Abstract
BACKGROUND
MRI is a surrogate biomarker for major neurodevelopmental disabilities in survivors of perinatal hypoxic-ischemic encephalopathy as injury to the basal ganglia/thalami is highly predictive of major neuromotor and cognitive problems. Major disabilities and the appearance of neonatal (R2-2) MRI are improved with therapeutic hypothermia. We evaluated neurodevelopmental outcomes when conventional MRI showed minimal or no brain injury.
METHODS
IRB-approved series of 62 infants (≥36 weeks; ≥1800 g; 34 male/28 female) cooled for hypoxic-ischemic encephalopathy from 2005-11 who underwent neonatal (R2-2) MRI and Bayley Scales of Infant and Toddler Development-III 22 +/− 7 (R2-9) months of age. MRI at 5–14 (mean 8) days was scored as normal (score=0), showing focal grey or white matter injury only (score =1), or basal ganglia/thalamic and/or watershed lesions with or without more extensive hemispheric injury (score =2). Sensitivity, specificity, and positive (PPV) and negative predictive values (NPV) for MR scores 0 and 1 and statistical interaction between MRI score and age at MRI were determined.
RESULTS
MR score=0 was seen in 35/62 patients; 26/35 (74%) were typically developing, 7 (20%) had moderate and 2 (6%) had severe delay. MR score=1 was seen in 17/62 (27%) patients; 5/17 (29%) were normal, 11/17 (65%) had moderate delay, and 1/17 (6%) had severe neurodevelopmental delay. Of the 52 patients with MR scores 0 and 1, 40% were abnormal. The NPV of a normal MRI was 74%. For score 1, sensitivity was 95% [CI 63%–83%], specificity 84% [CI 70%–90%], PPV 84% [CI 71%–93%], and NPV 74% [CI 62%–82%].
CONCLUSIONS
Caution is warranted when prognosticating about neurodevelopmental status in early childhood after HIE with cooling and longer follow-up studies are needed to determine the prognostic significance of a neonatal MRI (R2-4) showing no or minor degrees of brain injury.
Keywords: Hypoxic ischemic encephalopathy, hypothermia, infant, newborn, MRI, prognosis
Introduction
Perinatal hypoxic ischemic encephalopathy (HIE) continues to be an important cause of cerebral palsy and neurodevelopmental delay and pediatric neurologists may be called upon to prognosticate in this clinical setting (1). Therapeutic hypothermia is now widely used in term neonates with moderate-to-severe [HIE as several large trials have shown that cooling within 6 hours lowers mortality and lessens major neurodevelopmental disability at 18–24 months of age with a relative risk reduction of 0.76 (0.65– 0.89) (1–10). As in non-cooled infants, perinatal MRI is widely used as a short-term surrogate outcome measure, and as a biomarker of the treatment efficacy of cooling (11–15). Cooling appears to lessen the frequency and improve the severity of basal ganglia/thalami (BG/T) abnormalities compared to non-cooled term infants with HIE and may also be protective of the cerebral cortex (12–15). The patterns of brain injury in HIE relate to the degree and duration of the perinatal hypoxia and hypotension and have been extensively described by Barkovich, Rutherford, and others (16–18). The classic pattern of BG/T injury is due to profound hypotension and primarily affects the thalami, posterior putamen, hippocampi, and sensorimotor pathways with relative sparing of the remaining cortex while mild-moderate hypotension is reflected as injury to watershed regions of the brain (16–18). There has been extensive reporting on the high sensitivity and positive predictive value for disorders of tone, movement, and cognition seen with basal ganglia/thalamus (BG/T) and watershed injury (16–18). However, the clinical significance of a perinatal MRI showing no or minor degrees of brain injury; e.g. focal white matter and cortical injury, in cooled survivors of HIE has not been so well defined. The aim of this study was therefore to investigate the predictive value of no or minor degrees of brain injury for neurodevelopmental status in the domains of language, cognition, and motor at 24 months.
Materials and Methods
From 2005-11, there were 90 consecutive inborn term infants (50 males/40 females, ≥36 weeks gestation, birth weight ≥1800 g) with HIE who had been admitted to our neonatal intensive care unit. This NNICU serves an underinsured population for whom English is not the primary language spoken in the home. Whole body cooling is the standard of care for moderate and severe HIE and pediatric neurologists are often consulted to prognosticate prior to patient discharge. Newborns with perinatal acidemia had been screened for HIE using the National Institute Child Heath and Human Development Neonatal Research Network biochemical and clinical criteria (1). The criteria include: (1) a pH <7.0 or a base deficit > 16 mEq/L on umbilical cord blood or any postnatal blood sample within 1 hour of age; or (2) history of an acute perinatal event and either: (1) no blood gas available or (2) a pH from 7.01–7.15 or a base deficit from 10–15.9 mEq/L, along with a 10-minute Apgar score <5, or assisted ventilation initiated at birth and continued for at least 10 minutes. With documented or suspected seizures, total body cooling therapy was begun irrespective of neurological exam. In the absence of seizures, the indication for cooling were moderate or severe HIE as defined by a neurologic examination showing alterations in level of consciousness; lack of spontaneous activity; abnormalities of tone, posture, or primitive reflexes; and/or autonomic system dysfunction. The neurologic examinations were done by neonatologists with specific training in neurologic evaluation of neonates at risk for HIE. The infant and maternal medical records of the infants were reviewed and pertinent demographic, clinical, and laboratory data were recorded. Infants were cooled within 6 hours of life for 72 hours using a cooling blanket (Blanketrol II, Cincinnati Sub-Zero) and maintaining the esophageal temperature at 33.5°C. Informed parental consent was obtained for entry into the cooling study and for subsequent use of clinical and imaging data for research purposes; this study was IRB approved.
MRI
As part of clinical protocol, all neonates with HIE undergo MR imaging at an adjacent children’s hospital after rewarming, when clinically stable, and prior to discharge to depict the pattern and extent of brain injury; as close to 5 days of age as possible. MR imaging was done in the initial 40 patients using a 1.5T and the next 40 patients were imaged at 3T, which is now standard of care for neonates at our institution. MR was done after feeding and swaddling the infant (“bundle and feed”) with vacuum pillows (MedVac-Kohlbrat & Bunz, Radstadt, Austria); neonatal ear muffs (MiniMuffs; Natus Medical, San Carlos, CA) were used to dampen sound. A registered nurse continuously monitored heart rate and peripheral oxygen saturations. In cases where sedation was needed, a pediatric anesthesiologist administered propofol infusions and monitored the patient for the duration of the study. Images were acquired using a 6 or 8-channel head coil and parallel imaging. Sequences included sagittal and axial T1; coronal and axial T2 fast spin echo; and axial diffusion weighted images (DWI) with b=1000. Although axial T2 gradient echo or susceptibility weighted images, diffusion tensor imaging (DTI), and MR spectroscopy were acquired in many infants, these sequences are not acquired in all patients and results derived from these techniques are beyond the scope of this report. Images were scored by 3 pediatric neuroradiologists who reached consensus blinded to clinical outcome scoring MR findings as 0 = normal; 1= focal cortical or white matter lesions without involvement of BG/ T, WS lesions, or infarction; and 2 = BGT and/or watershed lesions with or without additional cerebral lesions.
Neurodevelopmental follow-up
Neurodevelopmental assessment was done using the Bayley Scales of Infant and Toddler Development-III (BSID-III) [19] at approximately 24 months of age, administered by a trained pediatric developmental specialist with 11 years of experience, in conjunction with a neurodevelopmental pediatrician with > 25 years’ experience. Both were blinded to the patients’ MR findings and neonatal course and were assisted by a certified translator-interpreter for non-English speaking subjects. Neurodevelopmental assessment was encouraged at follow-up well-child clinic visits for all patients regardless of the clinical status and without knowledge of MRI results. For those subjects who underwent serial neurodevelopmental assessments, the BSID-III closest to 24 months was used for this study. A moderate delay was defined by a BSID-III which was 1–2 standard deviations below the norm; i.e. the worst composite score of 70–84 in one or more of 3 domains: cognitive, language, and motor. Severe delay was defined as a BSID-III > 2 standard deviations below the norm; e.g. any of 3 composite scores < 70, or an inability to assign a score due to severe mental deficiency or cerebral palsy as assessed using the Gross Motor Function Classification System (20).
Statistics
Statistical analysis was performed using Sigma Plot 11.0 (SPSS, Chicago, IL). The predictive values of MR scores 0 and 1 for delay in 1 or more domains were calculated using sensitivity, specificity, and positive and negative predictive values. The statistical interaction between MRI score and age at time of scan was evaluated using the Kruskal-Wallis test. Inter-rater agreement (kappa) was determined for interpretation of MR studies. (R2-7)
Results
Of the 90 patients with HIE cooled over this time period; 10 died in the early perinatal period and prior to MR imaging. Of the 80 survivors, 10 (12.5 %) had moderate-severe HIE and 70 (87.5%) had moderate HIE; 2 patients were excluded from the study due to congenital brain malformations diagnosed on the neonatal MR study. Of the 78 patients who underwent neonatal MRI and were eligible for inclusion in this study, 62 (79%) patients (34 males/28 females) also underwent BSID-III, which was done at 22 +/− 7 months (mean 22 months); 16 of 78 patients were lost to follow-up. Neonatal MRI was done at 5–14 (mean 7) days.
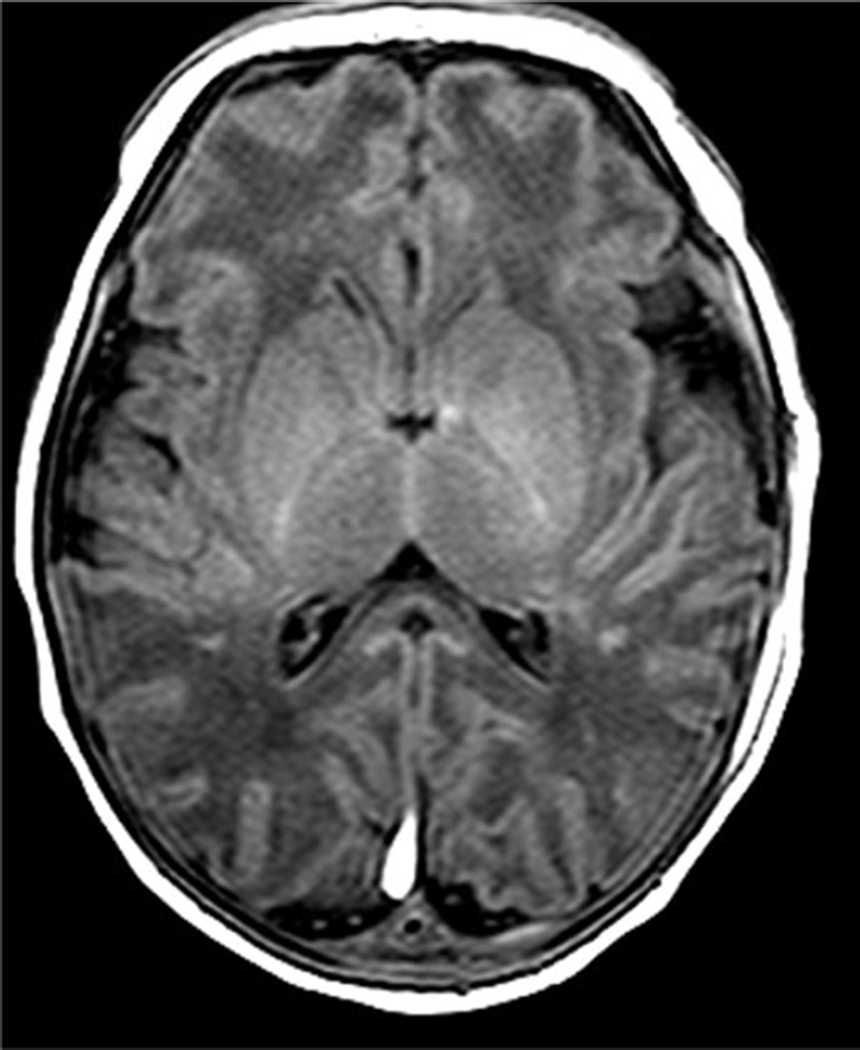
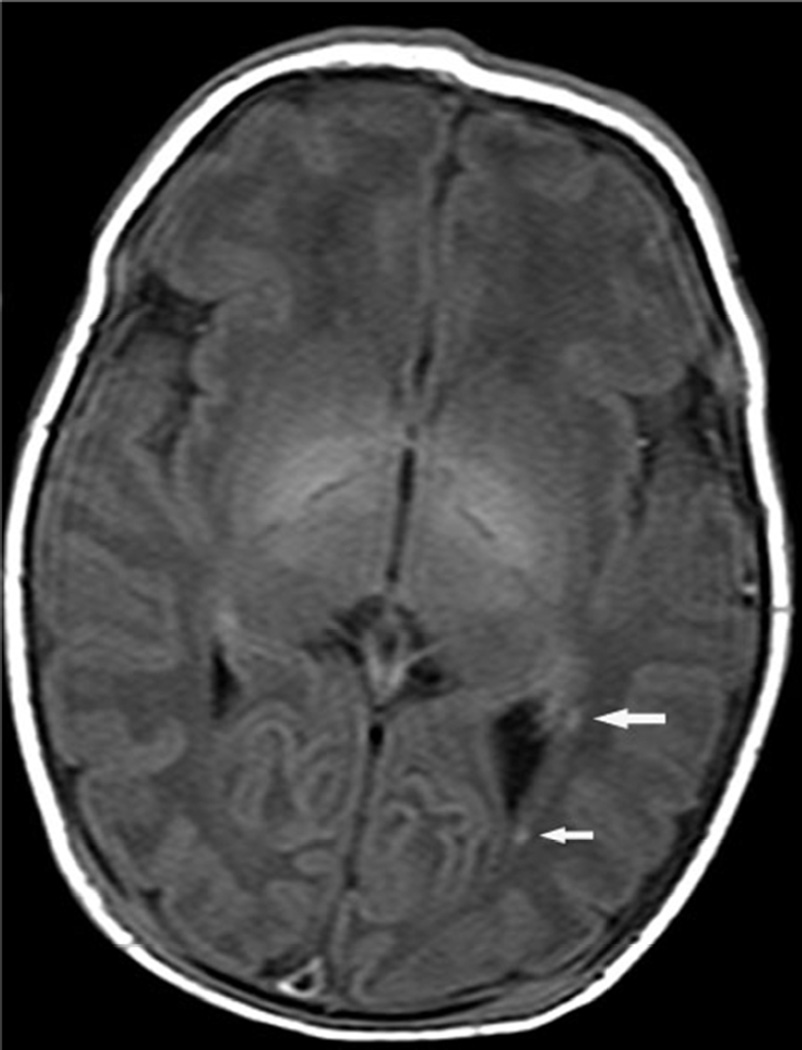
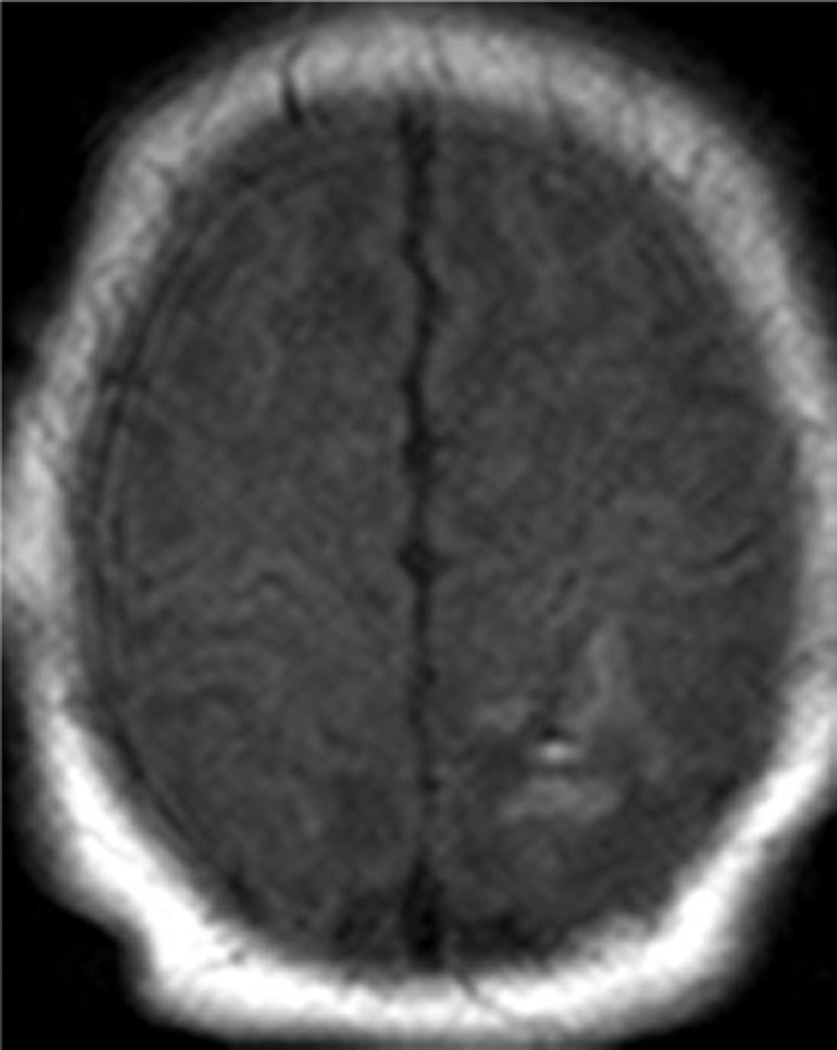
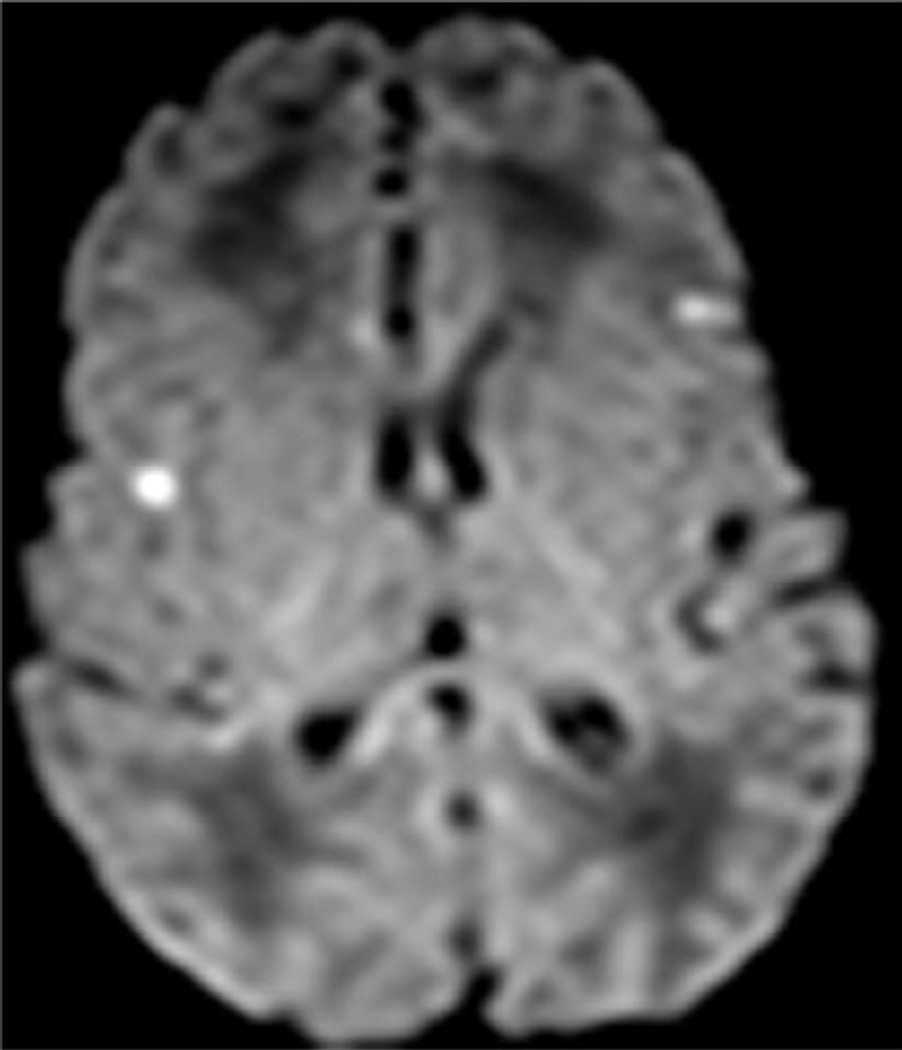
Examples of MR scores 1 are illustrated in Figures 1a–d. WMI was usually as seen regions of T1 shortening with or without corresponding areas of diminished signal intensity on T2 images, most often in the periventricular white matter and with variable areas of restricted diffusion. The cortical lesions were single in 2 and multiple in 7.
Fig. 1.
Examples of MRI showing minimal brain injury (score=1). White matter lesions most often involved the deep parietal white matter and tended to be bilateral.
a. Axial T1 image of a 7 day old infant shows multiple punctate areas of T1 shortening within the parietal white matter. The basal ganglia and thalami are normal
b. White matter lesions (arrows) were occasionally unilateral.
c. Isolated focal cortical infarct in 39 week infant imaged at day of life 7.
d. In another patient, there are scattered areas of restricted diffusion within the cerebral cortex.
Table 1 summarizes the degree of developmental delay seen in patients with MR scores 0–1. MR score=0 was seen in 35/62 patients; 26/35 (74%) had normal BSID-III, 7 (20%) had moderate and 2 (6%) had severe developmental delay. MR score=1 was seen in 17/62 (27%) patients; 5/17 (29%) were normal by BSID-III, 11/17 (65%) had moderate delay, and 1/17 (6%) had severe neurodevelopmental delay. Of the 52 patients with MR scores 0 and 1, 40% were developmentally delayed; 11 had isolated cognitive or language delay with normal motor skills and 10 had delays in cognitive and motor domains. Patients with MR score=2 consistently had abnormal outcomes with neuromotor deficits and abnormalities of tone. The NPV of a normal MRI was 74%; sensitivity 71%; specificity 84% and PPV 81%. For score 1, sensitivity was 95% [CI 63%–83%], specificity 84% [CI 70%–90%], PPV 84% [CI 71%–93%], and NPV 74% [CI 62%–82%]. For MR score 2, sensitivity was 28% [CI 19%–28%], specificity was 100% [CI 90%–100%], PPV was 100% [CI 68%–100%], and NPV was 54% [CI 48%–54%]. There was no statistical association between MRI score and age at MRI; exact Kruskal-Wallis p=0.24 [95% CI, 0.2352, 0.2520]. Kappa value was 0.89. (R2-7)
Table 1.
MR scores vs. neurodevelopmental status around 24 months for perinatal MR showing no or minor degrees of brain injury
| MRI |
Normal (BSID-III ≥85) |
Moderate Delay (BSID-III 70–84) |
Severe Delay (BSID-III <70) |
|---|---|---|---|
| Score 0 (n=35) | 26 (74%) | 7(20%) | 2 (6%) |
| Score 1 (n=17) | 5 (29%) | 11 (65%) | 1 (6%) |
DISCUSSION
We report herein an observational case series of 62 term infants with predominantly moderate HIE who underwent therapeutic whole body cooling, perinatal MRI, and standardized assessment of neurodevelopmental status in early childhood. MR studies were retrospectively scored by experienced pediatric neuroradiologists blinded to outcome and correlated with clinical outcome in early childhood to determine the negative predictive value of a normal MRI and the frequency with which seemingly minor degrees of brain injury by MR were associated with measurable deficits in either cognitive, language, or motor domains. A normal MRI; e.g. score 0, was seen in almost 56% of the 62 patients who underwent neurodevelopmental assessment and had a negative predictive value of 74%. MR score 1 was seen in about 27% of patients who underwent BSID-III and had PPV of 84% for developmental delay in early childhood which was most often mild-moderate, consistently involved cognitive domains, and which were usually not associated with cerebral palsy. Focal cortical injury with or without white matter lesions was more common than isolated focal white matter injury but was associated with similar incidence and severity of neurodevelopmental delay as focal isolated white matter injury. The classic patterns of HIE (score 2) were seen 13% of patients and had a PPV of 100% for deficits which were usually severe and associated with abnormalities of tone and motor. We found no statistical interaction between MRI score and day-of-life at time of scan although there is ongoing debate about optimal imaging time in neonatal HIE (11, 21–23). Given the imaging manifestations of HIE may vary even among patients of the same gestational age and day of life, serial MR imaging would be preferable but is impractical and cost-prohibitive.
We found lower NPV for a normal MRI in term neonates who had undergone cooling for HIE than has been reported non-cooled survivors of HIE (17, 23–25). Miller reported 20 non-cooled survivors with normal MR studies; all were developmentally normal by standardized testing at 12–30 months (17). Orkerafor reported 9/10 non-cooled infants with perinatal HIE and a normal perinatal MR appeared normally developing around12 months although not all underwent standardized neurodevelopmental assessment and 1 patient had obvious developmental delay (23). In Mercuri’s series of 12 non-cooled survivors with a normal MRI acquired between 1–4 weeks of life; all had normal developmental status at 12 months of age (25). In contrast, van Kooij found 61.5% of non-cooled survivors of HIE with no or mild white matter lesions on perinatal MR were found to have neurodevelopmental disabilities at 9–10 years of age and emphasized that standardized neurodevelopmental assessment before 18 months may be questionable (26). As developmental delays apparent in early childhood may resolve, especially with early childhood intervention, additional assessment later in childhood should be considered in these patients..
In 1998, Barkovich proposed a scoring system in perinatal asphyxia based on patterns of injury seen in circulatory arrest and less severe anoxic injury and included aspects of both patterns of injury (16). Higher points on the scoring system correlated with neuromotor and cognitive deficits in early childhood. Rutherford expanded on the Barkovich classification and suggested some infants with HIE may have focal white matter injury in the absence of basal ganglia/thalami lesions and that these white matter lesions give rise to tissue atrophy and cognitive impairment (21). The clinical significance of focal white matter injury has received comparatively less emphasis than BG/T and watershed injury in HIE; both in non-cooled and cooled patients with less emphasis on the clinical significance of focal cortical injury in cooled survivors of HIE (27–31). Barrett described a cohort of 36 non-cooled term infants with HIE; 4 (11%) had acute non cystic white matter and findings on follow-up MR studies were typical for the pattern of white matter injury seen in preterm infants and were associated with a poor outcome (27). Li described white matter injury in 11 of 48 non-cooled term newborns with HIE but did not provide details about neurodevelopmental outcome (28). In Li’s study, white matter injury varied from minimal to severe; more severe white matter injury was associated with earlier gestational ages. We did not observe any correlation between white matter injury and younger gestational ages in our cohort. Cowan reported isolated white matter injury in 16% of non-cooled infants with HIE; “mild” injury was associated with either a normal outcome at 2 years or mild language delay; “moderate” injury with cognitive delays and behavior problems but not with debilitating neuromotor problems and “severe” injury with more severe neuromotor problems and a higher incidence of seizures and behavioral problems (31). Mercuri described white matter abnormalities in 14 of 52 non-cooled term infants who underwent developmental assessment at 12 months (25). Of the 5 patients in Mercuri’s study with moderate white matter lesions, 2 (40%) were abnormal at 1 year while 100% of the 9 patients with severe white matter lesions were developmentally abnormal (25). There are at present no defined MR biomarkers for developmental delay or intellectual impairment in later childhood in cooled survivors of HIE with minor degrees of brain injury by MRI as hypothermia is relatively new, although now the standard of care for perinatal HIE in many neonatal intensive care units. Despite statistically significant improvements in the primary outcome measures used in clinical trials of therapeutic hypothermia; e.g. death or major neuromotor disability, newer studies have suggested cooling does not significantly affect developmental delay or intellectual impairment in later childhood (32) (R2-5) and the absence of neurologic deficits at time of hospital discharge and a MR showing no or minor degrees of brain injury does not equate with normal early childhood development.
Limitations of this study are the small number of subjects with focal white matter and/or cortical injury, lack of follow-up beyond early childhood, and lack of quantitative assessment of white matter integrity using techniques such as diffusion tensor imaging and MR spectroscopy. The effect of variable patient age at time of MR imaging on the sensitivity of MR is not clear and it would have been preferable to have all patients imaged on the same day of life. However, this was a retrospective study and age at time of imaging was beyond our control. Neonatal MR studies may be difficult to interpret for neuroradiologists who do not have a large volume of neonatal MRI but the pediatric neuroradiologists who reached consensus on the MR findings in this report have together >40 years collective experience.
In conclusion, in this longitudinal cohort study we investigated the predictive value of a simple scoring system for perinatal MRI acquired in term infants after cooling for HIE and found lower scores were associated with fewer motor and tone problems but a normal MRI did not consistently equate to normal cognitive and language measures around 24 months of age. That mild degrees of brain injury may be associated with developmental disabilities in early childhood suggests the pediatric neurologist should be cautious when counseling parents about prognosis after HIE with cooling, especially when perinatal MR showed seemingly mild degrees of focal white matter and cortical injury, and that these patients should be referred for standardized assessment of neurodevelopment in early childhood.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shankaran S, Pappas A, Laptook AR, McDonald SA, Ehrenkranz RA, Tyson JE, et al. Outcomes ofsafety and effectiveness in a multicenter randomized, controlled trial of whole-body hypothermia forneonatal hypoxic-ischemic encephalopathy. Pediatrics. 2008 Oct;122(4):e791–e798. doi: 10.1542/peds.2008-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns withhypoxic ischaemic encephalopathy. Cochrane Database of Systematic Reviews. 2013 doi: 10.1002/14651858.CD003311.pub3. Issue 1. Art.No.: CD003311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, et al. Wholebodyhypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005 Oct;353(15):1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 4.Azzopardi DV, Strohm B, Edwards AD, Dyet L, Halliday HL, Juszczak E, et al. Moderatehypothermia to treat perinatal asphyxia encephalopathy. N Engl J Med. 2009 Oct;361(14):1349–1358. doi: 10.1056/NEJMoa0900854. [DOI] [PubMed] [Google Scholar]
- 5.Zhou WH, Cheng GQ, Shao XM, et al. Selective head cooling with mild systemic hypothermia afterneonatal hypoxic-ischemic encephalopathy: a multicenter randomized controlled trial in China. J Pediatr. 2010 Sep;157(3):367–372. 372, e361–e363. doi: 10.1016/j.jpeds.2010.03.030. [DOI] [PubMed] [Google Scholar]
- 6.Simbruner G, Mittal RA, Rohlmann F, Muche R. Systemic hypothermia after neonatalencephalopathy: outcomes of neo.nEURO.network RCT. Pediatrics. 2010 Oct;126(4):e771–e778. doi: 10.1542/peds.2009-2441. [DOI] [PubMed] [Google Scholar]
- 7.Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates withhypoxic-ischemic encephalopathy. N Engl J Med. 2005 Oct 13;353(15):1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 8.Azzopardi D, Brocklehurst P, Edwards D, et al. The TOBY Study. Whole body hypothermia for thetreatment of perinatal asphyxial encephalopathy: a randomised controlled trial. BMC Pediatr. 2008;8:17. doi: 10.1186/1471-2431-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobs SE, Morley CJ, Inder TE, et al. Whole-body hypothermia for term and near-term newbornswith hypoxic-ischemic encephalopathy: a randomized controlled trial. Arch PediatrAdolesc Med. 2011 Aug;165(8):692–700. doi: 10.1001/archpediatrics.2011.43. [DOI] [PubMed] [Google Scholar]
- 10.Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermiaafter neonatal encephalopathy: multicentre randomised trial. Lancet. 2005 Feb 19–25;365(9460):663–670. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 11.Bonifacio SL, Glass HC, Vanderpluym J, Agrawal AT, Xu D, Barkovich AJ, et al. Perinatal eventsand early magnetic resonance imaging in therapeutic hypothermia. J Pediatr. 2011 Mar;158(3):360–365. doi: 10.1016/j.jpeds.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shankaran S, Barnes PD, Hintz SR, Laptook AR, Zaterka-Baxter KM, McDonald SA, et al. Braininjury following trial of hypothermia for neonatal hypoxic-ischaemic encephalopathy. Arch DisChild Fetal NeonatalEd. 2012 Nov;97(6):F398–F404. doi: 10.1136/archdischild-2011-301524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rutherford M, Ramenghi LA, Edwards AD, Brokolehurst P, Halliday H, Levene M, et al. Assessment of brain tissue injury after moderate hypothermia in neonates with hypoxic-ischaemicencephalopathy: a nested substudy of randomized controlled trial. Lancet Neurol. 2010 Jan;9(1):39–45. doi: 10.1016/S1474-4422(09)70295-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rutherford M, Azzopardi D, Whitelaw A, et al. Mild hypothermia and the distribution of cerebrallesions in neonates with hypoxic-ischemic encephalopathy. Pediatrics. 2005;116:1001–1006. doi: 10.1542/peds.2005-0328. [DOI] [PubMed] [Google Scholar]
- 15.Inder TE, Hunt RW, Morley CJ, et al. Randomized trial of systemic hypothermia selectively protectthe cortex on MRI in term hypoxic-ischemic encephalopathy. J Pediatr. 2004;145:835–837. doi: 10.1016/j.jpeds.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 16.Barkovich AJ, Hanau BL, Vigneron D, Sola A, Partridge JC, Allen F, et al. Prediction ofneuromotor outcome in perinatal asphyxia: evaluation of MR scoring systems. AJNR Am JNeuroradiol. 1998 Jan;19(1):143–149. [PMC free article] [PubMed] [Google Scholar]
- 17.Miller SP, Ramaswamy V, Michelson D, Barkovich AJ, Holshouser B, Wycliffe N, et al. Patterns ofbrain injury in term neonatal encephalopathy. J Pediatr. 2005 Apr;146(4):453–460. doi: 10.1016/j.jpeds.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 18.Rutherford M, Malamateniou C, McGuinness A, Allsop J, Biarge MM, Counsell S. Magneticresonance imaging in hypoxic-ischaemic encephalopathy. Early Hum Dev. 2010 Jun;86(6):351–360. doi: 10.1016/j.earlhumdev.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 19.Bayley N. Bayley Scales of Infant and Toddler Development, Psych Corp: Harcourt Assessment. 3rd edition. 2006. [Google Scholar]
- 20.Palisano RJ, Hanna SE, Rosenbaum PL, et al. Validation of a model of gross motor function forchildren with cerebral palsy. PhysTher. Oct. 2000;80(10):974–985. [PubMed] [Google Scholar]
- 21.Wintermark P, Hansen A, Soul J, Labrecque M, Robertson RL, Warfield SK. Early versus late MRIin asphyxiated newborns treated with hypothermia. Arch Dis Child Fetal NeonatalEd. 2011;96(1):F36–F44. doi: 10.1136/adc.2010.184291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rutherford M, Counsell S, Allsop J, Boardman J, Kapellou O, Larkman D, et al. Diffusionweightedmagnetic resonance imaging in term perinatal brain injury: a comparison with site of lesionand time from birth. Pediatrics. 2004 Oct;114(4):1004–1014. doi: 10.1542/peds.2004-0222. [DOI] [PubMed] [Google Scholar]
- 23.Okereafor A, Allsop J, Counsell SJ, Fitzpatrick J, Azzopardi D, Rutherford, et al. Patterns of braininjury in neonates exposed to perinatal sentinel events. Pediatrics. 2008 May;121(5):906–914. doi: 10.1542/peds.2007-0770. [DOI] [PubMed] [Google Scholar]
- 24.Barnett A, Mercuri, Rutherford M, et al. Neurological and perceptual-Motor Outcome at 5–6 yearsof age in children with neonatal encephalopathy: relationship with neonatal brain MRI. Neuropediatrics. 2002;33:242–248. doi: 10.1055/s-2002-36737. [DOI] [PubMed] [Google Scholar]
- 25.Mercuri E, Ricci D, Cowan FM, Lessing D, Frisone MF, Haataja L, et al. Head growth in infantswith hypoxic-ischemic encephalopathy: correlation with neonatal magnetic resonance imaging. Pediatrics. 2000 Aug;106(2 Pt 1):235–243. doi: 10.1542/peds.106.2.235. [DOI] [PubMed] [Google Scholar]
- 26.Van Kooij BJ, van Handel M, Nievelstein RA, Groenendaal F, Jongmans MJ, de Vries LS. SerialMRI and neurodevelopmental outcome in 9 to 10-year old children with neonatal encephalopathy. JPediatr. 2010;157(2):221–227. doi: 10.1016/j.jpeds.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 27.Barrett MJ, Donoghue V, Mooney EE, et al. Isolated acute non-cystic white matter injury in terminfants presenting with neonatal encephalopathy. Arch Dis Child Fetal NeonatalEd. 2012;98(2):F158–F160. doi: 10.1136/archdischild-2011-301505. [DOI] [PubMed] [Google Scholar]
- 28.Belet N, Belet U, Incesu L, Uysal S, Ozinal S, Keskin T, et al. Hypoxic-ischemic encephalopathy:correlation of serial MRI and outcome. Pediatr Neurol. 2004 Oct;31(4):267–274. doi: 10.1016/j.pediatrneurol.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 29.Li AM, Chau V, Poskitt KJ, et al. White matter injury in term newborns with neonatalencephalopathy. Pediatr Res. 2009;65(1):85–89. doi: 10.1203/PDR.0b013e31818912d2. [DOI] [PubMed] [Google Scholar]
- 30.Martinez-Biarge M, Bregant T, Wusthoff CJ, Chew AT, Diez-Sebastian J, Rutherford MA, Cowan FM. White matter and cortical injury in hypoxic-ischemic encephalopathy: antecedent factors and 2-year outcome. J Pediatr. 2012;161(5):799–807. doi: 10.1016/j.jpeds.2012.04.054. [DOI] [PubMed] [Google Scholar]
- 31.Cowan F, Dubowitz L, Mercuri E, Counsell S, Rutherford M. White matter injury can lead tocognitive without major motor deficits following perinatal asphyxia and early encephalopathy. DevMed Child Neurol Suppl. 2003;93:45–14. [Google Scholar]
- 32.Shankaran S, Pappas A, McDonald SA, Vohr BR, Hintz SR, Yolton K, et al. Childhood outcomesafter hypothermia for neonatal encephalopathy. N Engl J Med. 2012 May 31;366(22):2085–2092. doi: 10.1056/NEJMoa1112066. [DOI] [PMC free article] [PubMed] [Google Scholar]