Abstract
Background
Atrial fibrillation (AF) is the most common cardiac dysrhythmia and contributes significantly to health care expenditures. We sought to assess the frequency and predictors of hospitalization in patients with AF.
Methods
The ORBIT-AF registry is a prospective, observational study of outpatients with AF enrolled from June 29, 2010, to August 9, 2011. The current analysis included 9,484 participants with 1-year follow-up. Multivariable, logistic regression was used to identify baseline characteristics that were associated with first cause-specific hospitalization.
Results
Overall, 31% of patients with AF studied (n = 2,963) had 1 or more hospitalizations per year and 10% (n = 983) had 2 or more. The most common hospitalization cause was cardiovascular (20 per 100 patient-years vs 3.3 bleeding vs 17 noncardiovascular, nonbleeding). Compared with those not hospitalized, hospitalized patients were more likely to have concomitant heart failure (42% vs 28%, P < .0001), higher mean CHADS2 (1 point for congestive heart failure, hypertension, age ≥75, or diabetes; 2 points for prior stroke or transient ischemic attack) scores (2.5 vs 2.2, P < .0001), and more symptoms (baseline European Heart Rhythm Association class severe symptoms 18% vs 13%, P < .0001). In multivariable analysis, heart failure (adjusted hazard ratio [HR] 1.57 for New York Heart Association III/IV vs none, P < .0001), heart rate at baseline (adjusted HR 1.11 per 10-beats/min increase >66, P < .0001), and AF symptom class (adjusted HR 1.37 for European Heart Rhythm Association severe vs none, P < .0001) were the major predictors of incident hospitalization.
Conclusions
Hospitalization is common in outpatients with AF and is independently predicted by heart failure and AF symptoms. Improved symptom control, rate control, and comorbid condition management should be evaluated as strategies to reduce health care use in these patients.
Atrial fibrillation (AF) is the most common adult tachyarrhythmia, and its prevalence is projected to at least double over the next 30 years.1 In addition to significant morbidity and mortality,2,3 AF consequences include the expenditure of significant health care resources. It is estimated that annual care for AF exceeds $6 billion in the United States.4 Most of these costs result from inpatient care5 and are likely to grow. Some data suggest that AF hospitalizations have increased to overtake myocardial infarction (MI) and heart failure as the most common cause of cardiovascular admission globally.6-9
Although AF admissions are common, few studies have assessed all-cause and cause-specific hospitalization rates among US patients with prevalent AF. The objectives of the current study are as follows: (1) to assess the burden of hospitalization in patients with prevalent AF, (2) to describe the cause-specific rates of hospitalization, and (3) to identify baseline factors that significantly predict cause-specific hospitalization in US patients with AF.
Methods
We used data from the ORBIT-AF study to assess hospitalization burden in patients with AF. The ORBIT-AF study is a registry of US outpatients with AF, managed by primary care physicians, cardiologists, and/or electrophysiologists. An adaptive design was used to recruit a nationally representative sample of sites, with heterogeneity of geography and provider type. Site management and study coordination were performed by the Duke Clinical Research Institute. Sites enrolled consecutive patients, 18 years or older, with electrocardiographically documented AF. Patients were followed up every 6 months for at least 2 years and could not be included if AF was due to a reversible cause (eg, in the setting of cardiac surgery or hyperthyroidism) or if life expectancy was less than 6 months. A Web-based case report form was used to gather data, and the primary sources were the patients' medical record and treating physician.
Data elements included patient demographics, medical history, components of AF history (including prior treatment and symptoms), medical therapies, vital signs, laboratory and imaging measures, and incident procedures and adverse events. Detailed medication data were collected, including oral anticoagulation use, monitoring, and international normalized ratio (INR) data (time in therapeutic range [TTR] is calculated based on the Rosendaal method).10 End points included major adverse cardiovascular events (death, MI, revascularization, stroke or systemic embolism, new heart failure, intervention for AF, cardiac device implantation, or cardioversion), bleeding events, and cause-specific hospitalization.
The present analysis includes 1-year data from ORBIT-AF, and all patients with at least 1 follow-up visit were included.
Statistical methods
Classification of hospitalizations
The total number of hospitalizations during follow-up in the study cohort was tabulated. Hospitalization cause was determined by the site investigator and categorized as cardiovascular, bleeding, or other (noncardiovascular, nonbleeding). All-cause mortality according to hospitalization burden was also calculated. Additional details of the ORBIT-AF design and rationale have been previously described.11.
Occurrence of first, cause-specific hospitalization (number of first hospitalizations per 100 patient-years) is presented overall and, for subgroups, by age, sex, heart failure status, and CHADS2 score (1 point for congestive heart failure, hypertension, age ≥ 75, or diabetes; 2 points for prior stroke or transient ischemic attack). To compare rates within each subgroup, we present P values using a Wald-test.
Characteristics of patients hospitalized
Patients were stratified by burden of hospitalization: those with no hospitalizations and those with any hospitalization during the follow-up period. Baseline characteristics were compared between these groups. Continuous variables are presented as medians (Q1-Q3), and differences across 2 groupswere assessed using the Wilcoxon rank sum test. Categorical variables are presented as counts (proportions), and differences across hospitalization status were assessed using the χ2 test.
To summarize the end points of (a) time to first all-cause hospitalization, (b) time to first cardiovascular hospitalization, (c) time to first bleeding hospitalization, (d) time to first other hospitalization, we display the number of patients at risk and the probability of no hospitalization using a Kaplan-Meier plot. Similarly, probability of survival is shown by hospitalization burden (none, any, 1, >1) using a Kaplan-Meier plot.
In order to identify predictors of hospitalization in patients with AF, we created multivariable models for the end points of (a) time to first all-cause hospitalization and (b) time to first cardiovascular hospitalization. These results are presented as risk estimates (hazard ratio [HR]) and corresponding 95% CI and P values using Cox regression with the robust sandwich covariance estimates (accounting for correlation in the same site). The final regression models for first all-cause and first cardiovascular hospitalization were developed in the first imputed dataset based on selected risk factors from candidate baseline characteristics using backward selection, with an α for exclusion of .05. All continuous variables were tested for linearity, and nonlinear relationships were accounted for using linear splines. Missing covariate data in the regression analysis (<15%) were handled by multiple imputation using Markov Chain Monte Carlo and regression methods. Final estimates and associated SEs reflect the combined analysis for 5 imputed data sets. Discrimination of models was evaluated using c-index: values close to 1 indicate that the model correctly identifies patients at greater risk compared with those at decreased risk.
All patients provided written, informed consent, and the ORBIT-AF registry was approved by the Duke University Institutional Review Board. Each site also obtained institutional review board approval pursuant to local regulations. All analyses of the aggregate, de-identified data were performed by the Duke Clinical Research Institute using SAS software (version 9.3; SAS Institute, Cary, NC).
Results
The overall ORBIT-AF population included 10,132 patients from 174 sites. After excluding 648 patients who did not have 6- or 12-month follow-up, this yielded a study population of 9,484 patients from 174 sites, enrolled from June 29, 2010, to August 9, 2011. During the follow-up period, these patients experienced a total of 4,548 hospitalizations; 69% had no hospitalization, 21% had exactly 1 hospitalization, and 10.4% had 2 or more hospitalizations during the follow-up period (Figure 1). Overall, 2,963 (31%) patients had at least 1 hospitalization during follow-up; baseline characteristics, stratified by occurrence of any hospitalization, are shown in Table I. Patients hospitalized were slightly older (median 76 vs 75 years, P < .0001), tended to be female (44% vs 42%, P = .046), and were more likely to have a government payer (Medicare/Medicaid 73% vs 69%, P = .0002). Overall, patients who experienced hospitalization were more likely to have significant cardiovascular risk factors and noncardiovascular disease or comorbidities. Compared with patients not hospitalized, those hospitalized were more likely to have concomitant cardiovascular disease, including peripheral vascular disease, coronary artery disease, and cerebrovascular disease (P < .0001 for each).
Figure 1.
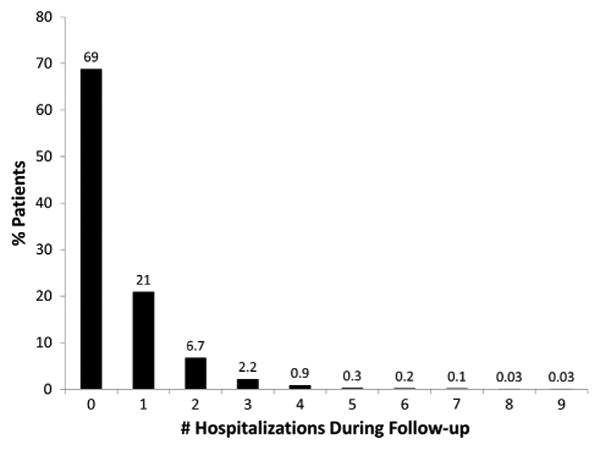
Distribution of number of hospitalizations at 1 year of follow-up.
Table I. Baseline characteristics, by incident hospitalization.
| Overall (n = 9484) |
No hospitalization (n = 6521) |
Any hospitalization (n = 2963) |
P | |
|---|---|---|---|---|
| Age (y) | 75 (67–82) | 75 (67–81) | 76 (68–82) | <.0001 |
| Female | 43 | 42 | 44 | .046 |
| Race | .17 | |||
| White | 90 | 89 | 91 | |
| Black or African American | 4.7 | 4.8 | 4.6 | |
| Hispanic | 4.1 | 4.3 | 3.6 | |
| Other | 1.4 | 1.5 | 1.2 | |
| Health insurance status | .0002 | |||
| Medicare or Medicaid | 70 | 69 | 73 | |
| Private | 25 | 26 | 22 | |
| Other | 4.7 | 4.8 | 4.4 | |
| Hypertension | 83 | 82 | 85 | .0006 |
| Hyperlipidemia | 73 | 72 | 74 | .12 |
| Diabetes | 29 | 27 | 35 | <.0001 |
| Peripheral vascular disease | 13 | 12 | 17 | <.0001 |
| Coronary artery disease | 33 | 29 | 39 | <.0001 |
| Heart failure | <.0001 | |||
| None | 67 | 72 | 57 | |
| NYHA I | 10 | 9.9 | 11 | |
| NYHA II | 15 | 13 | 20 | |
| NYHA III/IV | 7.4 | 5.5 | 11 | |
| Prior cerebrovascular events (stroke or TIA) | 15 | 14 | 17 | <.0001 |
| Left ventricular ejection fraction | <.0001 | |||
| >50% | 70 | 72 | 68 | |
| 40%–50% | 6.3 | 5.8 | 7.5 | |
| 30%–40% | 8.9 | 7.9 | 11 | |
| <30% | 4.3 | 3.6 | 5.7 | |
| Calculated glomerular filtration rate (mL/min per 1.73 m2 | 67 (53–82) | 68 (55–83) | 64 (50–79) | <.0001 |
| AF type at baseline | 0.54 | |||
| New onset | 4.4 | 4.3 | 4.7 | |
| Paroxysmal | 51 | 51 | 50 | |
| Persistent | 17 | 17 | 17 | |
| Long-standing persistent | 28 | 28 | 29 | |
| EHRA symptom class | <.0001 | |||
| None | 38 | 40 | 33 | |
| Mild | 45 | 45 | 46 | |
| Severe | 15 | 13 | 18 | |
| Disabling | 1.8 | 1.8 | 1.8 | |
| CHADS2 score, mean (SD) | 2.3 (1.3) | 2.2 (1.3) | 2.5 (1.3) | <.0001 |
| Antiarrhythmic therapy at baseline | 29 | 28 | 31 | .003 |
| Any oral anticoagulant | 77 | 76 | 78 | .14 |
| Oral anticoagulant* | .39 | |||
| None | 23 | 24 | 22 | |
| Warfarin | 72 | 71 | 73 | |
| Dabigatran | 4.8 | 4.9 | 4.8 | |
| Percent time in therapeutic range (INR 2-3), median (IQR) | 62 (41–80) | 65 (45–83) | 54 (36–73) | <.0001 |
| Percent time INR <2, median (IQR) | 19 (4–37) | 17 (0–34) | 25 (9–43) | <.0001 |
| Percent time INR >3, median (IQR) | 9 (0–23) | 9 (0–22) | 12 (0–26) | <.0001 |
Values are presented as % or median (interquartile range), unless noted otherwise. Time in therapeutic range was calculated using the Rosendaal method.10
Abbreviations: TIA, transient ischemic attack; IQR, interquartile range.
No patients were on rivaroxaban or apixaban at baseline.
Type of AF at baseline was not associated with hospitalization; however, patients hospitalized had worse European Heart Rhythm Association (EHRA) symptom scores12 (18% with severe symptoms vs 13%, P < .0001) and higher CHADS2 scores (mean 2.5 vs 2.2, P < .0001). Use of any oral anticoagulant (78% vs 76%, 0.14) was not different across patients based on hospitalization. Patients who experienced hospitalization were more likely to have warfarin monitored by an anticoagulation clinic (35% vs 30%, P = .0001), had their INR tested more frequently (4.7% monitored weekly vs 3.3%, P < .0001), and had lower TTR (54% vs 65%, P < .0001). Use of both aspirin (47% vs 43%, P = .001) and clopidogrel (9.5% vs 6.1%, P < .0001) was more common in patients with at least 1 hospitalization. Characteristics of patients hospitalized multiple times during the follow-up period, compared with those hospitalized none or one time, are shown in the Supplementary Material (Table S1).
Causes of hospitalization
Of 4,548 hospitalizations, 2,242 (49%) were for cardiovascular reasons, 345 (7.6%) were for bleeding, and 1,961 (43%) were for other reasons. Cause-specific hospitalization rates, by subgroup, are shown in Figure 2. Patients with heart failure had the highest rates of hospitalization, for any cause, and proportions of cardiovascular, bleeding, and other hospitalizations appeared stable across subgroups.
Figure 2.
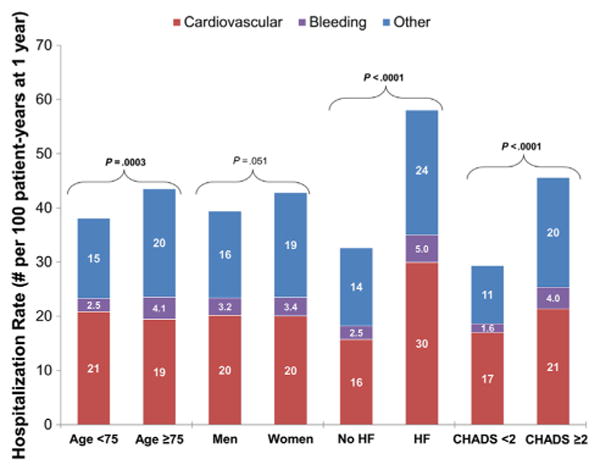
Rates of cause-specific hospitalization in patients with AF, by subgroup. P values presented are for the comparison of first all-cause hospitalization between the subgroups. HF, heart failure.
Mortality
Patients with any hospitalization during follow-up died at a rate of 10.81 events per 100 patient-years vs 1.92 deaths per 100 patient-years for patients without any hospitalization during follow-up. Mortality was highest among patients hospitalized multiple times during follow-up (14.8 per 100 patient-years), compared with 8.9 per 100 patient-years in patients hospitalized once during follow-up.
Predictors of first incident hospitalization
All-cause first hospitalization occurred in at a rate of 38.8 per 100 patient-years (cardiovascular: 20.1 per 100 patient-years; bleeding: 3.3 per 100 patient years; other: 17.5 per 100 patient-years). Kaplan-Meier curves for cause-specific first hospitalization, calculated independently, are shown in Figure 3.
Figure 3.
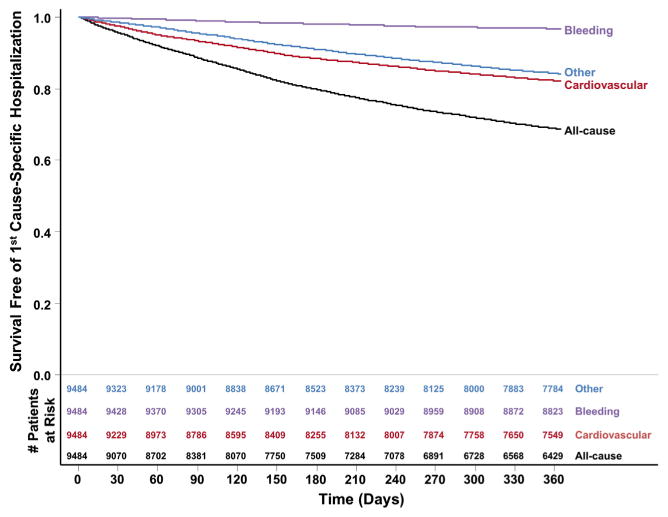
Kaplan-Meier rates of first hospitalization, by cause.
In multivariable analysis, patients with significant heart failure at baseline (New York Heart Association [NYHA] class II or III/IV) had the highest hazards of all-cause (adjusted HR 1.57 for NYHA III/IV, 95% CI 1.38-1.78) and cardiovascular (adjusted HR 1.72, 95% CI 1.46-2.04) hospitalization. Severe symptoms of AF by EHRA classification (those affecting daily activities)12 were also significantly predictive of all-cause (adjusted HR 1.37 EHRA severe symptoms, 95% CI 1.21-1.55), as was elevated heart rate at baseline (adjusted HR 1.11 per every 10-beats/min increase over 66, 95% CI 1.07-1.16). In addition, several measures of AF chronicity and severity (eg, left-atrial size, AF type), as well as several cardiovascular and noncardiovascular comorbidities, were all also significantly associated with hospitalization. Complete model details for first all-cause hospitalization are shown in Table II. In a model of first cardiovascular hospitalization, EHRA symptoms and heart rate persisted as significant factors (see Supplementary Material).
Table II. Predictors of first all-cause hospitalization.
| Adjusted HR (95% CI) |
t Value | P | |
|---|---|---|---|
| NYHA class III/IV | 1.57 (1.38–1.78) | 6.80 | <.0001 |
| Increased heart rate (>66), per 10 beats/min | 1.11 (1.07–1.16) | 5.36 | <.0001 |
| NYHA class II | 1.35 (1.21–1.51) | 5.28 | <.0001 |
| EHRA severe | 1.37 (1.21–1.55) | 5.09 | <.0001 |
| COPD | 1.23 (1.12–1.34) | 4.37 | <.0001 |
| Prior treatment with antiarrhythmic drug | 1.16 (1.07–1.27) | 3.62 | .0003 |
| Prior MI | 1.17 (1.07–1.29) | 3.28 | .001 |
| Peripheral vascular disease | 1.18 (1.07–1.31) | 3.31 | .0009 |
| History of PCI | 1.18 (1.07–1.30) | 3.34 | .0008 |
| Diabetes | 1.13 (1.05–1.22) | 3.22 | .001 |
| NYHA class I | 1.21 (1.07–1.36) | 3.13 | .002 |
| EHRA mild | 1.16 (1.06–1.28) | 3.22 | .001 |
| Not living independently | 1.24 (1.09–1.42) | 3.29 | .001 |
| Obstructive sleep apnea | 1.16 (1.05–1.28) | 2.94 | .003 |
| Severe LAE | 1.21 (1.07–1.36) | 3.03 | .003 |
| Anemia | 1.15 (1.05–1.26) | 2.95 | .003 |
| Hypothyroidism | 1.11 (1.03–1.20) | 2.77 | .006 |
| Cancer | 1.12 (1.03–1.21) | 2.70 | .007 |
| Frailty | 1.20 (1.05–1.39) | 2.61 | .009 |
| Moderate LAE | 1.11 (0.99–1.25) | 1.73 | .08 |
| Mild LAE | 1.07 (0.94–1.20) | 1.02 | .3 |
| EHRA disabling symptoms | 1.06 (0.78–1.45) | 0.39 | .7 |
| Increased heart rate (≤66), per 10 beats/min | 0.89 (0.80–0.99) | −2.07 | .04 |
| Increased diastolic blood pressure, per 10 mm Hg | 0.95 (0.91–0.99) | −2.42 | .02 |
| Increased hematocrit, per 10% | 0.83 (0.76–0.90) | −4.40 | <.0001 |
c Index = 0.63.
Abbreviations: COPD, chronic obstructive lung disease; PCI, percutaneous coronary intervention; CHF, congestive heart failure; LAE, left atrial enlargement.
We subsequently modeled predictors of first all-cause and cardiovascular hospitalization after excluding all patients with heart failure (n = 6,369 without heart failure; 67%). For both all-cause and cardiovascular hospitalization, the major predictors were EHRA severe symptoms (adjusted HR for all-cause hospitalization 1.42, 95% CI 1.19-1.69; adjusted HR for cardiovascular hospitalization 1.96, 95% CI 1.57-2.45) and increasing heart rate (adjusted HR per 10 beats/min for all-cause hospitalization 1.11, 95% CI 1.05-1.17; adjusted HR per 10 beats/min for cardiovascular hospitalization 1.17, 95% CI 1.10-1.25). Concomitant disease also contributed to risk of hospitalization among patients with AF without heart failure (see Supplementary Material).
Discussion
In this observational cohort of nearly 10,000 outpatients with AF, hospitalization is common, with nearly 1 in 3 patients hospitalized within a year. Most hospitalizations in patients with AF are for cardiovascular causes. Bleeding hospitalizations represented a significant minority, and patients experiencing any hospitalization had lower TTR than did those not hospitalized. Patients with highly symptomatic AF and heart failure are at particular risk for hospitalization, and in multivariable analyses, severe heart failure, AF symptoms, and elevated heart rate at baseline are significantly predictive of all-cause and cardiovascular hospitalization.
Prior data regarding AF and hospitalization has primarily focused on the overall rates of hospitalization AF; few data on the burden of hospitalization events among outpatients with AF exist. Naccarelli et al13 used MarketScan and Medicare claims data to assess frequency and cause of hospitalization in older Americans with AF. They found that more than one-third of patients with AF in Medicare had been hospitalized within 1 year, the majority for cardiovascular causes. However, patient-level characteristics were not available to identify factors most associated with hospitalization. Our data provide a more detailed look at such patients and represent the largest, prospective model of hospitalization events for outpatients with AF in the United States.
Patients with preexisting heart failure were at highest risk for hospitalization in our cohort. The combination of AF with heart failure is well known to be associated with particularly high risk of morbidity and mortality.14 Furthermore, heart failure alone represents a significant burden of inpatient hospitalization in the United States.15 Our data demonstrate that preexisting heart failure in patients with AF significantly predicts subsequent hospitalization, and such patients account for a disproportionate burden of hospitalizations relative to other subgroups. This difference was largely driven by an excess of cardiovascular hospitalizations in patients with heart failure. These data underscore the urgent need and ongoing efforts to improve the treatment of heart failure and reduce the frequency of recurrent hospitalization events in patients with concomitant AF and heart failure.
However, hospitalization was also common in patients with AF without heart failure, with more than 32 events per 100 patient-years. Additional independent predictors of hospitalization included several factors related to the complexity of patients' AF. Patients with more severe symptoms by ERHA score and those who were not functionally independent were at higher risk for hospitalization. Similarly, those treated with antiarrhythmic drug therapy and patients requiring electrophysiology management were also more likely to experience hospitalization. Lastly, left atrial enlargement, another measure of AF chronicity, was also a significant predictor of hospitalization and is consistent with prior data suggesting that left atrial measurements can predict subsequent cardiovascular events.16 When we modeled predictors of hospitalization in only these patients with AF without heart failure, symptom status remained a major driver of events. Although this may seem intuitive in retrospect, providers evaluating patients in clinic should recognize that symptom status is a marker of high risk for hospitalization.
Increased heart rate at baseline was also significantly associated with hospitalization, both in patients with and in patients without heart failure, and after adjustment for symptom class. Whether increased heart rate is a marker for another process or whether better rate control could mitigate hospitalization risk requires additional study.
Taken together, these data support the need to investigate strategies to aggressively manage patients with symptomatic and/or uncontrolled AF, in an effort to improve clinical outcomes. Preliminary data suggest that catheter ablation for AF may reduce subsequent health care use, in addition to improving symptoms.17,18 Although rhythm control strategies have not been shown to improve survival, they do improve quality of life and decrease hospitalization.19,20 Further studies of symptomatic management of AF are needed and may yield reductions in hospitalization events for these patients.
Although bleeding was the least common cause for hospitalization compared with cardiovascular and other causes, it represented a relatively high proportion by historical standards. It also represents a potentially modifiable cause of hospitalization. Most of this cohort was treated with warfarin. In these patients, both measures of warfarin management (frequency of INR testing and TTR) were significantly worse in patients experiencing hospitalization. Although this may reflect interruption of warfarin in relation to the hospitalization, it may also be a marker of instability leading to hospitalization. Regardless, TTR has been closely correlated with both bleeding and thromboembolic events,21,22 and strategies for maintaining high TTR are vital to the appropriate care of patients with AF managed with warfarin. Whether better management of INR or use of novel anticoagulants that do not require monitoring lead to reduced hospitalization remains to be proven.
Lastly, a significant number of hospitalizations in patients with AF were for noncardiovascular, nonbleeding causes, and several significant comorbidities were predictive of first all-cause hospitalization in multivariable analysis. Furthermore, patients who were more frequently hospitalized had increasing rates of all-cause mortality. These findings highlight the extensive, prevalent comorbidity present in an unselected population of patients with AF and the associated risk. They also demonstrate the potential opportunities for intervention in patients with significant disease burden, particularly those who are hospitalized. Many patients have concomitant, morbidity-altering illnesses that will increase their risk of adverse events and complicate management of AF. Treatment for the patient with AF should be considered in the context of the patient's other medical problems, with the over-arching goal to minimize morbidity and mortality from all causes using proven, evidence-based therapies.23
Limitations
The data for this analysis are derived from an observational cohort of patients in clinical practice with known AF and thus subject to the limitations inherent therein, including sampling and/or reporting biases. Furthermore, conclusions regarding hospitalization cause are limited in that the broad categories are investigator assigned and data regarding urgency of admission (elective, emergent) are not available. Residual measured or unmeasured confounding may influence some or all of these findings. Finally, although the ORBIT-AF registry was inclusive, this population is older (consistent with the epidemiology of AF in the United States) and overwhelmingly white (90%). Accordingly, these data may not be entirely generalizable to younger patients, minority populations, or patients cared for in settings that differ from those represented in ORBIT-AF.
Conclusions
Hospitalization is common in outpatients with AF, typically for cardiovascular or noncardiovascular, non-bleeding causes. Patients experiencing hospitalization have more comorbid disease, worse AF symptoms, and lower TTR. Heart failure symptom class and AF symptom class, independently, are significant predictors of hospitalization. Improved symptom control and heart failure/comorbid condition management should be targeted as the primary strategies to reduce health care use in these patients.
Supplementary Material
Table S1. Characteristics of patients hospitalized multiple times during follow-up
Table S2. Predictors of first cardiovascular hospitalization
Table S3. Predictors of first all-cause hospitalization among patients without heart failure
Acknowledgments
The ORBIT-AF registry is sponsored by Janssen Scientific Affairs, LLC, Raritan, NJ. Dr Steinberg was funded by NIH T-32 (Training Grant No. 5 T32 HL 7101-38).
Footnotes
Gust H. Bardy, MD, served as guest editor for this article.
Trial Registration clinicaltrials.gov Identifier: NCT01165710.
Dislosures: S. Kim, L. Thomas, B.J. Gersh have nothing to disclose. Other authors' disclosure information is as follows: B.A. Steinberg, other, modest: Medtronic, Inc. G.C. Fonarow, consultant/advisory board, modest: Ortho. J. Ansell, consultant/advisory board, modest: Boehringer Ingelheim, Alere, Bristol Myers Squibb, Pfizer, Janssen, and Daiichi. P.R. Kowey, consultant/advisory board (modest): Johnson & Johnson, Daiichi Sankyo, Sanofi, Boehringer Ingelheim, Merck, Bristol Myers Squibb, and Portola. K.W. Mahaffey, financial disclosures prior to August 1, 2013, can be viewed at http://www.dcri.org/about-us/conflict-of-interest/Mahaffey-COI_2011-2013.pdf; disclosures after August 1, 2013, can be viewed at http://med.stanford.edu/profiles/kenneth_mahaffey. E. Hylek, honoraria, modest: Boehringer-Ingelheim, Bayer, and consultant/advisory board, modest: Daiichi Sankyo, Ortho-McNeil-Janssen, Johnson & Johnson, Boehringer-Ingelheim, Bristol-Myers Squibb, and Pfizer G. Naccarelli, Consultant: Significant - Janssen, Pfizer, Bristol Myers Squibb, Boehringer Ingelheim, Sanofi, Glaxo Smith Kline, Modest - Xention, Otsuka, Daiichi Sankyo. J. Reiffel, received consultant fees from Medtronic, Merck, Boehringer Ingelheim, Sanofi, Gilead, Xention Discovery and speakers bureau from Boehringer Ingelheim, Sanofi, Janssen, BMS, Pfizer and also received research grant from Gilead, Medtronic and Janssen. P. Chang, employment, significant: Janssen Pharmaceuticals, Inc. E.D. Peterson, research grant, significant: American Heart Association, American College of Cardiology, Janssen Pharmaceutical Products, Eli Lilly & Company, and Society of Thoracic Surgeons; consultant/advisory board, modest: Merck & Co; consultant/advisory board, significant: Boehringer Ingelheim, Genentech, Sanofi-Aventis, and Janssen Pharmaceutical Products. J.P. Piccini, research grant, significant: Johnson & Johnson/Janssen Pharmaceuticals, and Boston Scientific Corporation; other research support, significant: Johnson & Johnson/Janssen Pharmaceuticals; consultant/advisory board, modest: Forest Laboratories, Inc and Medtronic Inc; and consultant/advisory board, significant: Johnson & Johnson/Janssen Pharmaceuticals.
References
- 1.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285(18):2370–5. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 2.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22(8):983–8. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Wolf PA, D'Agostino RB, et al. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98(10):946–52. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 4.Coyne KS, Paramore C, Grandy S, et al. Assessing the direct costs of treating nonvalvular atrial fibrillation in the United States. Value Health. 2006;9(5):348–56. doi: 10.1111/j.1524-4733.2006.00124.x. [DOI] [PubMed] [Google Scholar]
- 5.Wu EQ, Birnbaum HG, Mareva M, et al. Economic burden and comorbidities of atrial fibrillation in a privately insured population. Curr Med Res Opin. 2005;21(10):1693–9. doi: 10.1185/030079905X65475. [DOI] [PubMed] [Google Scholar]
- 6.Wong CX, Brooks AG, Leong DP, et al. The increasing burden of atrial fibrillation compared with heart failure and myocardial infarction: a 15-year study of all hospitalizations in Australia. Arch Intern Med. 2012;172(9):739–41. doi: 10.1001/archinternmed.2012.878. [DOI] [PubMed] [Google Scholar]
- 7.Christiansen CB, Olesen JB, Gislason G, et al. Cardiovascular and non-cardiovascular hospital admissions associated with atrial fibrillation: a Danish nationwide, retrospective cohort study. BMJ Open. 2013;3(1) doi: 10.1136/bmjopen-2012-001800. http://dx.doi.org/10.1136/bmjopen-2012-001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wattigney WA, Mensah GA, Croft JB. Increasing trends in hospitalization for atrial fibrillation in the United States, 1985 through 1999: implications for primary prevention. Circulation. 2003;108(6):711–6. doi: 10.1161/01.CIR.0000083722.42033.0A. [DOI] [PubMed] [Google Scholar]
- 9.Johnson BH, Smoyer-Tomic KE, Siu K, et al. Readmission among hospitalized patients with nonvalvular atrial fibrillation. Am J Health Syst Pharm. 2013;70(5):414–22. doi: 10.2146/ajhp120461. [DOI] [PubMed] [Google Scholar]
- 10.Rosendaal FR, Cannegieter SC, van der Meer FJ, et al. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69(3):236–9. [PubMed] [Google Scholar]
- 11.Piccini JP, Fraulo ES, Ansell JE, et al. Outcomes registry for better informed treatment of atrial fibrillation: rationale and design of ORBIT-AF. Am Heart J. 2011;162(4):606–612. doi: 10.1016/j.ahj.2011.07.001. e1. [DOI] [PubMed] [Google Scholar]
- 12.Kirchhof P, Auricchio A, Bax J, et al. Outcome parameters for trials in atrial fibrillation: recommendations from a consensus conference organized by the German Atrial Fibrillation Competence NETwork and the European Heart Rhythm Association. Europace. 2007;9(11):1006–23. doi: 10.1093/europace/eum191. [DOI] [PubMed] [Google Scholar]
- 13.Naccarelli GV, Johnston SS, Dalal M, et al. Rates and implications for hospitalization of patients >/=65 years of age with atrial fibrillation/flutter. Am J Cardiol. 2012;109(4):543–9. doi: 10.1016/j.amjcard.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 14.Neuberger HR, Mewis C, van Veldhuisen DJ, et al. Management of atrial fibrillation in patients with heart failure. Eur Heart J. 2007;28(21):2568–77. doi: 10.1093/eurheartj/ehm341. [DOI] [PubMed] [Google Scholar]
- 15.Fang J, Mensah GA, Croft JB, et al. Heart failure-related hospitalization in the U.S., 1979 to 2004. J Am Coll Cardiol. 2008;52(6):428–34. doi: 10.1016/j.jacc.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 16.Pritchett AM, Jacobsen SJ, Mahoney DW, et al. Left atrial volume as an index of left atrial size: a population-based study. J Am Coll Cardiol. 2003;41(6):1036–43. doi: 10.1016/s0735-1097(02)02981-9. [DOI] [PubMed] [Google Scholar]
- 17.Kusumoto F, Prussak K, Wiesinger M, et al. Radiofrequency catheter ablation of atrial fibrillation in older patients: outcomes and complications. J Interv Card Electrophysiol. 2009;25(1):31–5. doi: 10.1007/s10840-008-9346-7. [DOI] [PubMed] [Google Scholar]
- 18.Blandino A, Toso E, Scaglione M, et al. Long-term efficacy and safety of two different rhythm control strategies in elderly patients with symptomatic persistent atrial fibrillation. J Cardiovasc Electrophysiol. 2013;24(7):731–8. doi: 10.1111/jce.12126. [DOI] [PubMed] [Google Scholar]
- 19.Pedersen OD, Bagger H, Keller N, et al. Efficacy of dofetilide in the treatment of atrial fibrillation-flutter in patients with reduced left ventricular function: a Danish investigations of arrhythmia and mortality on dofetilide (diamond) substudy. Circulation. 2001;104(3):292–6. doi: 10.1161/01.cir.104.3.292. [DOI] [PubMed] [Google Scholar]
- 20.Alboni P, Botto GL, Baldi N, et al. Outpatient treatment of recent-onset atrial fibrillation with the “pill-in-the-pocket” approach. N Engl J Med. 2004;351(23):2384–91. doi: 10.1056/NEJMoa041233. [DOI] [PubMed] [Google Scholar]
- 21.Wan Y, Heneghan C, Perera R, et al. Anticoagulation control and prediction of adverse events in patients with atrial fibrillation: a systematic review. Circ Cardiovasc Qual Outcomes. 2008;1(2):84–91. doi: 10.1161/CIRCOUTCOMES.108.796185. [DOI] [PubMed] [Google Scholar]
- 22.Morgan CL, McEwan P, Tukiendorf A, et al. Warfarin treatment in patients with atrial fibrillation: observing outcomes associated with varying levels of INR control. Thromb Res. 2009;124(1):37–41. doi: 10.1016/j.thromres.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 23.Hess PL, Kim S, Piccini JP, et al. Use of evidence-based cardiac prevention therapy among outpatients with atrial fibrillation. Am J Med. 2013;126(7):625–632. doi: 10.1016/j.amjmed.2013.01.037. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Characteristics of patients hospitalized multiple times during follow-up
Table S2. Predictors of first cardiovascular hospitalization
Table S3. Predictors of first all-cause hospitalization among patients without heart failure


