Abstract
BACKGROUND
Optimal treatment for breast cancer often involves lengthy multimodality care including 5 to 6 weeks of radiotherapy, but few studies have evaluated adherence to radiotherapy outside the context of a therapeutic clinical trial.
METHODS
Using a SEER-Medicare database, the authors identified women age 66 years or older with Stage I to III breast cancer diagnosed between 1992 and 2002. They evaluated rates of completion of radiotherapy, defined as a minimum of 25 sessions. Multivariate logistic regression analyses were performed to determine factors associated with completion of radiotherapy, and Cox multivariate models were used to determine the impact of radiotherapy completion on local recurrence.
RESULTS
Some 24,510 patients were included in the study. Eighty-seven percent of patients completed 25 or more radiotherapy sessions. In multivariate logistic regression models, mastectomy (HR 1.26, 95% CI 1.10-1.43), hospitalization during treatment (2.87, 2.49-3.31), earlier year of diagnosis, and black race (1.36, 1.14-1.63) were associated with increased risk of non-completion of radiotherapy. Among 21,269 patients treated with breast conservation, incomplete radiotherapy was associated with higher risk of local recurrence. A total of 98.7% of patients who did not complete radiation therapy were free of recurrence at 5 years vs. 97.5% of patients who completed radiation therapy (HR 1.46, CI 1.09-1.95).
CONCLUSION
This study demonstrates relatively high rates of completion of radiation therapy among a population of older woman with breast cancer. However, those who did not complete a full course of radiotherapy had small but statistically significant higher risk of breast cancer recurrence. Future efforts should focus on intervening with women at high risk of not receiving adjuvant radiotherapy and increasing rates of radiotherapy completion.
Keywords: breast cancer, adjuvant radiotherapy completion, aging, recurrence
In 2007, approximately 178,000 women in the US will be diagnosed with breast cancer, and over 40,000 women will die as a result of this disease.1 Despite these grim statistics, progress is being made in the treatment of breast cancer as, over the past 30 years, standard therapy has evolved from surgery alone to a combination of surgery, chemotherapy, radiation therapy, and oral hormonal therapy. Benefits of multimodality therapy have been documented in multiple randomized clinical trials.2 In addition, the most recent Early Breast Cancer Trialist’s Collaborative Group’s meta-analysis of clinical trials investigating radiation3 showed that radiation use decreased breast cancer mortality for patients with invasive breast cancer treated with breast conservation therapy and patients with lymph node-positive disease treated with mastectomy. However, mortality varies substantially by geography and patient characteristics such as ethnicity and socioeconomic status.1 One unaddressed factor that could be affecting the effectiveness of treatment is adherence to therapy.
The patient’s ability to adhere to a lengthy prescribed regimen has become increasingly important, because treatment for breast cancer has become more complicated. Because most cancer treatments are administered in a monitored setting, adherence has been assumed to be very high. Very few studies have addressed actual patient adherence outside of therapeutic clinical trials, as cancer patients are considered to be highly motivated and unusually adherent because of the seriousness of their disease. Furthermore, variables associated with adherence to treatment have not been well defined. Such data are needed to develop effective targeted strategies to improve compliance rates.
Previous work has shown that many women with breast cancer do not receive adjuvant radiotherapy after breast-conserving surgery and those patients who are older, unmarried, and those who have poor access to care are at risk for undertreatment.4-9 However, little research has focused on the rates of adherence to a complete course of radiation treatment. We wished to evaluate whether nonadherence to adjuvant radiation therapy was a substantial problem among a population-based cohort of older women with breast cancer. By using the Surveillance, Epidemiology, and End Results (SEER)-Medicare linked data we studied rates and predictors of adherence to radiation therapy. We also wanted to evaluate whether a shortened course of radiation would increase the risk of local breast cancer recurrence.
MATERIALS AND METHODS
Data Source
We used the SEER-Medicare linked database for this study. The SEER program is a national population-based tumor registry that collects information on incident cancer cases. Under an agreement between the National Cancer Institute (NCI) and the Center for Medicaid and Medicare Services (CMS), SEER subjects who are eligible for Medicare have been linked to their Medicare records. Of persons who are reported by SEER as diagnosed with cancer at age 65 or older, 94% were matched with their Medicare enrollment records. Our study includes subjects diagnosed through 2002 with patients’ Medicare claims available through 2004. Patient demographics, dates of diagnosis, modified AJCC stage (19881), grade, estrogen receptor status, and surgical treatment are available through the SEER registry data and are found in the SEER-Medicare PEDSF file. Education and poverty levels are provided as census tract level variables and are defined as the percentage of individuals living in a census tract with younger than 12 years education or living below the poverty level. We evaluated only patients within SEER-Medicare rather than the entire SEER database because SEER data does not have sufficient details concerning radiation treatments to permit assessment of compliance questions.
Study Population
This study included women age 66 years or older with a diagnosis of AJCC modified third edition stage I-III breast cancer who were diagnosed between 1992 and 2002. We only included women who were treated with definitive surgical therapy (defined as breast-conserving surgery or mastectomy with or without axillary lymph node dissection) and adjuvant radiation therapy. Patients who had had a previous cancer or who developed a second cancer within the first year after diagnosis were excluded, as therapy would likely be affected by a previous or concurrent second malignancy. Patients who did not have full coverage of both Medicare Part A and B or who were members of health maintenance organizations for 1 year before and 1 year after diagnosis were excluded because their claims may not be complete. All patients who were dead within 12 months of diagnosis were also excluded from the study because they might not have survived long enough to receive a full course (up to 3 months) of radiation therapy (to be considered adjuvant radiotherapy, claims had to begin within 9 months of diagnosis). Only 328 patients were excluded for this reason.
Adjuvant radiotherapy use was identified through the Level 1 HCPCS/CPT-4 codes in Medicare physician or outpatient claims. The included codes of radiation treatment delivery were 77,401–77,404, 77,406–77,409, 77,411–77,414, and 77,416. For each patient the total number of radiotherapy sessions was counted over 90 days from the first radiotherapy claims.
By using ICD-9-CM diagnosis and procedure codes, comorbid conditions calculated from 12 months to 3 months before diagnosis of breast cancer were searched from Medicare inpatient, outpatient, and physician claim data. Comorbidity score was calculated using the Klabunde adaptation of the Charlson comorbidity index from the SAS macro provided by the NCI.10-12 Hospitalization within 3 months of first radiation therapy was identified through admission date in Medicare inpatient claims.
To determine whether an incomplete course of radiation therapy would increase the risk of local recurrence, we evaluated the risk of subsequent mastectomy or ipsilateral new tumor development among the subset of patients treated with breast conservation. We followed previously published methods of identifying local recurrence after breast conservation in claims.13 The outcome of local recurrence was defined as a subsequent ipsilateral in situ or invasive breast cancer reported by SEER and/or subsequent mastectomy reported by Medicare claims. The outcome was identified starting from 12 months after first diagnosis date and censoring at December 2003, loss of Medicare coverage, or death. Secondary mastectomy was determined through ICD-9 procedure code 85.41–85.48 in Medicare inpatient and outpatient claims and HCPCS code 19,180, 19,182, 19,184–19,187, 19,200–19,220, 19,240, 19,224–19,229, 19,250–19,255 in Medicare Durable Medical Equipment, physician and outpatient claims. We could not evaluate the risk of local recurrence for patients treated with an initial mastectomy because there are no validated claims-based methods for assessing this outcome.
Statistical Analyses
Patients’ demographic and tumor information were described with column percentages. The distribution of patients based on number of received radiotherapy sessions was calculated. Patients who received at least 25 sessions of radiotherapy were considered to have received a standard course of radiotherapy. We used a 25-treatment cutoff based on a standard of care at the time our population was treated—a number that was established in large randomized trials of radiation after breast surgery.14,15
To determine the factors associated with adherence to radiotherapy, multivariate logistic regressions were performed and the final model included surgery, hospitalization, patient age at diagnosis, year of diagnosis, ethnicity, education level, poverty level, residence, AJCC stage, grade, estrogen receptor status, and Charlson comorbidity indices. Analysis was done adjusting for SEER registry and marital status. For the census tract variables of education and poverty quartiles were calculated in increasing order. The categories for percentage of persons 25 years or older with less than 12-year education were: 0% to 7.68% (representing high educational level), 7.69% to 13.44% (medium), 13.45% to 21.36% (lower), 21.37% to 100% (lowest), and unknown. The categories for percentage of residents living below the poverty level were: 0% to 3.53% (representing lowest poverty level), 3.54% to 6.29% (lower), 6.30% to 11.34% (medium), >11.35% (high), and unknown. Census data from the 2000 files were supplemented with 1990 files if missing or unknown information was found.
Breast cancer recurrence analysis was performed with the Kaplan-Meier method on the population of patients who underwent breast-conserving surgery for breast cancer. Patients were observed from 12 months after first diagnosis and censored at December 2003, loss of Medicare coverage, or death. Three-year and 5-year risk-free (recurrence) probability was plotted stratified by radiation adherence and test of equality over strata was performed through a log-rank test.
Cox proportional hazard models were used to determine the relationship between adherence to radiation therapy and risk of developing breast cancer recurrence (secondary outcome 5 second breast cancer event) after adjusting for hospitalization, patient age, year of diagnosis, ethnicity, education level, poverty level, residence, AJCC stage, grade, estrogen receptor status, and comorbidity. Hazard ratios were also adjusted for SEER registry and martial status. Models were censored at 3 years and 5 years of follow-up time. All computer programming and analyses were performed with the SAS system (Cary, NC).
RESULTS
A total of 24,510 patients were included in the study. Patient demographics and tumor information are presented in Table 1. In all, 87% of patients underwent breast-conserving surgery (BCS). The majority of patients were white (88%). Most patients resided in large metropolitan areas (61%); 64% of patients had stage I, 30% stage II, and 6% stage III disease. In all, 73% of patients had estrogen receptor (ER)-positive tumors; 23% of patients had a comorbidity score of 1 or more. The number of patients with breast cancer registered per year increased from 1992 to 2002 because of expansions of the SEER registry.
Table 1. Patient Characteristics.
| All patients |
Patients treated with breast conservation |
||||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Total | 24,510 | 100 | – | – | |
| Surgery | Breast conserving | 21,269 | 86.78 | 21, 269 | 100 |
| Mastectomy | 3241 | 13.22 | – | – | |
| Hospitalization | No | 23,376 | 95.37 | 20,368 | 95.76 |
| Yes | 1134 | 4.63 | 901 | 4.24 | |
| Age | 66-70 | 7504 | 30.62 | 6470 | 30.42 |
| 71-75 | 7723 | 31.51 | 6793 | 31.94 | |
| 76-80 | 5754 | 23.48 | 4987 | 23.45 | |
| >80 | 3529 | 14.4 | 3019 | 14.19 | |
| Year of diagnosis | 1992 | 1337 | 5.45 | 1123 | 5.28 |
| 1993 | 1386 | 5.65 | 1183 | 5.56 | |
| 1994 | 1491 | 6.08 | 1285 | 6.04 | |
| 1995 | 1742 | 7.11 | 1523 | 7.16 | |
| 1996 | 1774 | 7.24 | 1567 | 7.37 | |
| 1997 | 1990 | 8.12 | 1746 | 8.21 | |
| 8 9 9 | 2022 | 8.25 | 1775 | 8.35 | |
| 1999 | 2109 | 8.6 | 1840 | 8.65 | |
| 2000 | 3424 | 13.97 | 2956 | 13.9 | |
| 2001 | 3571 | 14.57 | 3088 | 14.52 | |
| 2002 | 3664 | 14.95 | 3183 | 14.97 | |
| Ethnicity | White | 21,615 | 88.19 | 18,893 | 88.83 |
| Black | 1270 | 5.18 | 1011 | 4.75 | |
| Other | 1625 | 6.63 | 1365 | 6.42 | |
| Education | High | 6094 | 24.86 | 5478 | 25.76 |
| Medium | 6079 | 24.8 | 5326 | 25.04 | |
| Low | 6099 | 24.88 | 5228 | 24.58 | |
| Lowest | 6081 | 24.81 | 5095 | 23.96 | |
| Unknown | 157 | 0.64 | 142 | 0.67 | |
| Poverty | Lowest | 6073 | 24.78 | 5406 | 25.42 |
| Low | 6107 | 24.92 | 5402 | 25.4 | |
| Medium | 6088 | 24.84 | 5258 | 24.72 | |
| High | 6085 | 24.83 | 5061 | 23.8 | |
| Unknown | 157 | 0.64 | 142 | 0.67 | |
| Residence | Big Metro | 14,986 | 61.14 | 13,191 | 62.02 |
| Metro | 6879 | 28.07 | 5930 | 27.88 | |
| Urban | 1312 | 5.35 | 1112 | 5.23 | |
| Less Urban | 1075 | 4.39 | 834 | 3.92 | |
| Rural | 258 | 1.05 | 202 | 0.95 | |
| Stage | I | 15,734 | 64.19 | 15,387 | 72.34 |
| II | 7414 | 30.25 | 5605 | 26.35 | |
| III | 1362 | 5.56 | 277 | 1.3 | |
| Grade | 1 | 5368 | 219 | 5107 | 2401 |
| 2 | 9789 | 3994 | 8727 | 4103 | |
| 3 | 6280 | 2562 | 4849 | 228 | |
| Unknown | 3073 | 1254 | 2586 | 1216 | |
| Estrogen receptor | Negative | 3092 | 1262 | 2481 | 1166 |
| Positive | 17,925 | 7313 | 15,769 | 7414 | |
| Unknown | 3493 | 1425 | 3019 | 1419 | |
| Charlson | 0 | 18,796 | 7669 | 16,308 | 7667 |
| 1 | 4155 | 1695 | 3621 | 1702 | |
| 2+ | 1559 | 636 | 1340 | 63 | |
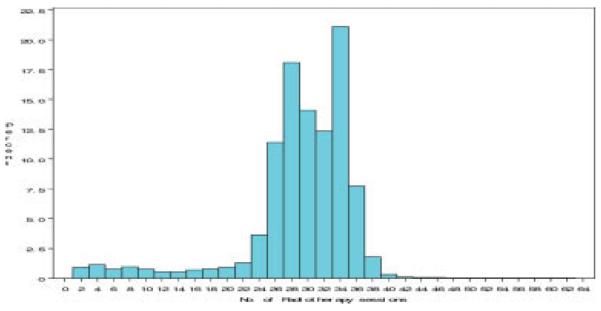
In all, 87% of patients completed 25 or more radiotherapy sessions, which was determined to define a complete course of radiotherapy; 89% of patients received between 24 and 38 treatments. The distribution of number of radiotherapy sessions is presented in Figure 1.
FIGURE 1.
Distribution of patients based on the number of received radiotherapy sessions.
We performed multivariate logistic regression models evaluating how various sociodemographic and clinical factors may influence completion of radiotherapy. The results are presented in Table 2. Patients who underwent mastectomy were less likely to complete radiotherapy than patients who underwent BCS (odds ratio [OR], 1.26; 95% confidence interval [CI], 1.10–1.43). Patients who were hospitalized during the course of their radiotherapy were less likely to complete therapy (2.87, 2.49–3.31). There was a correlation between later year of diagnosis and increased hazard of radiotherapy completion. Black women were less likely to complete therapy than white women (1.36, 1.14–1.63). Patients who lived in rural areas had the higher rates of radiotherapy completion when compared with women in big metro areas (0.51, 0.29–0.90). Notably, there were no differences in rates of completion between age groups, indicating that older patients are as likely to complete therapy as younger patients. Measures of education and poverty, clinical features of the primary breast cancer, and patient comorbidity were not associated with completion of radiation therapy.
TABLE 2. Multivariate Logistic Regression Analysis of Factors That May Influence Radiotherapy Completion*.
| Parameter | Variable | % Noncompletion rate |
Odds ratio (95% CI) |
|---|---|---|---|
| Surgery | Breast conserving | 12.4 | 1.00 |
| Mastectomy | 15.09 | 1.257 (1.103-1.434) | |
| Hospitalization | No | 12.06 | 1.00 |
| Yes | 27.07 | 2.870 (2.489-3.309) | |
| Age | 66-70 | 12.9 | 1.00 |
| 71-75 | 12.82 | 1.003 (0.910-1.106) | |
| 76-80 | 12.55 | 0.991 (0.891-1.103) | |
| >80 | 12.64 | 0.994 (0.876-1.128) | |
| Year of diagnosis | 1992 | 24.76 | 1.00 |
| 1993 | 19.7 | 0.744 (0.618-0.896) | |
| 1994 | 16.3 | 0.572 (0.474-0.692) | |
| 1995 | 13.2 | 0.444 (0.367-0.538) | |
| 1996 | 12.57 | 0.423 (0.349-0.512) | |
| 1997 | 13.92 | 0.475 (0.395-0.570) | |
| 1998 | 14.89 | 0.523 (0.436-0.627) | |
| 1999 | 12.47 | 0.398 (0.331-0.480) | |
| 2000 | 9.02 | 0.265 (0.221-0.318) | |
| 2001 | 8.93 | 0.264 (0.220-0.317) | |
| 2002 | 9.74 | 0.295 (0.246-0.353) | |
| Ethnicity | White | 12.45 | 1.00 |
| Black | 15.98 | 1.364 (1.144-1.626) | |
| Other | 14.22 | 1.117 (0.948-1.317) | |
| Education | High | 13.14 | 1.00 |
| Medium | 12.75 | 0.990 (0.885-1.107) | |
| Low | 11.89 | 0.908 (0.803-1.027) | |
| Lowest | 13.09 | 0.872 (0.755-1.006) | |
| Unknown | 18.47 | 1.202 (0.788-1.835) | |
| Poverty | Lowest | 13.14 | 1.00 |
| Low | 12.15 | 0.944 (0.842-1.058) | |
| Medium | 12.50 | 1.021 (0.901-1.157) | |
| High | 13.08 | 1.072 (0.923-1.245) | |
| Residence | Big Metro | 13.59 | 1.00 |
| Metro | 12.01 | 0.754 (0.666-0.854) | |
| Urban | 11.05 | 0.862 (0.702-1.058) | |
| Less Urban | 9.58 | 0.791 (0.611-1.025) | |
| Rural | 5.81 | 0.512 (0.293-0.897) | |
| Stage | I | 12.51 | 1.00 |
| II | 13.02 | 0.965 (0.880-1.058) | |
| III | 14.1 | 0.872 (0.717-1.061) | |
| Grade | 1 | 12.39 | 1.00 |
| 2 | 12.23 | 0.933 (0.841-1.036) | |
| 3 | 13.66 | 1.060 (0.941-1.195) | |
| Unknown | 13.21 | 0.873 (0.758-1.005) | |
| Estrogen receptor | Negative | 12.71 | 1.00 |
| Positive | 12.64 | 1.051 (0.930-1.187) | |
| Unknown | 13.4 | 1.023 (0.880-1.190) | |
| Charlson score | 0 | 12.94 | 1.00 |
| 1 | 11.94 | 0.921 (0.828-1.024) | |
| 2+ | 12.64 | 0.940 (0.800-1.105) |
Adjusted for SEER registry and marital status.
We next wished to evaluate whether incomplete therapy was associated with a higher risk of local recurrence. This analysis was limited to those patients who received radiotherapy after breast conservation, because recurrences can be identified in Medicare claims among these patients. A total of 21,269 patients were included in the analysis. The characteristics of this population are shown in Table 1.
Patients who did not complete radiation therapy had a small but statistically significant higher risk of developing breast cancer recurrence at 3 and 5 years when compared with patients who completed radiation therapy (log-rank P = .02 at 3 years, P = .02 at 5 years).
Of patients who did not complete radiation therapy, 98.1% were free of recurrence at 3 years and 96.6% were free of recurrence at 5 years versus 98.7% and 97.5% of patients who completed radiation therapy. In Cox multivariable analysis (Table 3), receiving less than 25 sessions of radiation therapy was associated with higher risk of breast cancer recurrence. At 3 years of follow-up the hazard ratio was 1.60, 95% CI, 1.11–2.29 and at 5 years, the hazard ratio was 1.46, 95% CI, 1.09–1.95. Hospitalization, year of diagnosis, and ethnicity were not associated with increased risk of local breast cancer recurrence.
TABLE 3. Cox Proportional Hazard Ratios for Developing Breast Cancer Recurrence at 3 and 5 Years*.
| Parameter | Variable | 3-year hazard ratio (95% CI) |
5-year hazard ratio (95% CI) |
|---|---|---|---|
| RT | 1-24 sessions | 1.595 (1.114-2.285) | 1.456 (1.086-1.954) |
| ≥25 sessions | 1.00 | 1.00 | |
| Hospitalization | No | 1.00 | 1.00 |
| Yes | 0.975 (0.511-1.861) | 0.908 (0.517-1.595) | |
| Age | 66-70 | 1.00 | 1.00 |
| 71-75 | 0.886 (0.641-1.224) | 0.819 (0.628-1.068) | |
| 76-80 | 0.737 (0.501-1.082) | 0.763 (0.560-1.040) | |
| >80 | 0.628 (0.383-1.030) | 0.736 (0.498-1.088) | |
| Year of diagnosis | 1992 | 1.00 | 1.00 |
| 1993 | 1.238 (0.627-2.445) | 1.334 (0.798-2.230) | |
| 1994 | 0.842 (0.404-1.754) | 1.014 (0.591-1.737) | |
| 1995 | 0.828 (0.406-1.688) | 0.893 (0.523-1.527) | |
| 1996 | 1.113 (0.567-2.186) | 1.427 (0.872-2.334) | |
| 1997 | 0.993 (0.502-1.963) | 1.053 (0.630-1.759) | |
| 1998 | 0.771 (0.379-1.568) | 0.810 (0.469-1.402) | |
| 1999 | 0.874 (0.437-1.749) | 0.931 (0.521-1.663) | |
| 2000 | 1.151 (0.603-2197) | 1.294 (0.740-2.262) | |
| 2001 | 1.104 (0.546-2.233) | 1.227 (0.656-2.294) | |
| 2002 | 1.489 (0.606-3.655) | 1.666 (0.721-3.849) | |
| Ethnicity | White | 1.00 | 1.00 |
| Black | 1.193 (0.672-2.119) | 1.295 (0.803-2.088) | |
| Other | 0.785 (0.398-1.547) | 0.892 (0.519-1.534) | |
| Education | High | 1.00 | 1.00 |
| Medium | 1.290 (0.849-1.962) | 1.265 (0.899-1.779) | |
| Lower | 1.217 (0.771-1.921) | 1.414 (0.989-2.021) | |
| Lowest | 1.565 (0.937-2.617) | 1.266 (0.830-1.933) | |
| Unknown | 1.545 (0.364-6.555) | 1.872 (0.666-5.266) | |
| Poverty | Lowest | 1.00 | 1.00 |
| Low | 0.967 (0.636-1.471) | 1.038 (0.746-1.445) | |
| Medium | 0.941 (0.597-1.484) | 0.917 (0.634-1.326) | |
| High | 0.876 (0.508-1.508) | 0.970 (0.628-1.499) | |
| Residence | Big Metro | 1.00 | 1.00 |
| Metro | 1.205 (0.779-1.865) | 1.163 (0.799-1.691) | |
| Urban | 1.543 (0.822-2.895) | 1.532 (0.906-2.592) | |
| Less Urban | 0.471 (0.176-1.262) | 0.571 (0.263-1.236) | |
| Stage | Rural I | 0.741 (0.165-3.319) 1.00 | 0.748 (0.219-2.555) 1.00 |
| II | 1.405 (1.046-1.887) | 1.372 (1.077-1.747) | |
| III | 3.083 (1.491-6.373) | 2.651 (1.392-5.047) | |
| Grade | 1 | 1.00 | 1.00 |
| 2 | 1.369 (0.895-2.095) | 1.294 (0.920-1.820) | |
| 3 | 1.691 (1.073-2.666) | 1.562 (1.080-2.259) | |
| Unknown | 1.401 (0.826-2.376) | 1.589 (1.064-2.374) | |
| ER | Negative | 1.00 | 1.00 |
| Positive | 0.390 (02.80-0.545) | 0.441 (0.333-0.584) | |
| Unknown | 0.439 (0.277-0.698) | 0.587 (0.409-8.44) | |
| Charlson | 0 | 1.00 | 1.00 |
| Comorbidity score | 1 | 1.126 (0.783-1.618) | 1.019 (0.750-1.386) |
| 2 | 1.368 (0.821-2.279) | 1.211 (0.770-1.904) |
RT indicates radiation therapy; ER, estrogen receptor.
Adjusted for SEER registry and marital status.
Tumor-related factors (advanced stage, advanced grade, and ER-negative receptor status) were associated with increased risk of recurrence.
DISCUSSION
In this study we evaluated rates and predictors of incomplete adjuvant radiotherapy among a large population-based cohort of older women with breast cancer. To our knowledge, this study is the first population-based study to evaluate rates and predictors of completing a course of adjuvant radiotherapy. Overall, our results are reassuring, in that 87% of patients completed a standard course of radiotherapy. However, given the high prevalence of breast cancer and the large number of women that are treated with adjuvant radiotherapy, this number still translates into a large absolute number of women who each year do not receive a prescribed course of treatment. Our results are in contrast to a much smaller study of 55 rural southern women, which indicated that 27% of patients did not complete radiotherapy after BCS,16 but the differences between studies are likely explained by the different patient populations.
Although the overall rates of completing radiotherapy were high, we were able to identify some groups of patients who were at increased risk of incomplete therapy.
The surgical treatment of breast cancer was related to length of radiotherapy. Patients treated with mastectomy were significantly less likely to complete therapy. After more radical surgery (compared with BCS) completing radiation therapy may be viewed by patients and physicians as less important, especially when the patient encounters side effects of treatment. We found that radiation adherence improved steadily with the year of diagnosis. Improved radiotherapy adherence over the years may be explained by improvement in radiation technique resulting in fewer side effects, improved supportive care, and increased awareness of importance of completing radiation therapy on breast cancer outcomes.
Hospitalization during the course of treatment was associated with shorter courses of radiotherapy. Hospitalization likely signifies illness, either related or unrelated to breast cancer therapy, which is likely to make it more difficult for patients to complete a lengthy course of radiation treatment. We suspect that most hospitalizations were not due to a toxicity of radiotherapy but nonetheless interfered with the completion of radiation. Notably, age and comorbidity were not associated with adherence to treatment, pointing out that older, sicker, and presumably less mobile patients were as likely to adhere to treatment as younger and healthier ones. This is in contrast to the study of patterns of chemotherapy for breast cancer in older woman in which age and comorbidity were associated with lower rates of chemotherapy use.5
We also found that black patients were less likely to receive a complete course of therapy. Other studies have previously reported that black women were less likely to receive radiotherapy,6,17 but no studies to our knowledge had evaluated completion of therapy. Although black women had a statistically higher chance of not competing therapy, the absolute difference in completion rates between black and white women was less than 5%. Interestingly, education and socioeconomic status were not associated with adherence to therapy, although we were only able to adjust for these factors at the level of census tract and not at the individual level.
It has been well established that radiation therapy, as compared with no radiation therapy, reduces the risk of local recurrence.2,13,18 In our study, among the women treated with breast conservation those who did not complete a full course of radiotherapy had statistically significant higher risk of local breast cancer recurrence. In our particular population, however, the differences in breast cancer recurrence, although statistically significant, are very small. Increased risk of recurrence was relatively modest because, unlike previous studies that compared radiation therapy to no radiation therapy, we compared complete to incomplete radiation therapy. Our findings are consistent with the previously cited much smaller study,16 in which women who did not adhere to radiotherapy were more likely to develop disease recurrence as compared with the adherent patients. Thus, completion of the standard radiation therapy program was important in minimizing the risk of local recurrence. Another reason for the relatively small benefit of radiation therapy in our study is that most likely the algorithm for detecting local recurrence using claims misses some cases. It can also be explained by the advanced age of the population and length of follow-up. In a younger population the benefits of adherence to radiation therapy are likely greater. As expected, tumor-related factors (advanced stage, advanced grade, and ER-negative receptor status) were associated with increased risk of recurrence—a finding that supports the validity of our data.
We used a 25-treatment cutoff based on a standard of care at the time our population was treated—a number that was established in large randomized trials of radiation after breast surgery.14,15 Since the years of our study, newer data suggest that shorter courses of radiotherapy may be sufficient.19 In addition, the development of newer techniques such as partial breast irradiation may continue to shorten the course of radiotherapy. We anticipate that shorter courses of therapy will continue to improve adherence and increase the proportion of women who receive radiotherapy.
There are several potential limitations of our study. First and foremost, we were unable to ascertain the reasons for incomplete therapy. The reasons likely included patient noncompliance, physician undertreatment, and early discontinuation due to radiation toxicity or other severe intercurrent illness. We identified the number of treatments through Medicare claims and billing errors could be present, although physicians and practices have strong incentives to bill correctly (both for reimbursement and to avoid Medicare fraud). Our study was limited to patients older then 65 years old and the results may not be applicable to a population of younger and in general healthier patients. Finally, as previously mentioned, the algorithm to detect local recurrence may have underdetected cases.
In summary, our study demonstrates relatively high adherence to radiation therapy among a population of older woman with breast cancer included in the SEER-Medicare database. These findings suggest that once women initiate adjuvant radiotherapy they are likely to complete it. We identified some groups of patients who were at increased risk of incomplete therapy. Among the women treated with breast conservation, those who did not complete a full course of radiotherapy had a small but statistically significant higher risk of breast cancer recurrence. Future efforts should focus on exploring why some women fail to complete radiotherapy after breast cancer surgery and intervening with patients at high risk of not receiving radiotherapy to reduce the number of undertreated women.
Acknowledgments
Dr. Giordano is supported by NIH 1K07 CA 109064-03.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 3.Clarke M. Meta-analyses of adjuvant therapies for women with early breast cancer: the Early Breast Cancer Trialists’ Collaborative Group overview. Ann Oncol. 2006;17((suppl)10):x59–62. doi: 10.1093/annonc/mdl238. [DOI] [PubMed] [Google Scholar]
- 4.Du X, Freeman JL, Nattinger AB, Goodwin JS. Survival of women after breast conserving surgery for early stage breast cancer. Breast Cancer Res Treat. 2002;72:23–31. doi: 10.1023/a:1014908802632. [DOI] [PubMed] [Google Scholar]
- 5.Du X, Goodwin JS. Patterns of use of chemotherapy for breast cancer in older women: findings from Medicare claims data. J Clin Oncol. 2001;19:1455–1461. doi: 10.1200/JCO.2001.19.5.1455. [DOI] [PubMed] [Google Scholar]
- 6.Giordano SH, Hortobagyi GN, Kau SW, Theriault RL, Bondy ML. Breast cancer treatment guidelines in older women. J Clin Oncol. 2005;23:783–791. doi: 10.1200/JCO.2005.04.175. [DOI] [PubMed] [Google Scholar]
- 7.Goodwin JS, Hunt WC, Samet JM. Determinants of cancer therapy in elderly patients. Cancer. 1993;72:594–601. doi: 10.1002/1097-0142(19930715)72:2<594::aid-cncr2820720243>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 8.Polednak AP. Trends in, and predictors of, breast-conserving surgery and radiotherapy for breast cancer in Connecticut, 1988-1997. Int J Radiat Oncol Biol Phys. 2002;53:157–163. doi: 10.1016/s0360-3016(01)02829-2. [DOI] [PubMed] [Google Scholar]
- 9.Silliman RA, Troyan SL, Guadagnoli E, Kaplan SH, Greenfield S. The impact of age, marital status, and physician-patient interactions on the care of older women with breast carcinoma. Cancer. 1997;80:1326–1334. [PubMed] [Google Scholar]
- 10.National Cancer Institiute SEER-Medicare: calculation of comorbidity weights. Available from: http://healthservices.cancer.gov/seermedicare/program/comorbidity.html.
- 11.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 12.Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol. 1993;46:1075–109. doi: 10.1016/0895-4356(93)90103-8. discussion, 81-90. [DOI] [PubMed] [Google Scholar]
- 13.Smith BD, Haffty BG, Buchholz TA, et al. Effectiveness of radiation therapy in older women with ductal carcinoma in situ. J Natl Cancer Inst. 2006;98:1302–1310. doi: 10.1093/jnci/djj359. [DOI] [PubMed] [Google Scholar]
- 14.Fisher B, Bauer M, Margolese R, et al. Five-year results of a randomized clinical trial comparing total mastectomy and segmental mastectomy with or without radiation in the treatment of breast cancer. N Engl J Med. 1985;312:665–673. doi: 10.1056/NEJM198503143121101. [DOI] [PubMed] [Google Scholar]
- 15.Fisher B, Redmond C, Fisher ER, et al. Ten-year results of a randomized clinical trial comparing radical mastectomy and total mastectomy with or without radiation. N Engl J Med. 1985;312:674–681. doi: 10.1056/NEJM198503143121102. [DOI] [PubMed] [Google Scholar]
- 16.Li BD, Brown WA, Ampil FL, Burton GV, Yu H, McDonald JC. Patient compliance is critical for equivalent clinical outcomes for breast cancer treated by breast-conservation therapy. Ann Surg. 2000;231:883–889. doi: 10.1097/00000658-200006000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mandelblatt JS, Hadley J, Kerner JF, et al. Patterns of breast carcinoma treatment in older women: patient preference and clinical and physical influences. Cancer. 2000;89:561–573. [PubMed] [Google Scholar]
- 18.Smith BD, Gross CP, Smith GL, Galusha DH, Bekelman JE, Haffty BG. Effectiveness of radiation therapy for older women with early breast cancer. J Natl Cancer Inst. 2006;98:681–690. doi: 10.1093/jnci/djj186. [DOI] [PubMed] [Google Scholar]
- 19.Whelan T, MacKenzie R, Julian J, et al. Randomized trial of breast irradiation Schedules after lumpectomy for women with lymph node-negative breast cancer. J Natl Cancer Inst. 2002;94:1143–1150. doi: 10.1093/jnci/94.15.1143. [DOI] [PubMed] [Google Scholar]