Abstract
Background
Substance use by youth living with HIV (YLWH) is a concern, given potential interactions with virus-associated immune suppression and adverse effects on risk behaviors, neurocognition, and adherence. Self-report substance use measures provide efficient cost-effective assessments. Analyses describe self-reported substance use among YLWH and examine agreement with toxicology assays.
Methods
Seventy-eight youth age 18–24 years (87% male, 71% African–American) with behaviorally acquired HIV-1 infection and 55 uninfected youth completed the Alcohol, Smoking, and Substance Involvement Screening Test to assess drug use frequency, including tobacco, marijuana, cocaine, and alcohol, over the prior three months. Elisa-based toxicology assays were used to detect 27 substances in plasma. Chi-square tests compared substance use between YLWH and uninfected youth; Kappa statistics compared agreement between self-report and toxicology.
Results
YLWH reported marijuana (49%), tobacco (56%), and alcohol (87%) use, with 20%, 28% and 3% reporting daily use of each substance, respectively; other substance use was uncommon. Uninfected youth reported less tobacco use but otherwise similar substance use. All youth who reported daily use of marijuana or tobacco had positive plasma toxicology results, while concordance decreased with less frequent self-reported use. Among youth reporting no substance use, few tested positive (4% YLWH, 2% uninfected youth for cannabis; 8%YLWH for tobacco).
Conclusions
Youth report high rates of marijuana, tobacco, and alcohol use. Concordance between self-report and toxicology for marijuana and tobacco use, particularly for daily users, supports self-report as a valid indicator of substance use in research studies of youth with or without HIV-1 infection.
Keywords: HIV, Adolescent, Substance use, Marijuana, Self-report, Toxicology
1. Introduction
Substance use is one of the most critical issues facing late adolescents and young adults (“youth”; Centers for Disease Control and Prevention, 2012) with or at risk for HIV infection and clinicians who work with them. Substance use is prevalent among youth in general, with a shift towards greater use and acceptance of marijuana (Brasseux et al., 1998), daily use of which is greater than at any other time in the last 30 years (Johnston et al., 2012) among high-school age youth.
A primary reason for concern regarding substance use among youth with HIV is its potential for contributing to disease progression, morbidity and transmission. Recent studies of changes in and patterns of substance use among youth with behaviorally acquired HIV, however, are lacking. Youth, who make up the largest group of new HIV infections (Centers for Disease Control and Prevention, 2011), may be at greater risk than adults for negative central nervous system outcomes related to substance use due to their ongoing brain development (Sowell et al., 2003) and the potentially greater impact of substance use during adolescence than adulthood (Squeglia et al., 2009). Interactions of the immune and central nervous system effects of cannabis and other substances of abuse with those of HIV in youth are a growing concern. Youth are also characterized by higher rates of sexual risk behaviors (Eaton et al., 2012) and poor medication adherence (Tanney et al., 2010); exacerbation of these risk behaviors by substance use has implications for HIV prevention and treatment outcome. In addition, tobacco use is higher among individuals with HIV than in the general population (Webb et al., 2007), and is an important contributor to non-AIDS-defining as well as AIDS-defining illness and mortality in this population (Helleberg et al., 2013; Shirley et al., 2013). Accurate assessment of substance use among youth with HIV has great importance for research on HIV treatment and outcomes as well as for clinical care.
Self-report is an efficient and cost effective means of assessing substance use and is particularly appropriate for studies where substance use is not the primary study outcome. However, concern that societal, family and legal pressures against drug and alcohol use may lead to underreporting by adolescents raises questions about the validity of using self-report. Concerns have been raised about under-reporting in studies to validate self-report substance use measures by comparison with biological measures in adults; however, a recent meta-analysis suggests validity of self-report for individual substances in mental health settings (Large et al., 2012). Relatively few studies have focused systematically on youth self-report, and those that have varied widely in methodology, drugs of focus, setting, and findings. The majority of studies have been conducted with youth who have or are at risk for substance use disorders. These studies have reported a range of findings, including under-reporting the frequency of cocaine use over six months compared to hair analysis (Delaney-Black et al., 2010), both under- and over-reported marijuana use over 48 h in comparison with urine toxicology screens (Akinci et al., 2001), and generally acceptable agreement (Solbergsdottir et al., 2004). A study of youth enrolled in or referred for substance abuse treatment showed both under-and over-reporting of substance use in structured interviews compared with urine toxicology, concluding that self-report has only fair validity (Williams and Nowatzki, 2005).
Efforts have been made to maximize likelihood of honest reporting by youth in research settings by measures designed to protect the confidentiality of their self-report. For example, Certificates of Confidentiality allow research sites to maintain privacy of substance use data. Computer-based interviews can transmit de-identified youth self-report directly to central data processing organizations so that site personnel are not aware of the participants’ responses to the interview. By using such self-report methods designed to minimize confidentiality concerns, Fendrich and colleagues (2005) compared self-report using audio computer-assisted self-interview (ACASI) with toxicology measures in an examination of tobacco use in a community sample of individuals age 18 and older. They found that under-reporting of tobacco use was uncommon, but was higher compared to previous studies, which the authors speculated might be related to increasing social undesirability of smoking.
Although under-reporting of substance use has been documented for adults with HIV (Hormes et al., 2012), few studies have reported validity of substance use self-report in an adolescent population with HIV. Murphy and colleagues (2000), in a study of high-risk youth age 13–20 both with and without HIV, used ACASI to query participants regarding the timing of their last marijuana use and compared their self-report with urine cannabinoid testing, about which participants had been informed in advance. This study found higher prevalence by self-report than by toxicological testing, and better agreement between self-report and urine toxicology when use was reported in the previous 5 or 7 than in the previous 2 day period. Agreement of self-report with toxicology was higher for HIV-infected than for uninfected participants. The authors concluded that self-report may more accurately reflect marijuana use over periods longer than 48 h than does urine cannabinoid testing, and for that reason has advantages for estimating prevalence of use; furthermore, self-report may be particularly advantageous for youth with HIV.
The purpose of the present analyses was to extend the findings of Murphy and colleagues by examining the relationship of self-report ACASI measures of substance use with plasma testing of a range of substances of abuse. The study cohort included individuals with HIV in the late adolescent/young adult age range (18–24) who were participating in a study of neurocognitive consequences of behaviorally acquired HIV infection. These analyses add to previous findings by addressing tobacco, alcohol and other drugs of abuse in addition to marijuana; using highly sensitive plasma toxicology assays; and assessing self-reported frequency of use over a longer time period. Because of concerns that youth who smoke cannabis in combination with tobacco would under-report tobacco use, analyses included co-use of marijuana and tobacco. Agreement of self-reported substance use and plasma toxicology also was analyzed for a group of similar HIV uninfected youth.
2. Methods
2.1. Participants
Youth aged 18–24 years with behaviorally acquired HIV-1 infection and CD4+ T cell counts >350 were enrolled from 15 Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN) and 12 International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) sites located in largely urban settings across the US and Puerto Rico into a prospective longitudinal study (ATN 061) of the preservation and expansion of T-cell subsets following initiation of antiretroviral therapy (ART). Among the 102 youth enrolled in ATN 061, 78 co-enrolled in a longitudinal study of neurocognitive functioning that included self-report assessment of substance use (ATN 071). Self-report, clinical and laboratory data from these 78 participants, who constitute the YLWH group, obtained at study entry were used for analyses. Exclusion criteria included prior ART experience (other than less than 6 month duration to prevent mother-to-child transmission of HIV), pregnancy, active substance use or dependence to a degree judged likely to interfere with meeting study requirements, psychosis, or significant non-HIV related cognitive or motor impairment (e.g., cerebral palsy or severe traumatic brain injury). Learning disabilities and Attention-Deficit/Hyperactivity Disorder (ADHD) were allowed. Fluency in English or Spanish was required. HIV uninfected youth of similar age, gender and ethnicity to the YLWH group were recruited from a single urban site in Florida. These youth were volunteer university students and youth followed at adolescent care clinics who were self-declared to be healthy. Inclusion criteria included age 18 to 24, HIV-uninfected, and no recent systemic illnesses, vaccinations, or pregnancy. CD4 T cell counts were obtained at entry for all subjects and HIV RNA viral load, for the YLWH group, was abstracted from medical records or study visit case report forms. The study was approved by the Institutional Review Board (IRB) at all participating institutions; participants provided written informed consent in accordance with local IRB requirements prior to enrollment.
2.2. Study evaluations
2.2.1. Substance use self-report
YLWH completed the World Health Organization Alcohol, Smoking, and Substance Involvement Screening Test (ASSIST; World Health Organization, 2002) via an audio computer-assisted self-interview (ACASI) conducted using a designated laptop computer in a private room. Data were transmitted immediately to a central data processing system and were not available to site personnel. The ASSIST was developed and validated as a substance use screening tool for individuals age 18 and above and has been used in studies of youth with HIV (Naar-King et al., 2010). It queries frequency of use during the past 3 months for tobacco products, alcohol, cannabis, cocaine, amphetamines, inhalants, sedatives, hallucinogens, opioids, and other, with frequency categories of “Never”, “Once or Twice”, “Monthly”, “Weekly”, and “Daily”. Other questions about consequences of substance use (e.g., substance use leading to health, social, legal or financial problems), estimated lifetime use, and age of first use were not included in these analyses. YLWH completed the ASSIST and had blood samples drawn as part of their participation in separate studies and were not aware that the two would be compared. Uninfected youth completed a written version of the ASSIST questionnaire. For uninfected youth, plasma samples were obtained at the same visit and they were informed that toxicology assays for drugs would be performed.
2.2.2. Toxicology assays
Plasma samples were analyzed by Immunalysis Corporation (Pomona, CA; Immunalysis.com), utilizing enzyme-linked immunosorbent assays (ELISA) for 27 analytes including common over-the-counter, prescription, and illicit drugs. The cannabinoids (Δ9-tetrahydrocannabinol, THC) ELISA assay detects acute (<1 day) and chronic use of marijuana. Tobacco is detected as cotinine, a nicotine metabolite, up to 7 days after use. Positive results emanating from passive inhalation of either marijuana or tobacco are unlikely (Wall et al., 1988). The alcohol assay, an enzymatic method to quantitate ethyl alcohol in plasma, was performed.
2.3. Statistical Analyses
Percentages of demographic characteristics between YLWH and uninfected groups were compared using chi-square tests. The median of age and clinical data (CD4 absolute and CD4%) were analyzed by Mann–Whitney tests between YLWH and uninfected groups. Proportions of substance use by toxicology between YLWH and uninfected groups were compared using chi-square tests, and proportions for frequency of use by self-report (“Never”, “Once or Twice”, “Monthly”, “Weekly”, and “Daily”) between YLWH and uninfected groups were analyzed by Wilcoxon tests. Proportions of substance use by toxicology and self-report between male and female groups were compared using Fisher’s exact test. Agreement between self-report and toxicology assays was evaluated using the Kappa statistic. All statistical analyses were performed by using R version 2.15.1(source; http://www.r-project.org) with significance set as p < 0.05.
3. Results
3.1. Participant demographics
The majority of participants were male, including two YLWH subjects self-identifying as transgender (Table 1). Most were African American but there was a higher proportion of Caucasians (p = 0.003) in the uninfected group and higher other/mixed race in the YLWH group (p = 0.002). YLWH were slightly younger, median age 21 years compared to 22 years for uninfected youth (p = 0.01) with more uninfected youth having completed high school or equivalent at the time of assessment (p = 0.005). For the YLWH group, median log10 viral load was 4.18 copies/mL (quartile range 3.83–4.50) and median CD4 T-cell count was 499 (quartile range 412–693) cells/µl, lower than the median CD4 T-cell count for the uninfected group of 701 (quartile range 497–875) cells/µl (p = 0.0007).
Table 1.
Demographic and disease severity characteristics of HIV-infected and uninfected groups.
| YLWH N = 78 | Uninfected N = 55 | P-value | |
|---|---|---|---|
| Count (%) | |||
| Gender (at birth) | |||
| Male* | 68 (87) | 40 (73) | 0.0607 |
| Female | 10 (13) | 15 (27) | 0.0607 |
| Transgender | 2 (3) | 0 (0) | 0.6361 |
| Race | |||
| African American | 55 (71) | 37 (67) | 0.8353 |
| Caucasian | 8 (10) | 18 (33) | 0.0027 |
| Other/Mixed race | 15 (19) | 0 (0) | 0.0015 |
| Completed high school or equivalent | 58 (74) | 52 (95) | 0.0051 |
| Median [Quartiles] | |||
| Age (years) | 21 [20,22] | 22 [20,23] | 0.0101 |
Includes transgender.
3.2. Self-report of substance use
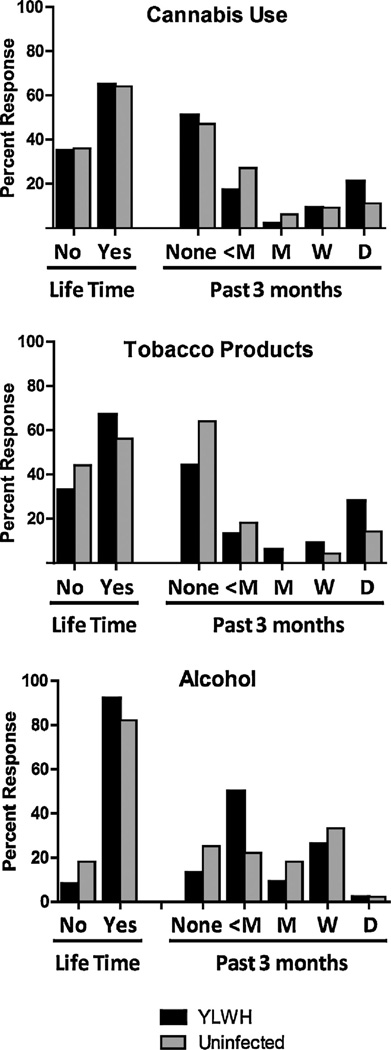
Fig. 1 shows frequency of self-reported use for cannabis, tobacco products, and alcohol. Self-reported use of other substances was less common in either group (YLWH, 18%; uninfected youth, 5%) and included self-reported use of opioids (heroin or prescription opioids including codeine, hydrocodone, hydromorphone, meperidine, morphine, oxycodone, and propoxyphene), amphetamines, barbiturates, benzodiazepines, inhalants, lysergic acid diethylamide, and ketamine. Positive self-reported use over the past three months among YLWH was 49% for cannabis (20% daily use), 56% for tobacco (28% daily use), and 87% for alcohol (3% daily use). Positive self-reported use for uninfected youth was 53% for cannabis (11% daily use), 36% for tobacco products (15% daily use), and 75% for alcohol (2% daily use).
Fig. 1.
Frequency of self-reported substance use, lifetime and in past three months.
3.3. Toxicology results
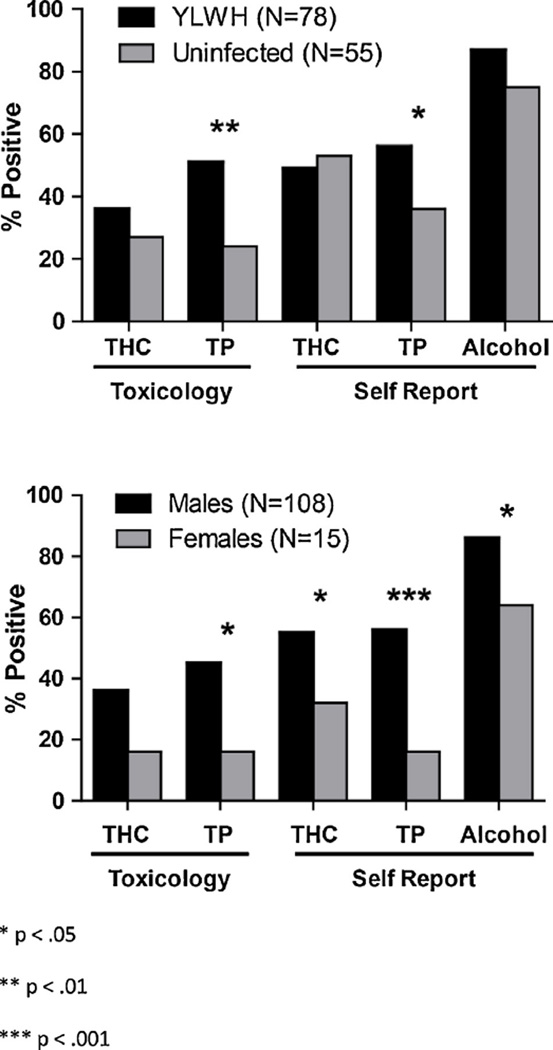
Positive plasma toxicology assays for YLWH were 36% for cannabinoids, 51% for cotinine, and 4% (one case) each for methamphetamine/amphetamine, benzodiazepines, and opioids. Positive toxicology assays for the uninfected group were 27% for cannabinoids, 24% for cotinine, and 5% (one case) each for methamphetamine/amphetamine, fluoxetine, oxycodone, and dextromethorphan. There were no positive assays for any other substances.
3.4. Differences by group and gender in substance use by self-report and toxicology
The YLWH and uninfected groups differed significantly in number of positive assays for cotinine (p = 0.0013; see Fig. 2). The YLWH group reported significantly higher tobacco product use (p = 0.023); self-report of cannabis and alcohol did not differ significantly (p = 0.65, p = 0.062). Male participants were significantly more likely than female participants to have positive assays for cotinine (p = 0.0067). Across groups, male participants were significantly more likely to report use of cannabis (p = 0.048), tobacco products (p = 0.00033), and alcohol (p = 0.018).
Fig. 2.
Reported use and differences between groups with and without HIV infection and between males and females in substance use as measured by self-report and toxicology for cannabinoids (THC), tobacco products (TP), and alcohol.
3.5. Concordance between self-report and toxicology results
Overall concordance between any self-reported use of cannabis and toxicology assay was 79% for YLWH and 87% for uninfected youth (κ = 0.587, p = 0.432). Similarly, concordance for tobacco products was 79% for YLWH and 84% for uninfected youth (κ = 0.588, p = 0.618). Concordance varied by self-reported frequency of use, with 100% of youth who reported daily use and 71–100% of youth reporting weekly use of either marijuana or tobacco having positive toxicology screens (see Table 2). Four percent of YLWH and 2% of uninfected youth denied use of cannabis but tested positive, and 8% of YLWH denied use of tobacco but tested positive. There were too few women in the study to examine concordance by gender.
Table 2.
Concordance of self-report and toxicology assays for THC and cotinine.
| YLWH N = 78 | Uninfected N = 55 | |||||
|---|---|---|---|---|---|---|
| Cannabis | ||||||
| Reported NO | Tested positive | Concordance | Reported NO | Tested positive | Concordance | |
| 40 | 3 | 93% | 26 | 1 | 96% | |
| Reported YES | Tested positive | Concordance | Reported YES | Tested positive | Concordance | |
| 38 | 25 | 29 | 14 | |||
| Daily | 16 | 16 | 100% | 6 | 6 | 100% |
| Weekly | 7 | 6 | 86% | 5 | 5 | 100% |
| Monthly | 2 | 0 | 0% | 3 | 1 | 33% |
| Once/twice | 13 | 3 | 23% | 15 | 2 | 13% |
| Overall κ = 0.587, p = 0.432 | ||||||
| Tobacco products | ||||||
| Reported no. | Tested positive | Concordance | Reported no. | Tested positive | Concordance | |
| 34 | 6 | 82% | 35 | 1 | 97% | |
| Reported yes | Tested positive | Concordance | Reported yes | Tested positive | Concordance | |
| 44 | 34 | 20 | 12 | |||
| Daily | 22 | 22 | 100% | 8 | 8 | 100% |
| Weekly | 7 | 5 | 71% | 2 | 2 | 100% |
| Monthly | 5 | 3 | 60% | 0 | 0 | – |
| Once/twice | 10 | 4 | 40% | 10 | 2 | 20% |
Overall κ = 0.588, p = 0.618.
3.6. Dual substance use
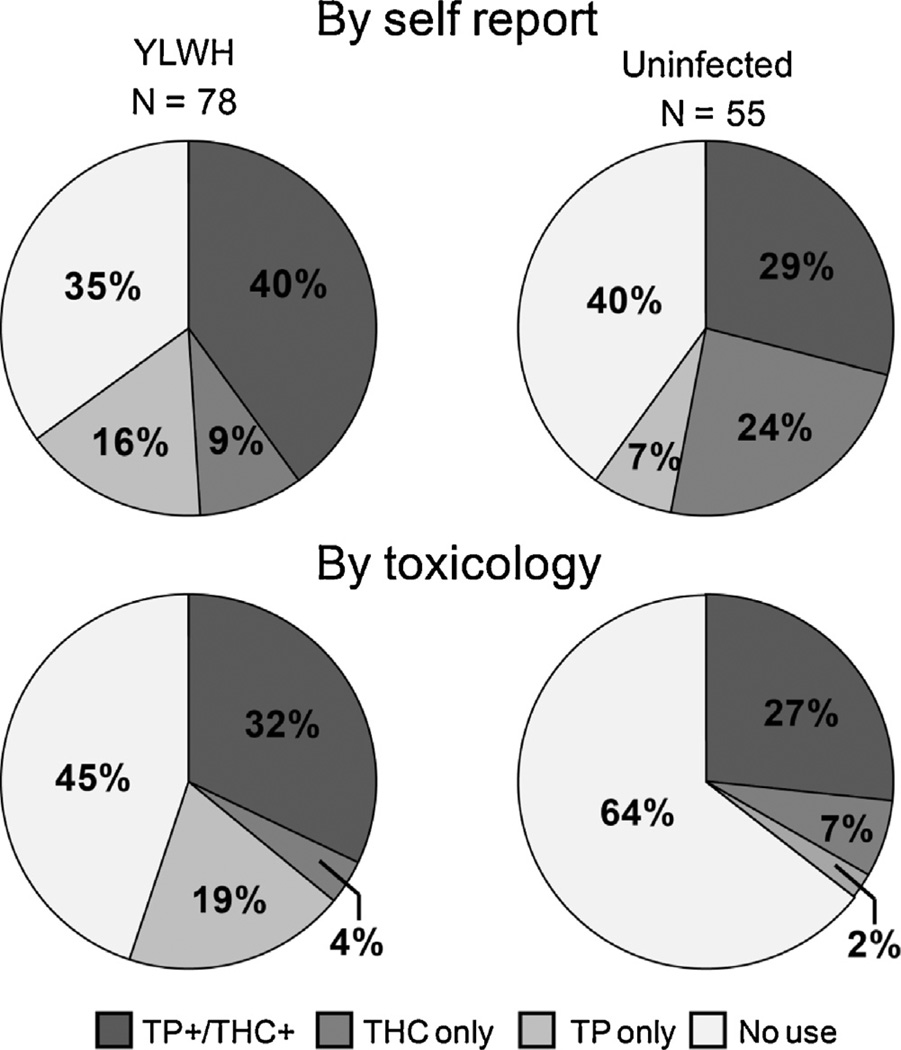
Use of both cannabis and tobacco products was reported by 40% of YLWH and 29% of uninfected youth (Fig. 3). There was no statistical difference between groups in proportion of either co-users or non-users by self-report (p = 0.21, p = 0.53). Toxicology assays were positive for both cannabinoids and cotinine for 32% of YLWH and 27% of uninfected youth (Fig. 2). Groups did not differ by rates of co-use, but uninfected youth had a significantly higher proportion of non-users compared to YLWH (p = 0.19, p = 0.0029).
Fig. 3.
Substance co-use by self-report and toxicology for cannabinoids (THC) and tobacco products (TP).
4. Discussion
The evaluation of substance use is critical in the care of HIV-infected and at-risk youth, and in research on intervention development and effectiveness in this population. This study provides a comparison of self-reported substance use, assessed in a manner that maximizes confidentiality, with sensitive plasma toxicology for a range of common and less common substances of abuse among YLWH. The degree of agreement between substance use self-report and toxicology assays observed in this study supports the use of self-report measures in the context of research studies of YLWH. The geographic and ethnic diversity of the YLWH who enrolled in this multicenter network study extends the generalizability of the findings. Although toxicology for alcohol has limited validity due to the rapid elimination of alcohol from the body, the concordance for other substances of abuse with higher stigma potential suggests that the self-report measure is likely to be valid for assessing alcohol use as well. In general, the results of this study suggest that an efficient self-report methodology can provide valid assessment of substance use for youth with HIV engaged in research studies, particularly for substances used most commonly in this population.
Several factors may account for differences of the findings of this study with previous ones that found substantial under-reporting of substance use as well as other risk behaviors by adolescents (Clark et al., 1997; D’Angelo et al., 1991). Decreased stigma and greater acceptance of marijuana use since the 1990s, when many studies were conducted, may contribute to greater accuracy of self-report for cannabinoids. Many prior studies reporting substance use in adolescents focused on youth with or at risk for substance abuse disorders, including pregnant young women, and found self-report to be unreliable. In contrast, the data presented here were collected within a study of HIV treatment strategies for which substance use was not part of the selection criteria unless the use was severe enough to interfere with meeting study requirements. Importantly, information about participants’ substance use was not shared with their medical providers, caregivers or others and did not influence their treatment, reducing motivation to under-report use. Further, the substance use evaluation was included as part of a large battery of assessments, rather than the major focus of the study, which may also increase accurate reporting. For participants with HIV, the use of the ACASI rather than a face-to-face interview measure provided confidentiality, eliminating concern about interviewer reactions; furthermore, participants were not aware that toxicology assays would be performed. However, it should be noted that similar concordance for most substances was seen for uninfected youth, who completed a written substance use questionnaire and knew that blood would be collected for the study.
The influence of frequency of use on agreement between self-report and toxicology is an important consideration. Although concordance between self-report and toxicology assays was moderate overall, it was very high for youth in both samples who reported frequent, particularly daily, use. This is not surprising since youth with more frequent use were more likely to have used the substance in close temporal proximity to drawing of the blood sample for toxicology assays. The results reported here are consistent with a small study comparing self-report and hair analysis of alcohol and substance use among adults with HIV in South Africa (Kader et al., 2012). That study, which found a higher rate of substance use for self-report than for hair analysis, concluded that not only was self-report more cost-effective, but it captured substance abuse of longer periods of time more accurately. Results are also consistent with those of Murphy and colleagues, who found greater sensitivity of self-report when marijuana use occurred more than 2 days prior to assessment, and with Malbergier and colleagues (2012), who found higher agreement of self-report with urinalysis in the context of more frequent self-reported cannabis or cocaine use. The results reported here offer further evidence that self-report is not only valid but is preferable for assessment of substance use over longer periods, as may be needed for research studies where study visits are conducted quarterly or even less frequently. The influence of use frequency, and the lack of perfect concordance, however, indicates a value for the use of multiple measures in research studies.
Of the substances assessed in our study, the lowest agreement occurred for report of tobacco use in YLWH. Tobacco use is a significant issue for youth and adults with HIV (Lifson and Lando, 2012), who may face increased cardiovascular risks as a result of HIV and its treatments (Mondy et al., 2011). Thus, maximizing accuracy of tobacco use reporting in this population is an important goal for future studies. Reluctance to self-report tobacco use may be related in part to increased negative public attitudes towards tobacco. However, in our study, most youth who showed positive toxicology for cotinine also reported using marijuana. The frequency of co-use of the two substances is important in its own right but also may indicate that youth are smoking combinations of marijuana and tobacco and do not necessarily view themselves as cigarette or pure tobacco smokers. Revising self-report measures to ask specifically about marijuana-tobacco combinations (e.g., smoking “blunts”) may provide greater accuracy of assessment. Although one might speculate that exposure to second-hand smoke also would contribute to discrepancies between self-report of tobacco use and toxicology assays, the assays used in this study are unlikely to have shown positive results due to passive inhalation (Wall et al., 1988).
The pattern of substance use observed in this group of YLWH enrolled in a research study reflects high level of use of cannabis, tobacco products, and alcohol. Uninfected youth reported significantly less tobacco use, but were otherwise similar to YLWH in their pattern of alcohol and cannabis use despite differences in assessment methodology and some demographic characteristics. Although the number of women in the study was small, results suggest that male participants have higher tobacco use by both toxicology and self-report, and higher cannabis and alcohol use as well by self-report. In general, the findings of this study suggest that use of substances other than tobacco among YLWH who are available and willing to participate in research studies is similar to that among a small group of uninfected youth, while tobacco use is higher among YLWH.
The results of this study reflect broader changes in adolescent drug use away from cocaine and other previously commonly used drugs, possibly due in part to changes in availability, and wider use and acceptance of marijuana including its use to manage symptoms of HIV and treatment side effects (Brasseux et al., 1998). Much of the literature regarding HIV and substances of abuse in adults has focused on methamphetamine and cocaine, substances with greater perceived risk; however, studies also have demonstrated alcohol effects on neurocognitive functioning in adults with HIV (Fama et al., 2009), and effects of both alcohol and cannabis on cognition and measures of brain integrity in uninfected adolescents (Squeglia et al., 2009, 2012), suggesting a basis for concern about the substance use pattern seen in our sample. The implications of this substance use take on particular significance for youth with HIV for several reasons: Its potential to facilitate secondary transmission of HIV by increasing likelihood of sexual risk behaviors or by reducing adherence and thus virologic control; the possibility that it may increase risk for neurocognitive impairment; and its potential impact on mortality both directly, through interactions with HIV, and indirectly through increased cardiovascular risk resulting from tobacco use.
This study has several limitations. The study cohort was largely male, and as such findings may not generalize to females. The group with HIV was comprised of youth with relatively good immune functioning (CD4 > 350) who were willing to enroll in a longitudinal research study; thus, they represent a select subset of the population of youth with HIV. In addition, youth with substance use judged by clinic personnel to be severe enough to interfere with adhering to study procedures were excluded from enrollment. The uninfected group, although similar in age range and proportion of African–American youth, differed from the group of YLWH in several respects and were recruited at a single University-based site, limiting generalizability; furthermore, unlike the YLWH, they were aware that toxicology testing would be performed. Youth in the study were from largely urban settings and may not represent youth age 18–24 in rural areas. The self-report measure focused on frequency and consequences of substance use but did not collect more detailed information regarding use patterns such as weekday versus weekend use of alcohol or prescribed versus illicit use of marijuana. The information provided by the toxicology assays was limited by the rapid elimination of the drugs and drug metabolites from blood. Further, agreement of toxicology and self-report for substances used less frequently in our sample (e.g., cocaine) should be replicated in samples with greater use of those substances.
Previous studies of self-report of sexually transmitted diseases, pregnancy, and substance use by adolescents have shown that self-report of these risk factors is often inaccurate, leading many investigators to question if this information could be substantiated for use in research studies (Clark et al., 1997; D’Angelo et al., 1991; O’Campo et al., 1992). However, this study shows that when self-report is done in a non-threatening venue the information provided is highly concordant with toxicology results. The results suggest that by using confidential self-report, such as the ACASI tools developed for this study, validity of future self-report evaluations of substance use is likely among youth with HIV engaged in research. Additional research is needed to confirm and extend these findings in larger and more diverse populations of youth with HIV and comparable youth without HIV.
Acknowledgments
This work was supported by The Adolescent Trials Network for HIV/AIDS Interventions (ATN; U01-HD040533) from the National Institutes of Health through the Eunice Kennedy Shriver National Institute of Child Health and Human Development (B. Kapogiannis, C. Worrell), and R01 DA031017 (M. Goodenow, J. Sleasman, S. Nichols, G. Goldberger, W. Hou), with supplemental funding from the National Institute of Drug Abuse (N. Borek, D. Lawrence), National Institute of Mental Health (P. Brouwers, S. Allison), Stephany W. Holloway University Chair for AIDS Research (M. Goodenow), and the Center for Human Immune Deficiency and Inflammation (M. Goodenow). The protocol was co-endorsed by the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT), supported by the National Institute of Allergy and Infectious Diseases (NIAID), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Institute of Mental Health (NIMH; U01 A1068632).
The study was scientifically reviewed by the ATN’s Behavioral Leadership Group. Network, scientific, and logistical support was provided by the ATN Coordinating Center (C. Wilson, C. Partlow) at the University of Alabama at Birmingham. Network operations and data management support was provided by the ATN Data and Operations Center at Westat, Inc. (J. Korelitz, B. Driver). Please note that listing in the acknowledgments section does not imply endorsement of the findings and conclusions of this manuscript. We acknowledge the contribution of the investigators and staff at the following ATN 071, 101, 061 and IMPAACT sites that participated in this study (listed in order of Site Principal Investigator, Study Coordinator, Psychologist). University of South Florida (P. Emmanuel, M.D., P. Julian, RN, T. Chenneville, Ph.D.); Children’s Hospital of Los Angeles (M. Belzer, M.D., M. Bradford, B.A., A. Hamilton, Ph.D., ABPP-CN); Children’s National Medical Center (L. D’Angelo, M.D., C. Trexler, RN, D. Marschall, Ph.D.); University of Pennsylvania and the Children’s Hospital of Philadelphia (M. Tanney, M.P.H., M.S.N., C.P.N.P., L. Hawkins, M.S., Ed.D.; J. Radcliffe, Ph.D.); Stroger Hospital and the CORE Center (J. Martinez, M.D., K. Bojan, D.N.P., H. Fuentes, Psy.D.); University Pediatric Hospital (I. Febo, M.D, H. Ayala-Flores, B.S.N., N. Scalley-Trifilio, M.A.); Montefiore Medical Center (D. Futterman, M.D., E. Bruce, M.D., E. Weiss, M.A.); Mount Sinai Medical Center, Adolescent Health Center (J. Steever, M.D., M. Geiger, M.P.H., M. Lehr, Ph.D.); University of California, San Francisco (B. Moscicki, M.D., L. Irish, B.S.N., R. Jeremy, Ph.D.); Tulane Medical Center (S. E. Abdalian, M.D., L. Kozina, RN, P. Sirois, Ph.D.); University of Maryland (L. Peralta, M.D., V. Tepper, Ph.D.; R. Gorle, M.P.H., T. Lee-Wilk, Ph.D.); University of Miami School of Medicine (L. Friedman, M.D., D. Maturo, M.S.N., A. Cuadra, Ph.D.); Children’s Diagnostic & Treatment Center (A. Puga, M.D., A. Inman, B.S., D. Patton, Ph.D.); St. Jude Children’s Research Hospital (P. Flynn, M.D., M. Dillard, B.S.N, P. Garvie, Ph.D., M. Wilkins, Ph.D.); Lurie Children’s Hospital (R. Garofalo, M.D., M.P.H., A. Sanders, M.P.H., A. Boyd, Ph.D.); University of Southern California (A. Kovacs, M.D., M. Aranda, M.P.H., M. Mejia, Ph.D.); Children’s Hospital of Michigan (E. Moore, M.D., A. Walters, RN, S. Cockern, Ph.D.); Children’s Hospital of Denver (E. McFarland, M.D., K. Hahn, B.S., CCRP, R. McEvoy, Ph.D.); Howard University (S. Rana, M.D., M. Deressa, M.P.H, E. Padilla, M.A.); Johns Hopkins University (A. G. Agwu, M.D., T. Noletto, M.P.H., L. Margolis, Ph.D.). We sincerely thank additional members of the ATN 071, 061 and 101 protocol teams, the ATN Community Advisory Board, and the youth who participated in the study.
Role of funding source
Nothing declared.
Footnotes
Contributors
Authors Nichols, Sleasman, Goodenow and Garvie conceived of and designed the study and with author Thornton wrote the study protocol. Authors Nichols, Garvie, Sleasman and Goldberger contributed to review of the literature. Authors Nichols, Lowe, Goldberger and Sleasman wrote the study methods, and authors Zhang and Hou performed statistical analyses and wrote the analytic methods. Authors Goodenow, Sleasman, and Lowe prepared the figures and tables. Author Sleasman screened and recruited the HIV-uninfected subjects. Author Nichols wrote the first draft of the manuscript. All authors have contributed to and approved the final manuscript.
Conflict of interest
No conflicts of interest or financial disclosures were reported.
References
- Akinci IH, Tarter RE, Kirisci L. Concordance between verbal report and urine screen of recent marijuana use in adolescents. Addict. Behav. 2001;26:613–619. doi: 10.1016/s0306-4603(00)00146-5. [DOI] [PubMed] [Google Scholar]
- Brasseux C, D’Angelo LJ, Guagliardo M, Hicks J. The changing pattern of substance abuse in urban adolescents. Arch. Pediatr. Adolesc. Med. 1998;152:234–237. doi: 10.1001/archpedi.152.3.234. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. CDC HIV/AIDS Facts December. Atlanta: CDC; 2011. HIV Among Youth. [Google Scholar]
- Centers for Disease Control and Prevention. Vital signs: HIV infection, testing, and risk behaviors among youths—United States. MMWR. 2012:61. [PubMed] [Google Scholar]
- Clark LR, Brasseux C, Richmond D, Getson P, D’Angelo LJ. Are adolescents accurate in self-report of frequencies of sexually transmitted diseases and pregnancies? J. Adolesc. Health. 1997;21:91–96. doi: 10.1016/s1054-139x(97)00042-6. [DOI] [PubMed] [Google Scholar]
- D’Angelo LJ, Getson PR, Luban NL, Gayle HD. Human immunodeficiency virus infection in urban adolescents: can we predict who is at risk? Pediatrics. 1991;88:982–986. [PubMed] [Google Scholar]
- Delaney-Black V, Chiodo LM, Hannigan JH, Greenwald MK, Janisse J, Patterson G, Huestis MA, Ager J, Sokol RJ. Just say “I don’t”: lack of concordance between teen report and biological measures of drug use. Pediatrics. 2010;126:887–893. doi: 10.1542/peds.2009-3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton DK, Kann L, Kinchen S, Shanklin S, Flint KH, Hawkins J, Harris WA, Lowry R, McManus T, Chyen D, Whittle L, Lim C, Wechsler H. Youth risk behavior surveillance—United States, 2011. MMWR Surveill. Summ. 2012;61:1–162. [PubMed] [Google Scholar]
- Fama R, Rosenbloom MJ, Nichols BN, Pfefferbaum A, Sullivan EV. Working and episodic memory in HIV infection, alcoholism, and their comorbidity: baseline and 1-year follow-up examinations. Alcohol.: Clin. Exp. Res. 2009;33:1815–1824. doi: 10.1111/j.1530-0277.2009.01020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendrich M, Mackesy-Amiti ME, Johnson TP, Hubbell A, Wislar JS. Tobacco-reporting validity in an epidemiological drug-use survey. Addict. Behav. 2005;30:175–181. doi: 10.1016/j.addbeh.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Helleberg M, Afzal S, Kronborg G, Larsen CS, Pedersen G, Pedersen C, Gerstoft J, Nordestgaard BG, Obel N. Mortality attributable to smoking among HIV-1-infected individuals: a nationwide, population-based cohort study. Clin. Infect. Dis. 2013;56:727–734. doi: 10.1093/cid/cis933. [DOI] [PubMed] [Google Scholar]
- Hormes JM, Gerhardstein KR, Griffin PT. Under-reporting of alcohol and substance use versus other psychiatric symptoms in individuals living with HIV. AIDS Care. 2012;24:420–423. doi: 10.1080/09540121.2011.608795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future National Results on Adolescent Drug Use: Overview of Key Findings, 2011. Ann Arbor, MI: Institute for Social Research, the University of Michigan; 2012. [Google Scholar]
- Kader R, Seedat S, Koch JR, Parry CD. A preliminary investigation of the AUDIT and DUDIT in comparison to biomarkers for alcohol and drug use among HIV-infected clinic attendees in Cape Town, South Africa. Afr. J. Psychiatry. 2012;15:346–351. doi: 10.4314/ajpsy.v15i5.43. [DOI] [PubMed] [Google Scholar]
- Large MM, Smith G, Sara G, Paton MB, Kedzior KK, Nielssen OB. Meta-analysis of self-reported substance use compared with laboratory substance assay in general adult mental health settings. Int. J. Methods Psychiatr. Res. 2012;21:134–148. doi: 10.1002/mpr.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifson AR, Lando HA. Smoking and HIV: prevalence, health risks, and cessation strategies. Curr. HIV/AIDS Rep. 2012;9:223–230. doi: 10.1007/s11904-012-0121-0. [DOI] [PubMed] [Google Scholar]
- Malbergier A, do Amaral RA, Cardoso LR, Castel S. Monitoring drug use among HIV/AIDS patients in Brazil: should we combine self-report and urinalysis? Curr. HIV Res. 2012;10:708–712. doi: 10.2174/157016212803901347. [DOI] [PubMed] [Google Scholar]
- Mondy KE, Gottdiener J, Overton ET, Henry K, Bush T, Conley L, Hammer J, Carpenter CC, Kojic E, Patel P, Brooks JT. High prevalence of echocardiographic abnormalities among HIV-infected persons in the era of highly active antiretroviral therapy. Clin. Infect. Dis. 2011;52:378–386. doi: 10.1093/cid/ciq066. [DOI] [PubMed] [Google Scholar]
- Murphy DA, Durako S, Muenz LR, Wilson CM. Marijuana use among HIV-positive and high-risk adolescents: a comparison of self-report through audio computer-assisted self-administered interviewing and urinalysis. Am. J. Epidemiol. 2000;152:805–813. doi: 10.1093/aje/152.9.805. [DOI] [PubMed] [Google Scholar]
- Naar-King S, Kolmodin K, Parsons JT, Murphy D. Psychosocial factors and substance use in high-risk youth living with HIV: a multi-site study. AIDS Care. 2010;22:475–482. doi: 10.1080/09540120903220279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Campo P, de Boer MA, Faden RR, Gielen AC, Kass N, Chaisson R. Discrepancies between women’s personal interview data and medical record documentation of illicit drug use, sexually transmitted diseases, and HIV infection. Med. Care. 1992;30:965–971. doi: 10.1097/00005650-199210000-00009. [DOI] [PubMed] [Google Scholar]
- Shirley DK, Kaner RJ, Glesby MJ. Effects of smoking on non-AIDS-related morbidity in HIV-infected patients. Clin. Infect. Dis. 2013;57:275–282. doi: 10.1093/cid/cit207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solbergsdottir E, Bjornsson G, Gudmundsson LS, Tyrfingsson T, Kristinsson J. Validity of self-reports and drug use among young people seeking treatment for substance abuse or dependence. J. Addict. Dis. 2004;23:29–38. doi: 10.1300/J069v23n01_03. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat. Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Squeglia LM, Jacobus J, Tapert SF. The influence of substance use on adolescent brain development. Clin. EEG Neurosci. 2009;40:31–38. doi: 10.1177/155005940904000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Pulido C, Wetherill RR, Jacobus J, Brown GG, Tapert SF. Brain response to working memory over three years of adolescence: influence of initiating heavy drinking. J. Stud. Alcohol Drugs. 2012;73:749–760. doi: 10.15288/jsad.2012.73.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanney MR, Naar-King S, Murphy DA, Parsons JT, Janisse H. Multiple risk behaviors among youth living with human immunodeficiency virus in five U.S. cities. J. Adolesc. Health. 2010;46:11–16. doi: 10.1016/j.jadohealth.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall MA, Johnson J, Jacob P, Benowitz NL. Cotinine in the serum, saliva, and urine of nonsmokers, passive smokers, and active smokers. Am. J. Public Health. 1988;78:699–701. doi: 10.2105/ajph.78.6.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb MS, Vanable PA, Carey MP, Blair DC. Cigarette smoking among HIV+ men and women: examining health, substance use, psychosocial correlates across the smoking spectrum. J. Behav. Med. 2007;30:371–383. doi: 10.1007/s10865-007-9112-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RJ, Nowatzki N. Validity of adolescent self-report of substance use. Subst. Use Misuse. 2005;40:299–311. doi: 10.1081/ja-200049327. [DOI] [PubMed] [Google Scholar]
- World Health Organization. The alcohol, smoking and substance involvement screening test (ASSIST): development, reliability and feasibility. Addiction. 2002;97:1183–1194. doi: 10.1046/j.1360-0443.2002.00185.x. [DOI] [PubMed] [Google Scholar]