3%, which is a more rapid increase than observed in other studies. The increased cancer incidence rate may be partially explained by changes in cancer classification, as exemplified by changes in the classification of hematologic malignancies in a study by Park et al. [26].
The increased incidence of carcinomas was primarily due to an increase in the incidence rates of thyroid carcinoma (APC = 17.9%). An increased incidence rate of thyroid carcinoma has also been noted among AYAs in Western countries [23], [27], [28]. However, the incidence of thyroid carcinoma among AYAs is more than three- to tenfold higher in Korea than in Canada [12], England [23], the United States [5], the Netherlands [3] and Portugal [22]. The reasons for the high incidence of thyroid carcinoma in Korean AYAs compared with other nationalities are unknown. Although the rapid increase and high incidence rate of thyroid cancer among older individuals worldwide might be attributable to the development of improved technologies for early detection [29], the exact cause of the increased incidence of most cancers in AYAs is unknown. Because of the difficulty in recommending thyroid cancer screening for AYAs solely based on incidence rates, further research to identify associated risk factors, such as family history, socioeconomic status, and environmental exposure, is needed.
In this study, a notable trend of increasing incidence was also observed for cervical carcinoma (APC = 6.2%) among female AYAs in Korea. Although the incidence of cervical carcinoma in Korean females of all ages is decreasing (APC = −4.3%) [6], the incidence of cervical carcinoma has been increasing among Korean females under 30 years of age [30]. A steady increase in cervical carcinoma in young women (20–29 years) has also been observed in England [31]. The increased incidence of cervical carcinoma among AYAs has been attributed to increases in human papillomavirus (HPV) infection [32], [33]. More specifically, an increase in sexual behavior among younger age groups has led to an increased rate of HPV infection [34], [35], and the prevalence rate of HPV has been reported to increase with decreasing age [32]. Therefore, since 2007, the Korean Society of Gynecologic Oncology and Colposcopy (KSGOC) has recommended the HPV vaccine for females aged 15–17 for the prevention of cervical carcinoma. In fact, certain recent studies have reported a decrease in the incidence of cervical carcinoma due to the use of the HPV vaccine at an earlier age [36], [37]. Therefore, the incidence of cervical carcinoma is expected to gradually decline among AYAs in Korea due to the HPV vaccine.
In terms of survival, our data are consistent with that reported for other geographic regions. Although the time period in our study differed, the overall cancer survival rate among AYAs in Korea was similar to the rate and significantly improvement reported in the U.S. and Germany. Improvements in relative survival rates among AYAs can be partially explained by advances in cancer detection, more intensive treatments, and increased expertise in adolescent oncology [38]. Additionally, access to effective protocols and the development of health infrastructures may have also contributed to improvements in survival rates [39].
However several important differences should be highlighted in lymphoma and leukemia. In the present study, the most significant improvements in survival were observed in leukemia and lymphoma patients, but the survival rates for leukemia and lymphoma were noticeably lower than in the U.S. and Germany [40], [41]. This reason for this difference in the survival rate by ethnic was that the incidence cases of subgroup of leukemia and lymphoma was different between U.S. AYAs [41] and Korea AYAs. More, ethnic disparities in tumor biology and clinical factors may influence cancer treatment and survival [42].
Compared with the survival of patients aged 1 to 10 years, overall survival and disease-specific survival are clinically significantly poorer among AYA patients with acute lymphoblastic leukemia [43]. The survival rates for leukemia among the Korean AYAs in our study have remained worse than among Korean children based on data from the KCCR [44].
Among AYAs, breast cancer accounts for approximately 7% and 4.9% of all cancers diagnosed in the United States [5] and Korea, respectively. In the United States, the 5-year survival rate for breast cancer is lower among AYAs (80.2%) than among patients in other age groups (30–39 years, 83.4%; 40–49 years, 88.9%), and particularly older patients [41]. Our study showed similar results. The relative survival rate for breast cancer among Korean females aged 15–29 years was 86.8% in 2006–2010, whereas the relative survival rate among Korean females aged ≥40 years was 91.0% based on a direct estimate from the KNCIDB.
One limitation of our study is that the follow-up period began relatively soon after the diagnosis of cancer, in contrast to the protocols in other studies [3]–[5]. Another limitation of this study is that we could not estimate the survival rates after adjusting for cancer stage and treatment because our registry database did not include information on cancer stage and treatment.
In conclusion, our study provides representative cancer statistics regarding temporal trends in the AYA population in Korea. In particular, the results showed an increasing trend in cancer incidence and an improving survival trend among AYAs in Korea. These results may support cancer control and prevention plans focusing on AYAs.
In the future, further research will help to identify factors affecting cancer incidence and responses to treatment among AYAs. In particular, research on the etiological factors related to the rapid increase in thyroid carcinoma in AYAs is needed.
Funding Statement
This work was supported by the National Cancer Center Grant (NCC-1310222). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bleyer A, Barr R, Hayes-Lattin B, Thomas D, Ellis C, et al. (2008) The distinctive biology of cancer in adolescents and young adults. Nature Reviews Cancer 8: 288–298. [DOI] [PubMed] [Google Scholar]
- 2. Cotterill SJ, Parker L, Malcolm AJ, Reid M, More L, et al. (2000) Incidence and survival for cancer in children and young adults in the North of England, 1968–1995: a report from the Northern Region Young Persons’ Malignant Disease Registry. British journal of cancer 83: 397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aben KK, van Gaal C, van Gils NA, van der Graaf WT, Zielhuis GA (2012) Cancer in adolescents and young adults (15–29 years): A population-based study in the Netherlands 1989–2009. Acta Oncologica: 1–12. [DOI] [PubMed]
- 4. Birch J, Alston R, Kelsey A, Quinn M, Babb P, et al. (2002) Classification and incidence of cancers in adolescents and young adults in England 1979–1997. British journal of cancer 87: 1267–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bleyer A, O’Leary M, Barr R, Ries L (2006) Cancer epidemiology in older adolescents and young adults 15 to 29 years of age, including SEER incidence and survival: 1975–2000. National Cancer Institue, NIH Pub No?06–5767Bethesda, MD 2006.
- 6. Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG, et al. (2013) Cancer statistics in Korea: incidence, mortality, survival and prevalence in 2010. Cancer Res Treat 45: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bleyer A (2007) Young adult oncology: the patients and their survival challenges. CA: A Cancer Journal for Clinicians 57: 242–255. [DOI] [PubMed] [Google Scholar]
- 8.Statistics Korea (2011), website. Available : http://kostat.go.kr. Accessed 2013 September 24.
- 9.Korea Central Cancer Registry. Cancer registry system in Korea. Available : http://www.ncc.re.kr. Accessed 2013 September 23.
- 10. Shin HR, Won YJ, Jung KW, Kong HJ, Yim SH, et al. (2005) Nationwide cancer incidence in Korea, 1999?2001; first result using the national cancer incidence database. Cancer Research and Treatment : Official Journal of Korean Cancer Association 37: 325–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cancer incidence in the Five Continents. Vol.X electronic version Lyon, IARC. website. Available : http://ci5.iarc.fr. Accessed 2013 October 23.
- 12.Canadian Cancer Society’s Steering Committee. Canadian Cancer Statistics 2009. Website. Available : http://www.cancer.ca/en/cancer-information/cancer-101/canadian-cancer-statistics-publication/past-editions-canadian-cancer-statistics/?region=qc. Accessed 2013 May 13.
- 13.Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, et al. (2000) International Classification of Diseases for Oncology. 3rd ed. Geneva, Switzerland: World Health Organization.
- 14. Barr RD, Holowaty EJ, Birch JM (2006) Classification schemes for tumors diagnosed in adolescents and young adults. Cancer 106: 1425–1430. [DOI] [PubMed] [Google Scholar]
- 15.Segi M (1960) Cancer mortality for selected sites in 24 countries (1950–1957). Sendai, Japan : Tohoku University School of Medicine.
- 16.Howlader N, Noone AM, Krapcho M, Garshell J, Neyman N, et al. SEER Cancer Statistics Review, 1975–2010, National Cancer Institute. Bethesda, MD, based on November 2012 SEER data submission, posted to the SEER web site, April 2013. website. Available : http://seer.cancer.gov/csr/1975_2010/. Accessed 2013 September 17.
- 17.Smith P (1992) Cancer Incidence in Five Continents. Comparison between registries: age-standardized rates. IARC scientific publications: 865–870. [PubMed]
- 18. Ederer F, Axtell LM, Cutler SJ (1961) The relative survival rate: a statistical methodology. National Cancer Institute monograph 6: 101–121. [PubMed] [Google Scholar]
- 19.Ederer F, Heise H Instructions to IBM 650 programmers in processing survival computations. Methodological note No.10. End Results Evaluation Section. Bethesda MD : National Cancer Institute. 1959.
- 20. Brenner H, Gefeller O (1997) Deriving more up-todate estimates of long-term patient survival. Journal of clinical epidemiology 50: 211–216. [DOI] [PubMed] [Google Scholar]
- 21. Desandes E, Lacour B, Belot A, Molinie F, Delafosse P, et al. (2013) Cancer Incidence and Survival in Adolescents and Young Adults in France, 2000–2008. Pediatric Hematology-Oncology 30: 291–306. [DOI] [PubMed] [Google Scholar]
- 22. Carreira H, Antunes L, Castro C, Lunet N, Bento MJ (2012) Cancer Incidence and Survival (1997–2006) Among Adolescents and Young Adults in the North of Portugal. Pediatric Hematology-Oncology 29: 663–676. [DOI] [PubMed] [Google Scholar]
- 23. Alston RD, Geraci M, Eden TO, Moran A, Rowan S, et al. (2008) Changes in cancer incidence in teenagers and young adults (ages 13 to 24 years) in England 1979–2003. Cancer 113: 2807–2815. [DOI] [PubMed] [Google Scholar]
- 24. Aben KK, van Gaal C, van Gils NA, van der Graaf WT, Zielhuis GA (2012) Cancer in adolescents and young adults (15–29 years): A population-based study in the Netherlands 1989–2009. Acta Oncologica 51: 922–933. [DOI] [PubMed] [Google Scholar]
- 25. Haggar FA, Preen DB, Pereira G, Holman CD, Einarsdottir K (2012) Cancer incidence and mortality trends in Australian adolescents and young adults, 1982–2007. BMC cancer 12: 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Park HJ, Park E-H, Jung K-W, Kong H-J, Won Y-J, et al. (2012) Statistics of hematologic malignancies in Korea: incidence, prevalence and survival rates from 1999 to 2008. The Korean Journal of Hematology 47: 28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marrett LD, Frood J, Nishri D, Ugnat AM (2002) Cancer in Young Adults in Canada Working G Cancer incidence in young adults in Canada: preliminary results of a cancer surveillance project. Chronic diseases in Canada 23: 58–64. [PubMed] [Google Scholar]
- 28. Hogan AR, Zhuge Y, Perez EA, Koniaris LG, Lew JI, et al. (2009) Pediatric thyroid carcinoma: incidence and outcomes in 1753 patients. Journal of Surgical Research 156: 167–172. [DOI] [PubMed] [Google Scholar]
- 29. Davies L, Welch HG (2006) Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA: the journal of the American Medical Association 295: 2164–2167. [DOI] [PubMed] [Google Scholar]
- 30.Oh CM, Jung KW, Won YJ, Shin A, Kong HJ, et al. (2013) Trends in the incidence of in? situ and invasive cervical cancer by age group and histological type in Korea from 1993 to 2009. (in press). Plos one. [DOI] [PMC free article] [PubMed]
- 31. Patel A, Galaal K, Burnley C, Faulkner K, Martin-Hirsch P, et al. (2012) Cervical cancer incidence in young women: a historical and geographic controlled UK regional population study. Br J Cancer 106: 1753–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim Y-T (2009) Current status of cervical cancer and HPV infection in Korea. Journal of gynecologic oncology 20: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Han MA, Choi KS, Lee H-Y, Jun JK, Jung KW, et al. (2012) Performance of papanicolaou testing and detection of cervical carcinoma in?situ in participants of organized cervical cancer screening in South Korea. PloS one 7: e35469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vaccarella S, Franceschi S, Herrero R, Muñoz N, Snijders PJ, et al. (2006) Sexual behavior, condom use, and human papillomavirus: pooled analysis of the IARC human papillomavirus prevalence surveys. Cancer Epidemiology Biomarkers & Prevention 15: 326–333. [DOI] [PubMed] [Google Scholar]
- 35. Shin H-R, Franceschi S, Vaccarella S, Roh J-W, Ju Y-H, et al. (2004) Prevalence and determinants of genital infection with papillomavirus, in female and male university students in Busan, South Korea. Journal of Infectious Diseases 190: 468–476. [DOI] [PubMed] [Google Scholar]
- 36. Cuzick J, Castanon A, Sasieni P (2010) Predicted impact of vaccination against human papillomavirus 16/18 on cancer incidence and cervical abnormalities in women aged 20–29 in the UK. British journal of cancer 102: 933–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Barnabas RV, Laukkanen P, Koskela P, Kontula O, Lehtinen M, et al. (2006) Epidemiology of HPV 16 and cervical cancer in Finland and the potential impact of vaccination: mathematical modelling analyses. PLoS Medicine 3: e138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Barr RD (2011) Adolescents, young adults, and cancer–the international challenge. Cancer 117: 2245–2249. [DOI] [PubMed] [Google Scholar]
- 39. Pritchard-Jones K, Kaatsch P, Steliarova-Foucher E, Stiller C, Coebergh J (2006) Cancer in children and adolescents in Europe: developments over 20 years and future challenges. European journal of Cancer 42: 2183–2190. [DOI] [PubMed] [Google Scholar]
- 40. Steliarova-Foucher E, Stiller C, Kaatsch P, Berrino F, Coebergh J-W, et al. (2004) Geographical patterns and time trends of cancer incidence and survival among children and adolescents in Europe since the 1970s (the ACCIS project): an epidemiological study. The Lancet 364: 2097–2105. [DOI] [PubMed] [Google Scholar]
- 41.Gondos A, Hiripi E, Holleczek B, Luttmann S, Eberle A, et al. (2013) Survival among adolescents and young adults with cancer in Germany and the United States: An international comparison. International Journal of Cancer. [DOI] [PubMed]
- 42. Shavers VL, Brown ML (2002) Racial and ethnic disparities in the receipt of cancer treatment. Journal of the National Cancer Institute 94: 334–357. [DOI] [PubMed] [Google Scholar]
- 43. Tricoli JV, Seibel NL, Blair DG, Albritton K, Hayes-Lattin B (2011) Unique characteristics of adolescent and young adult acute lymphoblastic leukemia, breast cancer, and colon cancer. Journal of the National Cancer Institute 103: 628–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.The Korea Central Cancer Registry, National Cancer Center. Annual report of cancer statistics in Korea in 2010, Ministry of Health and Welfare, 2012.