Abstract
The ability of hematopoietic stem cells (HSCs) to self-renew and differentiate into progenitors is essential for homeostasis of the hematopoietic system. The longevity of HSCs makes them vulnerable to accumulating DNA damage, which may be leukemogenic or result in senescence and cell death. Additionally, the ability of HSCs to self-renew and differentiate allows DNA damage to spread throughout the hematologic system, leaving the organism vulnerable to disease. In this review we discuss cell fate decisions made in the face of DNA damage and other cellular stresses, and the role of reactive oxygen species in the long-term maintenance of HSCs and their DNA damage response.
Keywords: Hematopoietic stem cells, DNA damage, Reactive oxygen species
Introduction
In human adults, the hematopoietic system is responsible for the replacement of more than 1011 cells that are removed daily due to damage and age [1; 2]. At the root of this highly proliferative system are hematopoietic stem cells (HSCs), a distinct population of cells that retain the ability to self-renew, both to maintain the stem cell population and to differentiate into multiple lineages in order to populate the mature hematologic system. In addition to their homeostatic role, HSCs are essential in handling severe injury to the organism, as evidenced by their ability to repopulate the hematopoietic system after lethal irradiation [3]. Most striking is the ability of a single HSC to repopulate the entire hematopoietic system for the lifetime of a mouse [4]. Experiments using HSCs are enabled and greatly improved by our ability to increase stem cell purity using a number of cell surface markers to identify cells containing greater potency and to remove cells that exhibit lineage commitment [5; 6]. The ability of a small population of identifiable HSCs to restore the complete hematopoietic lineage is at the center of clinical stem cell therapy in leukemia and various myelodysplastic conditions (e.g. myelodysplastic syndrome, MDS), as well as a central concern of the study of hematopoiesis, a paradigmatic system of stem cell biology [7].
Like stem cells in other tissues, HSCs are responsible for maintaining the genomic integrity of the hematopoietic system for the lifetime of an organism [8]. For this reason, the stem cell pool is maintained in the G0 phase of the cell cycle in the bone marrow niche [9; 10; 11]. The G0 phase is a non-dividing quiescent state resistant to inborn errors of genomic replication and the stresses of cellular metabolism [12]. Additionally, the bone marrow niche is thought to protect against various intrinsic and extrinsic insults that would be damaging to HSCs.
In this review we will discuss the characteristics of HSCs that predispose them to accumulating DNA damage and the conditions that result from genomic instability in the HSC compartment. We will then discuss the mechanisms through which DNA damage is sensed by HSCs, their resulting cell fate decisions, and the role of reactive oxygen species (ROS) in these pathways and in decisions following other cellular stresses. Finally, we will investigate the DNA damage response (DDR) in HSCs as compared to more differentiated hematologic cells and we will review new research into the effects of non-homologous end-joining (NHEJ).
DNA Damage in Hematopoietic Stem Cells
DNA damage is central to the development of cancer, aging and stem cell dysfunction, such as occurs in Fanconi anemia [13; 14; 15]. DNA is the largest cellular macromolecule, and there are only limited copies of each DNA molecule per cell. In addition, DNA must be retained for the lifetime of a cell, despite any damage that may accumulate. Thus, cells are particularly vulnerable to DNA damage [16]. Furthermore, DNA is sensitive to many damaging agents: spontaneous chemical reactions associated with the nuclear environment; reactive byproducts of metabolism, such as ROS, which can cause base oxidation and double strand breaks (DSBs); and from extrinsic agents such as ionizing radiation and chemicals [16; 17]. Additionally, DNA mutations can accumulate due to errors of replication in dividing cells. Cumulatively a cell might experience more than 105 lesions to its DNA per day [18].
The effect of cumulative DNA damage may be exaggerated in stem cell compartments due to the longevity of stem cells [19]. Furthermore, the ability of stem cells to self-renew and differentiate into progenitors allows for damage to be propagated throughout the stem cell and somatic compartments, affecting the entire tissue [20; 21].
The impact of accumulated DNA damage is particularly evident in the study of those diseases in which DNA damage repair is compromised, e.g., the human segmental progeroid syndromes. These syndromes result in a rapid decline in tissue function and maintenance, which is consistent with an inability of stem cells to carry out their homeostatic functions secondary to accumulated DNA damage [22]. The critical role of Fancd2, which is a member of the BRCA-mediated DNA repair pathway, in maintaining repopulation capacity of HSCs also supports the concept that genomic stability is essential for the maintenance of HSCs [23; 24]. Stem cell dysfunction can result either from damage that affects their ability to self-renew and differentiate, or from damage so severe that it causes the stem cells to be removed from the pool by senescence or apoptosis. Additionally, the progeroid syndromes also result in an increased risk for malignancy. It is well established that DNA damage is central to the development of malignancy, and the role of stem cells in establishing malignancy in the hematopoietic compartment and other tissues, has been widely recognized [25; 26; 27]. The accumulation of DNA damage has also been implicated in age-dependent stem cell failure, including in the adult human hematopoietic system [28; 29; 30]. With the proper function of stem cells being central to homeostasis and the disastrous consequences that result from their dysfunction, it seems likely that they would retain methods for resisting DNA damage and exhibit repair methods of high reliability and fidelity.
DNA Damage Sensing, Cell Fate and the Role of Reactive Oxygen Species
Identifying damage to the chromosome is the initial regulatory step in DNA damage response (DDR) signal transduction [20]. In the case of double strand breaks (DSBs), the damage sensing machinery includes PARP (poly ADP-ribose polymerase), Ku70/Ku80 and MRN (Mre11-Rad50-Nbs1). Once a DSB has been recognized, these proteins then recruit Ataxia-Telangiectasia Mutated (ATM) protein or Ataxia-Telangiectasia and Rad3-related (ATR) protein, both members of the phosphatidylinositol 3 kinase-related kinase (PIKK) family, to trigger phosphorylation and the activation of a cascade of repair enzymes. ATM phosphorylates a wide variety of proteins and serves as an alarm, warning the cell that a DSB is present, while ATR phosphorylates a smaller number of proteins and may be involved in maintaining proteins phosphorylated by ATM in their active or inactive states [31]. As is clear from its name, ATM is absent or mutated in the autosomal recessive condition Ataxia Telangiectasia (A-T) [32]. Cells derived from A-T patients are defective in DSB response and exhibit genomic instability and radiosensitivity, which is consistent with the clinically observed increased cancer risk and other symptoms in A-T patients [32; 33].
Reactive oxygen species (ROS) refers to those oxygen-containing byproducts of metabolism that are capable of causing oxidative damage to cellular components, including nucleic acids, proteins, lipids and carbohydrates [34; 35] In a 2007 meta-analytical study of 68 randomized clinical trails, Bjelakovic et al. reported, quite surprisingly, that increased mortality correlated with use of some dietary antioxidant supplements [36]. Despite this revelation, rigorous research has provided strong evidence for ROS mediated damage in the HSC compartment, and the contribution of ROS to HSC aging and senescence is well established. Hypoxic conditions in the bone marrow niche serve to reduce oxidative stress on HSCs and low oxygen niches may also serve to protect stem cells in other tissues [37; 38]. Additionally, it has been suggested that low ROS levels can be used to identify HSCs and that low intracellular ROS levels are crucial to the maintenance of HSC self-renewal [39].
In addition to activating DDR machinery, ATM is important in cell cycle delay and cell fate decisions following DNA damage. Investigation into the mechanism by which ATM participates in cell fate decisions has revealed ROS as a crucial player in the intersecting pathways that determine cell fate. Studies of A-T and Atm−/− models have demonstrated that there is deregulation of ROS homeostasis when ATM is dysfunctional. Clinical evidence of accumulated ROS-mediated damage in A-T is supported by observations of elevated ROS and oxidative stress response in Atm−/− mice, as well as investigators’ ability to reduce damage with treatment of N-acetyl cysteine (NAC), a powerful antioxidant [40; 41; 42; 43]. Furthermore, there is additional evidence for the activation of ATM by oxidative stress, in a DDR-independent manner [44; 45]. Thus, it is believed that ROS may serve both as an activator of ATM and a mediator of the ATM response. Recent work has begun to elucidate the mechanisms by which ATM regulates ROS levels and the role of ROS in the ATM-axis.
In 2004, the Suda group reported on a series of experiments that demonstrated a role for ATM and ROS in the maintenance of the HSC compartment, while demonstrating that telomere loss, though measurable in replating and reconstitution assays, is not primarily responsible for reduced reconstitution ability in Atm−/− HSCs [46]. Though differentiation of progenitors and mature hematopoietic cells was unaffected in Atm−/− mice, they noted both a reduction in the number of HSCs and early hematopoietic failure with serial bone marrow transplantation. Additionally, they noted elevation of the tumor suppressors p16INK4A and p19ARF in Atm−/− HSCs. p16INK4A and p19ARF are upstream activators of pathways that maintain the tumor suppressors Rb and p53, respectively, in their active states [47]. It is now known that derepression of the INK4A/ARF locus is associated with the loss of self-renewing HSCs by cellular senescence [47]. Treatment with NAC restored the repopulation capacity of Atm−/− HSCs, demonstrating that HSC loss secondary to Atm−/− was contingent on ROS elevation. NAC treatment also resulted in reduced p16INK4A and p19ARF expression in Atm−/− HSCs, indicating that they are downstream effectors of elevated ROS levels. Furthermore, both ectopic expression of Bmi1, a Polycomb transcriptional repressor of p16INK4A and p19ARF expression, and ectopic expression of the HPV-16 derived Rb and p53 inhibitors E7 and E6, respectively, demonstrated that HSC loss in Atm−/− mice is mediated by the p16INK4A-Rb pathway. The Suda group’s experiments demonstrated that ATM plays a role in the maintenance of HSC quiescence by regulating ROS levels and that Atm loss results in increased ROS and deregulation of the cell cycle, which can result in progressive HSC failure [48]. Interestingly, an earlier study demonstrated that neural stem cells also require Atm in order to avoid genomic instability, abnormal proliferation, and depletion [49]. These defects can be partially rescued by treating the mice with N-acetyl cysteine or a p38 mitogen-activated protein kinase (MAPK) inhibitor, and support the existence of the link between DNA repair, ROS levels and stem cell maintenance in multiple tissues.
While it is thus established that the p16INK4a-Rb pathway is strongly associated with the maintenance of HSC quiescence, additional research suggests that Mdm2-p53 pathway and its mediators are also essential in maintaining hematopoiesis [46; 50; 51; 52]. p53 is a well-known tumor suppressor that mediates the DDR by either causing cell cycle arrest when DNA damage is repairable, or inducing apoptosis or senescence when damage is too severe [53; 54; 55]. Though p53 transcript levels are high in HSCs, when the cells are free of stress, p53 protein is thought to be ubiquitinated by the E3 ubiquitin ligase Mdm2, leading to its degradation [56; 57; 58]. However, it is now known that some active p53 is necessary for the maintenance of HSC function. p53−/− mice have HSCs with reduced quiescence; they exhibit reduced repopulation capacity accompanied by an increase in HSC number, indicating that HSCs in p53−/− were readily entering the cell cycle, have reduced self-renewal capacity, and an inability to maintain hematopoiesis [59; 60; 61]. Previously, the Nimer group had shown that loss of the Mef transcription factor results in increased HSC quiescence, thus demonstrating that MEF (or ELF4) is a regulator of cell cycle entry for HSCs [62]. Liu et al. have demonstrated that Mef is a direct upregulator of Mdm2, indicating that p53 may play a role in the enhanced quiescence of HSCs in Mef / mice [59]. Interestingly, they showed that p53-mediated quiescence might be independent of some of p53’s more typical downstream effectors, such as p21, and implicated p38 MAPK and the PI3K-Akt pathway as mediators of p53 activity. Other studies suggest that low levels of active p53 reduce ROS levels and associated cellular stress, which is a surprising finding as p53 exhibits a pro-oxidant effect when it is present in the high levels necessary to mediate apoptosis [63]. Interestingly, another group also demonstrated that Mdm2 suppresses the p53 response to ROS, which promotes HSC survival and sustainable hematopoiesis by preventing excessive accumulation of spontaneous DNA damage [64]. p53’s role in the maintenance of HSC quiescence, as demonstrated by the Nimer group, may be essential in providing this protection against cellular stress.
Of course, we must not forget p53’s well-established role in mediating DDR. Milyavsky et al., in a study that will be discussed further in the “DNA Damage Repair in HSCs” section of this review, described two roles for p53 in human HSCs: a role in mediating the apoptotic response following ionizing radiation (IR), and a role in HSC maintenance that is independent of apoptosis mediation [65].
Interestingly, the Polycomb transcription factor Bmi1, has been studied independently for its role in maintaining HSC quiescence [66; 67; 68]. Bmi1−/− HSCs exhibit mitochondrial dysfunction and accumulation of ROS. As Ito et al. demonstrated, p16INK4A and p19ARF are downstream effectors of ROS, which may indicate that there is a positive feedback mechanism at play, whereby increased levels of ROS or activation of its effectors results in further increased ROS generation. In the hematopoietic system this leads to a failure of marrow reconstitution that is abrogated by NAC treatment, similar to that in Atm−/− mice. Chk2 is a cell cycle checkpoint protein that is phosphorylated by ATM in response to DSBs [69]. In Bmi1/Chk2 double knockouts HSC function is markedly improved, as compared to Bmi1−/− HSCs. Thus, Bmi1 appears to be necessary for the maintenance of HSC quiescence exclusively in the face of DNA damage or other ATM-activating stresses. Furthermore, the inability to achieve a complete rescue of HSC function in double knockout mice indicates that multiple pathways of the DDR, not just ATM-Chk2, are involved in cell-fate decisions, consistent with research discussed later.
The Suda group went on to fill-in the mechanism by which ATM regulates HSC differentiation and identified p38 MAPK as an intermediary between ROS and p16INK4A expression [70]. By interrupting p38 MAPK function, either with a small-molecule inhibitor or by interrupting MAP3K, an activator of p38 MAPK, they were able to restore repopulating capacity in serial transplantation of Atm−/− HSCs, which is consistent with clinical evidence for the use of p38 MAPK inhibitors in aplastic anemia [71]. p38 MAPK inhibition also reduced p16INK4A and p19ARF expression, demonstrating that p38 MAPK is an upregulator of these proteins.
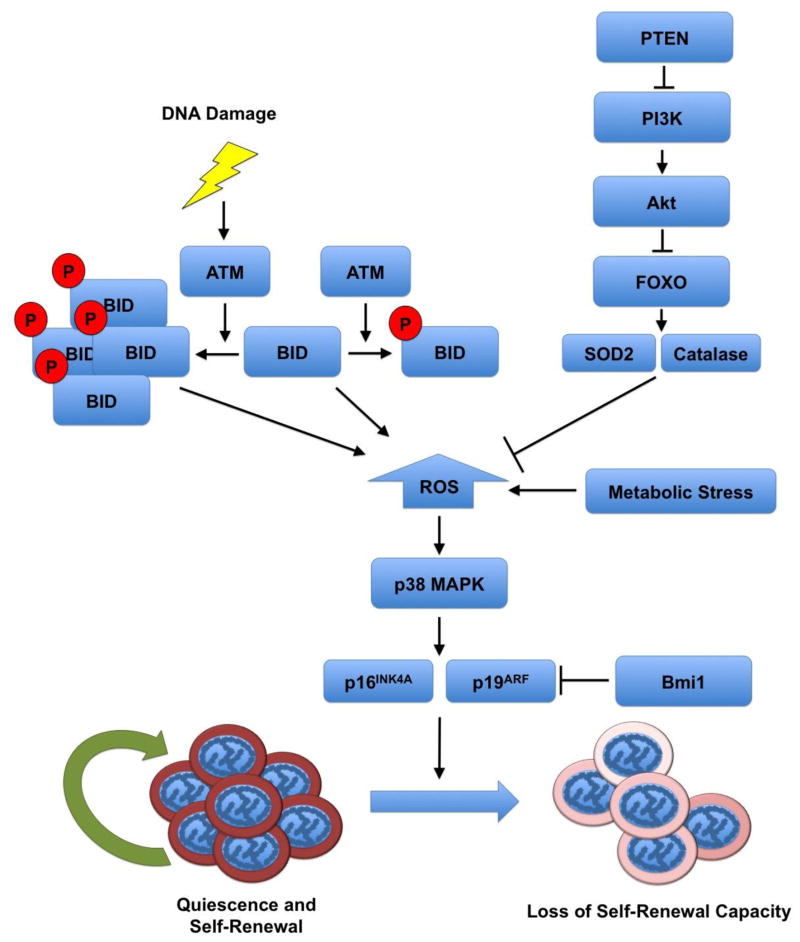
BID is a member of the BH3-only proapoptotic BCL-2 family [72]. BH3-only family members serve to communicate early death signals with BAX and BAK, the proapoptotic multidomain proteins, which activate a large number of substrates, thus mediating the apoptotic response. In back-to-back publications, Gross and Korsmeyer demonstrated that BID is involved in DDR through a mechanism that is distinct from its proapoptotic role and requires phosphorylation by ATM at serines 64 and 78 [73; 74]. Phosphorylation of BID by ATM results in inhibition of apoptosis and cell cycle arrest in vitro. The Gross group went on to demonstrate that BID is sequestered in the nucleus, but was able to exit the nucleus upon DNA damage by etoposide treatment [75]. Recently, the Gross group identified the role of the ATM-BID axis in the maintenance of HSC quiescence [76]. They generated knock-in BidS61A/S78A (BidAA) mice, which expressed a BID that could not be phosphorylated by Atm. Whereas BidWT mice are more resistant to total body irradiation (TBI), BidAA mice succumb to TBI quickly. Thus, phosphorylated BID conveys resistance to DNA damaging agents in an ATM-dependent manner. Competitive reconstitution assays demonstrated that BidAA HSCs are impaired in their ability to repopulate the bone marrow, though the ability to differentiate into various hematologic lineages was preserved, similar to Atm knockouts [46]. Furthermore, cell cycle profiling revealed that a lower percentage of BidAA HSCs are quiescent as compared to BidWT, demonstrating that BID phosphorylation is necessary for the maintenance of HSC quiescence. Additionally, ROS levels were high in BidAA HSCs and treatment with NAC abrogated the loss of HSC quiescence and protected HSCs from irradiation. Thus, BID phosphorylation by ATM is necessary for maintaining ROS homeostasis in HSCs and ROS elevation is the primary mediator of loss of HSC quiescence and IR susceptibility. Subcellular fractionation studies revealed that some BID localizes to the mitochondria, with in an increased amount of BID being found in the mitochondria in Atm−/− mice, which is consistent with the hypothesis that increased mitochondrial BID levels results in increased ROS generation. Interestingly, following TBI both BID and phosphorylated BID could be found localized to the mitochondria. Under normal conditions or conditions of minimal DNA damage, ATM phosphorylates BID, which may restrict it to the nucleus, resulting in maintained quiescence or cell cycle arrest. However, under conditions of severe DNA damage ATM phosphorylates a large amount of BID. A large amount of BID, including phosphorylated BID, manages to reach the mitochondria resulting in ROS accumulation and loss of HSC quiescence (Fig. 1). Finally, Gross and Korsmeyer observed increased levels of p16INK4A and p19ARF expression in BidAA mice, consistent with studies of Atm−/− mice [46; 70].
Figure 1. Stress Response Pathways Converge by Regulating ROS Levels.
Reactive oxygen species (ROS) initiate a common pathway by which hematopoietic stem cells abandon self-renewal and exit quiescence in favor of differentiation. Stress may be in the form of DNA damage, which is sensed by the ATM-BID pathway. Low levels of DNA damage may result in BID phosphorylation and sequestration in the nucleus, whereas severe DNA damage, such as in the case of double strand breaks, results in the accumulation of phosphorylated and unphosphorylated BID in the mitochondria and ROS accumulation. Stress may also result from the removal of cytokines and growth signals, which is sensed by the PI3K-Akt pathway. Additionally, oxidative stress can result in the accumulation of ROS, which is damaging to DNA and other cellular components, and may lead to differentiation of HSCs.
Together, these studies have begun to elucidate the role of ROS in HSC cell fate decisions. While ROS levels must be kept low to prevent tissue damage, it is becoming clear that low levels of ROS in HSCs allows ROS to be used as a signal for the loss of quiescence. When ROS levels are maintained in excess, the exit from quiescence leads to a progressive reduction of the functional HSC compartment. In this review we have examined a pathway whereby a DSB causes the activation of sensor proteins, which proceed to activate ATM. Under normal conditions phosphorylation by ATM may restrict BID to the nucleus, resulting in low ROS levels and the maintenance of HSC quiescence. However, following severe DNA damage, high levels of phosphorylated and unphosphorylated BID accumulate in the mitochondria and ROS levels increase. High levels of ROS lead to the activation of p38 MAPK, which activates p16INK4A, thus promoting the exit of the HSC from quiescence. While the ATM-BID axis is clearly important in ROS regulation, increasing evidence supports the idea that ROS levels are at the top of a common final pathway regulating cell fate decision and that this pathway has multiple inputs (Fig. 2). This is consistent with xenotransplantation experiments that demonstrate a role for ROS and ROS-mediated DNA damage in reducing the ability of human HSCs to self-renew, reducing their ability to engraft in recipient mice [77].
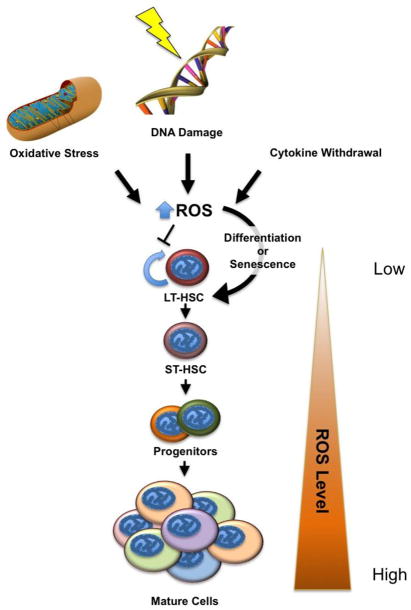
Figure 2. Cell Fate Decisions in Conditions of Stress.
Various forms of stress result in increasing levels of ROS. These include the loss of growth signaling, DNA damage, and oxidative stress due to metabolic deregulation or extrinsic factors. As ROS levels increase, LT-HSCs lose their self-renewal capacity, i.e. their stemness, choosing to differentiate or undergo senescence and depleting the stem cell pool. While stressed stem cells may differentiate properly and provide normal hematologic function, prolonged stress can result in hematopoietic failure. Low ROS levels are a hallmark of LT-HSCs, with ROS levels increasing as hematopoietic cells lose potency and differentiate. LT-HSC, long-term hematopoietic stem cell.
Loss of HSC Quiescence in the Face of Cellular Stress
Foxo3a appears to be another component necessary for HSC self-renewal. In 2007, Miyamoto et al. reported on the generation of Foxo3a−/− mouse whose HSCs demonstrate a diminished ability to reconstitute bone marrow on serial transplantation [78]. Earlier that same year, the Gilliland group reported on a FoxO1, FoxO3 and FoxO4 conditional knockout in the hematopoietic system (hereupon referred to as FoxO-deficient) [79; 80]. Foxo3a is one of four members of the forkhead family of transcription factors that, when active, reside in the nucleus and serve as proapoptotic signals [81; 82]. In response to cytokines and growth signals, FoxOs are inactivated by phosphorylation by the PI3K-Akt pathway, resulting in their exclusion from the nucleus. PI3K-Akt activation and FoxO exclusion from the nucleus are associated in vitro with loss of HSC quiescence and terminal differentiation [83].
In the Gilliland study, FoxO-deficient HSCs exhibited decreased number and an inability to reconstitute bone marrow in a recipient mouse after a single transplantation, secondary to an inability to maintain quiescence and self-renewal [79]. Furthermore, FoxO-deficient HSCs demonstrated oxidative stress and expressed fewer ROS reducing proteins as compared to wild-type (WT) HSCs, and treatment with NAC restored their repopulating capacity.
Foxo3a−/− mice displayed a milder phenotype as compared to FoxO-deficient mice [78]. Whereas FoxO-deficient HSCs were unable to repopulate bone marrow after a single transplantation, Foxo3a−/− mice showed impaired reconstitution capacity after the second and third transplantations, consistent with compensatory effects of FoxO family members in Foxo3a loss. Foxo3a−/− mice also had defects of ROS maintenance and showed reduced expression of ROS-regulating proteins, specifically superoxide dismutase 2 (SOD2) and catalase [84]. Additionally, Miyamoto et al. demonstrated that p38 MAPK activated by phosphorylation in more Foxo3a−/− HSCs than in wild type HSCs, and that p38 MAPK inhibition restores colony-forming capacity of Foxo3a−/− HSCs. As discussed previously, this suggests a common pathway whereby increased ROS levels, mediated by the ATM-BID and PI3K-Akt axes, activate p38 MAPK and its downstream effectors, resulting in loss of HSC quiescence and self-renewal ability. While it is not yet clear precisely how the ATM-BID axis regulates ROS homeostasis, it is clear that FoxOs upregulate transcription of antioxidant genes and that loss of FoxOs with PI3K-Akt activation results in decreased SOD2 and catalase expression, and thus decreased ROS removal.
This hypothesis is consistent with back-to-back articles from 2006 that detailed the role played by phosphatase and tensin homologue (PTEN) in HSC cell fate and leukemogenesis [85; 86]. PTEN, which is deleted, mutated or otherwise silenced in many hematological malignancies, is a tumor suppressor that dephosphorylates PI3K, thus negatively regulating the PI3K-Akt pathway [87; 88]. Interestingly, conditional Pten deletion results in HSC depletion, secondary to HSC hyperproliferation, and leukemogenesis by an mTOR (mammalian target of rapamycin) dependent mechanism, as treatment with rapamycin rescues Pten-deficient HSCs and depletes Pten-deficient leukemia-initiating cells [85]. Recently, it has been demonstrated that HSCs from mice in which Akt, a serine threonine kinase, is constitutively active also exhibit hyperproliferation preceding HSC depletion, as has been observed in Pten deficiency [89]. Concordantly, in mice lacking Akt1 and Akt2, HSCs tend to persist in the G0-phase and exhibit defects in hematopoietic differentiation [90]. These results are consistent with the previously discussed role of the PI3K-Akt pathway in FoxO phosphorylation and degradation.
The Passegué group recently reported on the importance of a protective autophagy program in HSCs [91]. Autophagy is an appealing candidate as a method by which HSCs deal with cellular stress, as it would serve to preserve the HSC pool, in contrast to apoptosis and senescence, which result in HSC depletion. Interestingly, Foxo3a, which has previously been shown to regulate HSC quiescence via ROS regulation, has been implicated in the autophagy pathway. By selectively inhibiting ATG12, an essential mediator of autophagy, in the hematopoietic compartment, the Passegué group demonstrated that autophagy is necessary for the maintenance of HSCs in response to starvation or cytokine withdrawal, whereas progenitor cells are apoptosis-prone and are not protected from stress by autophagy. This difference is secondary to increased expression of pro-autophagy genes in HSCs, rather than increased expression of the autophagy inhibitors, mTOR and phosho-S6, in progenitors. Using Foxo3a−/− mice, the group also demonstrated that Foxo3a, which is normally highly expressed and active in HSCs as compared to progenitors, promotes the activation of the autophagy response to stress. In Foxo3a−/− mice autophagy was delayed, but not absent, indicating that compensatory pathways, perhaps by other FoxO family members, may induce autophagy in conditions of cellular stress. It would be interesting to explore the autophagy response in FoxO-deficient mice [80]. The Passegué group also determined that old and young HSCs share similar capacity to upregulate autophagy in the face of cellular stress, though older HSCs have a higher basal level of autophagy. In fact, their work indicates that decreased nutrient uptake, not impaired autophagy, is responsible for reduced replating ability of old HSCs.
Together, the above results suggest that the FoxO family proteins, and specifically Foxo3a, work to maintain HSC quiescence and self-renewal ability by reducing ROS levels through increased expression of ROS scavenging proteins, and by promoting a protective autophagy response to cellular stress, saving HSCs from undergoing apoptosis.
DNA Damage Repair in HSCs
So far we have discussed the mechanism by which DNA damage is sensed by ATM-associated proteins, the role of ROS in cell fate decisions made in the face of stress, and other mechanisms by which a cell may deal with stress, namely autophagy. Here we will investigate the mechanism by which HSCs repair DSBs, and the advantages and pitfalls of this approach.
As mentioned previously, DNA is particularly susceptible to damage, which can occur at any point throughout the life of the cell. The cell responds to such damage through a variety of DDR pathways that which are chosen on the basis of the type of damage present and the stage in the cell cycle at which the damage occurred [17; 92]. DSBs represent a severe form of DNA damage that results in the activation of damage sensing proteins and ATM, a kinase that activates cell cycle checkpoint proteins, including p53. There are two primary mechanisms by which cells respond to DSBs: non-homologous end-joining (NHEJ) and homologous recombination (HR) [8; 93; 94]. While NHEJ can fix DSBs quickly, it can itself be damaging and highly error-prone, as it consists of the simple joining of two loose ends of DNA. Thus, NHEJ serves as the source for many reciprocal translocations and deletions, which are typical of malignancies [95; 96]. In contrast, homologous recombination (HR, sometimes called homology-directed repair) relies on sequence homology to direct its repair process, and repairs damage in the S or G2 phases of the cell cycle [97]. Stem cell quiescence generally serves to protect from metabolic stress and mistakes intrinsic to DNA replication [12]. However, in the face of DNA damage, quiescence is a bane to genomic integrity as NHEJ is the preferred method of DDR upon exiting G0, i.e. in G1 phase, due to the absence of sister chromatids and chromatin compaction, which complicate a homology search [17].
Recently, the Passegué and Dick groups each reported that HSCs display differential susceptibility to ionizing radiation (IR) as compared to progenitor cells, because they utilize stem cell-specific DNA repair mechanisms [65; 98]. The Passegué group demonstrated that murine HSCs are resistant to IR, as compared to progenitor cells, which is consistent with previous studies [99]. They speculate that this intrinsic resistance to IR is the result of the low levels of ROS in HSCs, which undergo growth arrest, rather than apoptosis, after IR exposure. They also report that radio-resistance coupled with the quiescent state of HSCs causes them to repair IR induced DNA damage using the error-prone NHEJ method. Using spectral karyotyping, a technique that utilizes fluorescently labeled probes, they observed a high frequency of genomic rearrangements and expansion of IR-damaged HSCs after transplantation. In support of the role of quiescence in NHEJ preference, the Passegué group also demonstrated that HSCs forced to enter the cell cycle prior to IR treatment were also radioresistant, but preferentially used HR in DNA repair [100].
In contrast to what is observed in murine HSCs, the Dick group has reported that human cord-blood HSCs are more sensitive to IR than progenitor cells [65]. By examining single-cell electrophoresis (“neutral comet assay”) and γH2AX foci formation, they have also demonstrated that progenitor cells underwent delayed double-strand joining after high level IR exposure, as compared to fibroblasts, and that joining in HSCs was even further delayed. They also demonstrated that apoptosis following delayed DSB repair in HSCs is p53 mediated and IR susceptibility in human HSCs is diminished when p53 levels are reduced, as evidenced by enhanced marrow reconstitution by xenotransplanted p53 knock-down (KD) HSCs exposed to IR. While p53 increases apoptosis of HSCs following damage, the Dick group also demonstrated that p53 function is necessary for HSC engraftment and self-renewal on serial transplantation. In mice serially transplanted with p53KD cord blood HSCs, the HSCs displayed persistent γH2AX foci, indicating DSBs, and decreased self-renewal capacity. This in contrast to their previous experiment in which p53KD rescued the reconstitution ability of primarily transplanted HSCs after IR exposure. Thus, Milyavsky et al. demonstrated two independent roles for p53 in HSC maintenance; causing apoptosis in the face of DNA damage by IR, and promoting genome integrity and self-renewal in expanding HSCs.
While both murine HSCs and human cord-blood HSCs utilize NHEJ to repair DSBs, the difference in the speed with which damage is repaired is striking. More surprising is the differential susceptibility of murine HSCs and human HSCs to IR, as compared to their respective progenitors [101]. In the effort to further qualify the DDR in HSCs, investigations into whether the difference in NHEJ kinetics is species specific or related to HSC ontogeny should be carried out.
Concluding Remarks
Stem cells have the ability to self-renew and to differentiate in order to maintain a tissue for the lifetime of an organism. Additionally, it is the responsibility of the stem cells to guard genomic integrity so that tissue function can be maintained and carcinogenesis can be avoided. For this reason, stem cells divide infrequently, most of them occupying the quiescent G0 phase of the cell cycle to protect them from the DNA damage inherent to DNA replication and damaging agents that result from cellular metabolism. Hypoxic stem cell niches, for example the endosteal bone marrow niche of hematopoietic stem cells, serve to protect stem cells from oxidative stress, which can result in damage to DNA and other cellular components. Though quiescence serves to protect stem cells from harm, when DNA damage does occur they are forced to undergo NHEJ, an error prone repair mechanism that can result in deletions, insertions and translocations. How, then, can stem cells protect genomic integrity and tissue function without high fidelity DNA repair mechanisms?
Without the wherewithal to faithfully repair DNA damage, damaged HSCs will preferentially sacrifice their ability to self-renew in favor of differentiating into short-lived progenitor cells. Progenitor cells do not have the capacity to self-renew and repopulate the bone marrow, thus protecting the hematopoietic compartment from inheriting and disseminating the provoking DNA damage.
We have focused on studies that identify ROS as a central player in the altruistic decision to differentiate when confronted with DNA damage and cellular stress. An environment of low oxidative stress, provided by the bone-marrow niche and low cellular metabolism, allows for HSCs to use ROS as a signal for differentiation. Interestingly, ROS is at the head of a final common pathway that leads to loss of HSC quiescence. This common pathway receives input for the DNA damage sensing ATM-BID axis, and from the PI3K-Akt axis, which regulates cell growth and apoptosis in response to growth signals. ROS does not only serve as a signaling molecule, but it is also damaging byproduct of cellular metabolism. Thus, DNA damage can lead to an increase in ROS levels and loss of self-renewal, and high levels of ROS can forewarn possible DNA damage, causing differentiation even before damage has occurred or been detected.
Hematopoiesis is the paradigmatic system for the study of stem cells and hierarchical differentiation. It is imperative that we further elucidate the role of ROS and oxidative stress in cell fate decisions and that we continue to search for those final participants in the decision to self-renew or differentiate.
Acknowledgments
We are thankful to the Ito lab members for comments and discussion on DNA damage responses in stem cells. K.I. is supported by grants from the NIH (R00CA139009, R01DK98263). C.W. is supported by an NIH MSTP training grant (T32-GM007288). Many original articles were omitted due to space limitations; for this, we apologize.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alberts B. Molecular biology of the cell. Garland Science; New York: 2002. [Google Scholar]
- 2.Attar EC, Scadden DT. Regulation of hematopoietic stem cell growth. Leukemia. 2004;18:1760–8. doi: 10.1038/sj.leu.2403515. [DOI] [PubMed] [Google Scholar]
- 3.Lemischka IR, Raulet DH, Mulligan RC. Developmental potential and dynamic behavior of hematopoietic stem cells. Cell. 1986;45:917–27. doi: 10.1016/0092-8674(86)90566-0. [DOI] [PubMed] [Google Scholar]
- 4.Osawa M, Hanada K, Hamada H, Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273:242–5. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- 5.Yilmaz OH, Kiel MJ, Morrison SJ. SLAM family markers are conserved among hematopoietic stem cells from old and reconstituted mice and markedly increase their purity. Blood. 2006;107:924–30. doi: 10.1182/blood-2005-05-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiel MJ, Yilmaz OH, Iwashita T, et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–21. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 7.Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132:631–44. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naka K, Hirao A. Maintenance of genomic integrity in hematopoietic stem cells. Int J Hematol. 2011;93:434–9. doi: 10.1007/s12185-011-0793-z. [DOI] [PubMed] [Google Scholar]
- 9.Cheshier SH, Morrison SJ, Liao X, Weissman IL. In vivo proliferation and cell cycle kinetics of long-term self-renewing hematopoietic stem cells. Proc Natl Acad Sci U S A. 1999;96:3120–5. doi: 10.1073/pnas.96.6.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kiel MJ, He S, Ashkenazi R, et al. Haematopoietic stem cells do not asymmetrically segregate chromosomes or retain BrdU. Nature. 2007;449:238–42. doi: 10.1038/nature06115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson A, Laurenti E, Oser G, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135:1118–29. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 12.Orford KW, Scadden DT. Deconstructing stem cell self-renewal: genetic insights into cell-cycle regulation. Nat Rev Genet. 2008;9:115–28. doi: 10.1038/nrg2269. [DOI] [PubMed] [Google Scholar]
- 13.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 14.Lombard DB, Chua KF, Mostoslavsky R, et al. DNA repair, genome stability, and aging. Cell. 2005;120:497–512. doi: 10.1016/j.cell.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 15.Bagby GC, Alter BP. Fanconi anemia. Semin Hematol. 2006;43:147–56. doi: 10.1053/j.seminhematol.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Hoeijmakers JH. DNA damage, aging, and cancer. N Engl J Med. 2009;361:1475–85. doi: 10.1056/NEJMra0804615. [DOI] [PubMed] [Google Scholar]
- 17.Branzei D, Foiani M. Regulation of DNA repair throughout the cell cycle. Nat Rev Mol Cell Biol. 2008;9:297–308. doi: 10.1038/nrm2351. [DOI] [PubMed] [Google Scholar]
- 18.Lindahl T. Instability and decay of the primary structure of DNA. Nature. 1993;362:709–15. doi: 10.1038/362709a0. [DOI] [PubMed] [Google Scholar]
- 19.Mandal PK, Blanpain C, Rossi DJ. DNA damage response in adult stem cells: pathways and consequences. Nat Rev Mol Cell Biol. 2011;12:198–202. doi: 10.1038/nrm3060. [DOI] [PubMed] [Google Scholar]
- 20.Ciccia A, Elledge SJ. The DNA damage response: making it safe to play with knives. Mol Cell. 2010;40:179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–8. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rossi DJ, Jamieson CHM, Weissman IL. Stems Cells and the Pathways to Aging and Cancer. Cell. 2008;132:681–696. doi: 10.1016/j.cell.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 23.Parmar K, Kim J, Sykes SM, et al. Hematopoietic stem cell defects in mice with deficiency of Fancd2 or Usp1. Stem Cells. 2010;28:1186–95. doi: 10.1002/stem.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang QS, Marquez-Loza L, Eaton L, et al. Fancd2−/− mice have hematopoietic defects that can be partially corrected by resveratrol. Blood. 2010;116:5140–8. doi: 10.1182/blood-2010-04-278226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clarke MF, Fuller M. Stem cells and cancer: two faces of eve. Cell. 2006;124:1111–5. doi: 10.1016/j.cell.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 26.Jamieson CH, Ailles LE, Dylla SJ, et al. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N Engl J Med. 2004;351:657–67. doi: 10.1056/NEJMoa040258. [DOI] [PubMed] [Google Scholar]
- 27.Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–70. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 28.Rossi DJ, Bryder D, Seita J, et al. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 2007;447:725–9. doi: 10.1038/nature05862. [DOI] [PubMed] [Google Scholar]
- 29.Rube CE, Fricke A, Widmann TA, et al. Accumulation of DNA damage in hematopoietic stem and progenitor cells during human aging. PLoS One. 2011;6:e17487. doi: 10.1371/journal.pone.0017487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J, Sun Q, Morita Y, et al. A differentiation checkpoint limits hematopoietic stem cell self-renewal in response to DNA damage. Cell. 2012;148:1001–14. doi: 10.1016/j.cell.2012.01.040. [DOI] [PubMed] [Google Scholar]
- 31.Barzilai A, Rotman G, Shiloh Y. ATM deficiency and oxidative stress: a new dimension of defective response to DNA damage. DNA Repair (Amst) 2002;1:3–25. doi: 10.1016/s1568-7864(01)00007-6. [DOI] [PubMed] [Google Scholar]
- 32.Meyn MS. Ataxia-telangiectasia and cellular responses to DNA damage. Cancer Res. 1995;55:5991–6001. [PubMed] [Google Scholar]
- 33.Shiloh Y. ATM and related protein kinases: safeguarding genome integrity. Nat Rev Cancer. 2003;3:155–68. doi: 10.1038/nrc1011. [DOI] [PubMed] [Google Scholar]
- 34.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–95. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 35.Suda T, Takubo K, Semenza GL. Metabolic regulation of hematopoietic stem cells in the hypoxic niche. Cell Stem Cell. 2011;9:298–310. doi: 10.1016/j.stem.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 36.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297:842–57. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 37.Cipolleschi MG, Dello Sbarba P, Olivotto M. The role of hypoxia in the maintenance of hematopoietic stem cells. Blood. 1993;82:2031–7. [PubMed] [Google Scholar]
- 38.Mohyeldin A, Garzon-Muvdi T, Quinones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell. 2010;7:150–61. doi: 10.1016/j.stem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 39.Jang YY, Sharkis SJ. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood. 2007;110:3056–63. doi: 10.1182/blood-2007-05-087759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takao N, Kato H, Mori R, et al. Disruption of ATM in p53-null cells causes multiple functional abnormalities in cellular response to ionizing radiation. Oncogene. 1999;18:7002–9. doi: 10.1038/sj.onc.1203172. [DOI] [PubMed] [Google Scholar]
- 41.Schubert R, Reichenbach J, Royer N, Pichler M, Zielen S. Spontaneous and oxidative stress-induced programmed cell death in lymphocytes from patients with ataxia telangiectasia (AT) Clin Exp Immunol. 2000;119:140–7. doi: 10.1046/j.1365-2249.2000.01098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takao N, Li Y, Yamamoto K. Protective roles for ATM in cellular response to oxidative stress. FEBS Lett. 2000;472:133–6. doi: 10.1016/s0014-5793(00)01422-8. [DOI] [PubMed] [Google Scholar]
- 43.Kamsler A, Daily D, Hochman A, et al. Increased oxidative stress in ataxia telangiectasia evidenced by alterations in redox state of brains from Atm-deficient mice. Cancer Res. 2001;61:1849–54. [PubMed] [Google Scholar]
- 44.Guo Z, Deshpande R, Paull TT. ATM activation in the presence of oxidative stress. Cell Cycle. 2010;9:4805–11. doi: 10.4161/cc.9.24.14323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guo Z, Kozlov S, Lavin MF, Person MD, Paull TT. ATM activation by oxidative stress. Science. 2010;330:517–21. doi: 10.1126/science.1192912. [DOI] [PubMed] [Google Scholar]
- 46.Ito K, Hirao A, Arai F, et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431:997–1002. doi: 10.1038/nature02989. [DOI] [PubMed] [Google Scholar]
- 47.Oguro H, Iwama A, Morita Y, et al. Differential impact of Ink4a and Arf on hematopoietic stem cells and their bone marrow microenvironment in Bmi1-deficient mice. J Exp Med. 2006;203:2247–53. doi: 10.1084/jem.20052477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheng T, Rodrigues N, Shen H, et al. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000;287:1804–8. doi: 10.1126/science.287.5459.1804. [DOI] [PubMed] [Google Scholar]
- 49.Allen DM, van Praag H, Ray J, et al. Ataxia telangiectasia mutated is essential during adult neurogenesis. Genes Dev. 2001;15:554–66. doi: 10.1101/gad.869001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Asai T, Liu Y, Bae N, Nimer SD. The p53 tumor suppressor protein regulates hematopoietic stem cell fate. J Cell Physiol. 2011;226:2215–21. doi: 10.1002/jcp.22561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pant V, Quintas-Cardama A, Lozano G. The p53 pathway in hematopoiesis: lessons from mouse models, implications for humans. Blood. 2012;120:5118–27. doi: 10.1182/blood-2012-05-356014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Asai T, Liu Y, Di Giandomenico S, et al. Necdin, a p53 target gene, regulates the quiescence and response to genotoxic stress of hematopoietic stem/progenitor cells. Blood. 2012;120:1601–12. doi: 10.1182/blood-2011-11-393983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fei P, El-Deiry WS. P53 and radiation responses. Oncogene. 2003;22:5774–83. doi: 10.1038/sj.onc.1206677. [DOI] [PubMed] [Google Scholar]
- 54.Vousden KH, Prives C. Blinded by the Light: The Growing Complexity of p53. Cell. 2009;137:413–31. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 55.Vousden KH, Lane DP. p53 in health and disease. Nat Rev Mol Cell Biol. 2007;8:275–83. doi: 10.1038/nrm2147. [DOI] [PubMed] [Google Scholar]
- 56.Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387:296–9. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 57.Collavin L, Lunardi A, Del Sal G. p53-family proteins and their regulators: hubs and spokes in tumor suppression. Cell Death Differ. 2010;17:901–11. doi: 10.1038/cdd.2010.35. [DOI] [PubMed] [Google Scholar]
- 58.Forsberg EC, Prohaska SS, Katzman S, et al. Differential expression of novel potential regulators in hematopoietic stem cells. PLoS Genet. 2005;1:e28. doi: 10.1371/journal.pgen.0010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu Y, Elf SE, Miyata Y, et al. p53 regulates hematopoietic stem cell quiescence. Cell Stem Cell. 2009;4:37–48. doi: 10.1016/j.stem.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Akala OO, Park IK, Qian D, et al. Long-term haematopoietic reconstitution by Trp53−/−p16Ink4a−/−p19Arf−/− multipotent progenitors. Nature. 2008;453:228–32. doi: 10.1038/nature06869. [DOI] [PubMed] [Google Scholar]
- 61.TeKippe M, Harrison DE, Chen J. Expansion of hematopoietic stem cell phenotype and activity in Trp53-null mice. Exp Hematol. 2003;31:521–7. doi: 10.1016/s0301-472x(03)00072-9. [DOI] [PubMed] [Google Scholar]
- 62.Lacorazza HD, Yamada T, Liu Y, et al. The transcription factor MEF/ELF4 regulates the quiescence of primitive hematopoietic cells. Cancer Cell. 2006;9:175–187. doi: 10.1016/j.ccr.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 63.Sablina AA, Budanov AV, Ilyinskaya GV, et al. The antioxidant function of the p53 tumor suppressor. Nat Med. 2005;11:1306–13. doi: 10.1038/nm1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Abbas HA, Maccio DR, Coskun S, et al. Mdm2 is required for survival of hematopoietic stem cells/progenitors via dampening of ROS-induced p53 activity. Cell Stem Cell. 2010;7:606–17. doi: 10.1016/j.stem.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Milyavsky M, Gan OI, Trottier M, et al. A distinctive DNA damage response in human hematopoietic stem cells reveals an apoptosis-independent role for p53 in self-renewal. Cell Stem Cell. 2010;7:186–97. doi: 10.1016/j.stem.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 66.Molofsky AV, Pardal R, Iwashita T, et al. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature. 2003;425:962–7. doi: 10.1038/nature02060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Park IK, Qian D, Kiel M, et al. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature. 2003;423:302–5. doi: 10.1038/nature01587. [DOI] [PubMed] [Google Scholar]
- 68.Liu J, Cao L, Chen J, et al. Bmi1 regulates mitochondrial function and the DNA damage response pathway. Nature. 2009;459:387–92. doi: 10.1038/nature08040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smith J, Tho LM, Xu N, Gillespie DA. The ATM-Chk2 and ATR-Chk1 pathways in DNA damage signaling and cancer. Adv Cancer Res. 2010;108:73–112. doi: 10.1016/B978-0-12-380888-2.00003-0. [DOI] [PubMed] [Google Scholar]
- 70.Ito K, Hirao A, Arai F, et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med. 2006;12:446–51. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- 71.Verma A, Deb DK, Sassano A, et al. Cutting edge: activation of the p38 mitogen-activated protein kinase signaling pathway mediates cytokine-induced hemopoietic suppression in aplastic anemia. J Immunol. 2002;168:5984–8. doi: 10.4049/jimmunol.168.12.5984. [DOI] [PubMed] [Google Scholar]
- 72.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–19. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 73.Kamer I, Sarig R, Zaltsman Y, et al. Proapoptotic BID is an ATM effector in the DNA-damage response. Cell. 2005;122:593–603. doi: 10.1016/j.cell.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 74.Zinkel SS, Hurov KE, Ong C, et al. A role for proapoptotic BID in the DNA-damage response. Cell. 2005;122:579–91. doi: 10.1016/j.cell.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 75.Oberkovitz G, Regev L, Gross A. Nucleocytoplasmic shuttling of BID is involved in regulating its activities in the DNA-damage response. Cell Death Differ. 2007;14:1628–34. doi: 10.1038/sj.cdd.4402181. [DOI] [PubMed] [Google Scholar]
- 76.Maryanovich M, Oberkovitz G, Niv H, et al. The ATM-BID pathway regulates quiescence and survival of haematopoietic stem cells. Nat Cell Biol. 2012;14:535–41. doi: 10.1038/ncb2468. [DOI] [PubMed] [Google Scholar]
- 77.Yahata T, Takanashi T, Muguruma Y, et al. Accumulation of oxidative DNA damage restricts the self-renewal capacity of human hematopoietic stem cells. Blood. 2011;118:2941–50. doi: 10.1182/blood-2011-01-330050. [DOI] [PubMed] [Google Scholar]
- 78.Miyamoto K, Araki KY, Naka K, et al. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell. 2007;1:101–12. doi: 10.1016/j.stem.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 79.Tothova Z, Kollipara R, Huntly BJ, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–39. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 80.Paik JH, Kollipara R, Chu G, et al. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell. 2007;128:309–23. doi: 10.1016/j.cell.2006.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brunet A, Bonni A, Zigmond MJ, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–68. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 82.Dijkers PF, Medema RH, Lammers JW, Koenderman L, Coffer PJ. Expression of the pro-apoptotic Bcl-2 family member Bim is regulated by the forkhead transcription factor FKHR-L1. Curr Biol. 2000;10:1201–4. doi: 10.1016/s0960-9822(00)00728-4. [DOI] [PubMed] [Google Scholar]
- 83.Yamazaki S, Iwama A, Takayanagi S, et al. Cytokine signals modulated via lipid rafts mimic niche signals and induce hibernation in hematopoietic stem cells. EMBO J. 2006;25:3515–23. doi: 10.1038/sj.emboj.7601236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kops GJ, Dansen TB, Polderman PE, et al. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature. 2002;419:316–21. doi: 10.1038/nature01036. [DOI] [PubMed] [Google Scholar]
- 85.Yilmaz OH, Valdez R, Theisen BK, et al. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441:475–82. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- 86.Zhang J, Grindley JC, Yin T, et al. PTEN maintains haematopoietic stem cells and acts in lineage choice and leukaemia prevention. Nature. 2006;441:518–22. doi: 10.1038/nature04747. [DOI] [PubMed] [Google Scholar]
- 87.Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–8. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 88.Dahia PL, Aguiar RC, Alberta J, et al. PTEN is inversely correlated with the cell survival factor Akt/PKB and is inactivated via multiple mechanismsin haematological malignancies. Hum Mol Genet. 1999;8:185–93. doi: 10.1093/hmg/8.2.185. [DOI] [PubMed] [Google Scholar]
- 89.Kharas MG, Okabe R, Ganis JJ, et al. Constitutively active AKT depletes hematopoietic stem cells and induces leukemia in mice. Blood. 2010;115:1406–15. doi: 10.1182/blood-2009-06-229443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Juntilla MM, Patil VD, Calamito M, et al. AKT1 and AKT2 maintain hematopoietic stem cell function by regulating reactive oxygen species. Blood. 2010;115:4030–8. doi: 10.1182/blood-2009-09-241000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Warr MR, Binnewies M, Flach J, et al. FOXO3A directs a protective autophagy program in haematopoietic stem cells. Nature. 2013;494:323–7. doi: 10.1038/nature11895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dasika GK, Lin SC, Zhao S, et al. DNA damage-induced cell cycle checkpoints and DNA strand break repair in development and tumorigenesis. Oncogene. 1999;18:7883–99. doi: 10.1038/sj.onc.1203283. [DOI] [PubMed] [Google Scholar]
- 93.Sancar A, Lindsey-Boltz LA, Unsal-Kacmaz K, Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem. 2004;73:39–85. doi: 10.1146/annurev.biochem.73.011303.073723. [DOI] [PubMed] [Google Scholar]
- 94.Mao Z, Bozzella M, Seluanov A, Gorbunova V. Comparison of nonhomologous end joining and homologous recombination in human cells. DNA Repair (Amst) 2008;7:1765–71. doi: 10.1016/j.dnarep.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Elliott B, Jasin M. Double-strand breaks and translocations in cancer. Cell Mol Life Sci. 2002;59:373–85. doi: 10.1007/s00018-002-8429-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Weinstock DM, Elliott B, Jasin M. A model of oncogenic rearrangements: differences between chromosomal translocation mechanisms and simple double-strand break repair. Blood. 2006;107:777–80. doi: 10.1182/blood-2005-06-2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Weinstock DM, Richardson CA, Elliott B, Jasin M. Modeling oncogenic translocations: distinct roles for double-strand break repair pathways in translocation formation in mammalian cells. DNA Repair (Amst) 2006;5:1065–74. doi: 10.1016/j.dnarep.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 98.Mohrin M, Bourke E, Alexander D, et al. Hematopoietic stem cell quiescence promotes error-prone DNA repair and mutagenesis. Cell Stem Cell. 2010;7:174–85. doi: 10.1016/j.stem.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Down JD, Boudewijn A, van Os R, Thames HD, Ploemacher RE. Variations in radiation sensitivity and repair among different hematopoietic stem cell subsets following fractionated irradiation. Blood. 1995;86:122–7. [PubMed] [Google Scholar]
- 100.Passegue E, Wagers AJ, Giuriato S, Anderson WC, Weissman IL. Global analysis of proliferation and cell cycle gene expression in the regulation of hematopoietic stem and progenitor cell fates. J Exp Med. 2005;202:1599–611. doi: 10.1084/jem.20050967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Seita J, Rossi DJ, Weissman IL. Differential DNA damage response in stem and progenitor cells. Cell Stem Cell. 2010;7:145–7. doi: 10.1016/j.stem.2010.07.006. [DOI] [PubMed] [Google Scholar]