Abstract
Over the past decade, public awareness of the long-term pathological consequences of traumatic brain injury (TBI) has increased. Such awareness has been stimulated mainly by reports of progressive neurological dysfunction in athletes exposed to repetitive concussions in high-impact sports such as boxing and American football, and by the rising number of TBIs in war veterans who are now more likely to survive explosive blasts owing to improved treatment. Moreover, the entity of chronic traumatic encephalopathy (CTE)—which is marked by prominent neuropsychiatric features including dementia, parkinsonism, depression, agitation, psychosis, and aggression—has become increasingly recognized as a potential late outcome of repetitive TBI. Annually, about 1% of the population in developed countries experiences a clinically relevant TBI. The goal of this Review is to provide an overview of the latest understanding of CTE pathophysiology, and to delineate the key issues that are challenging clinical and research communities, such as accurate quantification of the risk of CTE, and development of reliable biomarkers for single-incident TBI and CTE.
Introduction
Approximately 1% of the population of developed countries experiences traumatic brain injury (TBI) each year.1 The pathological consequences of TBI2 have received increasing media attention following reports of progressive neurological dysfunction in athletes who have been exposed to repetitive concussions in high-impact sports (initially boxing and, in the past 8 years, American football), and the increasing number of war veterans presenting with TBI caused by explosive blasts. Improved battlefield emergency care and, consequently, improved survival means that soldiers who might previously have died from polytrauma now survive and can go on to develop substantial cognitive deficits. This principle applies most clearly to veterans of the recent wars in Iraq and Afghanistan. The US Department of Defense terms TBI the ‘signature injury’ among veterans from these wars.3
In this article, we provide an overview of the acute and long-term pathophysiology of single-incident and repetitive TBI, focusing in particular on chronic traumatic encephalopathy (CTE). We briefly discuss imaging as well as plasma and cerebrospinal fluid (CSF) biomarkers for this disorder, and finally consider preventive measures that can be undertaken to avoid such injury. Understanding of the issues discussed will assist clinical and research communities to manage the risk of CTE and avoid disease.
Single-incident TBI
Acute sequelae
Many complications of TBI are evident immediately or soon after the injury.4–14 Acute post-traumatic sensory, motor and neurocognitive syndromes often occur owing to brain contusions, intracerebral haemorrhage, and axonal shearing. Skull fracture further complicates the pathobiology, leading to direct brain shearing and haemorrhage if the fracture is severe or depressed, and increasing the risk of ischaemia from vasoconstriction precipitated by blood products, seizures and infection. Seemingly mild ‘closed-head’ TBI, in which the skull is not fractured, can lead to diverse and sometimes disabling symptoms such as chronic headaches, dizziness and vertigo, difficulty in concentrating, word-finding problems, depression, irritability and impulsiveness. The duration of such symptoms is variable, but can be months or longer.
Post-traumatic stress disorder (PTSD) is a frequent accompaniment of military-related TBI, especially severe cases.5–14 Each syndrome can occur without the other: PTSD can occur following events of severe stress, and TBI can occur, even in combat, without resulting in PTSD. Their relationship, and how to differentiate and treat both disorders, is a growing area of research.
TBI sustained during combat is usually associated with an explosion, such as from artillery or an improvised explosive device, and these blasts often propel solders against a wall or the interior of a vehicle, which can result in a deceleration head injury. Although differentiation between the injury from the blast and the deceleration head trauma might not always be possible, investigations are under way not only to track severity of head trauma in soldiers by using accelerometers in ‘smart helmets’, but also to potentially counteract harmful effects of the blast.
Long-term sequelae
Cause and effect relationships for the delayed sequelae of single-incident severe TBI are not well understood, owing to the variable latent period between injury and subsequent neurological and neuropsychiatric dysfunction. Some closed-head injuries involve only brief loss of consciousness—that is, mild TBI—but memory, affective and executive dysfunction can nevertheless emerge and cause substantial impairment and life disruption. Even when no overt damage is apparent on neuroimaging, such symptoms can linger for months, causing disruption of learning with subsequent poor academic or work performance.
Severe single-incident TBI, with or without skull fracture, can lead to permanent brain damage, with incomplete recovery and residual sensory, motor and cognitive deficits. Unlike mild repetitive TBI, discussed below, severe single-incident TBI is associated with increased risk of late-onset Alzheimer disease (AD).15–20 As the late consequences of TBI generate pathology that is reminiscent of AD or pure tauopathy, we posit that these two disorders have a shared pathogenesis. However, we must keep in mind that the pathogenesis of spontaneous or genetic AD or tauopathy per se might not be equivalent to that of post-traumatic neurodegeneration.
A recent study found that some neurodegenerative causes of death (namely, AD and amyotrophic lateral sclerosis) were four times higher in US National Football League players than in the general population.21 No such trend in the prevalence of Parkinson disease was noted. Paradoxically, the overall mortality rate of these retired players was substantially lower than that of the general population. Independent replication of the study should help to clarify this puzzling dissociation of outcomes. Notably, differential diagnosis of AD versus CTE on clinical grounds is not entirely reliable, which highlights a key knowledge gap in this area: no compelling epidemio logical data are available to enable estimation of the incidence or prevalence of these neuro degenerative sequelae of TBI. Differential diagnosis in the future is likely to improve with the advent of neuropathology imaging—that is, amyloid imaging, which is already in clinical use;22,23 and tauopathy imaging, which is showing promise24,25 but is still in development.
Sequence of pathological events
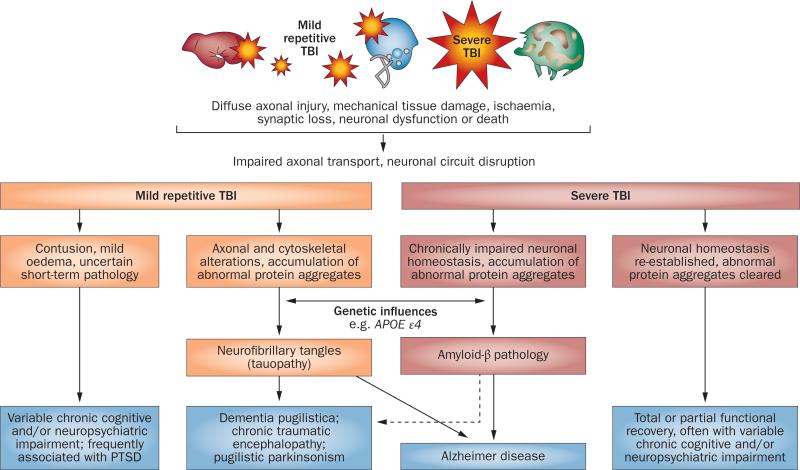
Extrapolating from the known pathological and clinical course of AD and other tauopathies, we can construct a hypothetical temporal sequence of events in TBI and CTE. The most frequently proposed mechanism in TBI is ‘diffuse axonal injury’ (Figure 1),which is associated with alterations in many physiological processes, and accumulation of abnormal protein aggregates in cells and the brain parenchyma.2 Altered proteolytic and proteostatic pathways are associated with changes that are evident at the histological level. Here, the similarity between pathways of idiopathic and post-traumatic neurodegeneration is most evident, in that both involve ac cumulation of apparently identical protein aggregates.
Figure 1.
Spectrum of pathological features and outcomes of mild and severe TBI. Abbreviations: APOE, apolipoprotein E; PTSD, post-traumatic stress disorder; TBI, traumatic brain injury.
Within hours of single-incident severe TBI in humans and experimental animals, dramatic accumulation of amyloid precursor protein is evident in damaged axons and cell bodies.26 Some studies in case series demonstrated that these changes are associated with increased brain concentrations of soluble amyloid-β42 (Aβ42) peptides and deposition of diffuse Aβ plaques in brain tissue in approximately 30% of patients with severe TBI, regardless of patient age (Figure 2).27–29 These plaques, which are predominantly Aβ42-immunoreactive, are similar to those observed in the earliest stages of AD pathology. An association between densities of such deposits and the apolipoprotein E (APOE) ε4 allele, which is in itself a risk factor for AD, has been established in boxers30,31 and patients with fatal head injuries.32 In addition, poly morphisms in the gene encoding neprilysin, an Aβ-degrading enzyme, probably influence the rate of Aβ degradation and clearance after TBI.33
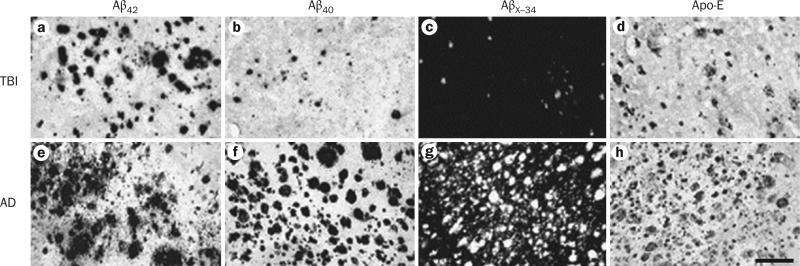
Figure 2.
Histopathology of the temporal cortex in TBI and AD. Brain sections from a–d | an individual with severe acute TBI and e–h | a patient with AD are labelled for Aβ42 (a,e), Aβ40 (b,f), AβX–34 (c,g), or Apo-E (d,h). In the TBI case, Aβ42 plaques (a) are more abundant than Aβ40 plaques (b). Frequency of plaques with AβX–34 (c) is closer to that of Aβ40, whereas distribution of Apo-E-positive plaques (d) is similar to Aβ42 plaques. The AD temporal cortex shows profusion of all plaque types (e–h), as well as neurofibrillary tangles, neuropil threads, and vascular amyloidosis (g). Scale bar: 200 μm. Abbreviations: Aβ, amyloid-β; AD, Alzheimer disease; Apo-E, apolipoprotein E; TBI, traumatic brain injury. Permission obtained from Elsevier Ltd © Ikonomovic, M. D. et al. Exp. Neurol. 190, 192–203 (2004).
Some patients with parkinsonism have a history of single-incident TBI.34,35 Genetic, environmental and physical factors presumably distinguish people who are destined to develop parkinsonism from those destined to manifest AD years after TBI. Notably, precise dose–response data linking injury severity with induction of pathology in sports associated with neurodegeneration are not available. Similarly, no sport-specific factors are known that can distinguish TBI associated with boxing versus American football, soccer, hockey and wrestling. Typically, TBI associated with these sports is combined under the rubric of ‘mild repetitive’ TBI.
Mild repetitive TBI
The classic clinicopathological correlation linking neuro cognitive effects of repetitive mild head injury with tauopathy was described in boxers by Corsellis.36,37 The pathology is distinct from the clinical and pathological sequelae of severe single-incident TBI. Landmark neuro pathology studies reported that dementia pugilistica, or ‘punch drunk’ syndrome, involves prominent tauo pathy, with patchy distribution of neurofibrillary tangles (NFTs) and neuropil threads throughout the neocortex.36–39 Diffuse Aβ plaques were present in 50–100% of cases depending on the series, whereas tauopathy was a universal finding across all case series.38,40
Dementia pugilistica
Dementia pugilistica typically involves NFT accumulation in the superficial grey matter, especially at the base of the sulci, with prominent perivascular and periventricular pathology. Subsequent immunohisto chemistry studies of the dementia pugilistica cases that were originally examined by Corsellis demonstrated that all cases had diffuse Aβ deposits (but not neuritic plaques) in addition to NFTs.38,39 The precise relationships between these lesions and clinical symptoms of dementia pugilistica is unknown, but may involve axonal injury, as traumatic tearing of neuronal connections (‘axonal shearing’, a component of diffuse axonal injury) is known to impair cortical and thalamic circuitry, thereby contributing to cognitive impairment and dementia.
As with single-incident TBI, a role for genetic factors in the development of AD neuropathology and dementia following mild repetitive TBI is emerging.30,41 The APOE ε4 allele is a well-established risk factor for amyloid plaque deposition and AD.42,43 Boxers who were APOE ε4 carriers were more likely to develop dementia pugilistica than those lacking the ε4 allele,30 suggesting that this allele confers greater vulnerability to brain pathology in trauma. Parkinsonism is also associated with dementia pugilistica. When rigidity and tremor dominate the clinical picture, the term ‘pugilistic parkin sonism’ is applied. An abundance of NFTs and the absence of Lewy bodies distinguishes pugilistic parkinsonism from idiopathic Parkinson disease. However, as in Parkinson disease, nigral neurons are lost in pugilistic parkinsonism, which probably underlies the clinical symptoms of this syndrome, although the precise mechanism is not known.
Chronic traumatic encephalopathy
In addition to studies in boxers, examination of the brains of several professional American football players and wrestlers has revealed an explanation for the little-discussed fact that football players who played for many years often suffered cognitive and neuropsychiatric decline in later life. Retired players with three or more reported concussions were found to have fivefold increased prevalence of mild cognitive disorders and threefold increased prevalence of substantial memory problems compared with healthy controls.44 Although later cognitive decline in long-time professional football players had been noted anecdotally for years, the first autopsy report on such a player appeared in the literature only recently.45 The pathology was indistinguishable from that of dementia pugilistica. This case had frequent diffuse neocortical Aβ plaques and sparse NFT pathology.45 To distinguish the aetiology from that of boxing, Omalu and DeKosky reinstated the term ‘chronic traumatic encephalopathy’,46,47 a term from the 1920s and 1950s that caught on with the popular press in the contexts of sports and military injury.
CTE is characterized by distinct patterns of Aβ and tau pathology, although the extent of pathology varies between CTE cases (Figures 2 and 3). McKee and colleagues48 noted that nearly 50% of CTE cases selected from the literature (on basis of the presence of NFT pathology) also had diffuse Aβ plaques, with nearly 30% of cases having classic AD (neuritic) plaques. Whereas boxing scoring includes records of knockouts, concussion histories in football players have been notoriously poor, owing to both absence of meticulous records and the culture of the sport, in which injuries are ignored by both players and coaching staff, to keep players in the game. The index case report of TBI in American football45 has since been followed by confirmatory case studies,49–52 which found that cognitive decline began years after cessation of play.
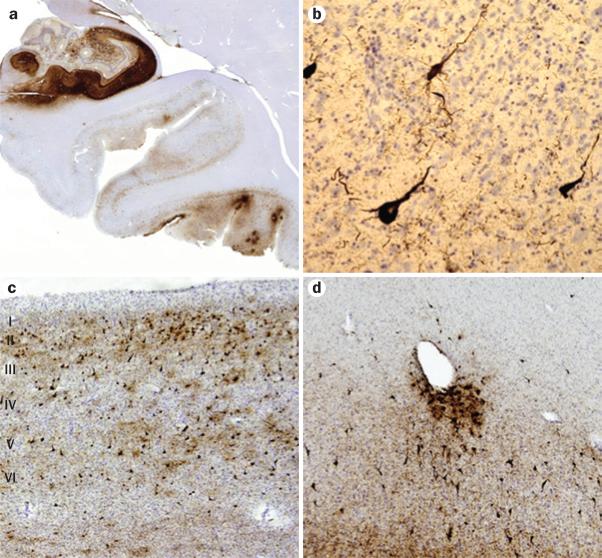
Figure 3.
Histopathological features of chronic traumatic encephalopathy in a former professional football player. All sections are immunostained for abnormally phosphorylated tau using an AT-8 monoclonal antibody that detects hyperphosphorylated tau (Ser202 and Thr205). a | Scanning view of the hippocampus and parahippocampal cortex. Note intense immunostaining of the entire Ammon horn and subiculum, with focal involvement at the depths of sulci of the inferior temporal lobe. Original magnification ×1. b | Appearance of individual neurofibrillary tangles in the neocortex. Original magnification ×160. c | Neurofibrillary tangles in the anterior insular cortex form preferentially in the superficial layers (layers II–III), rather than in deeper layers (layers V–VI) as is more common in Alzheimer disease. Original magnification ×30. d | Tendency for perivascular tau deposition and neurofibrillary tangle formation in the frontal cortex. Original magnification ×60. Permission obtained from the American Medical Association © Shively, S. et al. Arch. Neurol. 69, 1245–1251 (2012).
Cohort composition and player position might influence assessment and determination of the risk, diagnosis and mechanisms of CTE. In the ‘early days’ of professional football, players wore leather helmets, which were later replaced by plastic helmets with canvas strap-interiors and foam cushioning inside. Modern US football helmets, including face masks, have not prevented concussions or severe TBI with loss of consciousness. Modern technology involving ‘smart helmets’ that record the physics of impact will help us to understand and quantify such injuries.
Dementia pugilistica had been recognized for decades, whereas reports of a similar phenomenon in football emerged in the late 1990s and early 2000s. At the Professional Football Hall of Fame, long-standing players would gather every year, providing a unique opportunity to see high-level players from year to year. As the former players aged, increasing numbers of their members were noted to have memory problems. In addition to legal claims against the National Football League for the obvious orthopaedic injuries from playing football, claims for neurocognitive damage also began to emerge. One of the early players to sue for cognitive and neuropsychiatric injury was the first professional football player to undergo autopsy and can be regarded as the index case of sports-related CTE.44,45,53
Once the problem was identified, researchers and the National Football League, as well as other athletic organizing bodies, responded rapidly. Since the emergence of multiple cases from several independent pathology laboratories and clinics,40,47 and in other high-impact sports such as wresting,51 the phenomenon has been accepted by the medical and athletic authorities, with appropriate attempts to minimize its occurrence in contact sports. At least 12 former National Football League players have committed suicide over the past 25 years, many suffering from cognitive and affective symptoms. In two of the more recent suicides, the players shot themselves in the heart, which enabled their brains to be studied postmortem.53,55,56
Broad public awareness of CTE now exists. We have little idea, however, of the risk of developing CTE following TBI, or of the basis of interindividual differences in susceptibility to this disorder. APOE ε4 alleles30 might increase the risk of CTE in professional football players as they do in boxing, but conclusive evidence is lacking. Polymorphisms in the Aβ-degrading enzyme neprilysin have also been provisionally linked to CTE.33 The largest collected reports of CTE have not published genetic analyses. Coordinated national and international approaches to CTE research are needed.
CTE research centres could be modelled on the existing NIH Alzheimer Disease Centers and the Alzheimer Disease Neuroimaging Initiative (ADNI). Indeed, the US Department of Defense has recently initiated a ‘military TBI ADNI’, and the National Football League has teamed up with the NIH to launch new research into CTE. An important starting point will be lifelong TBI diary databases for those at highest risk for TBI and CTE, in order to obtain reliable estimates of the lifetime accumulation of TBI and lifetime risk of developing CTE. Not every boxer develops dementia pugilistica and not every football player develops CTE; given the current state of technology, whole-genome sequencing of patients with CTE could provide comprehensive genetic profiles of those who develop CTE as well as those who experience identical lifelong TBI histories but remain cognitively intact.
Many soldiers experience head trauma periodically in training. Omalu recently reported the index case of ‘military CTE’ in a veteran of the Iraq war who committed suicide,57 and suggested a link to the clinical PTSD phenotype, as mentioned above. These observations were recently confirmed and extended by an in dependent group of investigators.58
Biomarkers
Neuroimaging
Increasing evidence suggests that single-incident severe TBI and mild repetitive concussions are associated with a heterogeneous profile of neuropathological changes resembling those in chronic neurodegenerative disorders, including AD and Parkinson disease. Such a similarity supports the use of amyloid-binding PET ligands for in vivo monitoring of neuropathology after TBI. PET ligands for imaging of tauopathy and synucleinopathy are currently in development (Figure 4). Clinical diagnosis of AD can be aided by quantifying Aβ, tau, and phospho-tau levels in CSF,59 even in the absence of a positive amyloid PET scan.60 These biomarkers could be especially useful for differential diagnosis of CTE,61 as the Aβ deposits are primarily diffuse, and NFT distribution is different to that in AD and frontotemporal dementia. Prior to regulatory approval, these ligands must be validated against neuropathology to ensure that the imaging signal corresponds to the underlying amyloid or tau pathology, as already demonstrated for Pittsburgh compound B62 and florbetapir.23
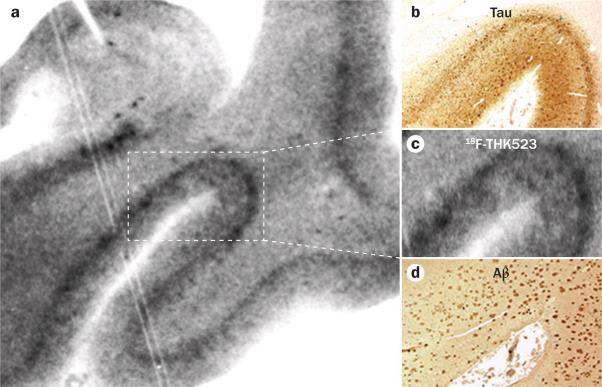
Figure 4.
18F-THK523 as a tauopathy marker. Autoradiography and microscopy analysis of hippocampal serial sections taken at autopsy from a 90-year-old patient with Alzheimer disease indicates that 18F-THK523 binds specifically to tau tangles, with no detectable binding to Aβ plaques. a | Low-magnification 18F-THK523 autoradiogram. b–d | Higher-magnification microscopy and autoradiogram images of three serial sections immunostained with antibodies to tau (b), which label neurofibrillary tangles; with 18F-THK523 (c); or with antibodies to Aβ (d), which label Aβ plaques. 18F-THK523 labelling seems to colocalize with tau immunostaining of neurofibrillary tangles, but not with plaques. Abbreviation: Aβ, amyloid-β. Permission obtained from Oxford University Press © Fodero-Tavoletti, M. T. et al. Brain 134, 1089–1100 (2011).
Body fluid biomarkers
The clinical diagnosis and evaluation of single-incident severe TBI, which involves the combination of clinical examination and MRI, is relatively straightforward. A substantial challenge for the clinician, however, is assessment of whether patients with milder forms of head trauma, and nonspecific symptoms such as dizziness and headache, have acquired permanent damage to the brain. Indeed, mild TBI can cause neuronal damage with selective swelling and disconnection of long axons in the white matter of the brain.63 Such challenges can be addressed by measurement of biochemical markers in body fluids, such as serum, plasma, urine or CSF.
Cerebrospinal fluid biomarkers
For brain disorders such as TBI, the advantage of CSF biomarkers is that the CSF is in direct contact with the brain extracellular fluid, which mirrors biochemical changes in the brain. As reviewed in another article in this issue, the most promising CSF biomarkers for acute TBI are axonal proteins, such as neurofilament light polypeptide and tau.61 The CSF levels of these proteins probably reflect the extent of axonal damage after acute TBI. Indeed, a marked increase in CSF total tau that correlates with clinical outcome is found in patients after severe TBI.64,65
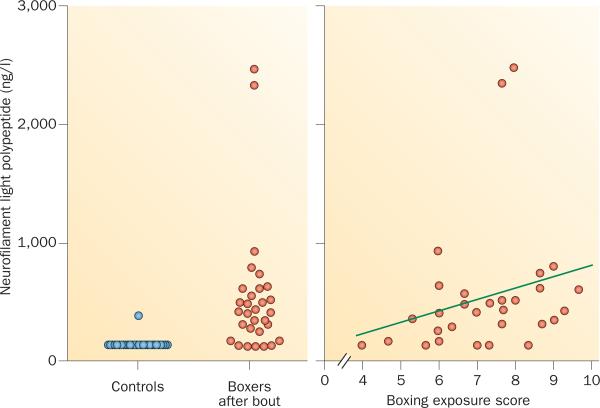
These biomarkers could be especially important in the evaluation of individuals with mild TBI such as boxers, who endure repeated TBI by head punches during sparring or bouts. Interestingly, a longitudinal study of amateur boxers showed a marked increase in levels of CSF neurofilament light polypeptide, together with a less pronounced increase in tau, after bouts.66 The increase in neurofilament light polypeptide was much more pronounced in boxers who sustained several head punches. These biomarker changes seem to be transitory, as CSF neurofilament light polypeptide levels returned to normal levels after a 3-month period of rest from bouts and sparring.67 Similar results have been reported in an independent study on Olympic boxers, confirming a direct correlation between increases in CSF biomarker levels and boxing exposure.68 These findings suggest that CSF levels of neurofilament light polypeptide and total tau could be valuable biomarkers for monitoring of axonal damage in boxers (Figure 5) and other TBI cases.
Figure 5.
CSF neurofilament light polypeptide in Olympic boxers after a bout. a | Individual CSF levels of neurofilament light polypeptide—a biomarker of axonal damage—in boxers after a bout and in age-matched controls. 25 of 30 (83%) of boxers had high CSF neurofilament light polypeptide levels, whereas 24 of 25 (96%) of controls had normal levels (below 125 ng/l), Wilcoxon signed rank test: P <0.001. b | Correlation between boxing exposure and CSF neurofilament light polypeptide levels. A lower score indicates fewer and easier fights, whereas higher scores indicate more, tougher fights with more head blows. Spearman's r = 0.40; P = 0.03. Abbreviation: CSF, cerebrospinal fluid. Figure is reproduced from Neselius, S. et al. PLoS ONE 7, e33606 (2012), which is published under an open-access license by the Public Library of Science.
Plasma biomarkers
Given that CSF samples must be obtained by invasive lumbar puncture, availability of biomarkers of brain damage that can be assayed in blood samples would be beneficial. Several protein biomarkers have been examined as potential blood markers of TBI.61 Of these, serum levels of the glial protein S-100B increased markedly in patients with severe TBI and correlated with clinical outcome.69,70 Reports are inconsistent, however, regarding the correlation between serum S-100B levels and intra cranial pathology as evaluated by CT or MRI scans, or clinical outcome in patients with mild TBI.69,70 A pilot study found an increase in plasma tau to more than 300% of control levels in amateur boxers following a bout.71 Tau levels in blood samples might, therefore, serve as a biomarker of mild TBI.
Several factors could contribute to the lack of plasma biomarkers of TBI: first, dilution of the brain-specific protein, both in the large volume of plasma and in the extracellular fluid of peripheral organs; second, degradation of the biomarker candidate by blood proteases; and third, clearance of the protein by hepatic metabolism or renal excretion. Furthermore, analyses of brain proteins in blood can be confounded by release of the same protein from peripheral tissues. For example, S-100B is released from fractured bones and injured skeletal muscles in patients with multiple trauma, thereby reducing the diagnostic power of this marker for TBI.69,70 Highly sensitive analytical techniques are needed to detect minute amounts of brain-specific proteins in peripheral blood as biomarkers for mild TBI. Measurement of tau protein in serum samples using a novel ultra-sensitive technique called digital ELISA, which involves single-molecule arrays, has recently been described.72 This assay has a limit of detection of 0.02 pg/ml, which is 1000-fold more sensitive than conventional immunoassays, and showed good performance in identifying brain damage in patients who had brain hypoxia following cardiac arrest.72 Studies are ongoing to examine whether serum levels of tau could serve as a blood biomarker of mild TBI.
Biomarker applications
Clinical studies of TBI biomarkers in professional contact sports such as boxing and martial arts, American foot ball, ice hockey and rugby, as well as in military personnel at risk of blast overpressure and direct traumatic head injuries, would be particularly valuable. Clinical evaluation and biomarker examination should ideally be performed at baseline (for example, before the football season or before military service in a combat zone) and after TBI. Such studies would provide important information both on pathogenic mechanisms in acute TBI and on the threshold of exposure that is needed to induce detectable neuronal damage.
CSF and other biomarkers of acute TBI could be important tools for medical counselling of concussed athletes and other risk groups, such as military personnel, providing both diagnostic information and prognostic guidance. Post-injury CSF biomarker levels might give information on the severity of axonal damage after a knockout or blast injury, and follow-up biomarker testing could be used to monitor when active brain-cell lysis has resolved—that is, when CSF biomarker levels return to normal—and, thereby, when athletes can return to their sport, or military personnel to service. Much research is needed before we come to this point in the medical care of patients with acute TBI. The ADNI study, mentioned above, provides an outstanding example of a multicentre, longitudinal study to understand pathogenic mechanisms, their interrelationships and their temporal evolution in AD, which could provide a useful model for future TBI research.73 These studies would not only provide data on the incidence and prevalence of chronic brain disorders, but also improve our understanding of the relationship between acute and long-term sequelae of repetitive TBI.
During life, clinical differentiation between delayed post-traumatic AD and CTE remains a challenge. CSF biomarker analysis or amyloid PET data are not currently available for autopsy-confirmed CTE cases, although the first case report of a negative amyloid scan in a retired National Football League player with the clinical diagnosis of probable CTE has recently been published.74 Current amyloid PET ligands detect fibrillar Aβ plaques, whereas imaging the diffuse Aβ deposits that are often part of CTE can be challenging. Advances have recently been made in PET imaging of tau, and accumulation of clinical experience with this imaging tool is expected to begin in 2013.24,25,75
Biomarkers would provide information on pathogenic mechanisms and the temporal evolution of different forms of pathology, which would also help to resolve the question of whether pathogenic mechanisms differ between dementia pugilistica and CTE. The temporal-sequence information will be highly informative for development and timing of medical interventions. Lastly, these studies would be important for formulation of widely accepted, evidence-based guideline s for the clinical diagnosis of CTE.
Regulation of contact sports
No treatment is available for dementia pugilistica or CTE, but these conditions—in contrast to most other neurodegenerative disorders—are preventable. In 2005, the World Medical Association (WMA) recommended banning boxing76 because of the basic intent of the sport to inflict bodily harm to the opponent. Several alternative ways to make the sport safer exist, including measures to reduce the number of head punches during a bout, use of protective equipment to limit the deleterious effects on the brain of repeated punches, and various approaches to prevent boxers from continuing a potentially dangerous fight, including education and action of the referees.
The tragic death of the professional boxer Kim Duk-Koo in 1982 owing to a subdural haematoma after being knocked out by Ray Mancini in round 14 served as an initiating factor to lower the number of rounds in professional boxing from 15 to 12. Following this change, a trend toward decreased mortality amongst professional boxers has been observed, although this trend might also be related to lower exposure to repetitive head trauma in this group of individuals, with shorter careers and fewer fights.77
Impact forces from punches have been studied experimentally, and can be comparable to the impact of a 6-kg bowling ball rolling at 20 mph.78 Boxing helmets and gloves reduce the impact from a punch.79 Protective headgear is mandatory in amateur boxing but not in professional boxing or mixed martial arts—a sport that is rising in popularity. Although helmets in sports reduce incidence of superficial facial injury and severe head injury, no clear evidence is available to show that they reduce the risk of concussion.80,81 Moreover, although protective equipment might reduce risk of CTE in amateur boxing, knockouts still occur, and around 20% of deaths due to brain damage occur in amateur boxing.82 Nevertheless, introduction of thicker gloves and headgear with thicker padding—as recommended by the WMA83—would probably reduce the risk of CTE and concussions.
Several international sports organizations, including the International Olympic Commission (IOC), the Fédération Internationale de Football Association, the International Rugby Board, and the International Ice Hockey Federation, have worked on consensus guidelines on sports-related concussion.81 These guidelines state that an athlete who shows any symptoms of concussion should be removed from play immediately and evaluated by a physician, and should not be allowed to return to play until symptoms have subsided.82–84 As the aim of boxing and martial arts sports is to inflict the opponent acute brain damage that is severe enough to win the bout by knockout, such guidelines are difficult to implement in these sports. Given that amateur boxing is an Olympic sport, this issue could present a delicate problem for the IOC.
Conclusions and future directions
The utility of the diagnosis of CTE remains to be studied and improved. Some researchers have questioned the identity of dementia pugilistica and CTE, given the apathetic clinical picture of the former in contrast to the frequently violent clinical picture of the latter (P. Davies, personal communication). Some individuals involved in organized sport remain unconvinced of the potential seriousness of the problem.85
Aβ plaques are more common in CTE than in acute TBI. The main distinguishing features of CTE are the disproportionate tauopathy relative to the amyloidosis and the concentration of CTE lesions at the base of the gyri. Research on TBI and CTE is aimed at elucidating the neuropathological basis of these differences.63 Laboratory modelling of TBI and CTE should facilitate elucidation of the cellular and molecular changes underlying these phenomena. The brain–skull–spine configurations of current rodent and swine models, however, are limited in their capacity to reflect the situation in humans, calling for design of improved models in other species. Despite this challenge, in the meantime, genetically modified rodent models provide powerful tools for delineation of TBI pathways. Several rodent models of CTE have been reported,58,86–93 but have yet to be confirmed. Using AD as a model for CTE progression, the acute phase is predicted to be largely Aβ-related, whereas the chronic phase may be more Aβ-independent and tauopathy-driven. Preliminary molecular pathology and experimental pharmacology data, however, suggest that at least some features of the AD model might or might not apply to TBI and CTE.87–91 Clearly, more research is required.
The incidence and prevalence of CTE and the role of genetic factors remains to be elucidated.94 Long-term follow-up of multiple cohorts of individuals, such as American football players and soldiers, who have sustained repetitive injuries of undefined number and severity, will be valuable for determination and replication of environmental, genetic and other risk factors that influence the natural history of TBI and CTE. TBI diaries will be helpful in determining the number and severity of head injuries, to allow estimation of cumulative risk of TBI to athletes.
Major clinical95 and neuroimaging96 efforts are under way, and these programmes will be facilitated by recent plans to place neurologists on the sidelines at National Football League games.97 These data can serve as models for prospective studies of the cognitive, neuropsychiatric and motor performance of soldiers, athletes and other exposed populations, and can inform behavioural and pharmacological interventions aimed at prophylaxis and therapy.
A future challenge will be translation of our improved understanding of TBI and CTE pathogenesis into rational, evidence-based changes in regulation of sports by governments and school boards that will minimize the public's exposure to TBI and its chronic neurodegenerative sequelae. In addition, the pathological cascades in TBI and CTE, in both acute and chronic stages, must be understood to enable identification of valid biomarkers and development of treatments.
Key points.
■ Traumatic brain injury (TBI) can lead to delayed-onset neurodegenerative syndromes that include Alzheimer disease (AD) and chronic traumatic encephalopathy (CTE)
■ CTE has gained attention owing to increasing media coverage of neuropsychiatric dysfunction in players of high-impact sport, such as boxing and American football
■ Brain pathology after single-incident severe TBI is similar to early amyloid pathology in AD, whereas repetitive TBI can produce tauopathy with or without amyloidosis that resembles pathology of boxers’ dementia
■ Estimation of the risk and prevalence of CTE remains challenging, and accurate prediction of TBI outcome and CTE risk for soldiers and players of high-impact sports is not yet possible
■ Several genetic risk factors for CTE have been proposed but remain to be established
■ Cerebrospinal fluid and neuroimaging biomarkers of TBI and CTE are emerging and hold promise for antemortem diagnosis of CTE, prediction of CTE risk, and monitoring of neuropathology progression
Review criteria.
Literature on dementia pugilistica and chronic traumatic brain injury published since 1950 was accessed via Internet-wide and database searches of both public and academic literature (that is, PubMed, PubMedCentral and MEDLINE). The search terms “brain trauma”, “brain injury”, and “dementia pugilistica” were used for broad searches, which were then narrowed by manual inspection. Reference lists from recent reviews of the topic were scanned for additional leads.
Acknowledgements
The authors gratefully acknowledge the support of NIH grants P01NS30318, P01AG14449 and P50AG05133 (S. T. DeKosky and M. D. Ikonomovic), and VA MERIT review grants 1I01BX000348 (S. Gandy) and 1I01RX000511 (M. D. Ikonomovic). S. Gandy also acknowledges the support of the Cure Alzheimer's Fund and of US NIH P50 AG05138. The authors thank P. Davies (North Shore-Hofstra) for helpful discussions.
Footnotes
Competing interests
The authors declare no competing interests.
Author contributions
All authors contributed to researching data for the article, discussion of the content, writing the article, and to review and/or editing of the manuscript before submission.
Contributor Information
Steven T. DeKosky, Office of the Dean and Department of Neurology, University of Virginia School of Medicine, P. O. Box 800793, Charlottesville, VA 22908, USA.
Kaj Blennow, Clinical Neurochemistry Laboratory, Institute of Neuroscience and Physiology, The Sahlgrenska Academy at University of Gothenburg, Sahlgrenska University Hospital, Mölndal SE-431 80, Sweden..
Milos D. Ikonomovic, Geriatric Research Educational and Clinical Center, VA Pittsburgh Healthcare System, and Departments of Neurology and Psychiatry, University of Pittsburgh School of Medicine, 200 Lothrop Street, Pittsburgh, PA 15213, USA.
Sam Gandy, Departments of Neurology and Psychiatry and Alzheimer's Disease Research Center, Icahn School of Medicine at Mount Sinai, James J. Peters Medical Center, New York, NY 10029, USA..
References
- 1.Centers for Disease Control and Prevention . Injury prevention and control: traumatic brain injury. Centers for Disease Control and Prevention; 2012. [online], http://www.cdc.gov/traumaticbraininjury/statistics.html. [Google Scholar]
- 2.DeKosky ST, Ikonomovic MD, Gandy S. Traumatic brain injury—football, warfare, and long-term effects. N. Engl. J. Med. 2010;363:1293–1296. doi: 10.1056/NEJMp1007051. [DOI] [PubMed] [Google Scholar]
- 3.US Department of Defense . Traumatic brain injury—Department of Defense special report. US Department of Defense; 2012. [online], http://www.defense.gov/home/features/2012/0312_tbi/ [Google Scholar]
- 4.Lange RT, Brickell TA, Ivins B, Vanderploeg R, French LM. Variable, not always persistent, postconcussion symptoms following mild TBI in U.S. military service members: a 5-year cross-sectional outcome study. J. Neurotrauma. doi: 10.1089/neu.2012.2743. http://dx.doi.org/10.1089/neu.2012.2743. [DOI] [PubMed]
- 5.Wall PL. Posttraumatic stress disorder and traumatic brain injury in current military populations: a critical analysis. J. Am. Psychiatr. Nurses Assoc. 2012;18:278–298. doi: 10.1177/1078390312460578. [DOI] [PubMed] [Google Scholar]
- 6.Capehart B, Bass D. Review: managing posttraumatic stress disorder in combat veterans with comorbid traumatic brain injury. J. Rehabil. Res. Dev. 2012;49:789–812. doi: 10.1682/jrrd.2011.10.0185. [DOI] [PubMed] [Google Scholar]
- 7.Vasterling JJ, et al. Neuropsychological outcomes of mild traumatic brain injury, post- traumatic stress disorder and depression in Iraq- deployed US Army soldiers. Br. J. Psychiatry. 2012;201:186–192. doi: 10.1192/bjp.bp.111.096461. [DOI] [PubMed] [Google Scholar]
- 8.Vanderploeg RD, et al. Health outcomes associated with military deployment: mild traumatic brain injury, blast, trauma, and combat associations in the Florida National Guard. Arch. Phys. Med. Rehabil. 2012;93:1887–1895. doi: 10.1016/j.apmr.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 10.Bazarian JJ, et al. The relation between posttraumatic stress disorder and mild traumatic brain injury acquired during operations enduring freedom and Iraqi freedom. J. Head Trauma Rehabil. 2013;28:1–12. doi: 10.1097/HTR.0b013e318256d3d3. [DOI] [PubMed] [Google Scholar]
- 11.Shively SB, Perl DP. Traumatic brain injury, shell shock, and posttraumatic stress disorder in the military—past, present, and future. J. Head Trauma Rehabil. 2012;27:234–239. doi: 10.1097/HTR.0b013e318250e9dd. [DOI] [PubMed] [Google Scholar]
- 12.Ruff RL, Riechers RG, 2nd, Wang XF, Piero T, Ruff SS. A case–control study examining whether neurological deficits and PTSD in combat veterans are related to episodes of mild TBI. BMJ Open. 2012;2:e000312. doi: 10.1136/bmjopen-2011-000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barnes SM, Walter KH, Chard KM. Does a history of mild traumatic brain injury increase suicide risk in veterans with PTSD? Rehabil. Psychol. 2012;57:18–26. doi: 10.1037/a0027007. [DOI] [PubMed] [Google Scholar]
- 14.Bogdanova Y, Verfaellie M. Cognitive sequelae of blast-induced traumatic brain injury: recovery and rehabilitation. Neuropsychol. Rev. 2012;22:4–20. doi: 10.1007/s11065-012-9192-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moretti L, et al. Cognitive decline in older adults with a history of traumatic brain injury. Lancet Neurol. 2012;11:1103–1112. doi: 10.1016/S1474-4422(12)70226-0. [DOI] [PubMed] [Google Scholar]
- 16.Shively S, Scher AI, Perl DP, Diaz-Arrastia R. Dementia resulting from traumatic brain injury: what is the pathology? Arch. Neurol. 2012;9:1–7. doi: 10.1001/archneurol.2011.3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sivanandam TM, Thakur MK. Traumatic brain injury: a risk factor for Alzheimer's disease. Neurosci. Biobehav. Rev. 2012;36:1376–1381. doi: 10.1016/j.neubiorev.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Esiri MM, Chance SA. Cognitive reserve, cortical plasticity and resistance to Alzheimer's disease. Alzheimers Res. Ther. 2012;4:7. doi: 10.1186/alzrt105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Norton MC, et al. Lifestyle behavior pattern is associated with different levels of risk for incident dementia and Alzheimer's disease: the Cache County study. J. Am. Geriatr. Soc. 2012;60:405–412. doi: 10.1111/j.1532-5415.2011.03860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson VE, Stewart W, Smith DH. Widespread τ and amyloid-β pathology many years after a single traumatic brain injury in humans. Brain Pathol. 2012;22:142–149. doi: 10.1111/j.1750-3639.2011.00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lehman EJ, Hein MJ, Baron SL, Gersic CM. Neurodegenerative causes of death among retired National Football League players. Neurology. 2012;79:1970–1974. doi: 10.1212/WNL.0b013e31826daf50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klunk WE, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann. Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 23.Clark CM, et al. Use of florbetapir-PET for imaging β-amyloid pathology. JAMA. 2011;305:275–283. doi: 10.1001/jama.2010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang W, et al. A highly selective and specific PET tracer for imaging of tau pathologies. J. Alzheimers Dis. 2012;31:601–612. doi: 10.3233/JAD-2012-120712. [DOI] [PubMed] [Google Scholar]
- 25.Chien DT, et al. Early clinical PET imaging results with the novel PHF-tau radioligand [F-18]-T807. J. Alzheimers Dis. doi: 10.3233/JAD-122059. http://dx.doi.org/10.3233/JAD-122059. [DOI] [PubMed]
- 26.Ciallella JR, et al. Changes in expression of amyloid precursor protein and interleukin-1β after experimental traumatic brain injury in rats. J. Neurotrauma. 2002;19:1555–1567. doi: 10.1089/089771502762300229. [DOI] [PubMed] [Google Scholar]
- 27.Ikonomovic MD, et al. Alzheimer's pathology in human temporal cortex surgically excised after severe brain injury. Exp. Neurol. 2004;190:192–203. doi: 10.1016/j.expneurol.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 28.DeKosky ST, et al. Association of increased cortical soluble Aβ42 levels with diffuse plaques after severe brain injury in humans. Arch. Neurol. 2007;64:541–544. doi: 10.1001/archneur.64.4.541. [DOI] [PubMed] [Google Scholar]
- 29.Nicoll JA, Roberts GW, Graham DI. Apolipoprotein E ε4 allele is associated with deposition of amyloid β-protein following head injury. Nat. Med. 1995;1:135–137. doi: 10.1038/nm0295-135. [DOI] [PubMed] [Google Scholar]
- 30.Jordan BD, et al. Apolipoprotein E ε4 associated with chronic traumatic brain injury in boxing. JAMA. 1997;278:136–140. [PubMed] [Google Scholar]
- 31.Jordan BD. Clinical spectrum of sports-related traumatic brain injury. Nat. Rev. Neurol. doi: 10.1038/nrneurol.2013.33. http://dx.doi.org/10.1038/nrneurol.2013.33. [DOI] [PubMed]
- 32.Horsburgh K, et al. β-amyloid (Aβ)42(43), aβ42, aβ40 and apoE immunostaining of plaques in fatal head injury. Neuropathol. Appl. Neurobiol. 2000;26:124–132. doi: 10.1046/j.1365-2990.2000.026002124.x. [DOI] [PubMed] [Google Scholar]
- 33.Johnson VE, et al. A neprilysin polymorphism and amyloid-β plaques after traumatic brain injury. J. Neurotrauma. 2009;26:1197–1202. doi: 10.1089/neu.2008.0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Factor SA, Weiner WJ. Prior history of head trauma in Parkinson's disease. Mov. Disord. 1991;6:225–229. doi: 10.1002/mds.870060306. [DOI] [PubMed] [Google Scholar]
- 35.Rugbjerg K, Ritz B, Korbo L, Martinussen N, Olsen JH. Risk of Parkinson's disease after hospital contact for head injury: population based case–control study. BMJ. 2008;337:a2494. doi: 10.1136/bmj.a2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corsellis JA, Brierley JB. Observations on the pathology of insidious dementia following head injury. J. Ment. Sci. 1959;105:714–720. doi: 10.1192/bjp.105.440.714. [DOI] [PubMed] [Google Scholar]
- 37.Corsellis JA. Boxing and the brain. BMJ. 1989;298:105–109. doi: 10.1136/bmj.298.6666.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roberts GW, Allsop D, Bruton C. The occult aftermath of boxing. J. Neurol. Neurosurg. Psychiatry. 1990;53:373–378. doi: 10.1136/jnnp.53.5.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tokuda T, Ikeda S, Yanagisawa N, Ihara Y, Glenner GG. Re-examination of ex-boxers’ brains using immunohistochemistry with antibodies to amyloid β-protein and tau protein. Acta Neuropathol. 1991;82:280–285. doi: 10.1007/BF00308813. [DOI] [PubMed] [Google Scholar]
- 40.McKee AC, et al. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J. Neuropathol. Exp. Neurol. 2009;68:709–735. doi: 10.1097/NEN.0b013e3181a9d503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gandy S, DeKosky ST. APOE ε4 status and traumatic brain injury on the gridiron or the battlefield. Sci. Transl. Med. 2012;4:134ed4. doi: 10.1126/scitranslmed.3004274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gandy S. The role of cerebral amyloid β accumulation in common forms of Alzheimer disease. J. Clin. Invest. 2005;115:1121–1129. doi: 10.1172/JCI25100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gandy S, DeKosky ST. Toward the treatment and prevention of Alzheimer's disease: rational strategies and recent progress. Annu. Rev. Med. 2013;64:367–383. doi: 10.1146/annurev-med-092611-084441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guskiewicz KM, et al. Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurgery. 2005;57:719–726. doi: 10.1093/neurosurgery/57.4.719. [DOI] [PubMed] [Google Scholar]
- 45.Omalu BI, et al. Chronic traumatic encephalopathy in a National Football League player. Neurosurgery. 2005;57:128–134. doi: 10.1227/01.neu.0000163407.92769.ed. [DOI] [PubMed] [Google Scholar]
- 46.Yi J, Padalino DJ, Chin LS, Montenegro P, Cantu RC. Chronic traumatic encephalopathy. Curr. Sports Med. Rep. 2013;12:28–32. doi: 10.1249/JSR.0b013e31827ec9e3. [DOI] [PubMed] [Google Scholar]
- 47.Clark EC, Harper EO. Electroencephalographic findings in 186 cases of chronic post traumatic encephalopathy. Electroencephalogr. Clin. Neurophysiol. 1951;3:9–14. doi: 10.1016/0013-4694(51)90049-1. [DOI] [PubMed] [Google Scholar]
- 48.McKee AC, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136:43–64. doi: 10.1093/brain/aws307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Omalu BI, Hamilton RL, Kamboh MI, DeKosky ST, Bailes J. Chronic traumatic encephalopathy (CTE) in a National Football League Player: case report and emerging medicolegal practice questions. J. Forensic Nurs. 2010;6:40–46. doi: 10.1111/j.1939-3938.2009.01064.x. [DOI] [PubMed] [Google Scholar]
- 50.Omalu BI, Bailes J, Hammers JL, Fitzsimmons RP. Chronic traumatic encephalopathy, suicides and parasuicides in professional American athletes: the role of the forensic pathologist. Am. J. Forensic Med. Pathol. 2010;31:130–132. doi: 10.1097/PAF.0b013e3181ca7f35. [DOI] [PubMed] [Google Scholar]
- 51.Omalu BI, Fitzsimmons RP, Hammers J, Bailes J. Chronic traumatic encephalopathy in a professional American wrestler. J. Forensic. Nurs. 2010;6:130–136. doi: 10.1111/j.1939-3938.2010.01078.x. [DOI] [PubMed] [Google Scholar]
- 52.Omalu B, et al. Emerging histomorphologic phenotypes of chronic traumatic encephalopathy in American athletes. Neurosurgery. 2011;69:173–183. doi: 10.1227/NEU.0b013e318212bc7b. [DOI] [PubMed] [Google Scholar]
- 53.Dwyre B. Dave Duerson's suicide could be a turning point for the NFL. Los Angeles Times; Feb 21, 2011. http://articles.latimes.com/2011/feb/21/sports/la-sp-dwyre-20110222. [Google Scholar]
- 54.Nelson E, Sherwood R. Chris Benoit's murder, suicide: was brain damage to blame? ABC News; Aug 26, 2010. http://abcnews.go.com/Nightline/chris-benoits-dad-son-suffered-severe-brain-damage/story?id=11471875. [Google Scholar]
- 55.Tierny M. Football player who killed himself had brain disease. B16. New York Times; Jul 27, 2012. [Google Scholar]
- 56.Kounang N, Smith S. Seau had brain disease that comes from hits to head, NIH finds. CNN; Jan 11, 2013. http://www.cnn.com/2013/01/10/health/seau-brain-disease/index.html. [Google Scholar]
- 57.Omalu B, et al. Chronic traumatic encephalopathy in an Iraqi war veteran with posttraumatic stress disorder who committed suicide. Neurosurg. Focus. 2011;31:E3. doi: 10.3171/2011.9.FOCUS11178. [DOI] [PubMed] [Google Scholar]
- 58.Goldstein LE, et al. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci. Transl. Med. 2012;14:134ra60. doi: 10.1126/scitranslmed.3003716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fagan AM, et al. Cerebrospinal fluid tau/β-amyloid42 ratio as a prediction of cognitive decline in nondemented older adults. Arch. Neurol. 2007;64:343–349. doi: 10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- 60.Cairns NJ, et al. Absence of Pittsburgh compound B detection of cerebral amyloid β in a patient with clinical, cognitive, and cerebrospinal fluid markers of Alzheimer disease: a case report. Arch. Neurol. 2009;66:1557–1562. doi: 10.1001/archneurol.2009.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zetterberg H, Smith DH, Blennow K. Biomarkers of mild traumatic brain Injury in cerebrospinal fluid and blood. Nat. Rev. Neurol. doi: 10.1038/nrneurol.2013.9. http://dx.doi.org/10.1038/nrneurol.2013.9. [DOI] [PMC free article] [PubMed]
- 62.Ikonomovic MD, et al. Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer's disease. Brain. 2008;131:1630–1645. doi: 10.1093/brain/awn016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Povlishock JT, Becker DP, Cheng CL, Vaughan GW. Axonal change in minor head injury. J. Neuropathol. Exp. Neurol. 1983;42:225–242. doi: 10.1097/00005072-198305000-00002. [DOI] [PubMed] [Google Scholar]
- 64.Franz G, et al. Amyloid beta 1–42 and tau in cerebrospinal fluid after severe traumatic brain injury. Neurology. 2003;60:1457–1461. doi: 10.1212/01.wnl.0000063313.57292.00. [DOI] [PubMed] [Google Scholar]
- 65.Öst M, et al. Initial CSF total tau correlates with 1-year outcome in patients with traumatic brain injury. Neurology. 2006;67:1600–1604. doi: 10.1212/01.wnl.0000242732.06714.0f. [DOI] [PubMed] [Google Scholar]
- 66.Zetterberg H, et al. Neurochemical aftermath of amateur boxing. Arch. Neurol. 2006;63:1277–1280. doi: 10.1001/archneur.63.9.1277. [DOI] [PubMed] [Google Scholar]
- 67.Neselius S, et al. CSF-biomarkers in Olympic boxing: diagnosis and effects of repetitive head trauma. PLoS ONE. 2012;7:e33606. doi: 10.1371/journal.pone.0033606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kövesdi E, et al. Update on protein biomarkers in traumatic brain injury with emphasis on clinical use in adults and pediatrics. Acta Neurochir. (Wien) 2010;152:1–17. doi: 10.1007/s00701-009-0463-6. [DOI] [PubMed] [Google Scholar]
- 69.Naeimi ZS, Weinhofer A, Sarahrudi K, Heinz T, Vecsei V. Predictive value of S-100B protein and neuron specific-enolase as markers of traumatic brain damage in clinical use. Brain Inj. 2006;20:463–468. doi: 10.1080/02699050600664418. [DOI] [PubMed] [Google Scholar]
- 70.Anderson RE, Hansson LO, Nilsson O, Dijlai-Merzoug R, Settergren G. High serum S100B levels for trauma patients without head injuries. Neurosurgery. 2001;48:1255–1258. doi: 10.1097/00006123-200106000-00012. [DOI] [PubMed] [Google Scholar]
- 71.Neselius S, et al. Olympic boxing is associated with elevated levels of the neuronal protein tau in plasma. Brain Inj. doi: 10.3109/02699052.2012.750752. (in press) [DOI] [PubMed] [Google Scholar]
- 72.Randall J, et al. Tau proteins in serum predict neurological outcome after hypoxic brain injury from cardiac arrest: results of a pilot study. Resuscitation. doi: 10.1016/j.resuscitation.2012.07.027. http://dx.doi.org/10.1016/j.resuscitation.2012.07.027. [DOI] [PubMed]
- 73.Weiner MW, et al. The Alzheimer's Disease Neuroimaging Initiative: a review of papers published since its inception. Alzheimers Dement. 2012;8(Suppl. 1):S1–S68. doi: 10.1016/j.jalz.2011.09.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mitsis E, et al. Florbetapir scanning excludes Alzheimer's disease in a retired NFL played with delayed cognitive impairment.. Presented at the 7 th Human Amyloid Imaging Conference; http://www.scribd.com/doc/117537451/HAI-2013-ConferenceBook#outer_page_83. [Google Scholar]
- 75.Fodero-Tavoletti MT, et al. 18F-THK523: a novel in vivo tau imaging ligand for Alzheimer's disease. Brain. 2011;134:1089–1100. doi: 10.1093/brain/awr038. [DOI] [PubMed] [Google Scholar]
- 76.World Medical Association . WMA statement on boxing. WMA; 2013. [online], http://www.wma.net/en/30publications/10policies/b6/index.html. [Google Scholar]
- 77.Baird LC, et al. Mortality resulting from head injury in professional boxing. Neurosurgery. 2010;67:1444–1450. doi: 10.1227/NEU.0b013e3181e5e2cd. [DOI] [PubMed] [Google Scholar]
- 78.Atha J, Yeadon MR, Sandover J, Parsons KC. The damaging punch. Br. Med. J. 1985;291:1756–1757. doi: 10.1136/bmj.291.6511.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bartsch AJ, Benzel EC, Miele VJ, Morr DR, Prakash V. Boxing and mixed martial arts: preliminary traumatic neuromechanical injury risk analyses from laboratory impact dosage data. J. Neurosurg. 2012;116:1070–1080. doi: 10.3171/2011.12.JNS111478. [DOI] [PubMed] [Google Scholar]
- 80.Jako P. Safety measures in amateur boxing. Br. J. Sports Med. 2002;36:394–395. doi: 10.1136/bjsm.36.6.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McCrory P, et al. Consensus statement on concussion in sport—the Third International Conference on Concussion in Sport held in Zurich, November 2008. Phys. Sportsmed. 2009;37:141–159. doi: 10.3810/psm.2009.06.1721. [DOI] [PubMed] [Google Scholar]
- 82.Svinth JR. Death Under the Spotlight: The Manuel Velazquez Boxing Fatality Collection. Electronic Journals of Martial Arts and Sciences. 2007 [online], http://ejmas.com/jcs/jcsartsvinth_a_0700.htm.
- 83.Miele VJ, Bailes JE. Objectifying when to halt a boxing match: a video analysis of fatalities. Neurosurgery. 2007;60:307–315. doi: 10.1227/01.NEU.0000249247.48299.5B. [DOI] [PubMed] [Google Scholar]
- 84.Cantu RC. Return to play guidelines after a head injury. Clin. Sports Med. 1998;17:45–60. doi: 10.1016/s0278-5919(05)70060-0. [DOI] [PubMed] [Google Scholar]
- 85.Epstein D. Conclusions? Too early. Sports Illustrated. 2013 Jan 21; http://sportsillustrated.cnn.com/vault/article/magazine/MAG1206738/index.htm.
- 86.Abrahamson EE, Ikonomovic MD, Dixon CE, DeKosky ST. Simvastatin therapy prevents brain trauma-induced increases in β-amyloid peptide levels. Ann. Neurol. 2009;66:407–414. doi: 10.1002/ana.21731. [DOI] [PubMed] [Google Scholar]
- 87.Loane DJ, et al. Amyloid precursor protein secretases as therapeutic targets for traumatic brain injury. Nat. Med. 2009;15:377–379. doi: 10.1038/nm.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tran HT, Sanchez L, Brody DL. Inhibition of JNK by a peptide inhibitor reduces traumatic brain injury-induced tauopathy in transgenic mice. J. Neuropathol. Exp. Neurol. 2012;71:116–129. doi: 10.1097/NEN.0b013e3182456aed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Magnoni S, et al. Tau elevations in the brain extracellular space correlate with reduced amyloid-β levels and predict adverse clinical outcomes after severe traumatic brain injury. Brain. 2012;135:1268–1280. doi: 10.1093/brain/awr286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tran HT, Sanchez L, Esparza TJ, Brody DL. Distinct temporal and anatomical distributions of amyloid-β and tau abnormalities following controlled cortical impact in transgenic mice. PLoS ONE. 2011;6:e25475. doi: 10.1371/journal.pone.0025475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tran HT, LaFerla FM, Holtzman DM, Brody DL. Controlled cortical impact traumatic brain injury in 3xTg-AD mice causes acute intra- axonal amyloid-β accumulation and independently accelerates the development of tau abnormalities. J. Neurosci. 2011;29:9513–9525. doi: 10.1523/JNEUROSCI.0858-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Garman RH, et al. Blast exposure in rats with body shielding is characterized primarily by diffuse axonal injury. J. Neurotrauma. 2011;28:947–959. doi: 10.1089/neu.2010.1540. [DOI] [PubMed] [Google Scholar]
- 93.Ojo JO, et al. Repetitive mild traumatic brain injury augments tau pathology and glial activation in aged hTau mice. J. Neuropathol. Exp. Neurol. 2013;72:137–151. doi: 10.1097/NEN.0b013e3182814cdf. [DOI] [PubMed] [Google Scholar]
- 94.Blennow K, Hardy J, Zetterberg H. The neuropathology and neurobiology of traumatic brain injury. Neuron. 2012;76:886–899. doi: 10.1016/j.neuron.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 95.Smith S. NFL players association, Harvard planning $100 million player study. CNN; Jan 30, 2013. http://www.cnn.com/2013/01/29/health/nfl-harvard-study/index.html. [Google Scholar]
- 96.Battista J. NFL joins with GE in effort to detect concussions. SP6. New York Times; Feb 3, 2013. [Google Scholar]
- 97.NFL expects to soon have neurological consultants on sidelines. National Football League. 2013 Jan 31; [No authors listed] [online], http://www.nfl.com/news/story/0ap1000000133506/article/nfl-expects-to-soon-have-neurological-consultants-on-sidelines.