Abstract
Background
The exact pathology of diabetic foot ulcers remains to be resolved. Evidence suggests that plantar shear forces play a major role in diabetic ulceration. Unfortunately, only a few manuscripts exist on the clinical implications of plantar shear. The purpose of this study was to compare global and regional peak plantar stress values in three groups; diabetic patients with neuropathy, diabetic patients without neuropathy and healthy control subjects.
Methods
Fourteen diabetic neuropathic patients, 14 non-neuropathic diabetic control and 11 non-diabetic control subjects were recruited. Subjects walked on a custom-built stress plate that quantified plantar pressures and shear. Four stress variables were analyzed; peak pressure, peak shear, peak pressure-time and shear-time integral.
Findings
Global peak values of peak shear (p=0.039), shear-time integral (p=0.002) and pressure-time integral (p=0.003) were significantly higher in the diabetic neuropathic group. Local peak shear stress and shear-time integral were also significantly higher in diabetic neuropathic patients compared to both control groups, in particular, at the hallux and central forefoot. Local peak pressure and pressure-time integral were significantly different between the three groups at the medial and lateral forefoot.
Interpretation
Plantar shear and shear-time integral magnitudes were elevated in diabetic patients with peripheral neuropathy, which indicates the potential clinical significance of these factors in ulceration. It is thought that further investigation of plantar shear would lead to a better understanding of ulceration pathomechanics, which in turn will assist researchers in developing more effective preventive devices and strategies.
Keywords: Plantar shear, plantar pressure, diabetic foot ulcers, plantar shear forces, plantar shear stresses, plantar ulcers, neuropathic foot ulcers, foot biomechanics, diabetic foot, diabetic foot biomechanics
INTRODUCTION
Estimated annual cost of diabetic foot ulcers and related amputations to the US healthcare system is over $30 billion (Rogers et al, 2008). Each year about 100,000 lower extremity amputations are performed on Americans with diabetes (Bloomgarden, 2008). Diabetic foot complications place a major burden not only on the US healthcare system but also on amputees’ quality of life.
The lifetime risk of developing a foot ulcer for diabetic patients is between 15–25% (Reiber, 1996; Lavery et al, 2003a). Diabetic patients with peripheral neuropathy are four times as likely to develop foot ulcers as those without neuropathy (Frykberg et al, 1998). In a cohort of 469 diabetic patients, cumulative incidence of ulceration was 20% and 3%, for individuals with and without peripheral neuropathy, respectively (Young et al, 1994). The exact pathology of diabetic foot ulcers is still not known. It is believed however that repetitive moderate mechanical stresses, in the presence of peripheral neuropathy, are the primary etiologic factors in plantar ulceration (Delbridge et al, 1985; Hall and Brand, 1979; Brand, 1978). Among these mechanical factors, horizontal component of the ground reaction forces (GRF), namely shear forces, and their relevance to diabetic ulcers have not been adequately studied. This is related to the technical challenges in the measurement of frictional shear force distribution under the foot (Perry et al, 2002). On the other hand, preliminary studies on plantar shear stresses have demonstrated the potential clinical significance of frictional shear in the pathology of diabetic foot lesions (Pollard and LeQuesne, 1983; Yavuz et al, 2007a; Yavuz et al, 2008). Furthermore, in an animal model application of frictional shear forces accelerated tissue breakdown (Goldstein and Sanders, 1998). Excessive frictional shear forces that act on soft tissue lead to hyperkeratosis (i.e. callosities), which have been previously associated with ulceration (Goldblum and Piper, 1954; McKenzie, 1974; Murray et al, 1996). In order to design better preventive devices and care, it is essential to understand the actual pathway to diabetic ulceration. Investigators deemed elevated plantar pressures responsible for diabetic foot lesions. However, efforts towards identifying a threshold pressure value for ulceration have failed. As a result pressure has been labeled as a “poor tool” in ulcer prediction (Armstrong et al, 1998a; Lavery et al, 2003b). Murray and associates (1996) reported that out of other risk factors, such as presence of calluses, high plantar pressures were the least predictive of ulcer formation. Therapeutic footwear, designed to redistribute pressures on the sole of the foot have been found only “meagerly” effective in preventing ulcer occurrences in a systematic review (Bus et al, 2008).
Therefore, revisiting the complicated pathology with a more extensive approach is crucial in order to minimize ulceration rates. Thus, the purpose of this study was to explore the clinical significance of plantar shear as well as pressure in ulceration by comparing global and regional stress data in diabetic neuropathic, diabetic non-neuropathic and a healthy control group. To our knowledge, this is also the first study that quantified plantar shear stresses in a diabetic non-neuropathic cohort that served as a control group.
RESEARCH DESIGN AND METHODS
Informed consent was obtained from 28 diabetic patients and 11 healthy volunteers who wanted to participate in the study, which was approved by the Institutional Review Board of the Kent State University College of Podiatric Medicine. The diabetic patients consisted of fourteen individuals with peripheral neuropathy and fourteen individuals without neuropathy. Exclusion criteria were having foot pain, prior surgeries in both feet and gross foot deformities. Inclusion criterion was the ability to walk along a 3.6m walkway multiple times without assistance.
Patients were recruited from Endocrinology and/or Podiatry Departments of various hospitals and clinics in the Greater Cleveland area (Ohio, USA). Peripheral neuropathy was assessed with a Biothesiometer (Biomedical Instrument Company, OH, USA) according to the task force report of the American Diabetes Association (Boulton et al, 2008). A vibration perception threshold of 25 volts was used to identify neuropathy. Based on neuropathy testing, diabetic patients were categorized as either neuropathic or non-neuropathic. The first cohort comprised the Diabetic Neuropathic Group (DN) whereas the second cohort comprised the Diabetic Control Group (DC). Group HC comprised healthy control individuals who were free of foot pain, prior surgeries and major foot deformities (Table 1).
Table 1.
Characteristics of subjects enrolled in the study. Values are mean (standard deviation), where applicable.
| DN | DC | HC | |
|---|---|---|---|
| No of subjects | 14 | 14 | 11 |
| Gender | 2 f, 12 m | 9 f, 5 m | 7 f, 4 m |
| Age (years) | 64.8 (6.8) | 52.4 (12.9) | 65.5 (6.0) |
| BMI | 32.0 (5.1) | 28.9 (7.4) | 27.8 (5.9) |
| Duration of diabetes (years) | 13.1 (11.4) | 14.2 (11.5) | n/a |
| Type 1/Type 2 diabetes | 2/12 | 5/9 | n/a |
| Vibration perception (volts) | 35.6 (9.1) | 11.7 (4.9) | n/a |
| Average gait speed (m/s) | 0.81 (0.24) | 0.88 (0.15) | 1.17 (0.15) |
Subjects were asked to walk at self-selected speeds multiple times on a custom-built pressure-shear plate, which was set flush on the 3.6 meter walkway. The device measures 11.4cm × 14.2cm with 1.5 mm space in between each of the 80 sensors that complement the plate. Each sensor measured 1.25cm × 1.25cm generating an effective surface area of 1.56cm2. Eighty transducers were arranged in an 8 × 10 array, which looked like a checker-board in appearance. Further specifications of the plate have been explained elsewhere (Yavuz et al, 2007b).
Data from three trials were averaged and used in the statistical analysis. Data was collected implementing the two-step method, which has been shown to produce similar pressure values to that of the mid-gait method (Bryant et al, 1999). Subjects were first asked to walk on the walkway a few times at self-selected gait speeds and their average step length was visually determined. Then the subjects were asked to position themselves about two steps before the stress plate. The volunteers then took a step with the non-dominant foot so that their dominant foot (in the case of a previous dominant foot surgery, vice versa) was on the stress plate. Subjects practiced this routine multiple times while the starting distance from the plate was adjusted as necessary until the subjects had their second step (forefoot) on the stress plate.
Four major stress variables were identified in each subject; peak pressure (PP), peak shear (PS), peak pressure-time integral (PTI) and peak shear-time integral (STI). Time-integral values were calculated by implementation of the trapezoidal rule over the stress-time curves using 99 subdivisions for each sensor. Then, spatial and temporal maximum values were identified as PTI or STI. Data analyses were based on global peak and regional peak values. For the regional stress analysis, pressure and shear profiles of the enrollees were masked into five forefoot regions by a custom-written Matlab (Mathworks, MA, USA) script; hallux, lesser toes, medial forefoot (first metatarsal head), central forefoot (second and third metatarsal heads) and lateral forefoot (fourth and fifth metatarsal heads). Forefoot was selected as the region of interest since most plantar ulcers develop in this area (Oyibo et al, 2001; Caselli et al, 2002).
Group characteristics were analyzed using analysis of variance (ANOVA). Plantar stress values were analyzed using ANOVA (global stress values) or analysis of covariance (ANCOVA) (global and regional stress values). While analyzing regional stress values, gait speed was used as the covariate. Significant group effects identified by ANOVA were further examined using Bonferroni post-hoc comparisons. For significant group effects identified by ANCOVA, simple linear contrasts (DN vs. DC, and DN vs. HC) were carried out. For all analyses, alpha was set to ≤ 0.05. IBM SPSS statistical software (v20, SPSS, Inc., Chicago, IL, USA) was used to analyze data.
RESULTS
Groups differed significantly on gait speed (p<.001) and age (p=.004), but not Body-Mass Index (BMI, p=.214). HC subjects walked about 25% faster than DC subjects at 1.17 m/s (p<.001), and about 30% faster than DN subjects (p=.001). The mean age of diabetic neuropathic patients was significantly higher than the mean age of diabetic control patients (p=.003); the mean age of DN and HC subjects was not significantly different. As expected, vibration perception threshold was significantly higher in DN subjects compared to DC subjects (p<.001).
Global Analyses
ANOVAs performed on the global data showed significant differences (Table 2) on PS, PTI, and STI (p<0.05), but not PP (p>0.05). For PS, STI, and PTI, differences between the DN and HC groups were significant (p=.035, .006, and .003, respectively), as assessed by Bonferroni post-hoc comparisons. For PTI, the difference between DN and DC groups was also significant (p=.010). When age and BMI were added separately as covariates to the analyses, neither accounted for a significant proportion of the variance, and the group differences for PS, STI, and PTI remained significant. When gait speed was added to the analyses as a covariate, it predicted a significant amount of the variance in PS and STI (14.6% and 17.6%, respectively), but not PP and PTI. At the same time, group differences in PS, STI, and PTI remained statistically significant.
Table 2.
Global peak results for three subject groups. Values are means (standard deviation).
| Variable | DN | DC | HC | p | Partial eta squared |
|---|---|---|---|---|---|
| PP (kPa) | 591.7 (113.1) | 506.2 (141.7) | 481.1 (109.8) | 0.069 | 0.138 |
| PS (kPa) | 91.3 (29.0) | 82.0 (26.4) | 64.6 (15.7) | 0.039* | 0.165 |
| STI (kPa.s) | 34.4 (19.2) | 20.3 (5.1) | 18.2 (2.8) | 0.002* | 0.282 |
| PTI (kPa.s) | 234.7 (72.3) | 167.4 (53.3) | 154.0 (32.7) | 0.003* | 0.299 |
denotes statistically significant difference
Regional Analyses
Since gait speed accounted for a statistically significant proportion of variance in the global analyses, it was used as the covariate in the ANCOVAs examining regional data. Gait speed accounted for a significant proportion of the variance only for STI at the hallux (p=0.006, partial eta squared=0.197) and central forefoot area (p=0.002, partial eta squared=0.246).
No significant difference was observed at the lesser toes (Table 3). DN-DC comparisons demonstrated statistical significance for PP at the medial forefoot (p=.001) and PS at hallux (p=.007), whereas STI was higher at hallux (p=.002), medial forefoot (p=.001) and central forefoot (p.002). PTI was higher at the medial forefoot (p=.001) and lateral forefoot (p=.002).
Table 3.
Results of planned contrasts for plantar loading variables at five forefoot regions (Data represents p values for group effects and for contrasts. Contrast values are not provided for non-significant differences at the group level)
| Site | Outcome | Group Effect |
Partial Eta Squared |
Contrast (DN vs. DC) |
Contrast (DN vs. HC) |
|---|---|---|---|---|---|
| Hallux | PP | .646 | .025 | ||
| PS | .003 | .277 | .007 | .002 | |
| STI | .001 | .367 | .002 | .001 | |
| PTI | .031 | .180 | .011 | ||
| Lesser Toes | PP | .447 | .045 | ||
| PS | .598 | .029 | |||
| STI | .213 | .085 | |||
| PTI | .481 | .041 | |||
| Medial Forefoot | PP | .001 | .358 | .001 | .001 |
| PS | .176 | .095 | |||
| STI | .003 | .285 | .001 | .006 | |
| PTI | .001 | .360 | .001 | .001 | |
| Central Forefoot | PP | .491 | .040 | ||
| PS | .046 | .161 | .050 | ||
| STI | .001 | .389 | .002 | .001 | |
| PTI | .052 | .156 | |||
| Lateral Forefoot | PP | .015 | .213 | .004 | |
| PS | .107 | .120 | |||
| STI | .089 | .129 | |||
| PTI | .001 | .425 | .002 | .001 |
DN-HC contrasts revealed statistical significance for PP at the medial forefoot (p=.001) and lateral forefoot (p=.004) as well as PS at the hallux (p=.002) and central forefoot (p=.050). STI was higher in the DN group at the hallux (p=.001), medial forefoot (p=.006) and central forefoot (p=.001). PTI, on the other hand was significantly different at the hallux (p=.011), medial forefoot (p=.001) and lateral forefoot (p=.001).
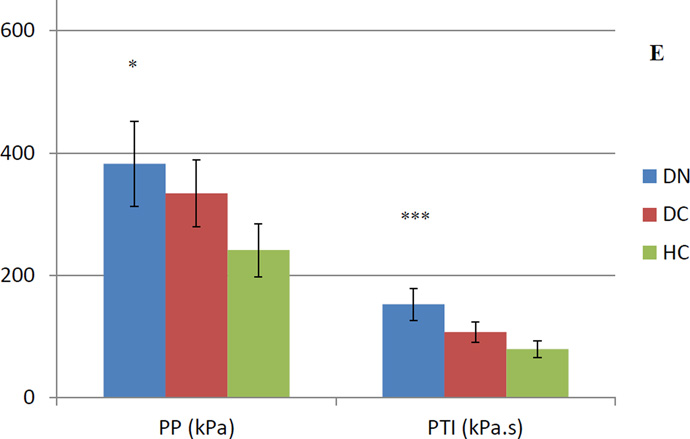
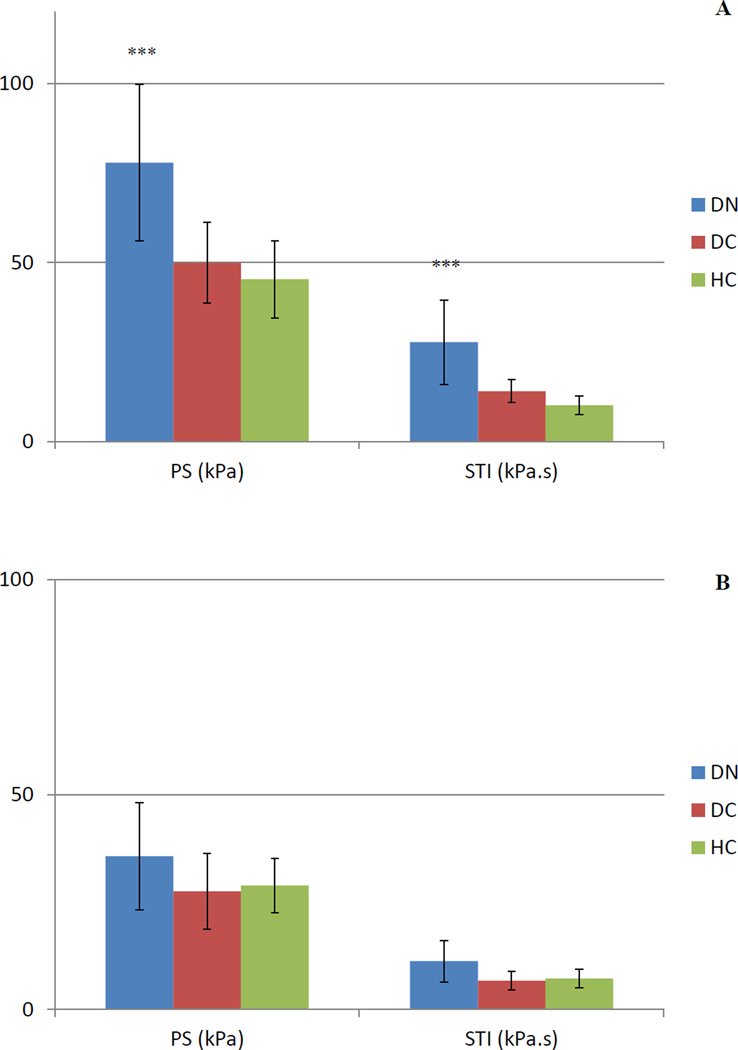
Highest regional PP (481.4 kPa) occurred under the central forefoot of DN patients (Figure 1). Maximum PS (77.9 kPa) in this group was seen under the hallux (Figure 2), which was significantly higher at this region. In DC and HC subjects, highest regional PP was 488.8 kPa and 444.3 kPa, respectively, both of which were experienced by the central forefoot. This site also experienced the highest regional PS in DC (77.6 kPa) and HC (61.1 kPa) groups. Maximum PTI was observed at the central forefoot in DN (192.4 kPa.s), DC (157.0 kPa.s) and HC (147.4 kPa.s) subjects. PTI was not significantly different at this region. Central forefoot was also the site for largest STI in DC (19.3 kPa.s) and HC (17.6 kPa.s) subjects. Similar to PS, regional maximum STI was also experienced by the hallux in DN patients (27.8 kPa.s), which was also significantly higher compared to both control groups.
Figure 1.
Peak Pressure (PP) and pressure-time integral (PTI) values for five foot regions. A) hallux, B) lesser toes, C) medial forefoot, D) central forefoot, E) lateral forefoot
Error bars represent standard deviation. * represents a group difference between DN-HC, ** represents a group difference between DN-DC, *** represents group differences between DN-HC and DN-DC.
Figure 2.
Peak shear (PS) and shear-time integral (STI) values for five foot regions. A) hallux, B) lesser toes, C) medial forefoot, D) central forefoot, E) lateral forefoot
Error bars represent standard deviation. * represents a group difference between DNHC, ** represents a group difference between DN-DC, *** represents group differences between DN-HC and DN-DC.
CONCLUSIONS
Plantar shear has been overlooked in the etiology of diabetic ulceration since readily available commercial equipment can only measure the distribution of vertical ground reaction forces (GRF) under the foot. Also, plantar shear force typically has lower magnitudes when compared with vertical GRF. Therefore, many investigators focused on quantifying and analyzing the normal component of GRF and its related stress, i.e. pressure, in identifying patients at risk of ulceration and when designing therapeutic footwear. However, reports by Ledoux et al (2005) and Hsi et al (1993) revealed that diabetic feet exerting elevated pressures may not ulcerate while feet with “normal” peak pressures may ulcerate. Moreover, in a prospective study by Murray et al (1996), it was reported that most (68%) ulcers do not develop at peak pressure locations. Lavery and associates (2003b) calculated the sensitivity and specificity values of peak pressure in identifying ulcers in a cohort of 1666 subjects. Based on these results, peak pressure was labeled as a “poor tool” in predicting diabetic ulcers by the authors. Therapeutic footwear designed to reduce peak plantar pressures and prevent diabetic ulcers have not been very successful. As a matter of fact, the effectiveness of such footwear in preventing ulcers is only “meager” according to the report by Bus et al (2008).
Potential clinical significance of plantar shear in diabetic ulceration has been demonstrated in earlier reports (Yavuz et al, 2007b; Yavuz et al, 2008). This significance stems from the facts that; (i) during a single stance phase, shear stresses apply twice in opposite directions on the plantar surface, which can be described as a biphasic character (Yavuz et al, 2008), (ii) peak plantar pressure and shear may occur at different sites under the same foot, which may explain why most ulcers do not develop at peak pressure locations, (iii) skin breakdown occurs faster in the presence of shear (Goldstein and Sanders, 1998), (iv) shear stresses previously have been associated with hyperkeratosis and callus formation, which is an increased risk factor for diabetic ulcers (Murray et al, 1996), (v) bunching, stretching and twisting effects generated by plantar shear, which were previously hypothesized by Davis (1993) and experimentally observed later, might accelerate tissue breakdown.
This study revealed for the first time that there are regional differences in plantar shear values among diabetic patients with and without neuropathy and healthy control subjects. Groups did not differ on any measure at the lesser toes. Significant differences were more likely to be detected between DN and HC groups than between DN and DC groups. This finding indicates, as expected, that pressure and shear values increase in diabetic patients on a continuous basis as the disease progresses and neuropathy onsets.
ANCOVA results indicated that the mean BMI and age of the groups did not influence the differences between the groups. However, gait speed accounted for a significant proportion of the variance for PS and STI in global stress analyses. Regional data analyses revealed that gait speed could explain a portion of the variance for only STI at the hallux and central forefoot area. The results are in accordance with earlier manuscripts that reported an association between gait speed and anteroposterior shear forces under the foot (Martin and Marsh, 1992; Schwartz et al, 2008). Also many other factors might influence the magnitudes and direction of plantar shear stresses such as frictional properties of skin, intrinsic muscle activity and moisture content of the skin. In the current study, in most of the DN patients, hallux and central forefoot area experienced the PS and STI. Identifying whether an upcoming ulcer would develop at a maximum shear bearing site was not the purpose of this study. However, a previous study indicated that about 37% of plantar forefoot ulcers develop at the hallux (Armstrong et al, 1998b). Lesser toes, medial, central and lateral forefoot experience respectively 12%, 27%, 10% and 14% of the ulcers according to the same report.
Significant increases were observed between DN-HC and DN-DC groups for both shear variables (PS and STI) at the hallux. The medial forefoot, the second leading site for ulcer occurrence, was the only site where three of the four stress variables were significantly different; DN-DC: PP, PTI and STI, DN-HC: PP, PTI and STI. An interesting finding was that PS did not differ significantly at the medial forefoot. At none of the foot sites were all four variables significantly different. At the central forefoot, which is another high-risk site for ulceration, STI was significantly higher in DN patients compared to both DC and HC subjects. Planned contrasts revealed a borderline value for PS when comparing DN patients to HC subjects. Another intriguing observation was that while at certain sites, such as the lateral forefoot, pressure values were significantly higher; at other sites such as the hallux and central forefoot, shear variables were significantly higher. These observations suggest that ulcers occurring at different sites of the plantar foot might occur via slightly different biomechanical pathways.
PTI was significantly higher in 5 out of the 10 possible regional comparisons, as opposed to PP, which was significantly higher in 3 comparisons. Similarly, STI was significantly higher in 6 regional comparisons, whereas PS was higher in 3 comparisons. This outcome implies that time-integral of plantar stresses might be a better tool in distinguishing diabetic patients who are at risk for ulceration.
Spatial resolution and the overall size of the platform are two instrumentation limitations of this study. Diabetic subjects recruited in the study did not have an ulcer history, preventing definite conclusions on the potential association between ulcer occurrence and peak shear and/or peak pressure. The relatively small sample size assessed in this study might have also prevented the statistical analysis from revealing differences in global mean PP, while statistical power was sufficient to reveal regional differences in PP. A number of research teams in the past were also not able to report significant differences in peak plantar pressure or peak force values at certain foot sites between DN patients with or without an ulcer history and control subjects. Ctercteko and associates (1981, N=68) and Pitei and associates (1999, N=57) assessed more patients than were assessed in this study. Moreover, in the study by Stess and associates (1996, N=48) and Rahman and associates (2006, N=75), global peak pressure was not significantly higher in diabetic neuropathic patients when compared to control subjects.
Only barefoot locomotion was assessed in this study. Several footwear studies revealed that pressure values in shod conditions are lower especially in the presence of insoles made of conforming materials (Bus et al, 2008). A few research groups have attempted to measure in-shoe shear stresses (Tappin et al, 1980; Akhlaghi and Pepper, 1996; Hosein and Lord, 2000; Razian and Pepper, 2003). Among these only Hosein and Lord (2000) reported shear stress magnitude data from multiple subjects. Their data revealed that mean peak shear occurred at the first metatarsal head (72.7 kPa), followed by the second metatarsal head (63.6 kPa), third metatarsal head (50.5 kPa) and fourth metatarsal head (39.4 kPa). These magnitudes are similar to the results reported in this article.
Unfortunately, the instrumentation developed in previous studies had major drawbacks. Some of these systems either required attachment of thick transducers to the plantar skin (Tappin et al, 1980), insoles with only a few sensors which needed to be customized for each subject (Akhlaghi and Pepper, 1996; Hosein and Lord, 2000; Razian and Pepper, 2003) or did not allow simultaneous triaxial force measurement (Akhlaghi and Pepper, 1996; Hosein and Lord, 2000). Another challenge with the proposed in-shoe shear sensing systems is that the introduction of a sensor between the plantar skin and footwear would change the coefficient of friction at the skin-footwear interface, which may yield to altered shear force readings. Therefore, at this time it is very difficult to correlate in-shoe plantar shear stresses to barefoot shear stresses. However, while in-shoe and barefoot shear stresses might have varying magnitudes, the locations of peak shear stress are thought to remain similar across the barefoot and in shod conditions.
Explaining diabetic ulcerations with elevated plantar pressures per se is believed to be an oversimplification of a very complicated pathology. Other factors, such as plantar shear, physical activity, skin temperature and material properties of the plantar tissue all play a major role in diabetic ulcers. Unfortunately, most of these factors have not been studied adequately. In order to minimize the burdening outcome of diabetic ulcers, it is crucial to have a better understanding of the complicated etiology by simultaneously assessing as many risk factors as possible. It is only then possible to develop more effective preventive devices and strategies.
To our knowledge this is the first study that has attempted to analyze site-specific plantar shear distribution and compare results between diabetic patients with and without peripheral neuropathy and healthy control subjects. Our results suggest that plantar shear is a promising tool in predicting diabetic ulcers and designing more effective therapeutic footwear. Future research should investigate a potential relationship between ulcer and peak shear stress sites.
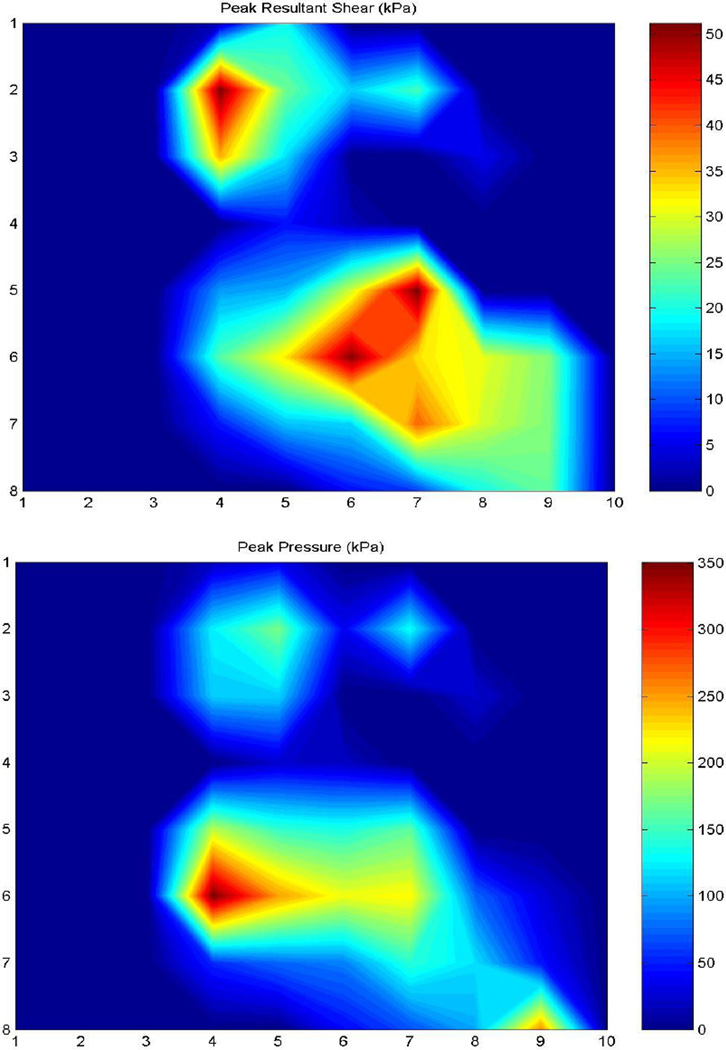
Figure 3.
Peak plantar shear (top) and pressure (bottom) profiles of a representative DN subject. Note the difference between the sites of PS (central forefoot) and PP (medial forefoot)
ACKNOWLEDGMENT
This research was possible due to support from the National Institutes of Health (1R15DK082962). The author would like to thank Andrew Franklin, Rebecca McGaha, Joseph Stuto, Vinai Prakash, Garneisha Torrence, Jessica Rispoli, Vincent Hetherington, DPM, and Leo Mallias of the Kent State University College of Podiatric Medicine. The author is also thankful for the efforts of Alan Glaros, PhD of the Kansas City University of Medicine and Biosciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Akhlaghi F, Pepper MG. In-shoe biaxial shear force measurement: the Kent shear system. Medical and Biological Engineering and Computing. 1996;34(4):315–317. doi: 10.1007/BF02511246. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong DG, Peters EJ, Athanasiou KA, Lavery LA. Is there a critical level of plantar foot pressure to identify patients at risk for neuropathic foot ulceration? Journal of Foot and Ankle Surgery. 1998a;37(4):303–307. doi: 10.1016/s1067-2516(98)80066-5. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong DG, Lavery LA, Harkless LB. Validation of a diabetic wound classification system. Diabetes Care. 1998b;21(5):855–859. doi: 10.2337/diacare.21.5.855. [DOI] [PubMed] [Google Scholar]
- 4.Bloomgarden ZT. The Diabetic Foot. Diabetes Care. 2008;31(2):372–376. doi: 10.2337/dc08-zb02. [DOI] [PubMed] [Google Scholar]
- 5.Boulton AJ, Armstrong DG, Albert SF, Frykberg RG, Hellman R, Kirkman MS, Lavery LA, et al. Comprehensive foot examination and risk assessment. A report of the Task Force of the Foot Care Interest Group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Diabetes Care. 2008;31(8):1679–1685. doi: 10.2337/dc08-9021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brand PW. Pathomechanics of pressure ulceration. In: Fredricks S, Brody GS, editors. Symposium on the Neurologic Aspects of Plastic Surgery; Houston, TX. 1978. [Google Scholar]
- 7.Bryant A, Singer K, Tinley P. Comparison of the reliability of plantar pressure measurements using the two-step and midgait methods of data collection. Foot Ankle Int. 1999;20(10):645–650. doi: 10.1177/107110079902001006. [DOI] [PubMed] [Google Scholar]
- 8.Bus SA, Valk GD, van Deursen RW, Armstrong DG, Caravaggi C, Hlavacek P, Bakker K, Cavanagh PR. The effectiveness of footwear and offloading interventions to prevent and heal foot ulcers and reduce plantar pressure in diabetes: a systematic review. Diabetes Metab Res Rev. 2008;24(S1):162–180. doi: 10.1002/dmrr.850. [DOI] [PubMed] [Google Scholar]
- 9.Caselli A, Pham H, Giurini JM, Armstrong DG, Veves A. The forefoot-to-rearfoot plantar pressure ratio is increased in severe diabetic neuropathy and can predict foot ulceration. Diabetes Care. 2002;25(6):1066–1071. doi: 10.2337/diacare.25.6.1066. [DOI] [PubMed] [Google Scholar]
- 10.Ctercteko GC, Dhanendran M, Hutton WC, Le Quesne LP. Vertical forces acting on the feet of diabetic patients with neuropathic ulceration. British Journal of Surgery. 1981;68:608–614. doi: 10.1002/bjs.1800680904. [DOI] [PubMed] [Google Scholar]
- 11.Davis BL. Foot ulceration: hypotheses concerning shear and vertical forces acting on adjacent regions of skin. Med Hypotheses. 1993;40(1):44–47. doi: 10.1016/0306-9877(93)90195-v. [DOI] [PubMed] [Google Scholar]
- 12.Delbridge L, Ctercteko G, Fowler C, Reeve TS, Le Quesne LP. The aetiology of diabetic neuropathic ulceration of the foot. Br J Surgery. 1985;72(1):1–6. doi: 10.1002/bjs.1800720102. [DOI] [PubMed] [Google Scholar]
- 13.Frykberg RG, Lavery LA, Pham H, Harvey C, Harkless L, Veves A. Role of neuropathy and high foot pressures in diabetic foot ulceration. Diabetes Care. 1998;21(10):1714–1719. doi: 10.2337/diacare.21.10.1714. [DOI] [PubMed] [Google Scholar]
- 14.Goldblum RW, Piper WN. Artificial lichenification produced by a scratching machine. J Invest Dermatol. 1954;22(5):405–415. doi: 10.1038/jid.1954.57. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein B, Sanders J. Skin response to repetitive mechanical stress: a new experimental model in pig. Arch Phys Med Rehabil. 1998;79(3):265–272. doi: 10.1016/s0003-9993(98)90005-3. [DOI] [PubMed] [Google Scholar]
- 16.Hall OC, Brand PW. The etiology of the neuropathic plantar ulcer. Journal of the American Podiatry Association. 1979;69(3):173–177. doi: 10.7547/87507315-69-3-173. [DOI] [PubMed] [Google Scholar]
- 17.Hosein R, Lord M. A study of in-shoe plantar shear in patients with diabetic neuropathy. Clinical Biomechanics (Bristol, Avon) 2000;15(4):278–283. doi: 10.1016/s0268-0033(99)00076-5. [DOI] [PubMed] [Google Scholar]
- 18.Hsi WL, Ulbrecht JS, Perry JE, Norkitis AJ, Cavanagh PR. Plantar pressure threshold for ulceration risk using the EMEDSF platform [abstract] Diabetes. 1993;42(S1):103a. [Google Scholar]
- 19.Lavery LA, Armstrong DG, Wunderlich RP, Tredwell J, Boulton AJ. Diabetic foot syndrome: evaluating the prevalence and incidence of foot pathology in Mexican Americans and non-Hispanic whites from a diabetes disease management cohort. Diabetes Care. 2003a;26(5):1435–1438. doi: 10.2337/diacare.26.5.1435. [DOI] [PubMed] [Google Scholar]
- 20.Lavery LA, Armstrong DG, Wunderlich RP, Tredwell J, Boulton AJ. Predictive value of foot pressure assessment as part of a population-based diabetes disease management program. Diabetes Care. 2003b;26(4):1069–1073. doi: 10.2337/diacare.26.4.1069. [DOI] [PubMed] [Google Scholar]
- 21.Ledoux WR, Cowley MS, Ahroni JH, Forsberg RC, Stensel VL, Shofer JB, Boyko EJ. No relationship between plantar pressure and diabetic foot ulcer incidence after adjustment for ulcer location. 65th Scientific Session of the American Diabetes Association; San Diego, CA. 2005. [retrieved on 01/23/2013]. http://professional.diabetes.org/Abstracts_Display.aspx?TYP=1&CID=49680. [Google Scholar]
- 22.MacKenzie IC. The effects of frictional stimulation on mouse ear epidermis. J Invest Dermatol. 1974;63(2):194–198. doi: 10.1111/1523-1747.ep12679356. [DOI] [PubMed] [Google Scholar]
- 23.Martin PE, Marsh AP. Step length and frequency effects on ground reaction forces during walking. J Biomechanics. 1992;25(10):1237–1239. doi: 10.1016/0021-9290(92)90081-b. [DOI] [PubMed] [Google Scholar]
- 24.Murray HJ, Young MJ, Hollis S, Boulton AJ. The association between callus formation, high pressures and neuropathy in diabetic foot ulceration. Diabetic Medicine. 1996;13(11):979–982. doi: 10.1002/(SICI)1096-9136(199611)13:11<979::AID-DIA267>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 25.Orendurff MS, Bernatz GC, Schoen JA, Klute GK. Kinetic mechanisms to alter walking speed. Gait Posture. 2008;27:603–610. doi: 10.1016/j.gaitpost.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Oyibo SO, Jude EB, Tarawneh I, Nguyen HC, Armstrong DG, Harkless LB, Boulton AJ. The effects of ulcer size and site, patient's age, sex and type and duration of diabetes on the outcome of diabetic foot ulcers. Diabetic Medicine. 2001;18(2):133–138. doi: 10.1046/j.1464-5491.2001.00422.x. 2001. [DOI] [PubMed] [Google Scholar]
- 27.Perry JE, Hall JO, Davis BL. Simultaneous measurement of plantar pressure and shear forces in diabetic patients. Gait Posture. 2002;15(1):101–107. doi: 10.1016/s0966-6362(01)00176-x. [DOI] [PubMed] [Google Scholar]
- 28.Pitei DL, Lord M, Foster A, Wilson S, Watkins PJ, Edmonds ME. Plantar pressures are elevated in the neuroischemic and the neuropathic diabetic foot. Diabetes Care. 1999;22(12):1966–1970. doi: 10.2337/diacare.22.12.1966. [DOI] [PubMed] [Google Scholar]
- 29.Pollard JP, Le Quesne LP. Method of healing diabetic forefoot ulcers. British Medical Journal (Clinical Research Ed.) 1983;286(6363):436–437. doi: 10.1136/bmj.286.6363.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rahman MA, Aziz Z, Acharya UR, Ha TP, Kannathal N, Law C, Subramaniam T, Shuen WY, Fang SC. Analysis of plantar pressure in diabetic type 2 subjects with and without neuropathy. ITBM-RBM. 2006;27:46–55. [Google Scholar]
- 31.Razian MA, Pepper MG. Design, development and characteristics of an in-shoe triaxial pressure measurement transducer utilizing a single element of piezoelectric copolymer film. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2003;11(3):288–293. doi: 10.1109/tnsre.2003.818185. [DOI] [PubMed] [Google Scholar]
- 32.Reiber GE. The epidemiology of diabetic foot problems. Diabetic Medicine. 1996;13(S1):6–11. [PubMed] [Google Scholar]
- 33.Rogers LC, Lavery LA, Armstrong DG. The right to bear legs--an amendment to healthcare: how preventing amputations can save billions for the US Health-care System. Journal of American Podiatric Medical Association. 2008;98(2):166–168. [PubMed] [Google Scholar]
- 34.Schwartz MH, Rozumalski A, Trost JP. The effect of walking speed on the gait of typically developing children. J Biomechanics. 2008;41:1639–1650. doi: 10.1016/j.jbiomech.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 35.Stess RM, Jensen SR, Mirmiran R. The role of dynamic plantar pressures in diabetic foot ulcers. Diabetes Care. 1997;20(5):855–858. doi: 10.2337/diacare.20.5.855. [DOI] [PubMed] [Google Scholar]
- 36.Tappin JW, Pollard JP, Beckett EA. Method of measuring shearing forces on the sole of the foot. Clinical Physics and Physiological Measurement. 1980;1:83–85. [Google Scholar]
- 37.Yavuz M, Erdemir A, Botek G, Hirschman GB, Bardsley L, Davis BL. Peak plantar pressure and shear locations: relevance to diabetic patients. Diabetes Care. 2007a;30(10):2643–2645. doi: 10.2337/dc07-0862. [DOI] [PubMed] [Google Scholar]
- 38.Yavuz M, Botek G, Davis BL. Plantar shear stress distributions: comparing actual and predicted frictional forces at the foot-ground interface. Journal of Biomechanics. 2007b;40(13):3045–3049. doi: 10.1016/j.jbiomech.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yavuz M, Tajaddini A, Botek G, Davis BL. Temporal characteristics of plantar shear distribution: relevance to diabetic patients. Journal of Biomechanics. 2008;41(3):556–559. doi: 10.1016/j.jbiomech.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Young MJ, Breddy JL, Veves A, Boulton AJM. The prediction of diabetic neuropathic foot ulceration using vibration perception thresholds. Diabetes Care. 1994;17(6):557–560. doi: 10.2337/diacare.17.6.557. [DOI] [PubMed] [Google Scholar]