Supplemental Digital Content is Available in the Text.
In a cohort of Medicare beneficiaries who underwent lumbar spinal fusion, we investigated the association of recombinant human bone morphogenic protein (rhBMP) and risk of subsequent cancer. After a mean follow-up of 4.7 years, rhBMP was not associated with overall cancer incidence or risk of individual tumor types.
Keywords: recombinant human bone morphogenic protein-2, spinal fusion, neoplasms, Medicare, aged, SEER Program
Abstract
Study Design.
Retrospective cohort study among Medicare beneficiaries with lumbar spinal fusion surgery.
Objective.
To determine the risk of subsequent cancer among patients who received recombinant human bone morphogenic protein (rhBMP) at surgery compared with those who did not.
Summary of Background Data.
rhBMP is commonly used to promote bone union after spinal surgery. BMP receptors are present on multiple cancer types, but the risk of cancer after receiving rhBMP has not been well studied.
Methods.
We identified 146,278 subjects aged 67 years and older who underwent surgery in 2003 to 2008 and were followed through 2010 for a new diagnosis of 1 of 26 cancers. Proportional hazards models were used to determine cancer risk associated with rhBMP use.
Results.
rhBMP was administered in 15.1% of the cohort. After an overall average follow-up of 4.7 years, 15.4% of rhBMP-treated and 17.0% of untreated patients had a new cancer diagnosis, with most commonly recorded types as prostate, breast, lung, and colorectal. In a multivariate proportional hazards model, there was no association of rhBMP with cancer risk (hazard ratio: 0.99, 95% confidence interval: 0.95–1.02). There was also no association of rhBMP with the risk of any individual cancer types. The results were consistent in analyses using 2 secondary definitions of incident cancer.
Conclusion.
In this large population-based analysis of Medicare beneficiaries, we found no evidence that administration of rhBMP at the time of lumbar fusion surgery was associated with cancer risk.
Level of Evidence: 4
The bone morphogenic proteins (BMPs) are a member of a large family of growth factors known as the transforming growth factor-β superfamily.1 Because of their ability to induce new bone formation, BMPs are used clinically as a substitute for iliac crest bone grafting in patients undergoing lumbar fusion surgery. One of these proteins, recombinant human bone morphogenetic protein-2 (rhBMP-2), is licensed in Europe and the United States for anterior lumbar spinal fusion and is delivered via an absorbable collagen sponge carrier. The combination of rhBMP-2 with absorbable collagen sponge is marketed as INFUSE Bone Graft (Medtronic Inc., Memphis, TN). A second product, rhBMP-7, is mixed with bovine collagen and reconstituted with saline and administered as a paste.
In addition to their effect on bone formation, BMPs also have roles in cell lineage commitment, differentiation, proliferation, and apoptosis, and receptors are present in multiple cell types, including cancer cells. A large number of laboratory-based in vitro and in vivo studies have examined the role of BMP in promoting tumorigenesis and metastasis and have yielded conflicting results.2
In the initial published clinical trials of the long-term safety of rhBMP, there seemed to be no association of rhBMP with subsequent cancer risk.3,4 However, because a postmarketing analysis indicated a nonsignificantly increased risk of pancreatic cancer in patients who received rhBMP, we previously performed a retrospective cohort study in the Medicare population.5 Although this analysis found no association of rhBMP with subsequent pancreatic cancer incidence, the study was restricted to 1 tumor type and had a relatively short duration of postsurgical follow-up. In addition, 2 recently published analyses of clinical trial data reported a higher rate of cancers in the rhBMP-treated patients than in those who had undergone bone grafts,6,7 but only 1 found the differences to be statistically significant.7 Given the discordant findings, our goal was to compare the incidence of all cancers after lumbar spinal fusion among a population-based sample of patients treated with rhBMP with the incidence among those who did not receive rhBMP.
MATERIALS AND METHODS
Patients
The cohort was obtained from all Medicare beneficiaries who underwent lumbar fusion surgery between October 2003 (first month in which Medicare provided reimbursement for rhBMP) and December 2008. The relevant files included the Medicare Provider Analysis and Review files, which included claims from inpatient hospitals; the Carrier file, which included claims from physicians and freestanding ambulatory surgical centers; and the Outpatient file, which included claims from institutional outpatient providers. Patients were identified if they had a procedure code for a lumbar fusion operation by one of the following International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) or Current Procedural Terminology, Fourth Edition (CPT-4) codes: ICD-9-CM 81.06, 81.07, 81.08, 81.36, 81.37, 81.38; CPT-4 22558, 22630, 22612.
To obtain complete claims history, patients were excluded if they were not continuously enrolled in fee-for-service Medicare for at least 2 years prior to the index surgery date. Patients who did not continuously participate in Medicare part B, which provides coverage for physician charges and outpatient services, were also excluded because their claims histories may have been incomplete. In addition, patients younger than 67 years were excluded—those younger than 65 years who were enrolled in Medicare because of end-stage renal disease or chronic disability not being representative of the general Medicare population and those aged 65 or 66 years with less than 2 years of enrollment data prior to the spinal surgery.
In addition, to exclude prevalent cases of cancer, as well as the inability to differentiate a newly treated cancer from the treatment of cancer recurrence, any patient with a claim indicating a previous malignant tumor diagnosis during the 2-year period prior to surgery was excluded. A 2-year cutoff was used to maximize the sensitivity of capturing and excluding patients who are long-term cancer survivors. Previous malignant neoplasm diagnoses were identified from one or more ICD-9-CM diagnosis codes in any file. Patients were also excluded if they had one or more ICD-9 diagnosis codes indicating a “personal history of a malignant neoplasm” (V10.00–10.9) or had one or more codes for radiation or chemotherapy.
Measures
Consistent with our previous analysis,5 a claim for rhBMP (ICD-9-CM 84.52) on the same day as fusion surgery was used as a surrogate for exposure, which cannot be ascertained directly using Medicare data. This code also includes the administration of rhBMP-7, but the overwhelming majority of procedures use rhBMP-2. Because Medicare did not provide additional reimbursement for these products until October 2003, to reduce exposure misclassification, we limited our study to patients who underwent fusion surgery from this date onward.
A diagnosis of a malignant neoplasm after surgery was the major outcome of interest and was identified by one or more of the ICD-9-CM codes listed in any of the Medicare files in follow-up (see Supplemental Digital Content Appendix 1, available at http://links.lww.com/BRS/A801). We included codes consistent with any of the 26 cancer types that are included in the National Cancer Institute's Surveillance Epidemiology and End Results (SEER) classification,8 and followed patients through the end of calendar year 2010. This time interval was consistent with that of 2 recently published systematic reviews and meta-analyses6,7 and would be more than adequate to detect a potential effect of rhBMP in promoting the growth of subclinical tumors.
Because a single code may not be valid and may reflect “rule out” or other diagnoses that were ultimately found to represent benign diseases, as in our pancreatic cancer analysis, we used 2 secondary definitions of cancer. These included (1) an ICD-9-CM diagnosis code for the same type of cancer on more than 1 date of service and (2) 2 or more ICD-9 diagnosis codes for the same type of cancer on different dates of service and at least 1 procedure code consistent with cancer therapy. The latter codes included site-specific procedure codes (see Supplemental Digital Content Appendix 1, available at http://links.lww.com/BRS/A801) as well as procedure codes for radiation therapy and chemotherapy. Per Centers for Medicare & Medicaid Services policy, because of patient confidentiality issues, any cell sizes with a frequency less than 11 were suppressed.
In addition to data about exposure and outcomes, we included potential confounders such as age (at the time of index surgery), sex, race (Caucasian, African American, other), and length of follow-up. The presence of comorbid conditions was measured using a previously validated index, which includes diagnoses present in Medicare Provider Analysis and Review, Outpatient, and Carrier files.9 In order to differentiate complications from comorbidities, only diagnoses that were present from 2 years through 30 days prior to date of surgery were included. Using this algorithm, a weighted score was assigned for each individual.
Analysis
Patients were followed from the date of index lumbar fusion surgery until the diagnosis of cancer, death, disenrollment, or end of the study period (December 31, 2010). Individuals who underwent an initial operation without rhBMP and a subsequent procedure with rhBMP were followed in the nonexposed group to the date of the second surgery and thereafter in the exposed group.
The association of demographic variables, comorbid conditions, and rhBMP administration with each cancer site and with overall cancer risk was examined using the primary cancer definition (one or more diagnoses). Chi-square analysis was used to determine statistical significance. In addition, to account for variable length of follow-up, a series of univariate Cox proportional hazards models were constructed to examine the association of rhBMP administration and risk of individual cancer types.
The independent association of rhBMP administration and cancer risk was then determined in multivariable analyses using Cox proportional hazards regression. In all models, covariates included demographic factors (age, race, and sex, if appropriate for that cancer), comorbidity score, and rhBMP administration.
Finally, to determine whether the observed incidence of cancer was different than expected from the general population, we used the standardized incidence ratio (SIR). The SIR determines the number of observed cases divided by the number of expected cases in both rhBMP-exposed and unexposed patient groups. The expected numbers were obtained by applying age- and sex-specific incidence rates for all cancers from the SEER Program8 to the corresponding person-time.
RESULTS
We initially identified 295,493 patients who underwent lumbar spinal fusion during the time period of interest. From that sample, patients were excluded for the following nonmutually exclusive reasons: not continuously enrolled in Medicare parts A and B (n = 69,398), enrolled in Medicare health maintenance organizations (n = 53,107), age less than 67 years (n = 56,699), and previous cancer diagnosis (n = 29,765). The remaining 146,278 patients were the subject of this analysis.
Characteristics of the cohort are shown in Table 1. The mean age was 74.5 ± 5.1 years, 66.5% were female, and 93.7% were Caucasian. Most patients had comorbidity scores of 0 or 1. A code for rhBMP administration was documented in 15.1% of surgical procedures. Compared with others, patients who received rhBMP were younger, somewhat more likely to be female or Caucasian, and had higher comorbidity scores. The proportion of patients who received rhBMP generally increased during the study period. The average length of follow-up was 4.8 ± 1.5 years (range: 1.23–7.25 yr) in the rhBMP-treated patients and 4.4 ± 1.3 years (range: 1.16–7.25 yr) in others. Death rates during the follow-up period were 3.27% in the rhBMP group and 3.45% in others.
TABLE 1. Characteristics of Patients According to Use of BMP.
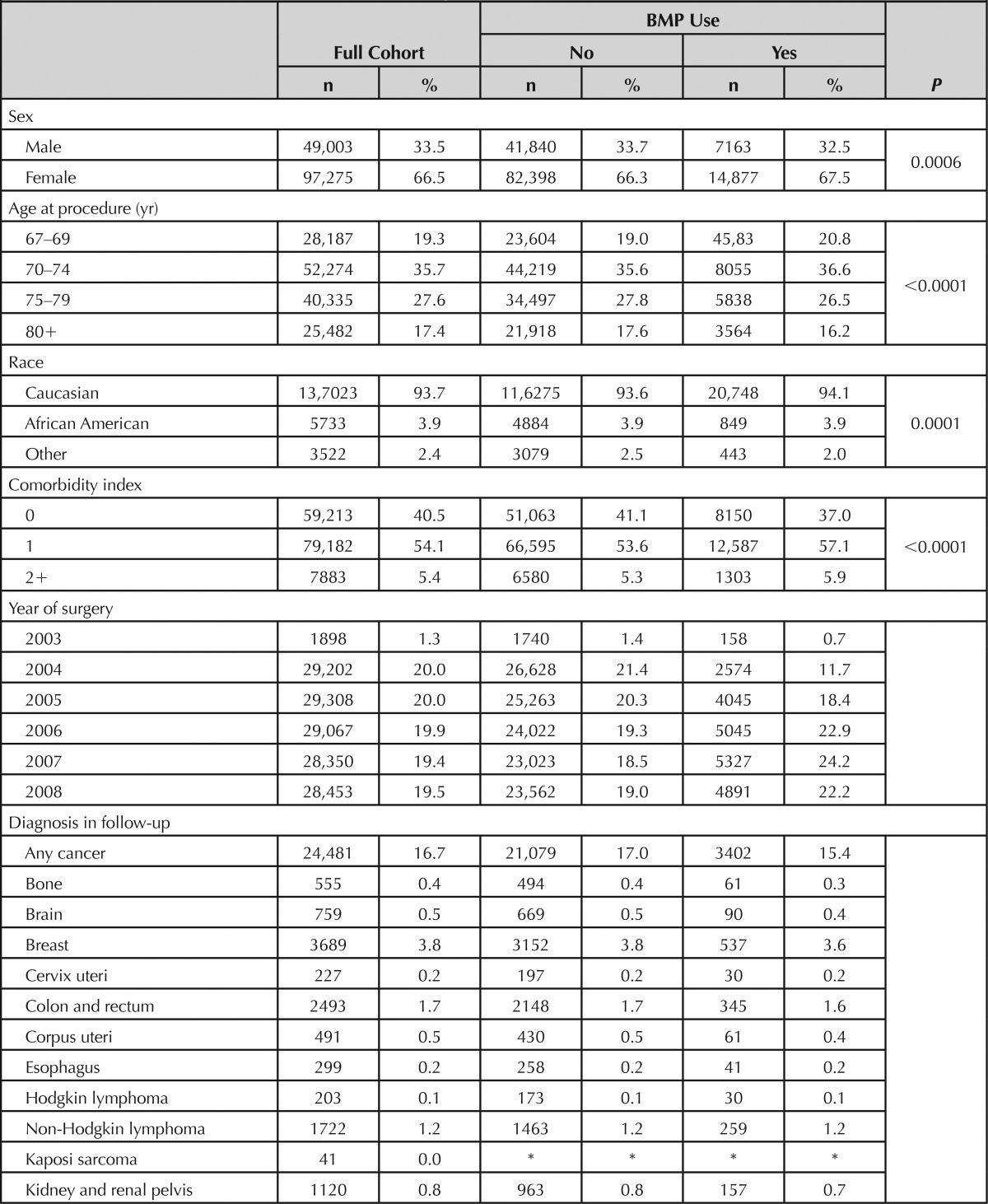
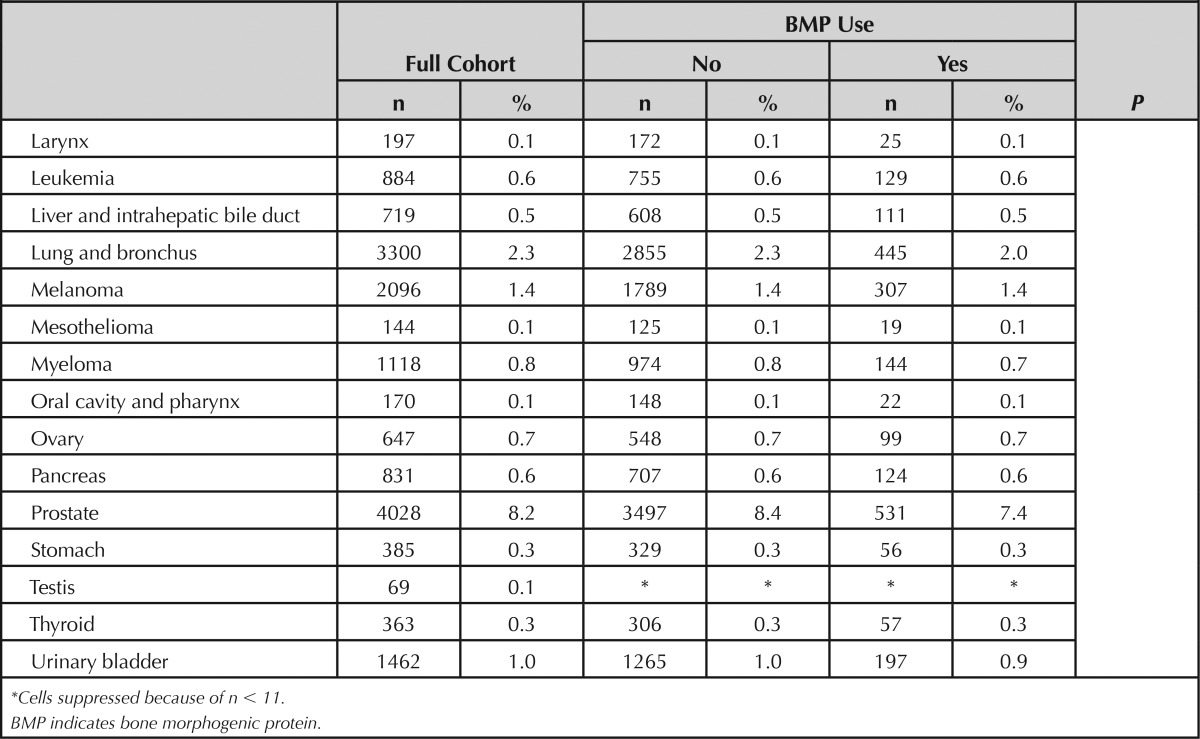
One or more diagnosis codes for cancer were documented in follow-up in 24,481 patients including 21,079 in the non-rhBMP group (17.0%) and 3402 in the rhBMP-treated patients (15.4%). Consistent with the known incidence of cancers in the older US population,8 the most commonly recorded cancer diagnoses were prostate, breast, lung, and colon and rectum.
Using a proportional hazards model, we determined the risk of cancer as a whole and within individual tumor types (Table 2). Overall, there was no association of rhBMP administration with cancer incidence (hazard ratio: 0.98, 95% confidence interval [CI]: 0.95–1.02). Similarly, when individual cancer sites were considered, there were no significant differences between the 2 groups. In an adjusted analysis, the risk of cancer was similar between rhBMP-treated patients and others (hazard ratio: 0.99, 95% CI: 0.96–1.03). As with the unadjusted analysis, there were no significant differences among specific cancer sites.
TABLE 2. Risk of Cancer Associated With Bone Morphogenic Protein Administration in Unadjusted and Adjusted Models.
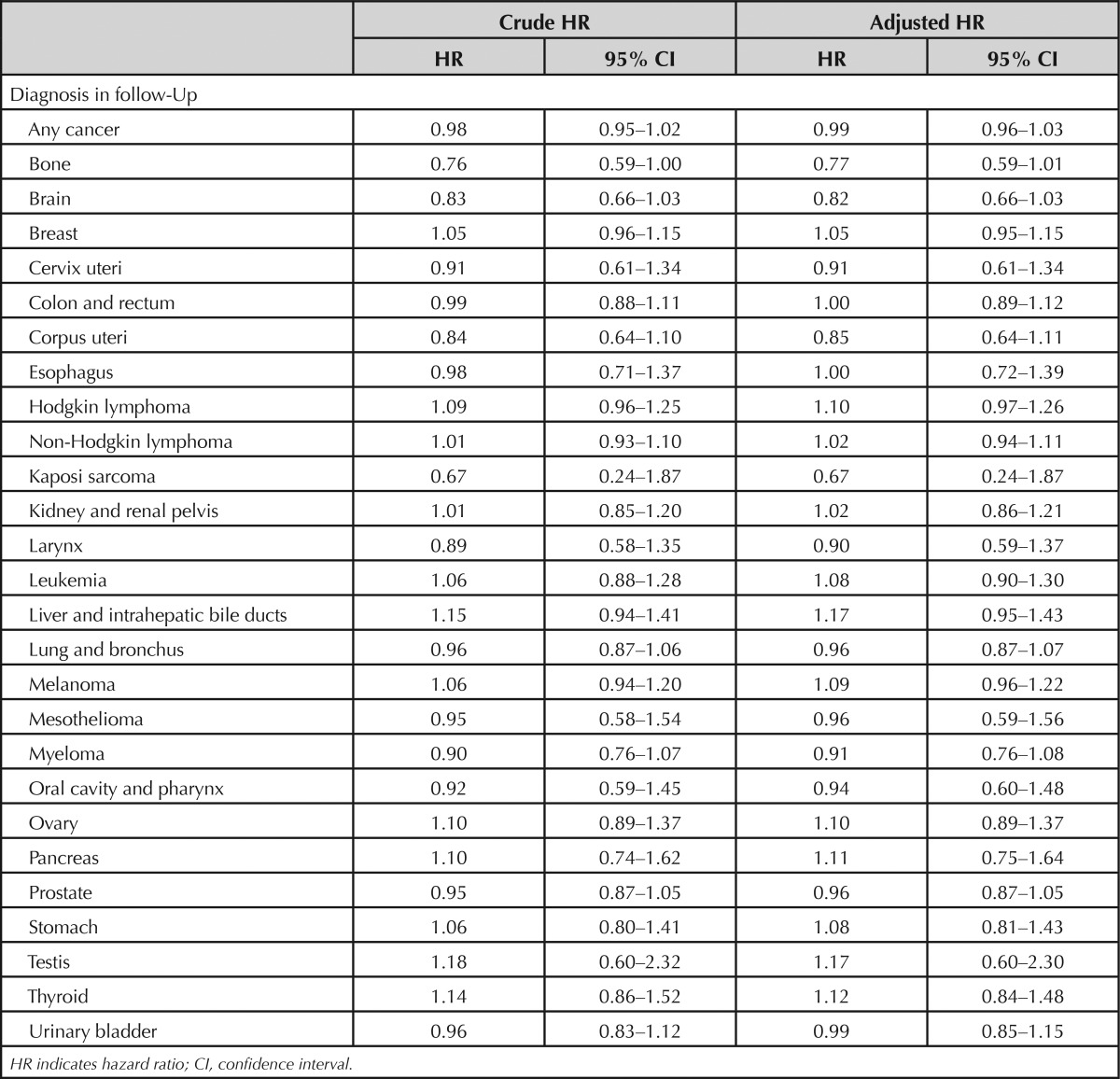
In a secondary analysis, we considered 2 other definitions of incident cancer. Using a criterion of a diagnosis on 2 or more different dates, we identified 18,942 cancer cases, with similar frequencies in rhBMP-treated (12.0%) and other patients (13.1%) (see Supplemental Digital Content Appendix 2, available at http://links.lww.com/BRS/A801). The overall cancer risk was similar in unadjusted (hazard ratio: 0.97, 95% CI: 0.93–1.01) and adjusted (hazard ratio: 0.98, 95% CI: 0.94–1.02) proportional hazards models. The risk was also similar among individual tumor types. Using the most stringent definition of 2 or more diagnoses and cancer treatment codes, we identified 14,362 cases with an incidence of 8.7% and 10.0% in rhBMP-treated and untreated patients, respectively (see Supplemental Digital Content Appendix 3, available at http://links.lww.com/BRS/A801). With this definition, there was a somewhat lower overall cancer risk with rhBMP use in both unadjusted (hazard ratio: 0.94, 95% CI: 0.89–0.98) and adjusted (hazard ratio 0.95, 95% CI: 0.90–0.99) proportional hazards analysis. When individual sites were examined, there was a lower risk of brain tumors in rhBMP-treated patients in unadjusted (hazard ratio: 0.65, 95% CI: 0.46–0.90) and adjusted (hazard ratio: 0.64, 95% CI: 0.46–0.90) models. No other significant differences were observed.
In the SIR calculations, using the primary case definition of 1 diagnosis code, the incidence of all cancers combined was higher than expected in both the rhBMP-treated and untreated groups. For rhBMP-treated patients, the SIR was 177.79 (95% CI: 172.00–185.00) and for untreated patients, the SIR was 177.00 (95% CI: 175.00–179.00). Consistent with more stringent diagnostic criteria, the SIRs were lower for both rhBMP-treated and untreated patients, using the other 2 case definitions. For a diagnosis code on 2 or more service dates, the SIR in the treated patients was 135.26 (95% CI: 130.00–141.00) and in untreated patients was 136.30 (95% CI: 134.00–138.00). For the criteria of 2 or more diagnosis codes as well as treatment, the SIRs closely approximated that of SEER. The SIR in rhBMP-treated patients was 96.99 (95% CI: 92.00–101.00) and in untreated patients was 101.66 (95% CI: 100.00–103.00).
DISCUSSION
rhBMP is commonly used as an adjunct to orthopedic surgical procedures to promote bone growth and is used as an alternative option to bone grafting. Although most safety reports have focused on local events such as bony overgrowth, wound healing, and neurological events,3,10–12 there is at least a theoretical concern about the increased risk of malignant tumors among patients treated with rhBMP. Cancer case ascertainment through analysis of clinical trial data is limited by issues of power and sample size as well as relatively short duration of follow-up. In this study, which included a population-based sample of a large number of surgical patients with a median follow-up of more than 4 years, we did not demonstrate an increased risk of malignancy across multiple tumor sites.
In theory, given the presence of BMP receptors of a variety of tumors, including lung, pancreatic, renal, brain, osteosarcoma, ovarian, breast, and prostate cancers,2 there is a potential risk of BMP in promoting tumorigenesis as well as metastases. In different models, BMP promotes angiogenesis,13,14 cancer cell growth,15 bone metastases,16 and cancer cell motility and invasiveness.16 However, BMP can also act as a growth and proliferation inhibitor and thus have antineoplastic effects.16,17 The only individual tumor type for which we found a significant difference in incidence was brain tumors, where there was a somewhat higher incidence in patients who were not treated with rhBMP-2. This finding is consistent with previous studies that showed an antitumor effect of rhBMP-2.18,19 Although clinical trials to date have failed to show a conclusively increased risk of malignancy, given the theoretical risk of cancer progression, rhBMP is not indicated in the vicinity of a resected or extant tumor in patients with active malignancy or undergoing treatment of malignancy.20 Our study results included the entire spectrum of SEER cancers and a relatively long follow-up period. However, if a potential risk of BMP is in mutagenesis, a longer observation period may be required to provide additional clinical evidence against the malignant potential of rhBMP.
As with other analyses, our methodology has a number of strengths and limitations. The strengths of the study include the large sample size, the representation of diverse practice sites, and the ability to follow patients for an average of 4.7 years after spinal surgery. Limitations include that the sample was restricted to patients aged 67 years and older, and generalizability of the findings to younger patients is uncertain. However, most cancers increase in incidence with age, and the older population accounts for a significant proportion of patients undergoing spinal fusion surgery. The study was also limited to fee-for-service beneficiaries who were enrolled in Medicare part B. The diagnoses of previous and subsequent cancer were ascertained through ICD-9-CM codes, which are used for billing purposes and not research. However, the results were consistent across 3 different definitions of incident cancer, increasing the face validity of the findings. The study lacked data on other risk factors such as smoking, alcoholism, obesity, and family history of cancer, which are either significantly under-reported or absent in Medicare data. As the study was observational and not randomized, there may have been systematic differences between groups that could have biased the results. However, in our previous analysis of pancreatic cancer risk,5 we found on medical record review that there was no association of rhBMP use with established cancer risk factors such as smoking and obesity. Despite the large sample size, the study lacked precision to measure the incidence of rare cancers in the elderly such as testicular cancer and Kaposi sarcoma. Finally, because we used a procedure code as a proxy for exposure to rhBMP-2, misclassification could be a concern. However, a previously conducted chart review study demonstrated a specificity of the code for rhBMP-2 (as opposed to other forms of rhBMP) of 95% and a positive predictive value of 100%.5
In summary, in this large cohort of older patients, the study provides evidence that treatment with rhBMP at the time of lumbar spinal fusion surgery does not increase the risk of subsequent malignancy. The results should be reassuring to providers and patients.
Key Points
Use of rhBMP has been postulated to increase the risk of subsequent cancer.
In a population-based sample of Medicare beneficiaries undergoing lumbar spinal fusion, we found no association of rhBMP administration with subsequent risk of cancer.
The lack of risk of cancer was consistent across all tumor types and different definitions of cancer incidence.
Supplementary Material
Acknowledgments
Supplemental digital content is available for this article. Direct URL citations appearing in the printed text are provided in the HTML and PDF version of this article on the journal's web site (www.spinejournal.com).
Footnotes
Address correspondence and reprint requests to Gregory S. Cooper, MD, Division of Gastroenterology, University Hospitals Case Medical Center, 11100 Euclid Ave, Wearn 244, Cleveland, OH 44106; E-mail: Gregory.cooper@UHhospitals.org
The device(s)/drug(s) is/are FDA approved or approved by corresponding national agency for this indication.
Medtronic, Inc. grant funds were received to support this work.
Relevant financial activities outside the submitted work: grant.
References
- 1.Rihn JA, Gates C, Glassman SD, et al. The use of bone morphogenic protein in lumbar spine surgery. J Bone Joint Surg Am 2008;90:2014–25. [PubMed] [Google Scholar]
- 2.Thawani JP, Wang AC, Than KD, et al. Bone morphogenic proteins and cancer: review of the literature. Neurosurgery 2010;66:233–46. [DOI] [PubMed] [Google Scholar]
- 3.Benglis D, Wang MY, Levi AD. A comprehensive review of the safety profile of bone morphogenic protein in spine surgery. Neurosurgery 2008;62:ONS423–31. [DOI] [PubMed] [Google Scholar]
- 4.Poynton AR, Lane JM. Safety profile for the clinical use of bone morphogenic proteins in the spine. Spine 2002;27(suppl):S40–8. [DOI] [PubMed] [Google Scholar]
- 5.Mines D, Gu Y, Kou TD, et al. Recombinant human bone morphogenic protein-2 and pancreatic cancer: a retrospective cohort study. Pharmacoepid Drug Saf 2011;20:111–8. [DOI] [PubMed] [Google Scholar]
- 6.Simmonds MC, Brown JVE, Heirs MK, et al. Safety and effectiveness of recombinant human bone morphogenic protein-2 for spinal fusion. Ann Intern Med 2013;158:877–89. [DOI] [PubMed] [Google Scholar]
- 7.Fu R, Selph S, McDonagh M, et al. Effectiveness and harms of recombinant human bone morphogenic protein-2 in spine fusion. Ann Intern Med 2013;158:890–902. [DOI] [PubMed] [Google Scholar]
- 8.Howlader N, Noone AM, Krapcho M, et al., eds. SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations). Bethesda, MD: National Cancer Institute; 2011. http://seer.cancer.gov/csr/1975_2009_pops09/. SEER data submission, posted to the SEER Web site. Accessed April 2012.
- 9.Klabunde CN, Warren JL, Legler JM. Assessing comorbidity using claims data. Med Care 2002;40(suppl):26–35. [DOI] [PubMed] [Google Scholar]
- 10.Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J 2011;11:471–91. [DOI] [PubMed] [Google Scholar]
- 11.Valdes MA, Thakur NA, Namdari S, et al. Recombinant bone morphogenic protein-2 in orthopaedic surgery: a review. Arch Orthop Trauma Surg 2009;129:1651–7. [DOI] [PubMed] [Google Scholar]
- 12.Glassman SD, Howard J, Dimar J, et al. Complications with recombinant human bone morphogenic protein-2 in posterolateral spine fusion. Spine 2011;36:1849–54. [DOI] [PubMed] [Google Scholar]
- 13.Langenfeld EM, Langenfeld J. Bone morphogenic protein-2 stimulates angiogenesis in developing tumors. Mol Cancer Res 2004;2:141–9. [PubMed] [Google Scholar]
- 14.Bieniasz M, Oszajca K, Eusebio M, et al. The positive correlation between gene expression of the two angiogenic factors: VEGF and BMP-2 in lung cancer patients. Lung Cancer 2009;66:319–26. [DOI] [PubMed] [Google Scholar]
- 15.Kleeff J, Maruyama H, Ishiwata T, et al. Bone morphogenic protein 2 exerts diverse effects on cell growth in vitro and is expressed in human pancreatic cancer in vivo. Gastroenterology 1999;116:1202–16. [DOI] [PubMed] [Google Scholar]
- 16.Singh A, Morris RJ. The Yin and Yang of bone morphogenic proteins in cancer. Cytokine Growth Factor Rev 2010;21:299–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soda H, Raymond E, Sharma S, et al. Antiproliferative effects of recombinant human bone morphogenic protein-2 on human tumor colony-forming units. Anticancer Drugs 1998;9:327–31. [DOI] [PubMed] [Google Scholar]
- 18.Piccirillo SG, Reynolds BA, Zanetti N, et al. Bone morphogenic proteins inhibit the tumorigenic potential of human brain tumor-initiating cells. Nature 2006;444:761–5. [DOI] [PubMed] [Google Scholar]
- 19.Hallahan AR, Pritchard JI, Chandraratna RA, et al. BMP-2 mediates retinoid-induced apoptosis in medulloblastoma cells through a paracrine effect. Nat Med 2003;9:1033–8. [DOI] [PubMed] [Google Scholar]
- 20.Infuse Bone Graft: Brief summary of indications, contraindications and warnings. https://www.infusebonegraft.com/infuse_indications.html. Accessed July 29, 2013.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


