Abstract
Objectives
Lung cancer is the leading cause of death among all cancers. An estimated 29% of the global population older than 15 years currently smokes tobacco. The presence of a high risk population, relatively asymptomatic nature of the disease in the early phase, and relatively good prognosis when discovered early makes screening for lung cancer an attractive proposition. We performed a systematic review and a meta-analysis of the baseline results of randomized controlled trials so far published, which included more than 14,000 patients. Analysis was used to determine whether data was for or against the screening of lung cancers using low-dose computed tomography (LDCT).
Design
Random effect meta regression model of meta-analysis and systematic review.
Methods
We performed a systematic review and a meta-analysis of the current literature to determine whether screening for lung cancer in a high-risk population with computed tomography improves outcomes. A search strategy using Medline was employed, studies selected based on preset criteria and application of exclusion criteria, and data collected and analyzed for statistical significance.
Results
Screening for lung cancer using LDCT resulted in a significantly higher number of stage I lung cancers (odds ratio 3.9, 95% confidence interval [CI] 2.0 –7.4), higher number of total non-small cell lung cancers (odds ratio 5.5, 95% CI 3.1–9.6), and higher total lung cancers (odds ratio 4.1, 95% CI 2.4 –7.1). Screening using LDCT also resulted in increased detection of false-positive nodules (odds ratio 3.1, 95% CI 2.6 –3.7) and more unnecessary thoracotomies for benign lesions (event rate 3.7 per 1000, 95% CI 3.5–3.8). For every 1000 individuals screened with LDCT for lung cancer, 9 stage I non-small cell lung cancer and 235 false-positive nodules were detected, and 4 thoracotomies for benign lesions were performed.
Conclusions
The baseline data from six randomized controlled trials offer no compelling data in favor or against the use of LDCT screening for lung cancer. We await the final results of these randomized controlled trials to improve our understanding of the effectiveness of LDCT in the screening for lung cancer and its effect on mortality.
Keywords: Lung cancer, Screening, Computed tomography, LDCT
Lung cancer is the leading cause of death among all cancers and continues to have a high mortality, despite advances in treatment.1,2 Increased incidence in smokers is well known, and prior studies have shown that tumors that are detected at an earlier stage show better 5-year survival rates.3,4 An estimated 29% of the global population older than 15 years currently smokes tobacco.5 A number of trials of screening for early lung cancer using chest radiography and sputum cytology have not shown a significant benefit from screening.6–14 Most early studies using low-dose computed tomography (LDCT) have been prospective cohort studies, the implication of these studies being limited by lead time bias.15 Screening for lung cancer has been a topic of debate, and both feasibility and benefit from such screening have been questioned.16–19 Although summaries of baseline findings from randomized controlled trials (RCTs) involving LDCT have been published, a meta-analysis has not been performed. Two systematic reviews have been published on LDCT in lung cancer screening,20,21 these included single-arm prospective cohort studies and did not present the baseline findings of RCTs.
Our objective was to perform a systematic review and a meta-analysis of the baseline results of the five RCTs published to date to determine whether screening for lung cancer with LDCT in comparison with no screening or chest radiography is effective in diagnosing lung cancers early in a high-risk population of smokers (PICO—Patients, Interventions, Comparison, Outcomes). Because the final results of these trials will not be available in the near future, we attempted to determine the effectiveness of screening by using a surrogate end point. Although lead time bias has been used as an argument against the use of detection of stage I cancers, it has been shown in studies that earlier detection of lung cancers results in a survival/mortality benefit.3 On the other hand, we also attempted to determine whether any benefit of detecting stage I lung cancers are offset by the harms of screening, detection of false-positive nodules, and thoracotomies for benign lesions.17,22–24 Pooling the results of a relatively homogeneous study population across studies in a meta-analysis provided data on 14,055 individuals.
METHODS
The PRISMA25,26 guidelines for systematic reviews were used (see Supplemental Figure 1, Supplemental Digital Content 1, http://links.lww.com/JTO/A22). We searched electronic databases from 1966 to February 2010 (MEDLINE, EMBASE, CINAHL, and Cochrane Library), Radiologic Society of North America (RSNA 2003–2009), European College of Radiology (ECR 2001–2009) meeting abstracts, major radiology and lung cancer textbooks, reference lists, and for completed trials not yet published. References and related articles from studies that fit the study population were reviewed. Articles were searched in the above resources with the following search concepts with their synonyms. Major search concepts included lung neoplasm, mass screening, computed tomography, and x-ray. These concepts were exploded to include all subheadings of Medical Subject Headings (MeSH) as well as text searches for articles not yet indexed. No other search filters were used. Non-English results were included. Attempt was made to find unpublished studies to avoid the file-drawer effect by searching for abstracts as well using our search strategy and also to include non-English studies to decrease funnel plot asymmetry. Only data from RCTs was used in our study. The types of participants included those at high risk for lung cancer, age group on average was 50 to 60 years, and the average smoking history was 20 to 30 pack-years. The intervention studied was LDCT in a high-risk population for lung cancer versus either no screening in three studies and chest x-ray in three others. The types of outcome measures included detection of stage I non-small cell lung cancer (NSCLC), all NSCLC, all lung cancers, detection of false-positive nodules (defined as noncalcified benign nodules more than 4 to 5 mm detected on initial LDCT), and rate of thoracotomy for benign lesions.
Validity assessment and assessment of risk of bias (study level/outcome level and in/across studies) was performed. All relevant articles were retrieved and independently assessed by two reviewers (M.G. and S.E.A.), with conflicts being resolved by a third independent review (J.J.G.). All articles that met these criteria were then exposed to a second stage of quality assessment. The U.S. Preventive Services Task Force guidelines for grading the validity of individual studies for use in systematic reviews was applied to all potential articles,27 and the Cochrane collaboration’s tool for assessing risk of bias28 was also used. This was a scale that evaluated generalizability, sample size, dropout rate, reproducibility, and statistical methodology of each study. No studies were excluded because of quality concerns.
The data abstraction was done by two independent investigators (M.G. and S.E.A.) using the search criteria above of the results of the RCTs of screening for lung cancer using LDCT. The comprehensive meta-analysis software version 2.2 was used for statistical analysis.29 Eight RCTs were identified comparing LDCT with no screening or chest x-ray (CXR) in a high-risk population for lung cancer.16,30–33 The baseline result of one RCT (NELSON34) was not available for analysis and was excluded from further review. The results of baseline and first repeat round have been published only for the trial Lung Screening Study33,35; for another five RCTs to date, we have the baseline round of results only. The enrolment procedure was volunteer-based for all trials. The percentage of dropout varied in the trials: 0 to 21%. Quantitative data synthesis was performed using the software Comprehensive Meta-Analysis with a random effects model approach, and the results are presented in Figures 1 through 4. Event rate and odds ratio were used as statistical end points for each end point: stage I NSCLC, total NSCLC, false-positive nodules, and thoracotomies for benign lesions. For each end point, 95% confidence intervals (CIs) were calculated. Heterogeneity among the RCTs predominantly involved the control arm, with three studies using CXR and three using no screening. This issue was addressed by first analyzing only studies comparing CXR and then analyzing all studies together.
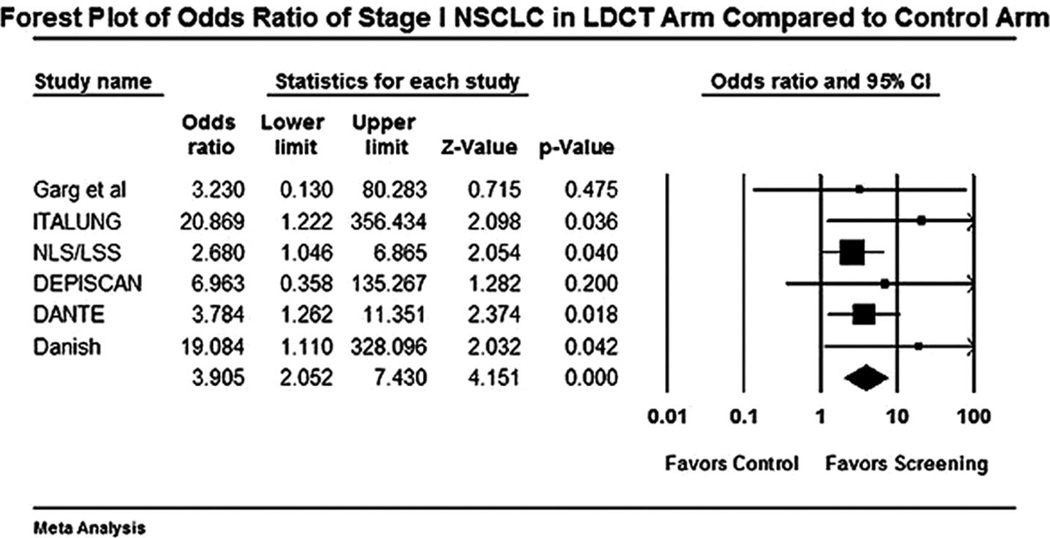
FIGURE 1.
Forest plot for detection of stage I non-small cell lung cancer (NSCLC) with low-dose computed tomography (LDCT) versus control.
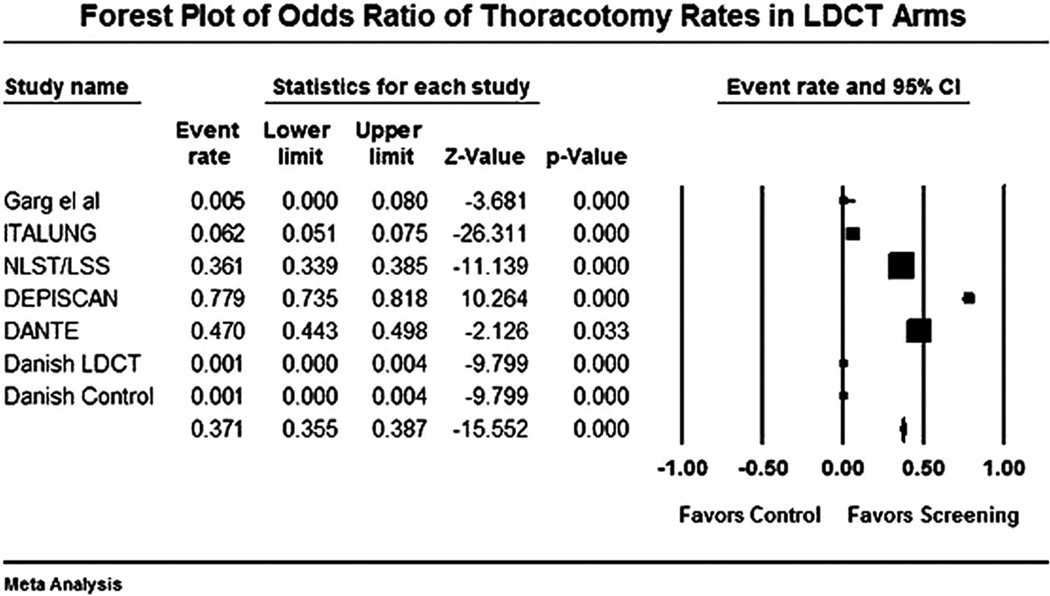
FIGURE 4.
Forest plot for performance of thoracotomy for benign lesions (thoracotomy × 100) in low-dose computed tomography (LDCT) arm versus controls.
RESULTS
Baseline characteristics of the study population in each arm, including age, gender and smoking history, length of screening, screening strategy employed (LDCT versus none and LDCT versus CXR), collimation of LDCT, and the year final results are expected are summarized in Table 1. In total, there were 7078 individuals in the LDCT arm and 6977 in the control arm, for a total of 14,055 individuals. Both the LDCT arm and control arm (CXR versus no imaging) were comparable in terms of age and smoking history. Collimation of LDCT scan varied from 0.6 to 5 mm among trials. Work-up of nodules detected varied between trials; fluorodeoxyglucose positron emission tomography was not routinely included in the nodule work-up; fine-needle aspiration was not always a frequent diagnostic procedure. Trials differ in some variables, such as offering usual care or chest radiograph to the controls, sample size, enrolment criteria, radiologic protocol, frequency screening regimen, CT findings work-up, data management, and the use of a computer-aided detection system for nodule detection and analysis. The control arm varied between the six studies; three studies compared LDCT with CXR whereas three studies compared LDCT with no screening. To assess for the effect of heterogeneity, the odds ratio for all six studies was compared with the odds ratio for studies comparing with CXR only for all end points. Table 2 summarizes the end points used in the meta-analysis. This includes the number of stage I NSCLC detected, total number of NSCLCs detected, total number of lung cancers detected, number of false-positive nodules detected, and number of thoracotomies performed for benign lesions in the LDCT arm compared with the control arm.
TABLE 1.
Baseline Characteristics of Included Studies
| Name of Study |
Screening Duration |
Sample Size | Trial Randomization |
Age (yr) |
Sex | Smoking History (yr)/ Ex-Smokers Quit (yr) |
Collimation of LDCT Scan (mm3) |
Year Final Results Expected |
|---|---|---|---|---|---|---|---|---|
| Garg/Colorado University16 | 2001 (1 yr) | 92 LDCT, 98 control (190) | LDCT vs. usual care | 50–80 | 97.4% male, 2.6% female | >30 | 5 | n/a |
| ITALUNG32 | 2004–2006 | 1613 LDCT, 1593 controls (3206) | LDCT vs. usual care | 55–69 | 64.7% male, 35.3% female | >20/<10 | 1–3 | 2012 |
| LSS33,35,45 | 2000–2004 | 1660 LDCT, 1658 CXR (3318) | LDCT vs. CXR | 55–77 | 59% male, 41% female | >30/<15 (NLST), <10 (LSS) | 0.6–2 (NLST)/5 (LSS) | 2011 |
| DEPISCAN31 | 2002–2004 | 385 LDCT, 380 controls (765) | LDCT vs. CXR | 50–75 | 71% male, 29% female | >15/<15 | 1–1.5 | n/a |
| DANTE30 | 2001–2006 | 1276 LDCT, 1196 controls (2472) | LDCT vs. usual care | 60–74 | Male only | >20/<10 | 5 | n/a |
| DANISH54 | 2004–2006 | 2052 LDCT, 2052 controls (4104) | LDCT vs. usual care | 49–74 | 55.2% male, 44.8% female | >20 | 3 | 2011 |
LDCT, low-dose computed tomography; NLST, National Lung Screening Trial; LSS, Lung Screening Study.
TABLE 2.
Summary of Surrogate End Points Studied in the Meta-Analysis
| Name of Study |
Screening Method |
Stage I NSCLC |
Total Cancers |
False-Positive Nodules |
Unnecessary Thoracotomies |
|---|---|---|---|---|---|
| Garg et al.16 | LDCT | 1 | 3 | 27 | 0 |
| None | 0 | 0 | 0 | ||
| ITALUNG32 | LDCT | 10 | 21 | 618 | 1 |
| None | 0 | 0 | 0 | ||
| LSS33,35,45 | LDCT | 16 | 30 | 295 | 6 |
| CXR | 6 | 7 | 145 | ||
| DEPISCAN31 | LDCT | 3 | 8 | 73 | 3 |
| CXR | 0 | 1 | 20 | ||
| DANTE30 | LDCT | 16 | 28 | 171 | 6 |
| CXR | 4 | 8 | 29 | ||
| DANISH54 | LDCT | 9 | 17 | 162 | 2 |
| None | 0 | 0 | 0 |
NSCLC, non-small cell lung cancer; LDCT, low-dose computed tomography; LSS, Lung Screening Study.
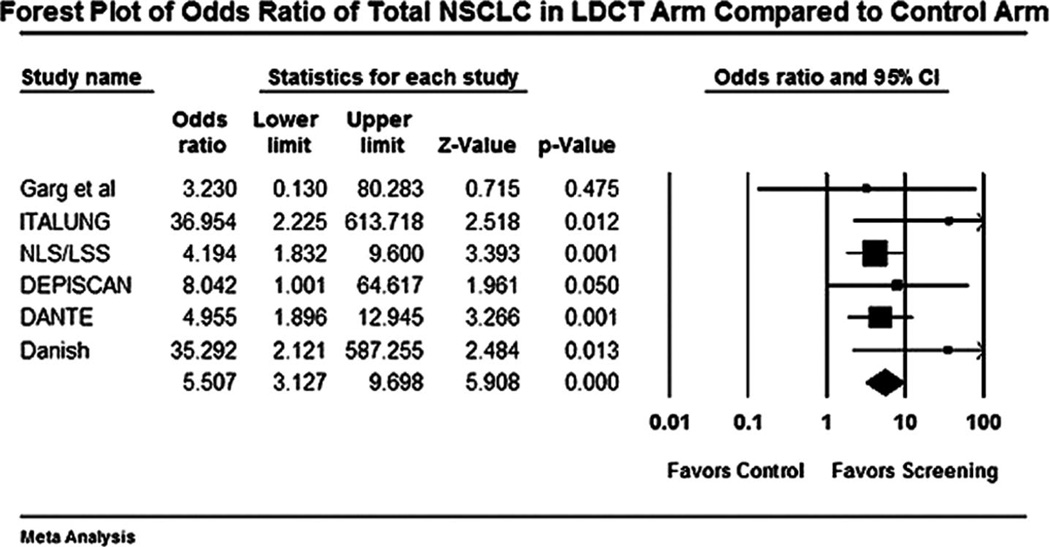
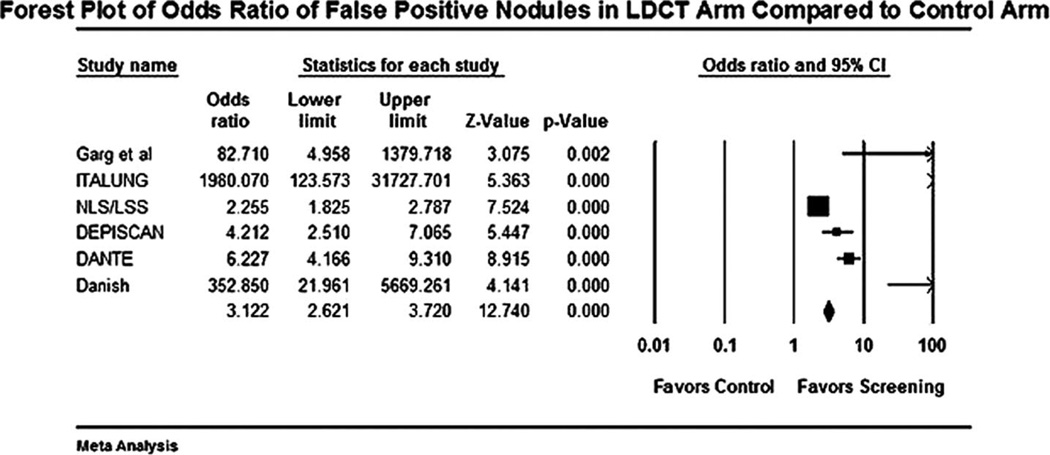
The forest plot for stage I NSCLCs in LDCT compared with control arm is shown in Figure 1. The odds of detecting a stage I NSCLC in the LDCT arm compared with control arm was 3.9 (95% CI 2.0 –7.4). The odds of detection of a NSCLC in the LDCT arm compared with the control arm was 5.5 (95% CI 3.1–9.6). The forest plot for this end point is shown in Figure 2. Forest plot for false-positive nodules is shown in Figure 3. An individual in the LDCT arm was 3.1 (95% CI 2.6 –3.7) times more likely to have a false-positive nodule compared with controls. The event rate was 3.7 per 1000 (95% CI 3.5–3.8) for performance of thoracotomy for a benign lesion in the LDCT arm (Figure 4). An individual was four times more likely to undergo a thoracotomy for a benign lesion in the LDCT arm compared with the control arm.
FIGURE 2.
Forest plot for detection of total non-small cell lung cancer (NSCLC) with low-dose computed tomography (LDCT) versus control.
FIGURE 3.
Forest plot for detection of false positive nodules with low-dose computed tomography (LDCT) versus control.
The effect of use of CXR in the control arm as opposed to no screening was estimated by performing the meta-analysis and calculating each end point for CXR studies only and comparing the results for all six studies. None of the end points differed significantly when compared as pooled results of CXR in screening as opposed to no screening. This also served as a measure of the effect of heterogeneity among the trials. The odds ratio and the event rate with both analyses were similar, and this is reflected in the forest plot for each outcome, with the two results comparable, with minimal effect of adding the studies with no screening in the control arm. We calculated the failsafe N statistic, which estimates the number of studies required to annul the results obtained in a meta-analysis.36 The failsafe N statistic was calculated to be 14 for the end point of stage I NSCLC and 279 for the end point of unnecessary thoracotomies.
DISCUSSION
A number of features make lung cancer an attractive option for early detection and these include the presence of a high-risk population, the relatively asymptomatic nature of the disease in the early phase, and relatively better prognosis when discovered early. Modalities to detect lung cancer include chest radiography, sputum cytology, and computed tomography. Screening with CXR and sputum cytology has been studied in numerous observational studies and RCTs; results have not shown any reduction in disease specific mortality, and the excess of lung cancers in the intervention group has been attributed to overdiagnosis.12,37,38 Advances in computed tomography technique have reduced the radiation exposure by the use of LDCT, which reported to have approximately the same radiation dose as mammography.39 The observation that CT picks up small, asymptomatic cancers led to increased interest in the use of CT in screening.40–42 In the 1990s, numerous observational studies that involved a single arm of screening, including ELCAP and I-ELCAP, were published, with CT picking up eight times more cancers than CXR.43,44 This led some to recommend immediate institution of lung cancer screening and others to await results of RCTs. One-arm prospective cohort studies evaluate screening in terms of cancer detection, interval cancer cases, tumor characteristics, and survival rates, whereas the aim of RCTs is to compare a group of individuals offered a screening regimen with a comparable, nonscreened, or differently screened group to demonstrate the reduction of mortality that would be achieved by the early diagnosis of lung cancers. RCTs are not affected by length bias and overdiagnosis; their results can reveal a real benefit of screening in mortality reduction by comparing occurrence of the disease and mortality in the active and control group.
Worldwide, today there are nine RCTs in screening for lung cancer ongoing or completed. The Lung Screening Study was the first RCT to start in the year 200033; the final analysis for lung cancer mortality is predicted for National Lung Screening Trial in 2011,45 for ITALUNG32 in 2012, and for NELSON46 in 2016. Our meta-analysis, to our knowledge, is the first to be performed of the available baseline results of the RCTs studying the effect of LDCT in screening for lung cancer.
Benefit of screening includes the detection of early stages of lung cancers with the possibility of surgical cure. Only 16% of lung cancers detected during routine care are stage I because individuals are diagnosed and worked up when they develop symptoms from lung cancers, which often is associated with later stages. The rate of detection of stage I lung cancers, in comparison, is 70% with LDCT screening. In our study, we found a significantly higher number of stage I lung cancers, higher number of total NSCLCs, and higher total lung cancers in the LDCT arm compared with the control arm. In our study, we found that individuals in the LDCT arm were 3.9 times more likely than controls to have a stage I lung cancer detected.
The benefits of detecting more stage I NSCLC may be offset by the harms of screening.47 Harms of screening include overdiagnosis, detection of false-positive lesions, and need for further work-up, which may include follow-up, repeat imaging, biopsy, or surgery. Individuals in the LDCT arm were 3.1 times more likely to have a false-positive nodule detected when compared with the control arm. In our study, we detected a rate of 4 of 1000 thoracotomies for benign lesions. This would translate to one unnecessary thoracotomy performed for every 250 high-risk individuals screened for lung cancer with LDCT.
Limitations of our study include problems inherent to all meta-analyses48 and heterogeneity among included studies, especially with CXR being used for screening in the control arm in three studies and no screening in the other three studies. Baseline results of one RCT (NELSON) were also not available for our analysis. Studies varied in protocol by which LDCT was obtained, methodology of follow-up, and work-up of noncalcified nodules detected. Other surrogate end points of harm, which were not analyzed in our study, are the number of unnecessary bronchoscopies performed and the number of transbronchial/transthoracic biopsies performed on benign lesions.
A number of unanswered questions remain with regard to screening for lung cancer with LDCT. The optimal frequency, length, and collimation of LDCT screening and the protocol for follow-up of noncalcified nodules are not known. In current smokers, primary prevention of lung cancer with smoking cessation should go hand in hand with screening for early-stage lung cancer if screening is implemented. positron emission tomography scanning has not been studied as a modality to screen for lung cancers but showed a sensitivity of 50% for detection of all cancers in one study.49
There are numerous limitations with lung cancer screening. One significant qualitative factor that is not studied in clinical trials is the psychologic impact of screening50,51; anxiety and mental issues can arise in individuals and their families who have suspicious lesions on initial screening who have to undergo repeat screening, especially with the high rate of false-positive nodules detected, as demonstrated in our meta-analysis. Occurrence of interval cancers and the performance of unnecessary procedures (biopsy/thoracotomy) are potential limitations with lung cancer screening. Some lung cancers detected by screening may never progress to cause symptoms or death in that individual’s lifetime and therefore may be overdiagnosed by screening.52 Finally, cost effectiveness will be an important determinant of the incorporation of screening strategies into national guidelines.53 The cumulative adverse end points of additional false positives, follow-up of those false positives, unnecessary procedures, cost, and psychologic burden point toward the possible superiority of baseline results over follow-up imaging.
LDCT seems to be better than CXR in detecting lung cancer, as shown in other studies and in our meta-analysis. Screening for lung cancer using LDCT resulted in detection of significantly more stage I lung cancers, more total NSCLCs, and more total lung cancers. Screening using LDCT also resulted in detection of more false-positive nodules and more unnecessary thoracotomies for benign lesions. For every 1000 individuals screened with LDCT for lung cancer, 9 stage I NSCLC and 235 false-positive nodules would be detected, and four thoracotomies for benign lesions would be performed. The systematic review and meta-analysis of the baseline data from six RCTs offer no compelling evidence either in favor or against LDCT screening for lung cancer. We await the final results of these RCTs to improve our understanding of the effectiveness of LDCT in the screening for lung cancer and its effect on mortality.
Footnotes
Disclosure: The authors declare no conflicts of interest.
Muralikrishna Gopal and Shaad Abdullah contributed to the study hypothesis, data collection, and manuscript preparation. James Grady contributed to statistical analysis. James Goodwin contributed to study supervision and manuscript preparation.
REFERENCES
- 1.WHO. World Health Organization; 2009. Global Burden of Lung Cancer. Fact sheet No. 297. [Google Scholar]
- 2.Cancer IAfRo. Worldwide Cancer Burden. World Cancer Report 2008. 2008:42–55. [Google Scholar]
- 3.Flieder DB, Port JL, Korst RJ, et al. Tumor size is a determinant of stage distribution in t1 non-small cell lung cancer. Chest. 2005;128:2304–2308. doi: 10.1378/chest.128.4.2304. [DOI] [PubMed] [Google Scholar]
- 4.Henschke CI. Survival of patients with clinical stage I lung cancer diagnosed by computed tomography screening for lung cancer. Clin Cancer Res. 2007;13:4949–4950. doi: 10.1158/1078-0432.CCR-07-0317. [DOI] [PubMed] [Google Scholar]
- 5.WHO. World Health Organization; 2007. Fact File: Tobacco and second-hand smoke. [Google Scholar]
- 6.Berlin NI, Buncher CR, Fontana RS, et al. The National Cancer Institute Cooperative Early Lung Cancer Detection Program. Results of the initial screen (prevalence). Early lung cancer detection: Introduction. Am Rev Respir Dis. 1984;130:545–549. doi: 10.1164/arrd.1984.130.4.545. [DOI] [PubMed] [Google Scholar]
- 7.Brett GZ. Lung cancer: diagnosis and survival. Br Med J. 1970;1:566. doi: 10.1136/bmj.1.5695.566-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flehinger BJ, Kimmel M, Polyak T, et al. Screening for lung cancer. The Mayo Lung Project revisited. Cancer. 1993;72:1573–1580. doi: 10.1002/1097-0142(19930901)72:5<1573::aid-cncr2820720514>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 9.Fontana RS. The Mayo Lung Project: a perspective. Cancer. 2000;89:2352–2355. doi: 10.1002/1097-0142(20001201)89:11+<2352::aid-cncr7>3.3.co;2-x. [DOI] [PubMed] [Google Scholar]
- 10.Frost JK, Ball WC, Jr, Levin ML, et al. Early lung cancer detection: results of the initial (prevalence) radiologic and cytologic screening in the Johns Hopkins study. Am Rev Respir Dis. 1984;130:549–554. doi: 10.1164/arrd.1984.130.4.549. [DOI] [PubMed] [Google Scholar]
- 11.Kubik A, Parkin DM, Khlat M, et al. Lack of benefit from semi-annual screening for cancer of the lung: follow-up report of a randomized controlled trial on a population of high-risk males in Czechoslovakia. Int J Cancer. 1990;45:26–33. doi: 10.1002/ijc.2910450107. [DOI] [PubMed] [Google Scholar]
- 12.Marcus PM, Bergstralh EJ, Zweig MH, et al. Extended lung cancer incidence follow-up in the Mayo Lung Project and overdiagnosis. J Natl Cancer Inst. 2006;98:748–756. doi: 10.1093/jnci/djj207. [DOI] [PubMed] [Google Scholar]
- 13.Melamed MR. Lung cancer screening results in the National Cancer Institute New York study. Cancer. 2000;89:2356–2362. doi: 10.1002/1097-0142(20001201)89:11+<2356::aid-cncr8>3.3.co;2-q. [DOI] [PubMed] [Google Scholar]
- 14.Stitik FP, Tockman MS. Radiographic screening in the early detection of lung cancer. Radiol Clin North Am. 1978;16:347–366. [PubMed] [Google Scholar]
- 15.Swensen SJ, Jett JR, Hartman TE, et al. CT screening for lung cancer: five-year prospective experience. Radiology. 2005;235:259–265. doi: 10.1148/radiol.2351041662. [DOI] [PubMed] [Google Scholar]
- 16.Garg K, Keith RL, Byers T, et al. Randomized controlled trial with low-dose spiral CT for lung cancer screening: feasibility study and preliminary results. Radiology. 2002;225:506–510. doi: 10.1148/radiol.2252011851. [DOI] [PubMed] [Google Scholar]
- 17.Bach PB, Jett JR, Pastorino U, et al. Computed tomography screening and lung cancer outcomes. JAMA. 2007;297:953–961. doi: 10.1001/jama.297.9.953. [DOI] [PubMed] [Google Scholar]
- 18.Field JK, Duffy SW. Lung cancer screening: the way forward. Br J Cancer. 2008;99:557–562. doi: 10.1038/sj.bjc.6604509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith RA, Field JK, Duffy SW. A global approach to cancer-screening trials. Lancet Oncol. 2008;9:908–909. doi: 10.1016/S1470-2045(08)70211-0. [DOI] [PubMed] [Google Scholar]
- 20.Yau G, Lock M, Rodrigues G. Systematic review of baseline low-dose CT lung cancer screening. Lung Cancer. 2007;58:161–170. doi: 10.1016/j.lungcan.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 21.Black C, de Verteuil R, Walker S, et al. Population screening for lung cancer using computed tomography, is there evidence of clinical effectiveness? A systematic review of the literature. Thorax. 2007;62:131–138. doi: 10.1136/thx.2006.064659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brenner DJ. Radiation risks potentially associated with low-dose CT screening of adult smokers for lung cancer. Radiology. 2004;231:440–445. doi: 10.1148/radiol.2312030880. [DOI] [PubMed] [Google Scholar]
- 23.Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 24.Swensen SJ, Jett JR, Sloan JA, et al. Screening for lung cancer with low-dose spiral computed tomography. Am J Respir Crit Care Med. 2002;165:508–513. doi: 10.1164/ajrccm.165.4.2107006. [DOI] [PubMed] [Google Scholar]
- 25.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 26.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris RP, Helfand M, Woolf SH, et al. Current methods of the US Preventive Services Task Force: a review of the process. Am J Prev Med. 2001;20:21–35. doi: 10.1016/s0749-3797(01)00261-6. [DOI] [PubMed] [Google Scholar]
- 28.Higgins JPT, Altman D. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.1. 2008. [updated September 2008]. [Google Scholar]
- 29.Borenstein M, Hedges LV, Higgins JPT, et al. Version 2. Englewood NJ: Biostat; 2005. Comprehensive Meta-Analysis. [Google Scholar]
- 30.Infante M, Lutman FR, Cavuto S, et al. Lung cancer screening with spiral CT: baseline results of the randomized DANTE trial. Lung Cancer. 2008;59:355–363. doi: 10.1016/j.lungcan.2007.08.040. [DOI] [PubMed] [Google Scholar]
- 31.Blanchon T, Bréchot J-M, Grenier PA, et al. Baseline results of the Depiscan study: a French randomized pilot trial of lung cancer screening comparing low dose CT scan (LDCT) and chest X-ray (CXR) Lung Cancer. 2007;58:50–58. doi: 10.1016/j.lungcan.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 32.Lopes Pegna A, Picozzi G, Mascalchi M, et al. ITALUNG Study Research Group. Design, recruitment and baseline results of the ITALUNG trial for lung cancer screening with low-dose CT. Lung Cancer. 2009;64:34–40. doi: 10.1016/j.lungcan.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 33.Gohagan JK, Marcus PM, Fagerstrom RM, et al. Final results of the Lung Screening Study, a randomized feasibility study of spiral CT versus chest X-ray screening for lung cancer. Lung Cancer. 2005;47:9–15. doi: 10.1016/j.lungcan.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 34.van Iersel CA, de Koning HJ, Draisma G, et al. Risk-based selection from the general population in a screening trial: selection criteria, recruitment and power for the Dutch-Belgian randomised lung cancer multi-slice CT screening trial (NELSON) Int J Cancer. 2007;120:868–874. doi: 10.1002/ijc.22134. [DOI] [PubMed] [Google Scholar]
- 35.Gohagan J, Marcus P, Fagerstrom R, et al. Baseline findings of a randomized feasibility trial of lung cancer screening with spiral CT scan vs chest radiograph: the Lung Screening Study of the National Cancer Institute. Chest. 2004;126:114–121. doi: 10.1378/chest.126.1.114. [DOI] [PubMed] [Google Scholar]
- 36.Carson KP, Schriesheim CA, Kinicki AJ. The usefulness of the “failsafe” statistic in meta-analysis. Educ Psychol Meas. 1990;50:233–243. [Google Scholar]
- 37.Manser R. Screening for lung cancer: a review. Curr Opin Pulm Med. 2004;10:266–271. doi: 10.1097/01.mcp.0000128432.79891.85. [DOI] [PubMed] [Google Scholar]
- 38.Black WC. Overdiagnosis: an underrecognized cause of confusion and harm in cancer screening. J Natl Cancer Inst. 2000;92:1280–1282. doi: 10.1093/jnci/92.16.1280. [DOI] [PubMed] [Google Scholar]
- 39.van Klaveren RJ, Habbema JDF, Pedersen JH, et al. Lung cancer screening by low-dose spiral computed tomography. Eur Respir J. 2001;18:857–866. doi: 10.1183/09031936.01.00076701. [DOI] [PubMed] [Google Scholar]
- 40.Iinuma T, Tateno Y, Matsumoto T, et al. Preliminary specification of X-ray CT for lung cancer screening (LSCT) and its evaluation on risk-cost-effectiveness. Nippon Igaku Hoshasen Gakkai Zasshi. 1992;52:182–190. [PubMed] [Google Scholar]
- 41.Takemura T, Sakai E, Kusumoto M, et al. Utility of helical CT for the secondary mass screening of lung cancer. Nippon Igaku Hoshasen Gakkai Zasshi. 1992;52:1322–1324. [PubMed] [Google Scholar]
- 42.Matsumoto M, Horikoshi H, Moteki T, et al. A pilot study with lung-cancer screening CT (LSCT) at the secondary screening for lung cancer detection. Nippon Igaku Hoshasen Gakkai Zasshi. 1995;55:172–179. [PubMed] [Google Scholar]
- 43.Henschke CI, McCauley DI, Yankelevitz DF, et al. Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet. 1999;354:99–105. doi: 10.1016/S0140-6736(99)06093-6. [DOI] [PubMed] [Google Scholar]
- 44.Henschke CI. Early lung cancer action project: overall design and findings from baseline screening. Cancer. 2000;89:2474–2482. doi: 10.1002/1097-0142(20001201)89:11+<2474::aid-cncr26>3.3.co;2-u. [DOI] [PubMed] [Google Scholar]
- 45.Clark KW, Gierada DS, Marquez G, et al. Collecting 48,000 CT exams for the lung screening study of the National Lung Screening Trial. J Digit Imaging. 2009;22:667–680. doi: 10.1007/s10278-008-9145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van den Bergh KA, Essink-Bot ML, Bunge EM, et al. Impact of computed tomography screening for lung cancer on participants in a randomized controlled trial (NELSON trial) Cancer. 2008;113:396–404. doi: 10.1002/cncr.23590. [DOI] [PubMed] [Google Scholar]
- 47.Black WC. Unexpected observations on tumor size and survival in stage IA non-small cell lung cancer. Chest. 2000;117:1532–1534. doi: 10.1378/chest.117.6.1532. [DOI] [PubMed] [Google Scholar]
- 48.Greenland S. Can meta-analysis be salvaged? Am J Epidemiol. 1994;140:783–787. doi: 10.1093/oxfordjournals.aje.a117326. [DOI] [PubMed] [Google Scholar]
- 49.Nishizawa S, Kojima S, Teramukai S, et al. Prospective evaluation of whole-body cancer screening with multiple modalities including [18F]fluorodeoxyglucose positron emission tomography in a healthy population: a preliminary report. J Clin Oncol. 2009;27:1767–1773. doi: 10.1200/JCO.2008.18.2238. [DOI] [PubMed] [Google Scholar]
- 50.Andersen BL, Farrar WB, Golden-Kreutz D, et al. Stress and immune responses after surgical treatment for regional breast cancer. J Natl Cancer Inst. 1998;90:30–36. doi: 10.1093/jnci/90.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Faller H, Bulzebruck H, Drings P, et al. Coping, distress, and survival among patients with lung cancer. Arch Gen Psychiatry. 1999;56:756–762. doi: 10.1001/archpsyc.56.8.756. [DOI] [PubMed] [Google Scholar]
- 52.Manser RL, Dodd M, Byrnes G, et al. Incidental lung cancers identified at coronial autopsy: implications for overdiagnosis of lung cancer by screening. Respir Med. 2005;99:501–507. doi: 10.1016/j.rmed.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 53.Mahadevia PJ, Fleisher LA, Frick KD, et al. Lung cancer screening with helical computed tomography in older adult smokers: a decision and cost-effectiveness analysis. JAMA. 2003;289:313–322. doi: 10.1001/jama.289.3.313. [DOI] [PubMed] [Google Scholar]
- 54.Pedersen JH, Ashraf H, Dirksen A, et al. The Danish randomized lung cancer CT screening trial—overall design and results of the prevalence round. J Thorac Oncol. 2009;4:608–614. doi: 10.1097/JTO.0b013e3181a0d98f. [DOI] [PubMed] [Google Scholar]