Abstract
Objective
To use a large population-based cohort to determine age-dependent short-term outcomes after pancreatic resection.
Methods
We identified all pancreatic resections in Texas from 1999 to 2005. Patients were stratified into 4 age groups (<60, 60–69, 70–79, and 80+ years). Bivariate and multivariate analyses were performed to determine the effect of age on mortality, discharge to home versus requiring inpatient nursing care, and length of stay.
Results
Three thousand seven hundred and thirty-six patients underwent pancreatic resection. Unadjusted in-hospital mortality increased with each increasing age group from 2.4% in patients <60 to 11.4% in patients 80 years and older (P < 0.0001). Likewise, postoperative lengths of stay increased with each increasing age group (P = 0.02). Age group independently predicted the need for discharge to an inpatient nursing unit rather than home (P < 0.0001), with the odds ration (OR) increasing with each increasing age group. With each increasing age group, patients were less likely to be resected at high-volume (H-V) hospitals (>10 pancreatic resections/y). Whereas low-volume (L-V) hospitals (≤10 pancreatic resections/y) had higher mortality rates (3.2% versus 7.3%, P < 0.0001), the difference in mortality between H- and L-V hospitals was more striking in older patients. With increasing age group, mortality increased from 3.0% to 9.5% to 11.4% to 14.7% at L-V hospitals. It increased from 2.0% to 3.5% to 4.5% to 8.7% at H-V hospitals (P < 0.0001). In the multivariate model controlling for gender, race, hospital volume, year of surgery, diagnosis, risk of mortality, severity of illness, admission status, and procedure type, older age group independently predicted increased mortality. The OR for patients 60–69 years was 2.5 (P = 0.0003), the OR for patients 70–79 years was 1.8 (P = 0.02), and the OR for patients 80+ years was 4.4 (P < 0.0001) when compared with patients <60 years.
Conclusions
In contrast to some previous single-institution studies, we found that increased age is an independent risk factor for mortality after pancreatic resection. For all ages, mortality rates were higher at L-V hospitals, but the difference worsened significantly with increasing age. Older patients had longer lengths of stay, were less likely to be discharged home, and more likely to require care at an inpatient nursing or acute care facility at the time of discharge.
Keywords: age, pancreatic resection, short-term outcomes
People aged 65 years and older account for the fastest growing subset of the United States population. Whereas the number of people under the age of 65 has tripled in the twentieth century, the number of people 65 years or older has increased by eleven times in the same time period.1–3 In 1990, people 65 or older comprised 1/25 of the population. By 1994, this increased to 1/8 of the population and by 2030 people over 65 are expected to account for 1/5 of the population. In addition, the eldest elderly, over 85 years of age, are expected to account for 24% of the elderly population and 5% of the overall population by 2050.1
Etzioni et al have previously described the potential impact of the growing elderly population on the surgical workforce.4 They examined national discharge and U.S. Census data and predict a 28% increase in General Surgery volume between 2001 and 2020. Advancing age is one of the well-known risk factors for the development of cancer including pancreatic and other periampullary cancers. The annual incidence of pancreatic cancer increases from 2 per 100,000 population in patients 40–44 years of age to 100 per 100,000 population in patients 80–84-year-old.5 Therefore, we can expect to see an increasing number of elderly patients presenting with pancreatic cancer in the coming decades.
Over the last decade, outcomes after pancreatic resection have improved with many series reporting in-hospital or 30-day mortality rates of less than 5%.6–9 Pancreatic resection still carries significant risk with reported morbidity rates exceeding 30%.6,7,10–17 Many surgeons believe that age alone is not a contraindication to pancreatic resection. However, the data are mixed. The age cutoffs for “elderly” vary significantly among the different studies with some studies using 65 years as a cutoff, some 70 years, and some 80 years. All of these studies are single-institution studies, often with limited number of patients. Few studies have enough patients to stratify them into multiple age groups.
The majority of studies report statistically higher morbidity rates in the designated older patients when compared with younger patients.11–15,18 Some studies show no difference in mortality,9,11–13,17 whereas other demonstrate higher mortality in the group they define as “older” patients.14,15,19 Likewise, some studies show that survival is the same in the elderly population after pancreatic resection,11,12,14 whereas others demonstrate lower survival rates in elderly patients.9 The lack of consistency in defining older patients, the single-institution nature of the studies, and the inconsistent findings make the studies difficult to compare and critically evaluate.
The goals of our study were to use a population-based dataset to compare outcomes after pancreatic resection in 4 different age groups of patients to examine trends in mortality, length of stay, and discharge status with increasing age. In addition, we explored the relationship between hospital volume, age, and patient outcomes. We used a multivariate analysis to determine the independent effect of age on outcomes after controlling for other important covariates.
METHODS
Data Source
Data from the Texas Hospital Inpatient Discharge Public Use Data File (PUDF) from the years 1999 through 2005 were used for this study. The data were collected by the Texas Department of State Health Services, Texas State Health Care Information Center (THCIC), Center for Health Statistics to develop administrative reports on the use and quality of hospital care in Texas.20 The database includes all discharge records for 466 participating nonfederal hospitals in Texas. It has 205 data fields in a base data file and 13 data fields in a detailed charges file. The data include patient demographics, hospital information, lengths of stay, ICD-9 diagnosis codes, ICD-9 procedure codes, hospital day of procedure, hospital charges, payer information, and discharge status.
Study Population/Patient Characteristics
For the years 1999 through 2005, all discharges with a primary procedure code for pancreatic resection (ICD-9 procedure codes, 52.6, 52.7, 52.51, 52.52, 52.53, and 52.59; Table 1) were selected. The codes included pancreatic head resection, distal pancreatic resection, total pancreatectomy, and pancreatic resections not otherwise specified. Pancreatic resection for any reason including periampullary adenocarcinoma, chronic pancreatitis, and other benign and malignant diseases of the pancreas were included.
TABLE 1.
ICD-9 Procedure Codes
| ICD-9 Procedure Code | Definition |
|---|---|
| 52.6 | Pancreatectomy (total) with synchronous duodenectomy |
| 52.7 | Pancreaticoduodenectomy, radical (one-stage) (two-stage) |
| 52.51 | Proximal pancreatectomy (head) (with part of body) (with synchronous duodenectomy) |
| 52.52 | Distal pancreatectomy (tail) (with part of body) |
| 52.53 | Radical/subtotal pancreatectomy |
| 52.59 | Pancreatectomy/Pancreaticoduodenectomy partial NEC |
Patients were divided into 4 age groups: <60 years, 60–69 years, 70–79 years, and 80+ years. The Texas Hospital Inpatient Discharge PUDF categorizes patients into 5-year age groups and does not provide the actual age. As a result, we do not report mean or median age of the cohort and all the analysis is performed with the categorical age group designations defined above. Bivariate and multivariate analysis analyses (see statistical methods below) were used to determine the effect of age on the outcome variables: (1) in-hospital mortality, (2) discharge status, and (3) length of stay. In-hospital mortality is used because this dataset does not provide 30 day mortality information. Patients surviving the index hospitalization were classified as having been discharged home (with or without home health) or discharged to a nursing facility or other acute inpatient unit. This includes skilled nursing facilities, intermediate nursing facilities, other inpatient acute care hospitals, and Medicare- and Medicaid-certified long-term care hospitals or nursing facilities.
The Texas Health Care Information Center calculates 2 variables for the purpose of risk adjustment, the “risk of mortality” and the “illness severity.” This methodology was designed by the 3M Corporation. These are calculated based on the All Patient Defined-Diagnosis Related Grouper (APR-DRG) and consider comorbidity, age, and certain procedures to give a score from 0 to 4, with 4 being the most severe. This methodology has been validated for risk adjustment in a study linking severity of illness to hospital costs.21 The “illness severity” and “risk of mortality” scores of 0 and 1 were collapsed into a single category because very few patients had illness severity scores of 0.
We also sought to determine the relationship of hospital volume and age in outcomes after pancreatic resection. A Texas hospital was included in the analysis if at least one pancreatic resection was performed there in the 6-year time period. Hospitals were classified into H-V and L-V providers based on the 2004 Leapfrog criteria of >10 cases per year.22 Because our study spanned multiple years we used the following criteria to determine hospital volume status: (1) a minimum volume of more than 10 pancreatic resections per year for 4 of the 7 years of the study and (2) an average volume during the 7-year period of >10 pancreatic resections.
Statistical Analysis
SAS Statistical Software, version 9.1.3 (Cary, N.C.) was used for all statistical analyses. Summary statistics were calculated for the entire cohort of patients. If data were missing in certain categories the reported percentages use the number known as the denominator. However, very few data points were missing throughout the dataset. The outcome variables of interest were then compared among the 4 age groups. χ2 analysis was used to compare proportions for all categorical data. The reported χ2 P-values represent an overall test for difference between any of the 4 groups and pairwise comparisons are not performed. The actual data are shown so that the reader can see where the differences exist. All means are reported as mean ± SD. Analysis of variance (ANOVA) was used to compare means among the 4 age groups for all continuous variables. For the single admission, total hospital charges and intensive care unit (ICU) were compared among the 4 groups. Pairwise comparisons were not reported. Again, P-values represent an overall test for any differences among groups. Significance was accepted at the P < 0.05 level.
Multivariate logistic regression models were used to assess the independent effect of age on in-hospital mortality and the requirement for ongoing inpatient nursing care at discharge (discharge other than to home). All of our multivariate models controlled for differences in demographic factors, year of operation, hospital volume, procedure type, diagnosis, risk of mortality, illness severity, and admission status (emergent versus elective) among the 4 age groups. The deviance per degree of freedom was used to assess model fit. For logistic regression models we report odds ratios and 95% confidence intervals (CI) relative to a chosen reference group. Type 3 P-values for each factor. Type 3 P-values represent the independent significance of each factor with all other factors already in the model.
RESULTS
Overall Cohort
Between January 1999 and December 2005, 3,736 patients underwent pancreatic resection in Texas. The total number of resections increased from 391 in 1999 to 654 in 2005. Demographic data, diagnoses, admission status, insurance status, procedure type, and number of patients resected at H-V hospitals (>10 pancreatic resections per year) are shown in Table 2. Patients were divided into 4 age groups for the analysis. Patients <60 years of age were 47.7%, 23.7% were 60–69 years of age, 22.9% were 70–79 years of age, and 5.7% were 80 or older. Female patients were 51.7%. The age group distribution remained constant over the 7 years of the study (P = 0.58). The majority of patients were white (63.0%) and there was a significant proportion of Hispanic patients (19.1%). The remaining racial distribution is shown in Table 2.
TABLE 2.
Demographics of Overall Cohort
| N (total = 3,736) | Percent | |
|---|---|---|
| Age distribution | ||
| <60 | 1,780 | 47.7% |
| 60–69 | 887 | 23.7% |
| 70–79 | 855 | 22.9% |
| 80+ | 214 | 5.7% |
| Gender (n = 60 missing) | ||
| Female | 1,901 | 51.7% |
| Male | 1,775 | 48.3% |
| Race | ||
| White | 2,352 | 63.0% |
| Black | 397 | 10.6% |
| Hispanic | 715 | 19.1% |
| Other | 272 | 7.3% |
| Diagnosis | ||
| Periampullary cancer | 2,167 | 58.0% |
| Chronic pancreatitis | 476 | 12.8% |
| Other malignant | 554 | 14.8% |
| Other benign | 539 | 14.4% |
| Procedure | ||
| Pancreatic head resection | 2,545 | 68.1% |
| Distal pancreatic resection | 936 | 25.1% |
| Pancreatectomy, unspecified | 255 | 6.8% |
| Elective operation (n = 15 missing) | 2,689 | 72.3% |
| Insured (n = 6 missing) | 3,486 | 93.5% |
| Resected at H-V hospital (>10 cases per year) | 2,264 | 60.6% |
The indications for pancreatic resection included periampullary cancer (58.0%), chronic pancreatitis (12.8%), and other benign (14.4%) and malignant (14.8%) lesions. Patients who underwent pancreaticoduodenal resection were 68.1%, 25.1% of patients underwent distal pancreatic resection, and 6.8% of patients underwent pancreatectomy not otherwise specified. These operations were elective in 72.3% of patients and urgent or emergent in the remaining 27.7%. Over 90% of patients were insured and 60.6% of patients had their pancreatic resection performed at H-V hospitals.
Mortality/discharge status data were available on 3,723 patients. There were 180 deaths after pancreatic resection for an overall in-hospital mortality rate of 4.8%. The distribution of “illness severity scores” and “risk of mortality” scores for the overall cohort is shown in Table 3. Both of these variables were positively correlated with mortality. As the scores increased, the mortality rates increased. This is also shown in Table 3. Of the patients who survived, 90.4% of the patients were discharged to their homes and 9.6% were discharged to skilled nursing facilities. Of those discharged home, 18.4% required a home health service. The mean length of stay was 16.6 ± 14.2 days (median 13 days).
TABLE 3.
Distribution of “Risk of Mortality” and “Illness Severity” Scores and Associated In-Hospital Mortality
| Number | Percent | Observed In-Hospital Mortality Rate* | |
|---|---|---|---|
| Risk of mortality score | |||
| 1 | 1,491 | 39.9% | 0.5% |
| 2 | 1,107 | 29.6% | 1.1% |
| 3 | 739 | 19.8% | 4.2% |
| 4 | 399 | 10.7% | 32.7% |
| Illness severity score | |||
| 1 | 207 | 5.6% | 0.5% |
| 2 | 688 | 18.4% | 0.9% |
| 3 | 1,693 | 45.3% | 2.0% |
| 4 | 1,148 | 30.7% | 12.2% |
P < 0.0001 for differences in in-hospital mortality by both “risk of mortality” and “illness severity” scores.
Demographics, Diagnoses, and Procedure by Age Group
The gender distribution differed by age group, with a higher percentage of female patients in the oldest and youngest groups (P = 0.04) is shown in Table 4. The percentage of white patients increased with each increasing age comprising only 56.6% of patients <60-year-old and 74.3% of patients over 80-year-old (P < 0.0001). Likewise, the number of insured patients increased with each increasing age group (P < 0.0001), with the distribution shown in Table 4. This difference was most obvious between the patients <60-year-old and patients 60–69-year-old, when Medicare coverage begins. With each increasing age group, periampullary cancer was more likely the indication for pancreatic resection, with 76.6% of patients 80 years or older and only 44.1% of patients <60-year-old having periampullary cancer (P < 0.0001).
TABLE 4.
Demographic Characteristics, Diagnoses, and Procedure Type by Age Group
| <60 yr (N = 1,780) | 60–69 yr (N = 887) | 70–79 yr (N = 855) | 80+ years (N = 214) | P | |
|---|---|---|---|---|---|
| Female | 53.9% | 49.0% | 49.4% | 54.7% | 0.04 |
| Caucasian | 56.6% | 66.1% | 70.2% | 74.3% | <0.0001 |
| Uninsured | 9.9% | 5.8% | 1.5% | 2.3% | <0.0001 |
| Periampullary cancer | 44.1% | 68.3% | 71.6% | 76.6% | <0.0001 |
| Resected at H-V hospital | 62.3% | 61.9% | 57.4% | 53.7% | 0.01 |
| Elective procedures | 69.0% | 75.7% | 75.7% | 71.4% | 0.0002 |
| Procedure | <0.0001 | ||||
| Head resection | 59.9% | 76.0% | 75.9% | 72.4% | |
| Distal resection | 31.9% | 17.8% | 19.0% | 22.4% | |
| Pancreatic resection, unspecified | 8.2% | 6.2% | 5.1% | 5.2% |
Resection at a H-V hospital was less common as patients got older. Patients <60 years 62.3%, 61.9% of patients 60–69 years, 57.4% of patients 70–79 years, and 53.7% of patients 80 years or older were resected at H-V centers (P = 0.01). However, as the age group increased, older patients were less likely to undergo emergent surgery than their younger counterparts, suggesting more careful selection of these patients. The distribution of procedures by age group is shown in Table 4. This relationship is complex with more patients in the youngest group undergoing distal pancreatic resection (P < 0.0001).
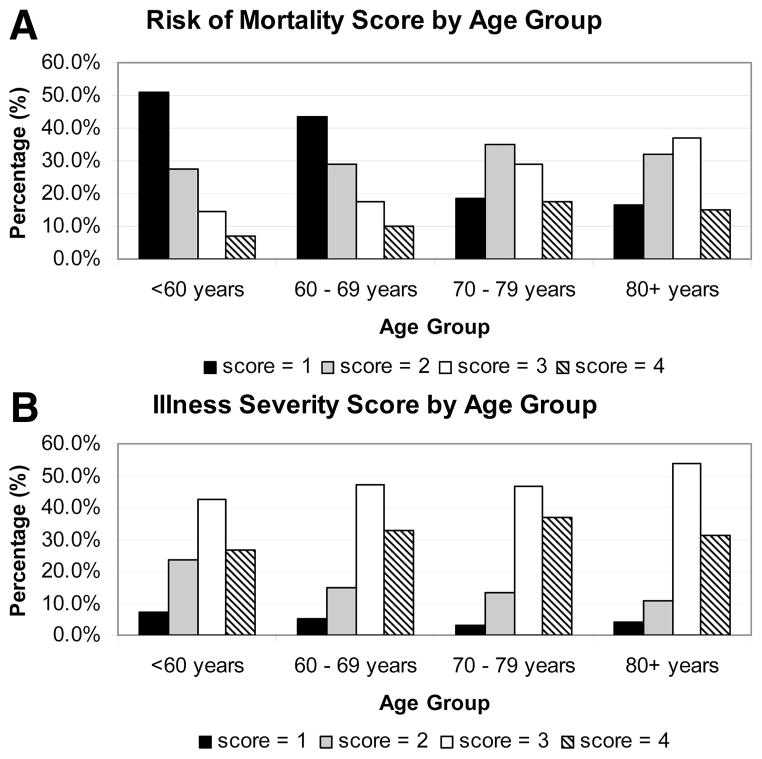
The distributions of “risk of mortality” and “severity of illness” scores differed between the age groups (P < 0.0001 for both scores) and are shown in Figure 1. For risk of mortality, as age group increased the distribution shifted toward the higher risk groups (Fig. 1A). The illness severity scores showed a similar, though less striking pattern (Fig. 1B).
FIGURE 1.
A. This bar graph represents the distribution of “risk of mortality” score within each age group category. The 4 possible scores are represented by different color bars (1 = black, 2 = gray, 3 = white, and 4 = striped). The x-axis shows the age group and the y-axis shows the percentage of patients with a particular score. For each age category, the 4 different color bars add up to 100%. With increasing age, the distribution of scores is shifted toward higher mortality risk. B. This bar graph represents the distribution of “illness severity” score within each age group category. The 4 possible scores are represented by different color bars (1 = black, 2 = gray, 3 = white, and 4 = striped). The x-axis shows the age group and the y-axis shows the percentage of patients with a particular score. For each age category, the 4 different color bars add up to 100%. With increasing age, the distribution of scores is shifted toward illness severity.
Outcomes by Age Group
In-hospital mortality increased from 2.4% to 5.8% to 7.4% to 11.4% with increasing age group (P < 0.0001) as is shown in Table 5. The median length of stay increased with increasing age group, with a median length of stay of 11 days for patients <60 years (mean = 16.0 ± 15.6 days), 13 days for patients 60–69 years (mean = 16.5 ± 12.4 days), 14 days for patients 70–79 years (mean = 17.6 ± 13.2 days), and 15 days for patients over 80 (mean = 17.9 ± 12.4 days). Data regarding length of stay include in-hospital mortalities. The median lengths of stay are unchanged when the mortalities are excluded.
TABLE 5.
Outcomes by Age Group
| <60 yr (N = 1,780) | 60–69 yr (N = 887) | 70–79 yr (N = 855) | 80+ years (N = 214) | P | |
|---|---|---|---|---|---|
| Mortality | 2.4% | 5.8% | 7.4% | 11.4% | <0.0001 |
| Discharge other than home* | 3.5% | 6.2% | 20.2% | 38.2% | <0.0001 |
| Discharge home with or without home health | 96.5% | 93.8% | 79.8% | 61.8% | <0.0001 |
| Discharge home with home health† | 13.8% | 18.7% | 27.8% | 31.3% | <0.0001 |
| Length of stay (median) | 11.0 d | 13.0 d | 14.0 d | 15.0 d | 0.02 |
| Total hospital charges (median) | $53,978 | $59,791 | $66,417 | $65,780 | 0.03 |
| ICU charges (median) | $3,131 | $3,750 | $5,520 | $6,077 | 0.009 |
Includes skilled nursing facilities, intermediate nursing facilities, other inpatient acute care hospitals, and Medicare- and Medicaid-certified long-term care hospitals or nursing facilities and excludes hospital mortalities.
Denominator is the number of patients discharged home.
At the time of discharge from the acute care facility performing the pancreatic resection, many patients required ongoing inpatient nursing care. The percentage of patients requiring continued inpatient nursing care either at a skilled nursing facility, intermediate nursing facility, another acute inpatient hospital, or other long-term care facility increased from 3.5% in patients <60 years, to 6.2% in patients 60–69 years, to 20.2% in patients 70–79 years, and to 38.2% in patients older than 80 years. Of the 90.4% patients discharged home, many required home health care. This percentage increased with age from 13.8% of patients <60 years discharged home to 31.3% of patients 80 or older discharged home (Table 5).
The total hospital charges and the ICU charges were compared among the 4 age groups. Median values are shown in Table 5. The ANOVA was statistically significant for an overall difference in total charges among the 4 groups (P = 0.03). Hospital charges increased with increasing age group for the first 3 groups, then remained relatively constant for the oldest group. For ICU charges, charges increased with each increasing age group and the overall comparison of means was statistically significant (P = 0.009).
Hospital Volume and Age Group
Overall, L-V hospitals had higher mortality rates (7.3% versus 3.2%, P < 0.0001). Furthermore, the difference in mortality between H- and L-V hospitals was accentuated with each increasing age group (P < 0.0001). For patients <60 years, the mortality at L-V hospitals was 3.0% whereas the mortality at H-V hospitals was 2.0%. In the 60–69 year age group, mortality increased to 9.5% at L-V hospitals and only 3.5% at H-V hospitals. For patients 70–79 years, mortality increased to 11.4% at L-V hospitals and 4.5% at H-V hospitals. Finally in patients older than 80 years, mortality increased to 14.5% at L-V hospitals and 8.7% at H-V hospitals.
Multivariate Analysis
We used logistic regression models to assess the independent effect of age group on mortality. The final model, shown in Table 6, controls for gender, race, year of surgery, diagnosis, admission status, “risk of mortality,” “illness severity,” hospital volume, and procedure type. When compared with patients <60 years of age, age group was an independent predictor of mortality after pancreatic resection (P = <0.0001), with patients age 60–69 being 2.53 times more likely to die (95% CI: 1.53 – 4.17), patients age 70–79 being 1.81 times more likely to die (95% CI: 1.12 – 2.93), and patients 80 and older being 4.45 times more likely to die (95% CI: 2.31 – 8.57) after pancreatic resection. Whereas gender (P = 0.17), race (P = 0.27), diagnosis (P = 0.07), and admission status (P = 0.06) did not independently predict survival, hospital volume (P = 0.0003), year of diagnosis (P = 0.01), “risk of mortality” score (P < 0.0001), and procedure type (P = 0.02) were independent predictors of mortality.
TABLE 6.
Multivariate Logistic Regression Analysis
| Factor | In-Hospital Mortality
|
Discharge Other Than Home
* |
||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Age group | ||||
| <60 | 1.00 | — | 1.00 | — |
| 60–69 | 2.53 | 1.53–4.17 | 1.91 | 1.27–2.87 |
| 70–79 | 1.81 | 1.12–2.93 | 5.61 | 3.95–7.96 |
| 80+ | 4.45 | 2.31–8.57 | 16.35 | 10.41–25.67 |
| Gender | ||||
| Male | 1.00 | — | 1.00 | — |
| Female | 0.77 | 0.54–1.11 | 1.16 | 0.90–1.50 |
| Race | ||||
| White | 1.00 | — | 1.00 | — |
| Black | 1.57 | 0.91–2.67 | 1.50 | 1.00–2.26 |
| Hispanic | 0.95 | 0.59–1.54 | 0.98 | 0.69–1.39 |
| Other | 1.48 | 0.752.91 | 1.35 | 0.82–2.52 |
| Hospital volume | ||||
| Low–volume (≤10/yr) | 1.00 | — | 1.00 | — |
| High–volume (>10/yr) | 0.50 | 0.34–0.73 | 0.46 | 0.35–0.60 |
| Year of operation | 0.89 | 0.81–0.97 | 1.07 | 1.00–1.15 |
| Diagnosis | ||||
| Periampullary cancer | 1.00 | — | 1.00 | — |
| Other | 1.47 | 0.97–2.21 | 1.27 | 0.93–1.72 |
| Risk of mortality | ||||
| 1 | 1.00 | — | 1.00 | — |
| 2 | 1.87 | 0.70–4.96 | 1.49 | 0.98–2.28 |
| 3 | 6.23 | 2.55–15.22 | 3.20 | 2.11–4.86 |
| 4 | 60.92 | 25.2–147.3 | 4.81 | 2.957.85 |
| Illness severity | ||||
| 1 | 1.00 | — | 1.00 | — |
| 2 | 1.19 | 0.14–10.49 | 3.94 | 0.50–30.78 |
| 3 | 0.57 | 0.07–4.97 | 9.63 | 1.27–72.97 |
| 4 | 1.03 | 0.12–8.97 | 15.00 | 1.96–115.12 |
| Admission status | ||||
| Elective | 1.00 | — | 1.00 | — |
| Urgent/emergent | 1.45 | 0.98–2.14 | 1.30 | 0.98–1.72 |
| Procedure | ||||
| Head resection | 1.00 | — | 1.00 | — |
| Distal resection | 0.46 | 0.25–0.85 | 0.66 | 0.44–0.99 |
| Pancreatectomy, not specified | 0.47 | 0.20–1.09 | 1.11 | 0.62–1.97 |
Includes skilled nursing facilities, intermediate nursing facilities, other inpatient acute care hospitals, and Medicare– and Medicaid–certified long–term care hospitals or nursing facilities and excludes hospital mortalities.
A separate logistic regression model was used to evaluate the independent effect of age group on discharge other than to home (including inpatient nursing care either at a skilled nursing facility, intermediate nursing facility, transfer to another acute inpatient hospital, or other long-term care facility). Again, age group was predictive, with patients 60–69 years being 1.91 times as likely to require continued inpatient care (95% CI: 1.27 – 2.87), patients 70–79 year being 5.61 times as likely to require continued inpatient care (95% CI: 3.95 – 7.96), and patients 80 years and older being 16.35 times more likely than patients less than 60-year-old to require continued inpatient care (95% CI: 10.4 – 25.7). Hospital volume (P < 0.0001), year of diagnosis (P = 0.04), “risk of mortality” score (P < 0.0001), and “illness severity” score (P < 0.0001) were significant independent predictors as well. The final model is also shown in Table 6.
DISCUSSION
This report is the first population-based study evaluating the effect of age on short-term outcomes after pancreatic resection. In contrast to previous studies that arbitrarily chose the cutoff to define old or elderly,11–19 our study is unique in that it analyzes 4 different age groups enabling us to demonstrate distinct trends with increasing age. To our knowledge, it is the first study to examine the effect of age on whether patients can be discharged home or require continued in-patient nursing care at discharge. In addition, it is the first study of significant size to allow the use of multivariate analysis to assess the independent effect of age on outcomes after pancreatic resection, thereby controlling for multiple covariates which may also influence the same outcomes.
Our study shows that increasing age group is a significant independent predictor for mortality after pancreatic resection after controlling for gender, race, year of surgery, diagnosis, admission status, “risk of mortality,” “illness severity,” hospital volume, and procedure type. The length of stay increased with increasing patient age group. Increasing age independently predicts the requirement for continued in-patient nursing care at discharge from the hospital from less than 4% in patients <60 years of age to over 38% in patients 80 and older. Likewise, in the patients discharged home, the need for home health care was higher with each increasing age group. Finally, this study demonstrates that with increasing age patients are less likely to be resected at H-V hospitals and that hospital volume disproportionately affects mortality as the age group increases.
With increasing age, ICU charges increased as might be expected. The same pattern was observed with total hospital charges for the first 3 age groups. The similar charges in the last 2 age groups imply careful selection of the most ideal patients in the oldest group. We did not perform a more in-depth economic analysis because we cannot determine readmission rates, charges from readmissions, charges at nursing facilities, or preoperative charges that occurred separate from the admission for surgery. In addition, we have data on hospital charges, but do not have actual cost data.
The observed discrepancies between our population-based data and many previous studies are likely multifactorial. All previous studies are single-institution studies with some having low numbers of patients. In many of these studies, there mortality rates were higher in the older age groups, but the study was underpowered to demonstrate a statistical difference.13,14,16,19 For example, Bathe et al retrospectively evaluated a total of 70 patients. Fifty-four were less 65–74-year-old and 16 were 75 years or older. Their older age group had a mortality rate of 25% compared with only 3.7% in the younger group, but this difference was not statistically significant.14 In addition, the age cutoff in most previous studies varied from 65 to 80 years. Some previous studies do not demonstrate a difference in length of stay.9,12,17 In 2 studies from the same institution, one shows a difference in length of stay11 whereas the other does not.12 The latter study was performed 8 years after the first and reported an overall decreased length of stay. The introduction of critical pathways for the care of patients undergoing pancreatic surgery during this time period likely explains the difference between the 2 studies. Our study demonstrates longer lengths of stay as age group increases, as others have reported.11,14
By multivariate analysis, we found that age was a significant independent predictor of mortality after pancreatic resection. However, when compared with patients <60-year-old, the odds ratio for in-hospital mortality in patients 60–69 was lower than the odds ratio for patients 70–79. We speculate that this may reflect increased aggressiveness with resection of larger tumors and subsequently more complications in younger patients.
We used the “risk of mortality” and “illness severity” scores calculated by the THCIC to control for patient comorbidities. The use of the “risk of mortality” and “illness severity” scores based on the 3M methodology has been validated for risk adjustment in a study linking severity of illness to hospital costs.21 These scores are further validated in the bivariate analysis in which both “risk of mortality” and “illness severity” scores are strongly positively correlated with in-hospital mortality rates. We did not use a Charlson comorbidity index,23 commonly used with administrative data, as this index is designed for longitudinal data. Our data represent data from single admissions making the Charlson score invalid for this purpose.
The year of operation was an independent predictor of mortality, with mortality decreasing with each increasing year of diagnosis. As the number of pancreatic resections has increased each year, this finding is likely the result of improved surgical technique, better ICU care and better management of complications with increased experience over the last decade. Conversely, with each increasing year of diagnosis, patients were more likely to require continued in-patient nursing care at discharge. This is not a result of older patients undergoing more pancreatic head resections, having more cancers, or being more severely ill, as the model controls for these factors (and others, see Table 6). We hypothesize that more patients are surviving the complications from these procedures. This is reflected in an increasing number of patients being unable to go home secondary to debilitation and increased nursing needs such as wound care, drain care, etc. Many patients who might have died in previous decades may now survive until discharge, but require too much nursing care to be discharged home.
We recognize that our study has several limitations. We are unable to evaluate readmission rates because the public uses the data file that does not provide a unique patient identifier allowing us to link admissions. Similarly, we cannot evaluate long-term survival with this data set. Because we are using administrative data, procedures are well coded, but overall morbidity rates and specific complications such as pancreatic fistula, delayed gastric emptying, and intraabdominal abscess are difficult to identify. Lastly, age is provided in 5-year categories, so we cannot provide mean and median age or evaluate age as a continuous variable.
To our knowledge this is one of the largest studies evaluating the effect of age on short-term outcomes after pancreatic resection. The population-based nature of the study increases generalizability over previous single institution reports. Increased age is an independent risk factor for mortality after pancreatic resection and the inability to be discharged home. Whereas age alone should not be a contraindication to pancreatic resection it is important for surgeons to recognize that older patients had higher inhospital mortality rates, longer lengths of stay, were less likely to be discharged home, and more likely to require care at an inpatient nursing or acute care facility at the time of discharge. Older patients were less likely to be resected at H-V hospitals and our data demonstrate that the effect of hospital volume on mortality is accentuated with increasing age. It will be important to develop algorithms to carefully select elderly patients for these procedures and to maximize referral to H-V centers for elderly patients having pancreatic surgery to optimize their outcomes. Another population-based analysis of long-term survival after pancreatic resection in cancer patients by age group is warranted as is a more in-depth longitudinal economic analysis.
Acknowledgments
Taylor S. Riall was supported by the Dennis W. Jahnigen Career Development Award.
References
- 1.United States Census Bureau. [Accessed 3/14/08];Sixty-five plus in the United States. 2001 http://www.census.gov/population/socdemo/statbriefs/agebrief.html.
- 2.U.S. Department of Commerce, U.N. Department of Public Information. An aging world 2001, DP/2264. Mar, 2002. [Google Scholar]
- 3.Anderson RN, DeTurk PB. United States Life Tables, 1999. National Vital Statistics Report. 2002;50:33. [PubMed] [Google Scholar]
- 4.Etzioni DA, Liu JH, Maggard MA, et al. The aging population and its impact on the surgery workforce. Ann Surg. 2003;238:170–177. doi: 10.1097/01.SLA.0000081085.98792.3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lillemoe KD. Pancreatic disease in the elderly patient. Surg Clin North Am. 1994;74:317–344. [PubMed] [Google Scholar]
- 6.Winter JM, Cameron JL, Campbell KA, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: A single-institution experience. J Gastrointest Surg. 2006;10:1199–1210. doi: 10.1016/j.gassur.2006.08.018. discussion 1210–1211. [DOI] [PubMed] [Google Scholar]
- 7.Balcom JHT, Rattner DW, Warshaw AL, et al. Ten-year experience with 733 pancreatic resections: changing indications, older patients, and decreasing length of hospitalization. Arch Surg. 2001;136:391–398. doi: 10.1001/archsurg.136.4.391. [DOI] [PubMed] [Google Scholar]
- 8.Yeo CJ, Sohn TA, Cameron JL, et al. Periampullary adenocarcinoma: analysis of 5-year survivors. Ann Surg. 1998;227:821–831. doi: 10.1097/00000658-199806000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fong Y, Blumgart LH, Fortner JG, et al. Pancreatic or liver resection for malignancy is safe and effective for the elderly. Ann Surg. 1995;222:426–434. doi: 10.1097/00000658-199522240-00002. discussion 434–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geer RJ, Brennan MF. Prognostic indicators for survival after resection of pancreatic adenocarcinoma. Am J Surg. 1993;165:68–72. doi: 10.1016/s0002-9610(05)80406-4. discussion 72–73. [DOI] [PubMed] [Google Scholar]
- 11.Sohn TA, Yeo CJ, Cameron JL, et al. Should pancreaticoduodenectomy be performed in octogenarians? J Gastrointest Surg. 1998;2:207–216. doi: 10.1016/s1091-255x(98)80014-0. [DOI] [PubMed] [Google Scholar]
- 12.Makary MA, Winter JM, Cameron JL, et al. Pancreaticoduodenectomy in the very elderly. J Gastrointest Surg. 2006;10:347–356. doi: 10.1016/j.gassur.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 13.Scurtu R, Bachellier P, Oussoultzoglou E, et al. Outcome after pancreaticoduodenectomy for cancer in elderly patients. J Gastrointest Surg. 2006;10:813–822. doi: 10.1016/j.gassur.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 14.Bathe OF, Levi D, Caldera H, et al. Radical resection of periampullary tumors in the elderly: evaluation of long-term results. World J Surg. 2000;24:353–358. doi: 10.1007/s002689910056. [DOI] [PubMed] [Google Scholar]
- 15.Brozzetti S, Mazzoni G, Miccini M, et al. Surgical treatment of pancreatic head carcinoma in elderly patients. Arch Surg. 2006;141:137–142. doi: 10.1001/archsurg.141.2.137. [DOI] [PubMed] [Google Scholar]
- 16.al-Sharaf K, Andren-Sandberg A, Ihse I. Subtotal pancreatectomy for cancer can be safe in the elderly. Eur J Surg. 1999;165:230–235. doi: 10.1080/110241599750007090. [DOI] [PubMed] [Google Scholar]
- 17.Hodul P, Tansey J, Golts E, et al. Age is not a contraindication to pancreaticoduodenectomy. Am Surg. 2001;67:270–275. doi: 10.1016/s0016-5085(00)81967-8. discussion 275–276. [DOI] [PubMed] [Google Scholar]
- 18.Richter A, Schwab M, Lorenz D, et al. Surgical therapy of pancreatic carcinoma in elderly patients over 70. Langenbecks Arch Chir Suppl Kongressbd. 1996;113:492–494. [PubMed] [Google Scholar]
- 19.Bottger TC, Engelmann R, Junginger T. Is age a risk factor for major pancreatic surgery? An analysis of 300 resections. Hepatogastroenterology. 1999;46:2589–2598. [PubMed] [Google Scholar]
- 20.Texas Department of State Health Services THCIC. http://www.dshs.state.tx.us/thcic/Hospitals/HospitalData.shtm.
- 21.Averill RF, McGuire TE, Manning BE, et al. A study of the relationship between severity of illness and hospital cost in New Jersey hospitals. Health Serv Res. 1992;27:587–606. discussion 607–12. [PMC free article] [PubMed] [Google Scholar]
- 22.Birkmeyer JD, Dimick JB. Potential benefits of the new Leapfrog standards: effect of process and outcomes measures. Surgery. 2004;135:569–575. doi: 10.1016/j.surg.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Baldwin LM, Klabunde CN, Green P, et al. In search of the perfect comorbidity measure for use with administrative claims data: does it exist? Med Care. 2006;44:745–753. doi: 10.1097/01.mlr.0000223475.70440.07. [DOI] [PMC free article] [PubMed] [Google Scholar]