Abstract
BACKGROUND
Although warfarin therapy reduces stroke incidence in patients with atrial fibrillation (AF), the rate of warfarin use in this population remains low. In 2008, the Medicare Part D program was expanded to pay for medications for Medicare enrollees.
OBJECTIVE
To examine rates and predictors of warfarin use in Medicare Part D beneficiaries with AF.
METHODS
This population-based retrospective cohort study used claims data from 41,447 Medicare beneficiaries aged 66 and older with at least 2 AF diagnoses in 2007 and at least 1 diagnosis in 2008. All subjects had continuous Medicare Part D prescription coverage in 2008. Statistical analysis using χ2 was used to examine differences in warfarin use by patient characteristics (age, ethnicity, sex, Medicaid eligibility, comorbidities, contraindications to warfarin, and whether they visited a cardiologist or a primary care physician [PCP]), CHADS2 score (congestive heart failure, hypertension, age, diabetes, and stroke or transient ischemic attack; higher scores indicate higher risks of stroke), and geographic regions. Using hierarchical generalized linear models restricted to subjects without warfarin contraindications (n = 34,947), we examined the effect of patient characteristics and geographic regions on warfarin use.
RESULTS
The overall warfarin use rate was 66.8%. The warfarin use rates varied between hospital referral regions, with highest rates in the Midwestern states and lowest rates in the South. The regional variation persisted even after adjustment for patient characteristics. Multivariable analysis showed that the odds of being on warfarin decreased significantly with age and increasing comorbidity, in blacks, and among those with low income. Seeing a cardiologist (OR 1.10; 95% CI 1.05–1.16), having a PCP (OR 1.23; 95% CI 1.17–1.29), and CHADS2 score of 2 or greater (OR 1.09; 95% CI 1.01–1.17) were associated with increased odds of warfarin use.
CONCLUSIONS
Warfarin use rates vary by patient characteristics and region, with higher rates among residents of the Midwest and among patients seen by cardiologists and PCPs. Preventing stroke-related disability in AF requires implementation of evidence-based initiatives to increase warfarin use.
Stroke is a leading cause of serious, long-term disability and the third leading cause of death in the US.1 Atrial fibrillation (AF) increases stroke risk 5-fold and accounts for approximately 15% of all strokes.2,3 AF affects 12% of adults aged 75 years and older and its prevalence is expected to double by 2050.2,4–6 Warfarin, an oral anticoagulant, reduces annual risk of ischemic stroke risk by approximately two thirds in patients with AF, from 4.5% to 1.4%.7–9 Except for patients at a very low risk for stroke, practice guidelines published by the Ameri-can College of Cardiology Foundation, American Heart Association, and other scientific bodies recommend warfarin therapy for stroke prevention in AF patients without contraindications.2,10 Despite this recommendation, the use of warfarin in AF patients remains low, with rates ranging from 39% to 65%.11–14 Increasing the use of interventions (eg, warfarin and other anticoagulants) to prevent stroke is an important public health issue.
Warfarin is a complex drug to use. The required frequent blood testing and dose adjustments, along with the perceived risk of bleeding (especially gastrointestinal and intracranial bleeds) are common barriers to warfarin prescribing and optimal patient adherence.15–19 Recently introduced oral anticoagulant agents (dabigatran [a direct thrombin inhibitor] and apixaban and rivaroxaban [factor Xa inhibitors]) have potential to reduce these barriers because they have fixed doses and require no blood testing.20–22 Clinical trials on these new agents showed similar or better efficacy in stroke prevention and a better adverse effect profile compared with warfarin.20–22 As more AF patients use these warfarin alternatives, it is important to understand the magnitude of potential warfarin underuse and the reasons for such underuse. Such understanding may help us anticipate (and plan for) therapeutic challenges (eg, toxicity misperceptions and anticoagulant underprescription) that may arise from use of these new anticoagulants. For example, regardless of availability of and access to oral anticoagulants, the decision to use warfarin is often based on the risks versus benefits perceived by the physician,15,23 which vary by patient. A retrospective cohort study has shown that physicians were less likely to use warfarin therapy after patient exposure to any adverse bleeding event, as compared to before the event.23 This lack of precision in practice patterns in warfarin prescribing contributes to variability in warfarin’s use.
There is regional variation in stroke prevalence. Although studies show little evidence linking regional variation in stroke to variation in stroke risk factors, the quality of management of such risk factors as AF, hypertension, smoking, and diabetes may explain some regional and racial variation.4,15,24,25 However, little is known about regional and statewide variations in the use of warfarin and other oral therapies for stroke prevention in Medicare enrollees with AF, in part because of the lack of a large nationwide database for outpatient oral drugs. The few studies conducted have small sample size or have been limited to restricted Medicare populations such as long-term care residents, managed-care beneficiaries, hospitalized patients, or patients in specific regions or health care settings.11–15,26
In 2006 the Medicare Part D program was implemented; in 2008 the program paid for outpatient prescription medications for approximately 27 million enrollees. The existence of this nationwide outpatient medication database allowed for the examination of national patterns of warfarin use in a large population-based sample. With the expanded coverage of medications by the Medicare Part D program, we wanted to know the rates and predictors of warfarin use in older patients with AF. We thus assessed the national rates of warfarin use across the US by patient characteristics and geographic location. This investigation is an important step in improving our understanding of the previously reported regional differences in stroke rate and in determining whether such regional differences parallel regional variations in the management of AF, a key risk factor for stroke.
Methods
DATA SOURCES
Claims from 2007 and 2008 for a 5% national sample of Medicare beneficiaries were used, including Medicare beneficiary summary files, Medicare Provider Analysis and Review (MedPAR) files, Outpatient Standard Analytic Files (OUTSAF), Medicare Carrier files, and Prescription Drug Event (PDE) files.
STUDY COHORT ESTABLISHMENT
Medicare beneficiaries with at least 2 AF diagnoses in 2007 and at least 1 in 2008 were selected (N = 108,777). The AF diagnoses included patients with AF and atrial flutter, identified by International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code 427.3. We excluded beneficiaries who were enrolled in health maintenance organizations (HMOs), did not have continuous Medicare Parts A and B coverage during 2007 and 2008, did not have continuous Medicare Part D coverage in 2008, or were younger than 66 years in 2008 (n = 66,970). Using ICD-9-CM code V66.7, we further excluded beneficiaries who had encounters for palliative care (including end-of-life care, hospice care, and terminal care) in 2008 (n = 81), and those who were ever on enoxaparin, fondaparinux, dalteparin, or tinzaparin at some time in 2008, but not on warfarin (n = 279), leaving 41,447 subjects in the study cohort. The University of Texas Medical Branch Institutional Review Board on human protection and research ethics approved the study.
MEASURES
We categorized beneficiaries by age, sex, and race/ethnicity using Medicare beneficiary summary files. We used the Medicaid indicator in 2008 as a proxy of low socioeconomic status. We calculated the CHADS2 (congestive heart failure, hypertension, age, diabetes, and stroke or transient ischemic attack) score in 2007 by adding 1 point for each of the following conditions: congestive heart failure (ICD-9-CM codes 398.91, 428.x), hypertension (401.x-405.x, 437.2), age 75 years or older, diabetes (250.x), and 2 points for stroke or transient ischemic attack (TIA, 342.x, 433.x-438.x, V12.54). CHADS2 is a validated risk score for predicting stroke in the setting of untreated AF, with higher scores indicating higher risks. In the multivariable analysis, we used a modified CHADS2 score by excluding the age criterion, since we were interested in studying the direct effect of age on warfarin use. Excluding age from the CHADS2 score also eliminated the collinearity between age and the CHADS2 score in the multivariable analysis.
A contraindication to anticoagulation was defined as having at least 1 diagnosis (in any position of the listed diagnoses by the Medicare providers) associated with hospitalization or at least 2 diagnoses (in any position) at least 30 days apart from Carrier/OUTSAF. The contraindications were based on the ICD-9-CM codes. The ICD-9-CM codes identifying the contraindications in 2007 are as follows: falls (E884.9, E887, E888.8, E888.9, E929.3, V15.88); gastrointestinal hemorrhage/disorders (456.0, 456.1 456.2, 531.x, 532.x, 533.x, 534.x, 569.3, 578.x); nongastrointestinal hemorrhage/disorders (287, 360.43, 363.61, 363.62, 363.72, 364.41, 372.72, 459.0, 568.81, 596.7, 599.7, 626.6, 627.1, 719.1x, 782.7, 784.7, 786.3); intracranial hemorrhage (430, 431, 432.x); endocarditis, pericarditis, and ruptured or dissecting aorta (421, 423, 441); and liver diseases and other associated conditions (303, 571, 572, 573).8,11,13,14,17 Claims with the aforementioned ICD-9-CM codes were obtained from the MedPAR, OUTSAF, and Carrier files. A modified Elixhauser comorbidity score27 in 2007 excluding conditions associated with the modified CHADS2 score (congestive heart failure, paralysis/stroke, diabetes, and hypertension) was calculated using claims from 2007.
Cardiologist visits in 2007 were determined by Part B claims in the Carrier files, with cardiology being the physician specialty. To find the primary care physician (PCP) for the cohort subjects, we first identified outpatient visits using American Medical Association Current Procedural Terminology evaluation and management codes 99201 to 99205 (new patient encounters) and 99211 to 99215 (established patient encounters). Individual providers were differentiated by using their Unique Physician Identification Number or National Provider Identifier. Physician specialty was based on Part B claims in the Carrier files. Those with multiple specialty codes were assigned the specialty that appeared most often in their claims. A PCP was defined as a general practitioner, family physician, internist, or geriatrician who saw the patient on 2 or more occasions in an outpatient setting in 2007. If a beneficiary had multiple PCPs in 2007, the PCP with the highest number of visits was assigned as that beneficiary’s PCP. In cases of ties in visits, the most recently visited PCP was assigned.
STUDY OUTCOMES
Our study outcome was having at least 2 warfarin prescriptions filled on different dates in 2008. This was determined by examining the PDE records for the study cohort.
STATISTICAL ANALYSES
The percentage of beneficiaries who received warfarin in 2008 was calculated and stratified by patient characteristics. χ2 Testing was used to examine differences in warfarin use rates by characteristics.
To account for the clustering effect of patients within Hospital Referral Regions (HRRs), hierarchical generalized linear models were used to examine the association of patient characteristics with the likelihood of receiving warfarin. The multilevel analysis excluded beneficiaries with diagnoses known to be contraindications to warfarin use. All analyses were performed with SAS version 9.2 (SAS Inc., Cary, NC). The Dartmouth Atlas of Health Care was accessed to establish HRRs to analyze regional variation.28 The map showing warfarin use rate in HRRs was constructed using ArcMap 9.3 for the HRRs that had at least 20 cohort subjects.
Results
Table 1 presents the overall warfarin use rate and results of bivariate (unadjusted) analysis of variations in rate according to patient characteristics, region, and CHADS2 score. The overall rate of warfarin use in the entire study cohort was 66.8%. Unadjusted correlates of lower rates of warfarin use are increasing age, being nonwhite, female sex, having low income, and increasing number of comorbid conditions. Seeing a cardiologist or PCP was associated with higher rates of warfarin use. Unexpectedly, warfarin use rate was lower in those with CHADS2 scores of 2 or more compared to those with a CHADS2 score of 0. Additional analysis (table not shown) revealed that patients with a CHADS2 score of 2 or more are older (mean age ± SD 81.5 [6.8]) and have more comorbidities (mean Elixhauser comorbidity score 1.8 [1.6]) than those with a CHADS2 score of 0 (mean age 70.6 [2.6] and mean Elixhauser comorbidity score 0.7 [1.0]). The low rate of warfarin use likely reflects the reluctance of clinicians to prescribe warfarin to the elderly and those with multiple comorbidities, regardless of CHADS2-based treatment guide-line recommendations.
Table 1.
Characteristics of Patients with Diagnoses of Atrial Fibrillation and Warfarin Use Rate
| Characteristic | Pts., n (%) | Warfarin Use (%) | p Valuea |
|---|---|---|---|
| Overall | 41,447 (100) | 66.8 | |
| Age in 2008, years | |||
| 66–69 | 4,283 (10.3) | 70.2 | |
| 70–74 | 7,060 (17.0) | 71.3 | |
| 75–79 | 9,287 (22.4) | 70.3 | |
| 80–84 | 9,817 (23.7) | 68.3 | |
| 85–89 | 7,175 (17.3) | 62.6 | |
| 90–94 | 3,088 (7.5) | 53.3 | |
| ≥95 | 737 (1.8) | 38.9 | <0.001 |
| Race/ethnicity | |||
| non-Hispanic white | 37,770 (91.1) | 67.3 | |
| black | 1,532 (3.7) | 59.0 | |
| Hispanic | 1,205 (2.9) | 64.6 | |
| other/unknown | 940 (2.3) | 60.9 | <0.001 |
| Sex | |||
| male | 16,404 (39.6) | 68.7 | |
| female | 25,043 (60.4) | 65.6 | <0.001 |
| Census division | |||
| New England | 2,874 (6.9) | 69.7 | |
| Middle Atlantic | 6,350 (15.3) | 67.6 | |
| East North Central | 6,642 (16.0) | 68.5 | |
| West North Central | 3,929 (9.5) | 71.6 | |
| South Atlantic | 8,145 (19.7) | 65.2 | |
| East South Central | 2,967 (7.2) | 65.3 | |
| West South Central | 4,203 (10.1) | 62.7 | |
| Mountain | 1,854 (4.5) | 68.6 | |
| Pacific | 4,483 (10.8) | 64.2 | <0.001 |
| Contraindications for warfarin in 2007 | |||
| 0 | 34,947 (84.3) | 68.6 | |
| 1 | 5,745 (13.9) | 58.2 | |
| ≥2 | 755 (1.8) | 51.3 | <0.001 |
| Cardiologist visit in 2007 | |||
| no | 15,921 (38.4) | 64.1 | |
| yes | 25,526 (61.6) | 68.5 | <0.001 |
| Medicaid eligibility in 2008 | |||
| no | 32,432 (78.2) | 68.3 | |
| yes | 9,015 (21.8) | 61.3 | <0.001 |
| Elixhauser comorbidity score | |||
| 0 | 3,090 | 74.0 | 0 |
| 1–2 | 15,524 (37.5) | 70.0 | 1 |
| 3–4 | 12,145 (29.3) | 67.1 | ≥2 |
| ≥5 | 10,688 (25.8) | 59.8 | <0.001 |
| PCP visit in 2007 | |||
| no | 12,689 (30.6) | 63.0 | |
| yes | 28,758 (69.4) | 68.5 | <0.001 |
| CHADS2 score in 2007b | |||
| 0 | 1,903 (5.5) | 69.3 | |
| 1 | 7,398 (21.2) | 71.0 | |
| ≥2 | 25,646 (73.4) | 67.8 | <0.001 |
CHADS2 = congestive heart failure, hypertension, age, diabetes, and stroke or transient ischemic attack; PCP = primary care physician.
χ2 Test.
Use rates in this category were from patients without contraindication for warfarin.
Table 2 presents the results of a multivariable analysis of the association between patients’ characteristics and the odds of their using warfarin in 2008. Patients with contraindications to warfarin were excluded from this analysis. The modified CHADS2 score (without the age criterion) was used in the analysis to study the independent effect of age on warfarin use. Compared with non-Hispanic whites, blacks were less likely to use warfarin (OR 0.86; 95% CI 0.76–0.97). There was no significant difference by sex. Patients aged 85 years or older, Medicaid beneficiaries, and those with multiple comorbidities were also less likely to use warfarin. Factors associated with significantly increased odds of warfarin use were seeing a cardiologist in 2007 (OR 1.10; 95% CI 1.05–1.16), having a PCP in 2007 (OR 1.23; 95% CI 1.17–1.29), and having a modified CHADS2 score of 2 or greater (OR 1.09; 95% CI 1.01–1.17).
Table 2.
Effect of Patient Characteristicsa on Warfarin Use by Multilevel Model
| Characteristic | OR (95% CI) |
|---|---|
| Age in 2008, years | |
| 66–69 | Reference |
| 70–74 | 1.05 (0.95–1.15) |
| 75–79 | 1.05 (0.96–1.15) |
| 80–84 | 0.95 (0.87–1.04) |
| ≥85 | 0.64 (0.59–0.70) |
| Race | |
| non-Hispanic white | Reference |
| black | 0.86 (0.76–0.97) |
| Hispanic | 0.96 (0.84–1.11) |
| other/unknown | 0.81 (0.69–0.95) |
| Sex | |
| male | 1.04 (0.99–1.09) |
| female | Reference |
| Census division | |
| New England | 1.49 (1.26–1.76) |
| Middle Atlantic | 1.39 (1.22–1.59) |
| East North Central | 1.33 (1.17–1.50) |
| West North Central | 1.62 (1.40–1.86) |
| South Atlantic | 1.13 (1.01–1.28) |
| East South Central | 1.09 (0.93–1.26) |
| West South Central | Reference |
| Mountain | 1.27 (1.07–1.50) |
| Pacific | 1.08 (0.94–1.23) |
| Cardiologist visit in 2007 | |
| no | Reference |
| yes | 1.23 (1.17–1.29) |
| CHADS2 scoreb in 2007 | |
| 0 | Reference |
| 1 | 0.92 (0.86–0.99) |
| ≥2 | 1.09 (1.01–1.17) |
| Medicaid eligibility in 2008 | |
| no | Reference |
| yes | 0.89 (0.84–0.95) |
| Elixhauser comorbidity scorec | |
| 0 | Reference |
| 1–2 | 0.88 (0.84–0.93) |
| 3–4 | 0.67 (0.63–0.72) |
| ≥5 | 0.56 (0.51–0.63) |
CHADS2 = congestive heart failure, hypertension, age, diabetes, and stroke or transient ischemic attack; PCP = primary care physician.
Patients with contraindications were excluded.
The age criteria from the CHADS2 score were removed so that the effect of age alone could be studied.
Excluding conditions associated with the CHADS2 score (congestive heart failure, paralysis, diabetes, and hypertension) to prevent collinearity.
Figure 1 shows the rates of warfarin use in 2008 among patients living in the 306 HRRs in the US. The patients with contraindications to warfarin use were not included in this analysis. In general, patients in the Midwest had the highest rate of warfarin use by HRR. The South had the lowest rate of warfarin use, with less than a 60% use rate in many HRRs. These regional variations persisted even after adjusting for ethnicity and other patient characteristics (Table 2).
Figure 1.
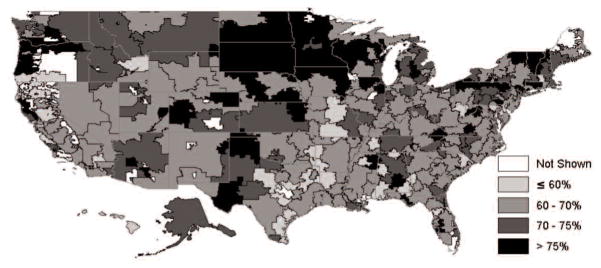
Warfarin use rate by hospital referral region. Patients with contraindication were excluded. Rates for Hospital Referral Regions with less than 20 eligible cohort subjects are not shown (n = 8).
Discussion
Past studies of the rate of warfarin use in older Americans with AF have focused on specific populations such as HMO enrollees, nursing home residents, or single-site health care facility inpatients.11,15 The recent availability of Medicare Part D data allowed us to examine rates and predictors of warfarin use in Medicare fee-for-service beneficiaries with diagnoses of AF across the US. We found an overall use rate of 66.8%. Previous studies of patients with AF reported a warfarin use rate ranging from 39% to 65%.11–14 The wide variations in previously reported rates in part reflect differences in settings (community hospitals vs Veterans Administration hospitals), characteristics of the population (nursing home residents vs community-dwelling HMO enrollees), and methods of determining warfarin use (medical chart review vs pharmacy database).11,14
Our findings of lower warfarin use among the very old and blacks are consistent with prior findings.8,11 Warfarin use declines with age.14,15,26 This may reflect concern about the risk of intracranial hemorrhage, a risk cited in several studies as a major reason for not choosing warfarin therapy in the elderly.11,16,29 Warfarin use has also been reported to be lower among black and Hispanic Medicare patients with AF.8,11 The unexpected finding of a lower unadjusted warfarin use rate among patients with a CHADS2 score of 2 or greater can be attributed to the higher percentage of the very old and those with high comorbidities in this group compared to the group with a lower CHADS2 score. However, when we adjusted for age and comorbidities in the multivariable model, those with a modified CHADS2 score (without the age criterion) of greater than 2 had higher odds of being on warfarin compared to those with a modified CHADS2 score of 0. Our findings suggest that clinicians might be relying more on age and comorbidity burden and less on CHADS2 score in determining eligibility for warfarin use in patients with AF. Future study is needed to examine whether this therapeutic inertia based on advanced age and multimorbidity will continue or decline with the newly approved oral anticoagulants (eg, dabigatran), given their more standardized dosing and no indication for routine blood testing.
Our findings of lower warfarin use among ethnic minorities and those with no PCP or cardiologist visits have public health implications with regard to reducing stroke-related disability. Nonwhites (ethnic minorities) have higher rates of stroke and stroke risk factors (eg, diabetes and hypertension, but excluding AF) than whites.4,6,30,31 Despite the lower risk of AF reported among blacks compared to whites,6 blacks have a higher risk of AF-related stroke, suggesting suboptimal management of stroke risk factors such as AF. Data from the REGARDS (Reasons for Geographic and Racial Differences in Stroke) study also show that blacks have lower odds of being aware of their AF compared to whites (OR 0.32; 95% CI 0.20–0.52).4 A similar low awareness rate has been reported for those with hyperlipidemia.31 Our findings of lower warfarin use in blacks suggest that stroke reduction policies (eg, increased access to PCPs or cardiologists) may help reduce the excess stroke risk in ethnic minorities, perhaps by increasing awareness and treatment of AF and other cardiovascular risk factors.
Analysis by HRR revealed that the Midwest has the highest rates of warfarin use. Past surveys of physicians have shown warfarin use in AF to vary by region, with the lowest rate in the South and the highest in the Midwest.26,32 Geographic and patient variation in warfarin use is a public health issue. This issue is a microcosm of a larger problem: stroke risk and mortality vary by geographic region in the US, with the highest rates in the Stroke Belt (Alabama, Arkansas, Georgia, Indiana, Kentucky, Louisiana, Mississippi, North Carolina, South Carolina, Tennessee, and Virginia).30,31 Identifying the pattern of underutilization of warfarin is a critical first step in addressing disparities in the use of this important treatment among older adults. Planning such efforts will require a better understanding of multilevel factors (patients, providers, health systems, environment, and cultures) in these states where age-adjusted stroke mortality rates are 10% or more above the national mean. This excess prevalence was accounted for by race/ethnicity, socioeconomic status (education and income), risk factors (smoking and obesity), and suboptimally managed chronic diseases (hypertension, hyperlipidemia, diabetes, coronary heart disease).4,30,31 This variation by region may also reflect a difference in quality of treatment of stroke risk factors (especially AF) and/or unawareness of such risk factors.4
One important consideration is the potential impact of the newly introduced oral anticoagulant drugs on patterns of undertreatment of patients with AF. While these drugs show similar or even better efficacy than warfarin in preventing stroke in AF, they are expensive.20–22,33 Overall, warfarin has lower out-of-pocket costs for patients. Although frequent blood testing adds to the health care system’s overall cost, Medicare patients do not pay for the tests. However, data from the RE-LY (Randomized Evaluation of Long-Term Anticoagulation Therapy) and other anticoagulation trials showed dabigatran to be more cost-effective than warfarin for stroke prevention among AF patients aged 65 years or older with a CHADS2 score of 1 or higher.33 With the current study findings of low income predicting underuse of warfarin, future study is needed to examine whether the new oral anticoagulants (warfarin alternatives) would actually change patients’ adherence, clinicians’ reluctance to prescribe an anticoagulant, and the overall rate of anticoagulant use for stroke prevention in AF.
As Medicare Part D data on the new anticoagulant users accumulate, it is important to revisit long-term trends in the rate of anticoagulant (old and new) use and examine the impact of patient and physician factors on such trends. Potential safety concerns (eg, increased risk of myocardial infarction34 and lack of antidote for bleeding complications) associated with the newly introduced anticoagulants may also impact long-term anticoagulant use rate. The emerging safety concerns with new agents underscore the need for more comparative studies of efficacy, safety, and cost effectiveness of new agents versus older agents. It is particularly important that such future cost-benefit and cost-effectiveness studies of warfarin alternatives go beyond using warfarin as the drug of comparison. The comparison should also extend to newly introduced oral anticoagulants versus no anticoagulant use; the omission of anticoagulation for stroke prevention is still highly prevalent in patients with AF, as shown in our study.
Our study has several limitations. First, Medicare Part D claims data did not include information about a Medicare beneficiary’s preference regarding use of warfarin or the extent to which patients adhered to their prescribed regimen. Second, we have no information about out-of-pocket payments and the ability of patients to afford warfarin, despite warfarin being an inexpensive generic drug. However, we adjusted for this by using Medicaid eligibility as a marker of low income. Third, we were unable to assess factors affecting physicians’ decisions as to whether to prescribe warfarin treatment for different subpopulations, even in the face of a high CHADS2 score. Was avoidance of warfarin treatment attributable to the perceived higher risks in such populations, additional primary care cost and time, and/or administrative burden of managing the frequent visits for prothrombin testing? Future study is needed to answer these questions.
Fourth, our study did not analyze clinical outcomes (incidence of stroke or bleeding) stratified by warfarin use. The outcome in our study was rate of warfarin use in AF patients and which factors (patient and geographic characteristics) predicted such use, using 2007–2008 Medicare Part D claims data. As more years of Medicare Part D data become available, future studies are needed on how warfarin use versus nonuse, time in therapeutic INR, and frequency of warfarin prescription fillings affect incidence of outcomes such as stroke, transient ischemic attack, and bleeding complications. Such outcome studies might require the use of recently validated schemes (eg, HAS-BLED [Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile International Normalized Ratio, Elderly, Drugs/Alcohol Concomitantly]) to estimate risk of bleeding prior to anticoagulation and a new stratification scoring system (CHADS2-VASc) to predict risk of stroke.35,36 CHADS2-VASc has been shown to be better at predicting stroke than the CHADS2 score, especially in AF patients traditionally classified as being at low risk (CHADS2 score of 0 or 1). The CHADS2-VASc includes additional criteria of vascular disease, age 65 to 75 years, and sex category.35 Finally, our sample population is mostly white, with exclusion of those younger than 66 years of age or enrollees in Medicare HMOs. Thus, we cannot extrapolate our findings to these populations. Despite these limitations, our study has several strengths, including its large cohort size, availability of Medicare Part D data on outpatient prescriptions, information on comorbidities, and a wide geographic representation of the Medicare population.
In summary, our study of 41,447 Medicare Part D program enrollees aged 66 and older with AF showed that warfarin use rates vary by region and by patient characteristics, with higher rates among residents of the Midwest and in patients seen by cardiologists and PCPs. We found lower rates among persons aged 85 years or older, in persons with low income, in those with multimorbidity, and in blacks. Our findings of lower warfarin use in ethnic minorities, a population known to have high risk of stroke and stroke risk factors, suggest that policies that increase access to PCPs or cardiologists may reduce the excess stroke risk in this vulnerable population with AF. Improving awareness and treatment of stroke risk factors such as AF and increasing access to primary care are key to reducing stroke-related disability and mortality in older Americans.
Acknowledgments
Funding: This study was supported in part by National Institutes of Health Grants R01 AG033134, K05 CA134923, and P30 AG024832. Matthew Lowery received support from Summer Research Training in Aging for Medical Students grant NIH-T35-AG026778. The sponsors had no role in the design, methods, participant recruitment, data collection, and analysis, or in preparation of the manuscript.
Footnotes
Reprints/Online Access: www.theannals.com/cgi/reprint/aph.1R515
Conflict of interest: Authors reported none
Contributor Information
Mukaila A Raji, Professor & Director, Division of Geriatrics; Department of Internal Medicine, Sealy Center on Aging, University of Texas Medical Branch (UTMB), Galveston, TX.
Matthew Lowery, Sealy Center on Aging, UTMB.
Yu-Li Lin, Biostatistician, Sealy Center on Aging, UTMB.
Yong-Fang Kuo, Associate Professor, Sealy Center on Aging, UTMB.
Jacques Baillargeon, Associate Professor, Sealy Center on Aging, Department of Preventive Medicine & Community Health, UTMB.
James S Goodwin, Professor & Director, Sealy Center on Aging, UTMB.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics–2011 update. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lip GY, Tse HF, Lane DA. Atrial fibrillation. Lancet. 2012;379:648–61. doi: 10.1016/S0140-6736(11)61514-6. [DOI] [PubMed] [Google Scholar]
- 3.Wolf PA, Abbot RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–8. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 4.Meschia JF, Merril P, Soliman EZ, et al. Racial disparities in awareness and treatment of atrial fibrillation: the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Stroke. 2010;41:581–7. doi: 10.1161/STROKEAHA.109.573907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–25. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 6.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–5. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 7.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have non-valvular atrial fibrillation. Ann Intern Med. 2007;146:857–67. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 8.Birman-Deych E, Radford MJ, Nilasena DS, Gage BF. Use and effectiveness of warfarin in Medicare beneficiaries with atrial fibrillation. Stroke. 2006;37:1070–4. doi: 10.1161/01.STR.0000208294.46968.a4. [DOI] [PubMed] [Google Scholar]
- 9.Aguilar MI, Hart R. Oral anticoagulants for preventing stroke in patients with non-valvular atrial fibrillation and no previous history of stroke or transient ischemic attacks. Cochrane Database Syst Rev. 2005;3:CD001927. doi: 10.1002/14651858.CD001927.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuster V, Ryden LE, Cannom DS, et al. ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation. Circulation. 2011;123:e269–e367. doi: 10.1161/CIR.0b013e318214876d. [DOI] [PubMed] [Google Scholar]
- 11.Srivastava A, Hudson M, Hamoud I, Cavalcante J, Pai C, Kaatz S. Examining warfarin underutilization rates in patients with atrial fibrillation: detailed chart review essential to capture contraindications to warfarin therapy. Thromb J. 2008;6:6. doi: 10.1186/1477-9560-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darkow T, Vanderplas AM, Lew KH, Kim J, Hauch O. Treatment patterns and real-world effectiveness of warfarin in nonvalvular atrial fibrillation within a managed care system. Curr Med Res Opin. 2005;21:1583–94. doi: 10.1185/030079905X61956. [DOI] [PubMed] [Google Scholar]
- 13.Weisbord SD, Whittle J, Brooks RC. Is warfarin really underused in patients with atrial fibrillation? J Gen Intern Med. 2001;16:743–9. doi: 10.1111/j.1525-1497.2001.10432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Go AS, Hylek EM, Borowsky LH, Phillips KA, Selby JV, Singer DE. Warfarin use among ambulatory patients with nonvalvular atrial fibrillation: the Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. Ann Intern Med. 1999;131:927–34. doi: 10.7326/0003-4819-131-12-199912210-00004. [DOI] [PubMed] [Google Scholar]
- 15.Bungard TJ, Ghali WA, Teo KK, McAlister FA, Tsuyuki RT. Why do patients with atrial fibrillation not receive warfarin? Arch Intern Med. 2000;160:41–6. doi: 10.1001/archinte.160.1.41. [DOI] [PubMed] [Google Scholar]
- 16.Fang MC, Go AS, Hylek EM, et al. Age and risk of warfarin-associated hemorrhage: the Anticoagulation and Risk Factors in Atrial Fibrillation Study. J Am Geriatr Soc. 2006;54:1231–6. doi: 10.1111/j.1532-5415.2006.00828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fang MC, Go AS, Hylek EM, et al. Incidence of intracranial hemorrhage in patients with atrial fibrillation who are prone to fall. Am J Med. 2005;118:612–7. doi: 10.1016/j.amjmed.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 18.Man-Son-Hing M, Laupacis A. Balancing risks of stroke and upper gastrointestinal tract bleeding in older patients with atrial fibrillation. Arch Intern Med. 2002;162:541–50. doi: 10.1001/archinte.162.5.541. [DOI] [PubMed] [Google Scholar]
- 19.Wysowski DK, Nourjah P, Swartz L. Bleeding complications with warfarin use: a prevalent adverse effect resulting in regulatory action. Arch Inten Med. 2007;167:1414–9. doi: 10.1001/archinte.167.13.1414. [DOI] [PubMed] [Google Scholar]
- 20.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 21.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–92. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 22.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–91. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 23.Choudhry NK, Anderson GM, Laupacis A, Ross-Degnan D, Normand SL, Soumerai SB. Impact of adverse events on prescribing warfarin in patients with atrial fibrillation: matched pair analysis. BMJ. 2006;332:141–5. doi: 10.1136/bmj.38698.709572.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Howard G, Cushman M, Prineas RJ, et al. Advancing the hypothesis that geographic variations in risk factors contribute relatively little to observed geographic variations in heart disease and stroke mortality. Prev Med. 2009;49:129–32. doi: 10.1016/j.ypmed.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cushman M, Cantrell RA, McClure LA, et al. Estimated 10-year stroke risk by region and race in the United States: geographic and racial differences in stroke risk. Ann Neurol. 2008;64:507–13. doi: 10.1002/ana.21493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stafford RS, Singer DE. National patterns of warfarin use in atrial fibrillation. Arch Intern Med. 1996;156:2537–41. [PubMed] [Google Scholar]
- 27.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 28.The Dartmouth Atlas of Health Care. [accessed 2011 Jun 7];Zip code crosswalk files of 2007. http://www.dartmouthatlas.org/tools/downloads.aspx.
- 29.Fang MC, Chang Y, Hylek EM, et al. Advanced age, anticoagulation intensity, and risk for intracranial hemorrhage among patients taking warfarin for atrial fibrillation. Ann Intern Med. 2004;141:745–52. doi: 10.7326/0003-4819-141-10-200411160-00005. [DOI] [PubMed] [Google Scholar]
- 30.Liao Y, Greenlund KJ, Croft JB, Keenan NL, Giles WH. Factors explaining excess stroke prevalence in the US Stroke Belt. Stroke. 2009;40:3336–41. doi: 10.1161/STROKEAHA.109.561688. [DOI] [PubMed] [Google Scholar]
- 31.Zweifler RM, McClure LA, Howard VJ, et al. Racial and geographic differences in prevalence, awareness, treatment and control of dyslipidemia: the reasons for geographic and racial differences in stroke (REGARDS) study. Neuroepidemiology. 2011;37:39–44. doi: 10.1159/000328258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stafford RS, Singer DE. Recent national patterns of warfarin use in atrial fibrillation. Circulation. 1998;97:1231–3. doi: 10.1161/01.cir.97.13.1231. [DOI] [PubMed] [Google Scholar]
- 33.Freeman JV, Zhu RP, Owens DK, et al. Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in atrial fibrillation. Ann Intern Med. 2011;154:1–11. doi: 10.7326/0003-4819-154-1-201101040-00289. [DOI] [PubMed] [Google Scholar]
- 34.Uchino K, Hernandez AV. Dabigatran association with higher risk of acute coronary events: meta-analysis of noninferiority randomized controlled trials. Arch Intern Med. 2012;172:397–402. doi: 10.1001/archinternmed.2011.1666. [DOI] [PubMed] [Google Scholar]
- 35.Olesen JB, Lip GY, Hansen ML, et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ. 2011;342:d124. doi: 10.1136/bmj.d124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lane DA, Lip GY. Use of the CHA2DS2-VASc and HAS-BLED scores to aid decision making for thromboprophylaxis in nonvalvular atrial fibrillation. Circulation. 2012;126:860–5. doi: 10.1161/CIRCULATIONAHA.111.060061. [DOI] [PubMed] [Google Scholar]


