Abstract
Microbial populations stochastically generate variants with strikingly different properties, such as virulence or avirulence and antibiotic tolerance or sensitivity. Photorhabdus luminescens bacteria have a variable life history in which they alternate between pathogens to a wide variety of insects and mutualists to their specific host nematodes. Here, we show that the P. luminescens pathogenic variant (P form) switches to a smaller-cell variant (M form) to initiate mutualism in host nematode intestines. A stochastic promoter inversion causes the switch between the two distinct forms. M-form cells are much smaller (one-seventh the volume), slower growing, and less bioluminescent than P-form cells; they are also avirulent and produce fewer secondary metabolites. Observations of form switching by individual cells in nematodes revealed that the M form persisted in maternal nematode intestines, were the first cells to colonize infective juvenile (IJ) offspring, and then switched to P form in the IJ intestine, which armed these nematodes for the next cycle of insect infection.
Pathogenic and mutualistic bacteria can exist in different states in their host to survive sudden environmental shifts such as antibiotic exposure or host immune activation (1, 2). Photorhabdus luminescens bacteria are bioluminescent symbionts of Heterorhabditis bacteriophora nematodes, and the two organisms (as a mutualistic pair) infect, kill, and reproduce inside insects. Nematodes in the infective juvenile (IJ) stage penetrate an insect host and regurgitate their intestinal symbionts in the insect hemocoel (3), which leads to the death of the insect and the release of nutrients that support nematode reproduction (4). Inside the insect, the bacteria grow exponentially and secrete potent Tc and Mcf insecticidal toxins (5–7). The symbionts also produce crystalline inclusion proteins (Cips) with high levels of essential amino acids that are required for nematode reproduction, as well as antimicrobials that defend the insect cadaver from microbial competitors (8, 9). Nematode-bacterial associations were identified, and pulse-chase methodologies were performed (to limit symbionts) as described previously (10). The symbionts are maternally transmitted to IJ offspring developing inside the nematode’s body (10). Mutualism is initiated when Photorhabdus phase variants express maternal adhesion (Mad) fimbriae and adhere to the nematode intestine (11). Fimbriae are proteinaceous surface filaments that function in bacterial cell adherence during host-cell colonization (12). Transmission proceeds through a series of steps involving adherence, invasion, and intracellular growth (10).
By visualizing individual cells inside nematode intestines, we discovered that Photorhabdus switched to a distinct small-cell form to initiate mutualism (M form), different from the insect pathogenic (P-form) cells that were only transiently present inside intestines (Fig. 1A and figs. S1 and S2). The M-form cells lacked visible CipA and CipB inclusions that were produced by the P form and are essential for nematode reproduction (8) (fig. S2). The M form grew as translucent small-colony variants (13) consisting of small cells, with opaque sectors of larger P-form cells irrupting after 48 hours and eventually dominating the colony (fig. S3). Before our observations, the biological significance of small-colony variants was unknown, and the P form was considered the “wild type” because it is the predominant form isolated from entomo-pathogenic nematodes and infected insects, and it produces the antibiotics, bioluminescence, Cips, and insecticidal toxins normally associated with Photorhabdus bacteria (5–9, 14). Observing the development of this microbial symbiosis inside the nematodes revealed that the P form switches to the M form, which site-specifically colonizes the maternal nematode intestine to initiate mutualism.
Fig. 1.
Photorhabdus cells that initiate mutualism in the nematode intestine are small-cell variant M-form cells. (A) Photorhabdus cells that initiate mutualism (green) on the ninth intestinal ring cells, left and right, INT9L and INTR, posterior intestinal cells and transients (red) are present throughout the lumen of the nematode. (B)M-form cells develop small colonies, and transient P-formcells develop large colonies. (C) Small colonies consist of primarily small cells, and large colonies consist of primarily larger cells.
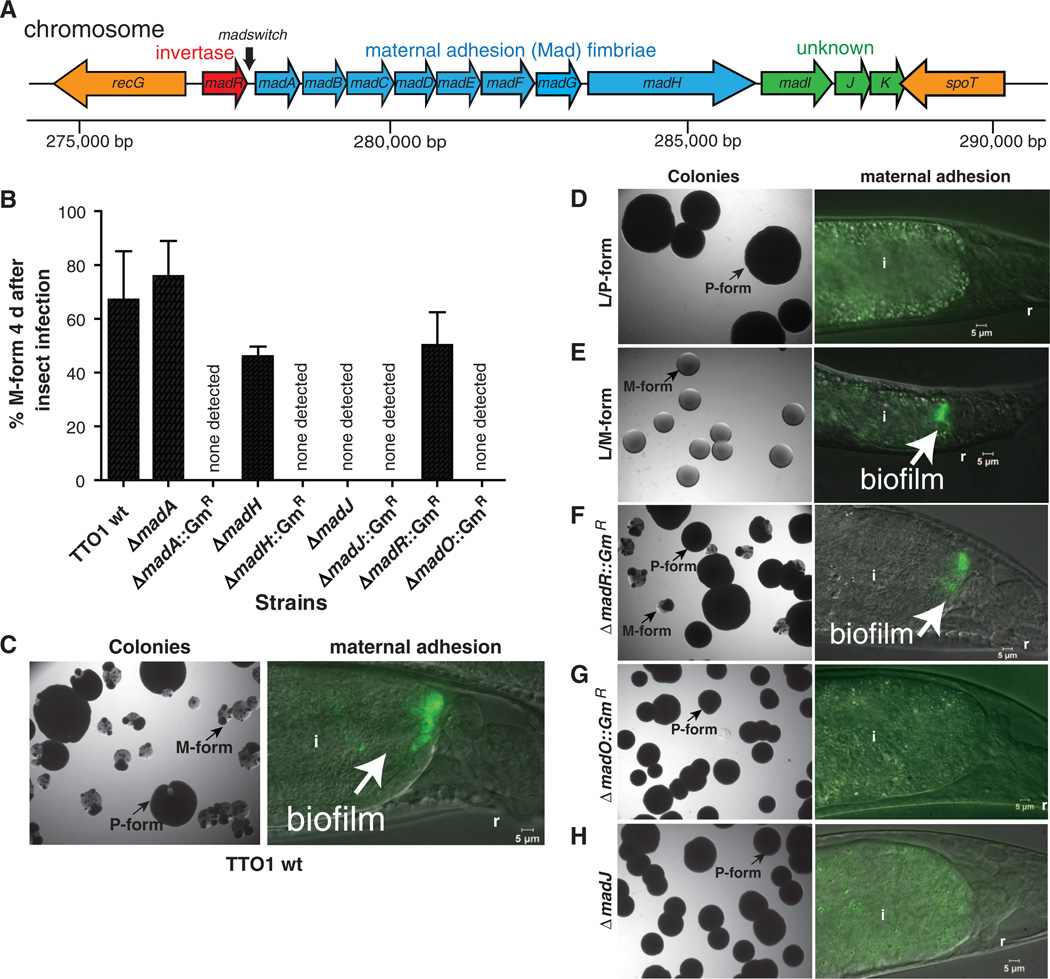
Switching from P to M form correlated with promoter inversion from OFF to ON orientations and, consequently, the expression of the Mad fimbrial locus. This switch enabled aminority variant population to selectively adhere to the posterior maternal intestine (fig. S4) (11). Inversion of the madswitch promoter occurring between two 36–base pair (bp) inverted repeats flanking 257 bp (Fig. 2) regulates mad, in an ON or OFF type of phase variation (15, 16). Cells stochastically express mad, which indicates that it is a highly mutable contingency locus (1). M-form cells transcribed madA from the madswitch when oriented ON and linked the M form with promoter inversion (fig. S4). We determined previously that P-form colonies aged up to 60 days in a Petri dish predominantly switched to the madswitch oriented ON(11).Viable cells isolated from these colonies then grew as the M form (fig. S5). However, the M form prevailed much sooner (4 days) in vivo after P-form infection of Galleria mellonella insect larvae, and this assay was subsequently used to examine form switching independent of mutualism (Fig. 2 and fig. S5). We developed a recombination methodology to manipulate the Photorhabdus genome (fig. S6). The original disruptions in madA and madH predicted to encode the major subunit and usher-assembled fimbrial proteins, respectively, failed to generate the M form, which indicated that mad expression was required for M formation (Fig. 2). However, in-frame madA and madH deletion mutants still generated the M form, while retaining observable defects in nematode mutualism, including no detectable adhesion to the maternal nematode intestine or colonization of IJ nematodes. Taken together, these results suggest that the original mutations prevented M formation because of polarity on a distal regulator rather than on fimbriae structural genes.
Fig. 2.
Expression of the mad fimbrial locus and madO are required for M formation. (A) Physical map of the mad locus required for maternal adhesion, which is an initial step of nematode mutualism. madA to K are co-transcribed and expressed by inversion of the madswitch promoter located between madR and madA. madR is predicted to encode a FimB-type site-specific recombinase (i.e., invertase). (B) Expression of mad is essential for M formation. The majority of TT01 wild-type P-form cells switch to the M form 4 days after insect infection. Marked mutations (e.g., ΔmadA::GmR) of madA, H, J, and an orphan FimB-type recombinase madO failed to switch to the M form at detectable levels. ゔmadR::GmR switched to the M form. In-frame deletions in madA and madH, but not madJ, led to a switch to the M form. Error bars represent standard deviation. (C to H) (Left) Colonies 4 days after P-form infection of insects, except the locked strains (D and E); (right) selection of the M form during maternal adhesion in nematodes. (C) TT01 wild type switched to the M form in insects and in nematodes. (D) No M-form colonies (N = 883) and nomaternal adhesion were detected in the locked L/P form. (E) Locked L/M form grew as M-form colonies (N = 1656) lacking sectors of the P form and adhering to the posterior nematode intestine. (F) Deleting the MadR invertase did not result in loss of switching to the M form in insects or nematodes. (G) Deleting the MadO invertase prevented switching to the M form in insects and nematodes. (H) Deleting MadJ resulted in no detectable M form in insects and 54%of maternal nematodes lacking adherent bacteria. i, intestine; r, rectum.
One distal mad gene, madJ, was an attractive target as the possible cause of the M form because it encodes a homolog of CaiF (17) and of GrlA (18), transcriptional activators of entero-hemorrhagic Escherichia coli. Deletion of madJ prevented M formation and severely compromised mutualism, which supported a functional role for MadJ as the regulatory output for M formation (Fig. 2, B and H, and Table 1). Half of the maternal nematodes associated with ΔmadJ lacked detectable persistent bacteria; the other half had visibly fewer persistent bacteria and transmitted symbionts to only 21.5%of IJ progeny (Table 1). Attempts to complement the ΔmadJ mutation by expressing madJ in trans from a broad host range plasmid were unsuccessful, because the plasmid was not maintained (see methods in the supplementary material). To further test the effect of madswitch inversion on mutualism and M formation, we deleted an invertase (i.e., a tyrosine-type site-specific recombinase) gene, madR, adjacent to the madswitch, but this failed to disrupt M formation or mutualism, which indicated that MadR is not involved in switching or that its function is redundant with another invertase that functions as the ON switch (Fig. 2, B and F). However, deleting a single orphan invertase gene, madO (plu1991), located 575 bp up-stream of fruB (fig. S1), prevented M formation andmutualism (Fig. 2, B, G, and H, and Table 1), which indicated that MadO is required to switch the madswitch ON and to allow madJ expression. The expression levels of MadO could control the rate of promoter inversion from OFF to ON, or other factors may limit switching.
Table 1.
M formation, maternal adhesion, and transmission of Photorhabdus mad mutants. Column 1, M-form cells in insects 4 days after injection of P-form cells. Column 2, adhesion in the intestines of maternal nematodes at 38 to 40 hours; n = 40 to 60 animals. Column 3, transmission in the intestines of 7-day-old IJ offspring; n = 400 to 500 animals. All values ± standard deviation of the mean; ND, none detected.
| Strains | M form (%) | Maternal adhesion (%) | Percent transmission to IJs (%) |
|---|---|---|---|
| TT01 (wild type) | 67.1 ± 18 | 98.4 ± 1.3 | 94.4 ± 2.9 |
| L /P form | ND* | ND | ND |
| L /M form | 100* | 97.3 ± 1.6 | 92.1 ± 6.4 |
| ΔmadR::GmR | 50.2 ± 12.2 | 98.6 ± 1.9 | 90.6 ± 3.2 |
| ΔmadA::GmR | ND | ND | ND |
| ΔmadA | 75.8 ± 13.1 | ND | ND |
| ΔmadH::GmR | ND | ND | ND |
| ΔmadH | 46.0 ± 3.6 | ND | ND |
| ΔmadJ::GmR | ND | 41.4 ± 8.0 | 19.1 ± 3.5 |
| ΔmadJ | ND | 46.1 ± 8.0 | 21.5 ± 6.4 |
| ΔmadO::GmR | ND | ND | ND |
Determined from colonies from an overnight liquid LBP culture; n = 883 for L /P form, n = 1656 for L /M form.
To test the effects of madswitch promoter inversion on phenotype switching, we genetically locked the madswitch to either the ON or OFF orientation (fig. S7). The madswitch was locked ON and OFF by recombining the switch in either orientation while deleting the upstream inverted repeat and madR (fig. S7). The madswitch reporter was similarly constructed, which resulted in madA-FRT-gfp-madB. M formation was assayed by injecting P-form cells into Galleria mellonella larvae, and then 4 days later determining the percentage of M-form colonies. Deleting the upstream inverted repeat in P-form cells locked the madswitch OFF and prevented the locked (L)/P-form (which failed to initiate mutualism) from being transmitted to IJ nematodes; cells that switched to the M-form phenotype were not detected (Fig. 2, B and D, and Table 1). Conversely, deleting the same repeat while inverting the madswitch ON switched the P form to L/M-form colonies without P-form sectors (Fig. 2, B and E; fig. S3; and Table 1). All recombinants had the L/M-form phenotype; several of these were frozen, and one was used for further study. The L/M form initiated mutualism and was transmitted to IJ nematode offspring (Fig. 2 Dand E, and Table 1). Thus, the orientation of the madswitch promoter determines the phenotype and life-style of Photorhabdus.
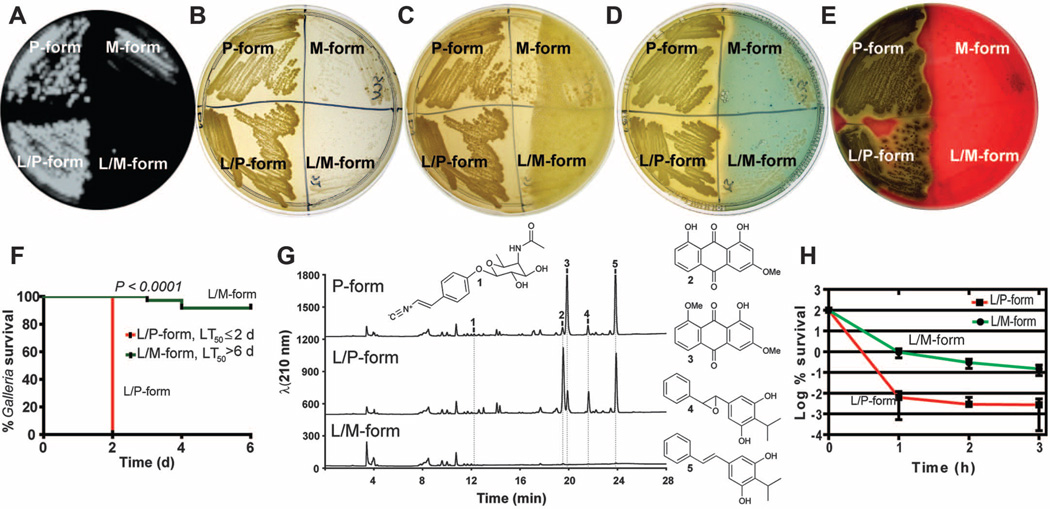
Having locked M and P forms (fig. S7) enabled us to determine their many phenotypic differences. TheM-form cells were shorter (length = 1.2 versus 4.4 µm), were smaller in diameter (0.8 versus 1.2 µm) and volume (0.7 versus 4.8 µm3), and contained no visible Cips compared with the P form (fig. S3). The M form grew more slowly [μ= 0.09 ± 0.003 (SEM)] than the P form [μ = 0.13 ± 0.02 (SEM)] and was outgrown by the P form [competitive index = 106.3 900B1; 26.6 (SD)] (fig. S8) after 24 hours of growth at 28°C in lysogeny broth plus sodium pyruvate (LBP). The competitive disadvantage of the M form in co-culture was more than expected on the basis of the differences in growth rates. The M form produced less bio-film on polystyrene and was nonmotile (fig. S8). It also produced less bio-luminescence, pigment, antibiotic, siderophore, and hemolysin (Fig. 3, A to E) and only minor amounts of rhabduscin, anthraquinone pigments, and multipotent stilbenes (19). Stilbenes have been shown to act as antibiotics, insect phenoloxidase inhibitors, and nematode development signals (20, 21). All of these molecules were robustly produced by the P form (Fig. 3G). The M form produced more cinnamate, a precursor to stilbenes, after 48 hours of growth (fig. S9) likely because of less stilbene synthesis and cinnamate hydrolysis (22, 23). IJ nematodes resumed development and reproduced less on the Mform than on the P form (fig. S8). The M form had an altered capsule, absorbed less methylene blue and Congo red dyes, and exhibited a lower ability to reduce tetrazolium chloride dye (fig. S10). Finally, the M form was avirulent to insect Galleria mellonella larvae [time in which 50%of larvae die (LT50) > 6 days, dose of ~100 cells] relative to the P form (LT50 < 2 days) (Fig. 3F). These radical differences likely reflect the dedicated function of each form (i.e., initiating nematode mutualism or insect pathogenicity).
Fig. 3.
Change in phenotypes, pathogenicity, secondary metabolite production, and persistence between P and M forms. (A) to (E) Quadrants on Petri dishes containing the P form (left), M form (right), unlocked forms (top), and locked forms (bottom). (A) Bioluminescence produced by the P form, which is absent in the M form (note that bioluminescence from P-form revertants is visible in the M form). (B) The P form is yellow and opaque, and the M form is unpigmented and transparent. (C) Antimicrobial activity produced at 48 hours of growth on LBP by the P form and not the M form against a softagar overlay containing Micrococcus luteus indicator bacteria. (D) Production of siderophore iron-chelating activity by the P form and not the M form indicated as a zone of clearing as iron is removed from chrome azural S chelator. (E) Hemolytic activity on sheep blood agar produced by the P form but not the M form. (F) Virulence of the L/P form and not the L/M form after injection into Galleria mellonella. (G) Metabolite analysis detected the production of rhabduscin (1), two anthraquinone pigment molecules (2 and 3) and two hydroxystilbene molecules (4 and 5), which were mostly absent in the L/M form. (H) There are 50 times more persister cells tolerant to ciprofloxacin in the L/M form than in the L/P form.
Slow-growing small-colony variants and dormant persister cells are known to occur during chronic infections (24, 25). Because M forms emerge from prolonged aging of P-form colonies, we reasoned that the M form develops a higher incidence of persister cells (a high persister, hip phenotype). Indeed, the M form exhibited a hip phenotype, with >0.15% of cells tolerant to 1 µg/ml ciprofloxacin after 3 hours of exposure, which was 49.3 times the 0.003% tolerance of P-form cells (P = 0.01) (Fig. 3H). The M form also exhibited more (8.7-fold, P = 0.005) tolerance to streptomycin aminoglycoside antibiotic (fig. S11). This degree of resistance provides further evidence that the M form is biased toward dormancy, which could be advantageous inside the nematode.
Furthermore, comparison of the transcriptomes of the locked M and P forms revealed that >10% of the genome, or 250 up-regulated genes (≥2-fold, P ≤ 0.05) and 265 down-regulated genes (≤0.5-fold, P ≤ 0.05), was differentially expressed in LBP at 24 hours and 28°C (tables S1 and S2). One locus highly expressed in the L/M form was that of clustered regularly interspaced short palindromic repeats (CRISPR)–associated sequences (CASs), which may function in gene silencing with other CAS-CRISPR sequences (26) (table S1). Other strongly up-regulated genes included madA, which was validated by quantitative reverse transcription polymerase chain reaction (fig. S12), and hexA, which is known to repress P-form phenotypes (27). Notable down-regulated genes were those encoding CipA and CipB (8), luciferase, PrtA protease (28), and Tc insecticidal toxins (5) (tables S1 and S2). The down-regulation of genes involved in heme and menaquinone biosynthesis (hemD, hemE, menA, aroF, and aroQ) indicates a reduced electron potential in the M form, like that of many clinical small-colony variants (24) (table S2).
In organisms that switch between phenotypic variants, analogous to cooperating and cheating, the abundance of each type is a result of switching frequencies and variant fitness (29). The P to M form switching frequency (1.21 × 10ȡ3 per cell per generation) was greater than the M to P form switching frequency (4.30 × 10ȡ5 per cell per generation), which indicated that, although the P form switches to the M form 28 times more often than the reverse, the superior fitness of the P form enables it to remain dominant in culture (fig. S8). The higher fitness of the P form explains why M-form colonies are overtaken by the P form and why P-form colonies remain P-form colonies during laboratory growth (i.e., LBP, at 28°C, for 48 hours).
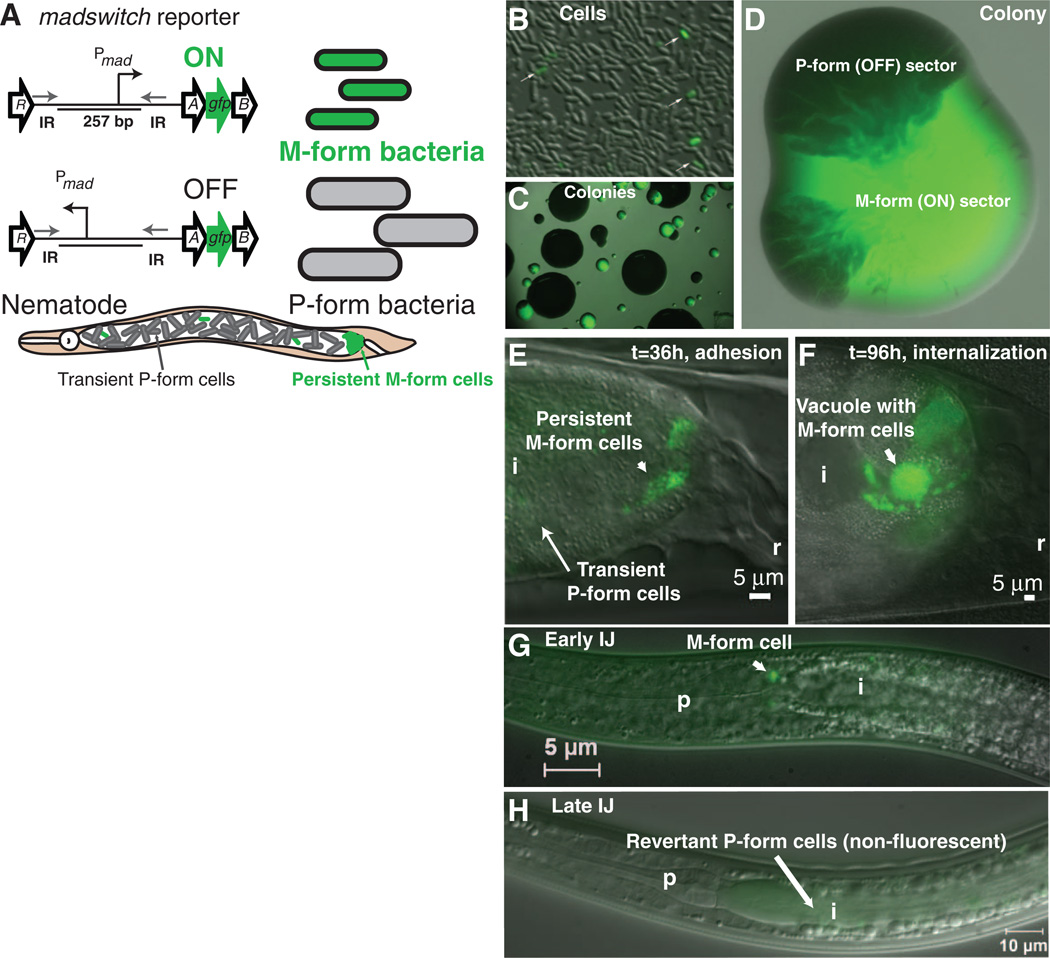
To visualize M formation at single-cell resolution, a green fluorescent protein gfp reporter was inserted between madA and madB (Fig. 4A). Few M-form cells were present in culture, and the resultant M-form colonies developed non-fluorescent sectors of P-form revertants (Fig. 4, B to D). Cells initiating nematode mutualism were green, whereas most cells transiently present in the nematode intestine were not (Fig. 4E). The M form prevailed during intracellular growth and the initial step of IJ colonization (Fig. 4, F and G). However, bacterial cells in the fully colonized IJ intestine were not green and grew mostly as P-form colonies, which indicated that the IJs had become armed with the P form (Fig. 4, H and I, and fig. S13). Because the MadO invertase is required to flip the madswitch from OFF to ON, repressing MadO in IJ cells may favor MadR flipping of the madswitch from ON to OFF (fig. S1). Data supporting this model include the observation that 92.5% of colonies from IJs colonized by the ΔmadR::GmR mutant were the M form, whereas only 0.29% were M form from IJs associated with P form (fig. S13).
Fig. 4.
Single-cell reporter studies of the madswitch during the development of symbiosis in nematodes. (A) A gfp gene was inserted between madA and madB so as not to disrupt expression of downstream mad genes. The madswitch ON cells are small, green fluorescent cells capable of maternal adhesion, and the madswitch OFF cells are large, non-fluorescent cells that are the majority of cells transiently present in the maternal nematode intestine. (B) Few cells (white arrows) have the madswitch oriented ON in culture. (C) Small colonies have the madswitch oriented ON, and large colonies have the madswitch oriented OFF. (D) An isolated colony of the M form (green) develops dark, opaque sectors that are madswitch oriented OFF P form. (E) madswitch-ON cells adhere to the posterior maternal nematode intestine, and most cells transiently present are not fluorescent, with the madswitch OFF. (F) Most adherent cells that invade and grow inside vacuoles of the rectal gland cells have the madswitch oriented ON. (G) One or two cells on or inside the pharyngeal intestinal valve cells have the madswitch oriented ON. (H) Seven days after the symbionts fully colonize the IJs, all cells are not fluorescent with the madswitch OFF, and again, the insect pathogenic P form is arming the nematode for insect infection. i, intestine; r rectum; p, pharynx.
Our examination of a multipartite interaction among bacteria, nematodes, and insects in a tractable model system provides key molecular insight into the drivers of phenotypic behaviors of Photorhabdus bacteria in their nematode and insect hosts. A stochastic promoter inversion switch controls the bacterial phenotypes, i.e., the M-form and P-form variants with dramatically different physiologies, required for initiating mutualistic or pathogenic life-styles, respectively. During initiation of nematode mutualism, most cells inside the maternal nematode intestine were P-form transients, but although fewer M-form cells persisted, they were preferentially transmitted to IJ offspring developing inside the maternal nematode. However, bacterial cells in fully colonized IJs switched back to the P form and armed these nematodes for insect infection. Chance promoter inversion appears to be an efficient mechanism in Photorhabdus to reversibly switch between conflict and cooperation with two different animal hosts.
Supplementary Material
Acknowledgments
We thank L. R. Kroos and L. R. Snyder for helpful discussions and comments on the manuscript and K. Lewis and J. Landgraf for technical assistance. This study was supported by Michigan State University Research for Excellence Fund Center for Microbial Pathogenesis, AgBioResearch, and startup funds (to T.A.C.) and by NIH (grant R01 GM086258 to J.C.). During the course of the work, J.M.C. was supported by a Damon Runyon Cancer Research Foundation fellowship (DRG-2002-09) and an NIH Pathway to Independence award (grant 1K99 GM097096-01 to J.M.C.). The microarray data discussed in this publication have been deposited in NCBI’s Gene Expression Omnibus (GEO) (30) and are accessible through GEO Series accession no. GSE32088 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc = GSE32088).
Footnotes
Supplementary Materials
www.sciencemag.org/cgi/content/full/337/6090/88/DC1
Materials and Methods
Figs. S1 to S13
Tables S1 to S4
References (31–42)
References and Notes
- 1.Moxon ER, Rainey PB, Nowak MA, Lenski RE. Curr. Biol. 1994;4:24. doi: 10.1016/s0960-9822(00)00005-1. [DOI] [PubMed] [Google Scholar]
- 2.Balaban NQ, Merrin J, Chait R, Kowalik L, Leibler S. Science. 2004;305:1622. doi: 10.1126/science.1099390. [DOI] [PubMed] [Google Scholar]
- 3.Ciche TA, Ensign JC. Appl. Environ. Microbiol. 2003;69:1890. doi: 10.1128/AEM.69.4.1890-1897.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waterfield NR, Ciche T, Clarke D. Annu. Rev. Microbiol. 2009;63:557. doi: 10.1146/annurev.micro.091208.073507. [DOI] [PubMed] [Google Scholar]
- 5.Bowen DJ, Ensign JC. Appl. Environ. Microbiol. 1998;64:3029. doi: 10.1128/aem.64.8.3029-3035.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowen D, et al. Science. 1998;280:2129. doi: 10.1126/science.280.5372.2129. [DOI] [PubMed] [Google Scholar]
- 7.Daborn PJ, et al. Proc. Natl. Acad. Sci. U.S.A. 2002;99:10742. doi: 10.1073/pnas.102068099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bintrim SB, Ensign JC. J. Bacteriol. 1998;180:1261. doi: 10.1128/jb.180.5.1261-1269.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richardson WH, Schmidt TM, Nealson KH. Appl. Environ. Microbiol. 1988;54:1602. doi: 10.1128/aem.54.6.1602-1605.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciche TA, Kim KS, Kaufmann-Daszczuk B, Nguyen KC, Hall DH. Appl. Environ. Microbiol. 2008;74:2275. doi: 10.1128/AEM.02646-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Somvanshi VS, Kaufmann-Daszczuk B, Kim KS, Mallon S, Ciche TA. Mol. Microbiol. 2010;77:1021. doi: 10.1111/j.1365-2958.2010.07270.x. [DOI] [PubMed] [Google Scholar]
- 12.Nuccio SP, Bäumler AJ. Microbiol. Mol. Biol. Rev. 2007;71:551. doi: 10.1128/MMBR.00014-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hurlbert RE, Xu J, Small CL. Appl. Environ. Microbiol. 1989;55:1136. doi: 10.1128/aem.55.5.1136-1143.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akhurst RJ. J. Gen. Microbiol. 1980;121:303. [Google Scholar]
- 15.Zieg J, Hilmen M, Simon M. Cell. 1978;15:237. doi: 10.1016/0092-8674(78)90098-3. [DOI] [PubMed] [Google Scholar]
- 16.van der Woude MW, Bäumler AJ. Clin. Microbiol. Rev. 2004;17:581. doi: 10.1128/CMR.17.3.581-611.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eichler K, Buchet A, Lemke R, Kleber HP, Mandrand-Berthelot MA. J. Bacteriol. 1996;178:1248. doi: 10.1128/jb.178.5.1248-1257.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deng W, et al. Proc. Natl. Acad. Sci. U.S.A. 2004;101:3597. [Google Scholar]
- 19.Crawford JM, Kontnik R, Clardy J. Curr. Biol. 2010;20:69. doi: 10.1016/j.cub.2009.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joyce SA, et al. Angew. Chem. Int. Ed. Engl. 2008;47:1942. doi: 10.1002/anie.200705148. [DOI] [PubMed] [Google Scholar]
- 21.Eleftherianos I, et al. Proc. Natl. Acad. Sci. U.S.A. 2007;104:2419. [Google Scholar]
- 22.Williams JS, Thomas M, Clarke DJ. Microbiology. 2005;151:2543. doi: 10.1099/mic.0.28136-0. [DOI] [PubMed] [Google Scholar]
- 23.Chalabaev S, et al. Appl. Environ. Microbiol. 2008;74:1717. doi: 10.1128/AEM.02589-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Proctor RA, et al. Nat. Rev. Microbiol. 2006;4:295. doi: 10.1038/nrmicro1384. [DOI] [PubMed] [Google Scholar]
- 25.Lewis K. Annu. Rev. Microbiol. 2010;64:357. doi: 10.1146/annurev.micro.112408.134306. [DOI] [PubMed] [Google Scholar]
- 26.Makarova KS, et al. Nat. Rev. Microbiol. 2011;9:467. doi: 10.1038/nrmicro2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joyce SA, Clarke DJ. Mol. Microbiol. 2003;47:1445. doi: 10.1046/j.1365-2958.2003.03389.x. [DOI] [PubMed] [Google Scholar]
- 28.Bowen DJ, et al. Microbiology. 2003;149:1581. doi: 10.1099/mic.0.26171-0. [DOI] [PubMed] [Google Scholar]
- 29.Acar M, Mettetal JT, van Oudenaarden A. Nat. Genet. 2008;40:471. doi: 10.1038/ng.110. [DOI] [PubMed] [Google Scholar]
- 30.Edgar R, Domrachev M, Lash AE. Nucleic Acids Res. 2002;30:207. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.