Abstract
Background
A strong volume-outcome relationship has been demonstrated for pancreatic resection, and regionalization of care to high-volume centers (>11 resections/year) has been recommended. However, it is unclear if volume alone should be the sole criteria for regionalization. The objective of this study is to evaluate variability in outcomes among high-volume hospitals (>11 resections/year).
Methods
We used the Texas Hospital Inpatient Discharge Database from 1999 through 2005 to evaluate variability in outcomes after pancreatic resection among high-volume hospitals in Texas. The outcome variables of interest were mortality, length of stay, discharge to a skilled nursing facility, operation within 24 hours of hospital admission, and total hospital charges. Unadjusted and adjusted models were performed.
Results
A total of 12 high-volume hospitals were in Texas. The number of resections at each hospital ranged from 78–608 cases for the 7-year time period studied. In unadjusted models, there was significant variability in mortality (range, 0.7%–7.7%, P < .0001), duration of stay (range of medians, 9–21 days, P < .0001), the need for ongoing nursing care at discharge (range, 0.7%–41.4%, P < .0001), operation within 24 hours of admission (range, 41%–96%, P < .0001), and total hospital charges (median range, $38,318–$110,860, P < .0001). There were significant differences in the demographics, risks of mortality, and illness severity among the 12 high-volume hospitals. Therefore, multivariate models were used to control for age group, sex, race/ethnicity, risk of mortality, illness severity, admission status, diagnosis, procedure, and insurance status. In the multivariate models, the particular hospital at which the pancreatic surgery was performed was a significant independent predictor of every outcome variable except mortality.
Conclusions
For pancreatic resection, there is significant variability in outcomes even among high-volume providers. Individual hospitals likely account for much of the variability not explained by hospital volume. Although the structure measure of hospital volume is easy to measure, these data suggest that it is not a reliable single measure of quality or outcomes after pancreatic surgery. (Surgery 2008;144:133-40.)
The Donabedian Model has been used to define, categorize, and measure quality in health care delivery. The model has three components: structure measures, process measures, and outcomes measures.1,2 Structure measures are a broad group of measures that define the setting in which health care is delivered. Hospital volume for a given surgical procedure is a structure measure. Process measures reflect the particular details of the care that patients receive. For example, did a patient receive appropriate prophylactic antibiotics for a given procedure? Outcomes measures, by far the most important and most difficult to measure, reflect how the patient does following some type of medical intervention. Although hospital volume is easy to measure and clearly related to improved patient outcomes after pancreatic resection,3-14 hospital volume is not the sole determinant of patient outcome.
A recent study by Meguid et al15 demonstrated that the volume cutoff for pancreatic resection was arbitrary, as a difference in perioperative mortality was observed regardless of the volume cutoff used. A sensitivity analysis determined that a volume cutoff of 31 pancreatic resections per year was the optimal cutoff. However, hospital volume in their model explained less than 2% of the variance in perioperative death after pancreatic resection.
In this era of cost containment and quality improvement, hospitals and surgeons are under increased pressure to provide evidence of the quality of care that they deliver. For example, the Leapfrog Group, which is a coalition of more than 150 large public and private health care purchasers, is making efforts to concentrate selected surgical procedures in centers that have the best results. In January 2004, pancreatic resection was added to the Leapfrog Group’s list of procedures targeted for evidence-based referral. For pancreatic resection, the Leapfrog Group’s standard for evidence-based referral is based strictly on the process measure of annual volume of procedures performed. They recommend a minimum volume of more than 10 cases per year.16 Although other surgical procedures on Leapfrog’s list include process measures in addition to volume (such as the use of beta-blockade in cardiac surgery), the recommendations for pancreatic surgery referral are based entirely on volume.
The use of volume as the sole criteria for referral of pancreatic resection is controversial. Proponents of regionalization of pancreatic resection quote data on improved mortality, durations of stay, long-term survival, and hospital costs documented in volume-outcome studies.3-13 How-ever, the benefits of regionalization must be weighed against the potential detriments. Inconveniences for patients including increased travel costs, loss of time from work, and limitations on where they can receive care are important.17 In addition, there is the potential for overwhelming high-volume centers, increased mortality at low-volume hospitals as a result of regionalization, the decreasing quality of urgent-related procedures at low-volume hospitals, and reduced access to surgical care if low-volume hospitals cannot recruit qualified surgeons.18
Volume alone is not the key determinant of good outcomes. Individual low-volume providers can have good outcomes,19-21 and the process measures at high-volume centers can be exported to low-volume centers with acceptable morbidity and mortality.22 Although outcomes are improved when high-volume providers are considered as a group, we hypothesize that outcomes vary significantly among high-volume providers. The objective of this study is to evaluate variability in outcomes among high-volume provider in Texas using the Texas Hospital Inpatient Discharge Files.
MATERIALS AND METHODS
Data source
Data from the Texas Hospital Inpatient Discharge Public Use Data File from the years 1999 through 2005, inclusive, are used for this study. The data are collected by the Texas Health Care Information Collection Center for Health Statistics of the Texas Department of State Health Services to develop administrative reports on the use and quality of hospital care in Texas.23 The database includes all discharge records for 466 participating non-federal hospitals in Texas. It has 205 data fields in a base data file and 13 data fields in a detailed charges file. The data include patient demographics, hospital information, durations of stay, International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) diagnosis codes, ICD-9-CM procedure codes, hospital day of procedure, hospital charges, payer information, and discharge status.
Study population/patient characteristics
For the years 1999 through 2005, all discharges with a primary procedure code for pancreatic resection (ICD-9-CM procedure codes: 52.6, 52.7, 52.51, 52.52, 52.53, and 52.59; Table I) were selected. Pancreatic resection for any reason including periampullary adenocarcinoma, chronic pancreatitis, and other benign and malignant diseases of the pancreas were included.
Table I.
International classification of diseases, 9th revision, clinical modification (ICD-9-CM) procedure and diagnosis codes.
|
ICD-9-CM
procedure code |
Definition |
|---|---|
| 52.6 | Pancreatectomy (total) with synchronous duodenectomy |
| 52.7 | Pancreaticoduodenectomy, radical (one-stage) (two-stage) |
| 52.51 | Proximal pancreatectomy (head) (with part of body) (with synchronous duodenectomy) |
| 52.52 | Distal pancreatectomy (tail) (with part of body) |
| 52.53 | Radical /subtotal pancreatectomy |
| 52.59 | Pancreatectomy / Pancreaticoduodenectomy partial NEC |
ICD-9-CM, International Classification of Diseases, 9th Revision, Clinical Modification; NEC, Not elsewhere classifiable.
In this study, the hospital was the unit of analysis. A Texas hospital was included in the analysis if at least 1 pancreatic resection was performed there in the 7-year time period included in this study. Hospitals were classified into high-volume and low-volume providers based on the 2004 Leapfrog criteria of more than 10 cases per year.16 The criteria to qualify as a high-volume provider were a minimum volume of more than10 pancreatic resections per year for four of the seven years of the study and an average volume during the 6-year period of more than 10 pancreatic resections. Only the high-volume hospitals were analyzed to evaluate for variability in outcomes among the high-volume providers. The hospitals were numbered arbitrarily 1 through 12 consistently in the accompanying Tables and Figures to protect their identities.
The Texas Health Care Information Collection Center calculates 2 variables for the purpose of risk adjustment. The “risk of mortality” and the “illness severity” are calculated with All Patient Refined-Diagnosis Related Groups (APR-DRG) software; comorbidity, age, and certain procedures are considered to produce a score from 0–4. Initially, we used both variables to control for patient comorbidities, but they demonstrated significant colinearity. As a result, we only used “risk of mortality” results did not differ if “illness severity” was used.
Statistical analysis
Overall summary data were obtained for the entire cohort. Patients at the 12 hospitals were compared to one another to identify any heterogeneity in the patient populations treated. The outcomes at the 12 high-volume hospitals were compared to one another. The outcome measures of interest were in-hospital mortality, the need for ongoing nursing care at discharge, total duration of stay, postoperative duration of stay, the performance of surgery within 24 hours of admission, and total hospital charges.
SAS Statistical Software, version 9.1.3 (Cary, NC) was used for all statistical analyses. Summary statistics were calculated for the entire cohort of patients. The outcome variables of interest were then compared among the high-volume providers. Chi-square analysis was used to compare proportions for all categorical data. Each analysis included 12 hospitals. The reported chi-square P values represent an overall test for difference between any of the groups. The actual data are shown such that the reader can see where the differences exist; however, pairwise comparisons were not performed given the number of groups. Analysis of variance was used to compare means among the 12 hospitals for the continuous variables. Again, P values represent an overall test for any differences among groups. Significance was accepted at the P < .05 level.
Because the demographic factors, operative factors, and patient comorbidities varied among hospitals, we used a series of multivariate models to assess the independent effect of the individual hospital on the following outcomes: in-hospital mortality, the need for ongoing nursing care at discharge, operation within 24 hours of admission, duration of stay, and postoperative duration of stay. This approach allowed us to control for observed differences in demographic factors, procedure, and patient risk of mortality among the hospitals. Multivariate logistic regression models were used to model the likelihood of mortality, discharge to a SNF, and surgery within 24 hours of admission. For the continuous outcome variables of duration of stay and postoperative duration of stay, Poisson regression models were used to determine the independent effect of each hospital. For all models, we do not report the individual beta estimates for each hospital, but the overall type 3 analysis of effects P value, which tests the significance of each hospital with all other control variables are in the model.
RESULTS
Overall cohort
From 1999 through 2005, there were 2481 pancreatic resections performed at the 12 high-volume hospitals identified. A total of 2015 (81.2%) were performed at hospitals doing ≥ 20 pancreatic resections per year. The number of pancreatic resections at high-volume hospitals increased from 250 in 1999 to 409 in 2005. Pancreatic resections were performed on Texas residents in 86.2% of cases. Of the patients, 17.4% were aged 18–44 years, 18.6% were 45–54 years, 25.8% were 55–64 years, 24.9% were 65–74 years, and 13.3% were 75 years or older. Male patients comprised 51.5% of the cohort. The race/ethnicity distribution was non-Hispanic white in 68.6%, non-Hispanic black in 10.8%, Hispanic in 12.3%, and other races in 8.1%. For the entire cohort, 80.5% of patients were admitted electively, and 93.1% were insured. The overall mortality rate was 2.8%. For those who did not die in the hospital, 75.5% were discharged home and 21.7% went to a SNF.
A pancreatic head resection was performed in 73.5%, a distal resection in 20.2%, and the type of resection was unspecified in 6.3% of cases. Resections were performed for pancreatic or periampullary cancer in 59.1%, chronic pancreatitis in 13.8%, other malignant pancreatic diseases in 12.9%, and other benign diseases in 14.2% of patients.
Differences in demographics, procedures, and diagnoses among hospitals
The number of pancreatic resections at each hospital varied from 78 to 608 in the 7-year time period included in the study. There were significant differences in the demographics, risks of mortality, and illness severity among the 12 high-volume hospitals. The total number of pancreatic resections performed and the demographic factors including age, sex, race, insurance status, and percentage of elective admissions are shown in Table II. The percentage of patients aged 75 years or older ranged from 6.3% to 29.2% among the different high-volume hospitals (P < .0001). Similarly, the sex distribution varied among hospitals with the percentage of female patients ranging from 37.4% to 56.3% (P = .02). The racial/ethnic distribution also varied significantly, with the percentage of non-Hispanic white patients ranging from 27.4% to 83.3% (P < .0001).
Table II.
Demographics, procedure, and tumor location by hospital
| Hospital | ≥75 y (%) | Male (%) | White (%) | Insured (%) | Elective (%) | PHR (%) | Cancer* (%) |
|---|---|---|---|---|---|---|---|
| 1 | 6.3 | 62.6 | 52.1 | 82.3 | 66.8 | 76.4 | 44.4 |
| 2 | 10.9 | 56.2 | 79.6 | 97.5 | 95.0 | 80.6 | 82.1 |
| 3 | 11.7 | 43.7 | 63.0 | 96.9 | 79.0 | 74.7 | 51.2 |
| 4 | 15.4 | 52.3 | 62.4 | 87.8 | 77.8 | 68.1 | 53.1 |
| 5 | 8.3 | 53.0 | 34.9 | 77.1 | 64.2 | 57.8 | 45.9 |
| 6 | 8.5 | 46.0 | 81.4 | 96.1 | 65.9 | 57.4 | 48.1 |
| 7 | 13.5 | 54.0 | 69.2 | 95.5 | 80.5 | 63.2 | 27.1 |
| 8 | 13.2 | 48.1 | 76.7 | 92.7 | 79.5 | 73.1 | 61.6 |
| 9 | 9.8 | 50.8 | 61.5 | 95.1 | 57.0 | 81.8 | 33.6 |
| 10 | 18.4 | 48.4 | 83.3 | 97.5 | 91.5 | 77.0 | 61.0 |
| 11 | 7.4 | 46.3 | 27.4 | 73.7 | 46.2 | 64.2 | 48.4 |
| 12 | 29.2 | 46.1 | 67.4 | 98.9 | 89.3 | 77.0 | 56.2 |
| P value | <.0001 | .02 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 |
PHR, pancreatic head resection.
Periampullary cancer.
The risk of mortality is reported in the Texas Hospital Inpatient Discharge Public Use Files. This variable is based on the APR-DRG software and considers comorbidity, age, and certain procedures to calculate the “risk of mortality” on a 0–4 scale, with 4 being the most severe. As only 2 patients had risk of mortality scores of 0, these were combined with the scores of 1 for the purpose of the analysis. The distribution of risk of mortality scores differed among the 12 hospitals and is shown in Table III.
Table III.
APR-DRG risk of mortality by hospital*
|
Risk of mortality
|
||||
|---|---|---|---|---|
| Hospital | 1 (%) | 2 (%) | 3 (%) | 4 (%) |
| 1 | 43.1 | 34.7 | 16.7 | 5.5 |
| 2 | 42.4 | 34.1 | 17.1 | 6.4 |
| 3 | 39.5 | 29.0 | 17.9 | 13.6 |
| 4 | 39.8 | 32.6 | 18.3 | 9.3 |
| 5 | 55.9 | 19.3 | 16.5 | 8.3 |
| 6 | 42.6 | 31.8 | 18.6 | 7.0 |
| 7 | 46.6 | 27.8 | 11.3 | 14.3 |
| 8 | 41.6 | 29.2 | 16.4 | 12.8 |
| 9 | 44.1 | 27.9 | 12.6 | 15.4 |
| 10 | 31.9 | 39.4 | 21.3 | 7.4 |
| 11 | 48.4 | 21.0 | 19.0 | 11.6 |
| 12 | 36.5 | 24.2 | 30.3 | 9.0 |
APR-DRG, All Patient Refined-Diagnosis Related Groups.
P < .0001.
There was variability in outcome measures among the 12 high-volume providers. The unadjusted mortality ranged from 0.7%–7.7% (P < .0001). For those patients who did not die in the hospital, most were able to go home, but some required ongoing nursing care at discharge. The percentage of patients discharged requiring ongoing nursing care at discharge varied among high-volume hospitals, ranging from 0.7% to 41.4% (P < .0001).
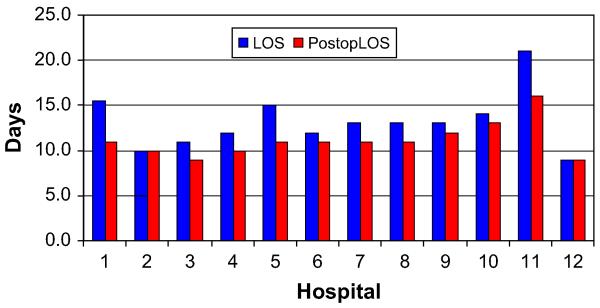
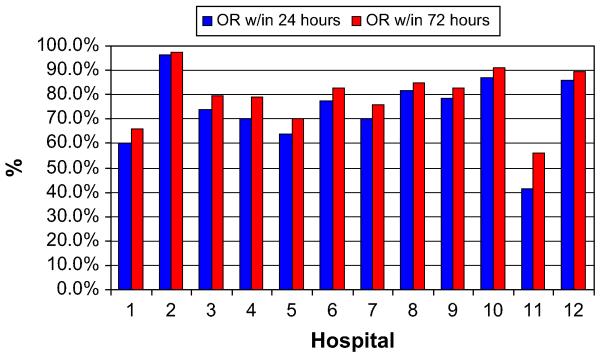
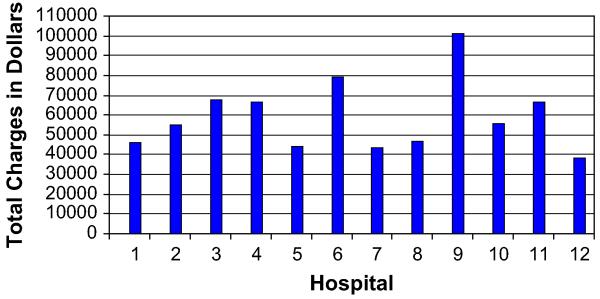
As single outliers skewed the mean, medians were used for duration of stay and charge data. The median total durations of stay and the post-operative durations of stay also varied among high-volume hospitals. The median total duration of stay ranged from 9–21 days (P < .0001), whereas the postoperative duration of stay ranged from 9–16 days (P < .0001, Fig 1). We also evaluated the percentage of patients operated on within 24 hours of admission or within 72 hours of admission. Hospitals varied in their preoperative durations of stay and their ability to operate on patients within 24 or 72 hours of admission, as shown in Fig 2. The median total charges ranged from $38,318–$100,860 (P < .0001) and are shown in Fig 3.
Fig 1.
Total and postoperative length of stay by hospital.
Fig 2.
Percentage of patients operated on within 24 or 72 hours.
Fig 3.
Total charges by hospital.
Multivariate analysis
All multivariate models controlled for age group, sex, race/ethnicity, risk of mortality, admission status, diagnosis, procedure, and insurance status. The particular hospital at which pancreatic surgery was performed was a significant predictor of every outcome variable except mortality. For in-hospital mortality, the type 3 analysis of effects P value for individual hospitals was 0.08 after controlling for age group, sex, race/ethnicity, risk of mortality, illness severity, admission status, diagnosis, procedure, and insurance status.
For need for ongoing nursing care, 1 of the 12 hospitals had only one patient in this category. As this was a significant outlier, it was excluded from in the multivariate analysis. In addition, hospital mortalities were excluded from the analysis. In this model, the individual hospital was a significant predictor of the need for ongoing nursing care, with a type 3 P value of <.0001. When compared to hospital 2, the individual hospitals had odds ratios ranging from 2.1 (95% confidence interval [CI], 1.01-4.39) to 8.80 (95% CI, 5.3-14.6), predicting increased likelihood the need for ongoing nursing care (Table IV) after controlling for age group, sex, race/ethnicity, risk of mortality, illness severity, admission status, diagnosis, procedure, and insurance status.
Table IV.
Logistic regression models for effect of individual hospital
|
Mortality (P = .08) |
Discharge to a SNF (P < .0001) |
Operating room within 24 h (P < .0001) |
||||
|---|---|---|---|---|---|---|
| Hospital ID | OR (95% CI) | P value | OR ( 95% CI) | P value | OR (95% CI) | P value |
| 1 | 1.00 (n/a) | – | – | – | 1.00 (n/a) | – |
| 2 | 0.23 (0.05-1.01) | 0.05 | 1.00 (n/a) | – | 8.75 (4.35-17.59) | <.0001 |
| 3 | 0.29 (0.06-1.42) | 0.12 | 3.39 (2.13-5.41) | <.0001 | 1.28 (0.65-2.55) | .48 |
| 4 | 0.56 (0.14-2.33) | 0.43 | 2.08 (1.34-3.23) | .001 | 0.92 (0.50-1.69) | .79 |
| 5 | 1.99 (0.34-12.1) | 0.45 | 5.32 (2.92-9.69) | <.0001 | 1.13 (0.52-2.45) | .75 |
| 6 | 0.93 (0.18-4.91) | 0.93 | 4.98 (3.02-8.21) | <.0001 | 3.18 (1.49-6.78) | .003 |
| 7 | 0.27 (0.05-1.51) | 0.13 | 7.57 (4.66-12.29) | <.0001 | 0.81 (0.40-1.63) | .55 |
| 8 | 0.59 (0.14-2.49) | 0.47 | 2.19 (1.39-3.45) | .0008 | 2.72 (1.36-5.45) | .005 |
| 9 | 1.28 (0.31-5.29) | 0.74 | 8.80 (5.30-14.62) | <.0001 | 5.56 (2.67-11.57) | <.0001 |
| 10 | 0.12 (0.02-0.79) | 0.03 | 2.93 (1.97-4.37) | <.0001 | 2.04 (1.05-3.98) | .04 |
| 11 | 0.86 (0.17-4.48) | 0.86 | 2.11 (1.01-4.39) | .05 | 0.44 (0.20-0.96) | .04 |
| 12 | 0.67 (0.15-2.98) | 0.60 | 4.61 (2.93-7.25) | <.0001 | 2.37 (1.10-5.12) | .03 |
OR, Odds ratio; CI, confidence interval; SNF, skilled nursing facility.
The individual hospital was also an independent predictor of operation within 24 hours of admission. The odds ratios for the different hospitals varied widely. Compared to hospital 1, other hospitals ranged from 8.75 times (95% CI, 4.35-17.59) as likely to 0.44 times (95% CI, 0.20-0.96) as likely to operate on patients within 24 hours of admission (P < .0001, Table IV).
Poisson regression models were used to test the independent effect of the individual hospital on duration of stay and postoperative duration of stay. The type 3 analysis of effects P values were <.0001 for hospitals in both models, implying that the hospital at which pancreatic surgery was performed influenced duration of stay and postoperative duration of stay.
Although each hospital was high volume by the Leapfrog criteria, the number of procedures performed annually at each hospital varied widely. We entered hospital volume into the multivariate models (as a continuous variable) to determine if the effect of an individual hospital would no longer be significant if volume was taken into account. In all cases where it was previously significant, the effect of the individual hospital remained significant after hospital volume was added. Moreover, individual hospital volume was not a significant predictor of mortality, but it was a significant predictor of the need for ongoing nursing care, ability to operate within 24 hours of admission, and total and postoperative duration of stay.
DISCUSSION
For pancreatic resection, there is significant variability in outcomes even among high-volume providers. Although the structure measure of hospital volume is easy to measure, these data suggest that hospital volume alone is not a reliable single measure of quality or outcomes after pancreatic surgery. Although the in-hospital mortality is similar among high-volume hospitals, the need for ongoing nursing care at discharge, the ability to operate within the first 24 hours of admission, the total and postoperative durations of stay, and the total hospital charges vary significantly even after controlling for patient demographics, risk of mortality, procedure, and diagnosis in multivariate models.
Because of the criteria used to determine hospital volume status (high-volume providers had a minimum volume of >10 pancreatic resections per year for four of the seven years of the study and an average volume during the 6-year period of >10 pancreatic resections), 11 of the 12 hospitals were considered “high volume” throughout the study period. Only 1 hospital truly became high volume, doing only 6 cases in 1999 to more than 30 in 2005. Several others had individual years with less than 11 resections, but they fluctuated around 11 and met the above criteria. In the 1 hospital in question, outcomes improved over the first and last time periods, suggesting that increased volume played a role.
Because mortality did not vary significantly among high-volume hospitals, it is possible that endpoints such as the need for ongoing nursing care discharge, duration of stay, and operation with 24 hours of admission reflect differences in practice patterns or geographic variation. Geographic variation is less likely to explain these differences because all of these hospitals are in Texas and all are in or nearby medium to large cities. Although practice patterns may differ, some recent studies of implementation of critical pathways have established guidelines, or at least goals, for these endpoints for which we should strive.
In their 2007 paper, Kennedy et al24 imported the Johns Hopkins pancreaticoduodenectomy critical pathway to Thomas Jefferson University in Philadelphia. They demonstrated a decrease in duration of stay from 13 days to 7 days after pathway implementation.24 A recent review of 1423 pancreaticoduodenectomies performed at the Johns Hopkins Hospital demonstrated a median duration of stay of 9 days.25 Although some practice variation will remain, these data suggest that 21-day total durations of stay and 16-day postoperative durations of stay are probably greater than necessary.
Despite the similar adjusted in-hospital mortality rates, the need for ongoing nursing care at discharge ranged from 0.7% to 41.4% among high-volume hospitals. Inability to be discharged home clearly affects quality of life and is an important outcome measure. The need for ongoing nursing care at discharge is not commonly reported for pancreatic resection. A recent population-based analysis of the California data demonstrated that in hospitals with a general surgery residency program, 6.5% of patients undergoing pancreatic resection were discharged to another acute care facility or skilled nursing facility. In hospitals that did not have a general surgery residency program, this number increased to 13.0% of patients.26 From the limited data available on the need for on-going nursing care, the rate should probably be less than 41%, and guidelines need to be developed based on the nationwide rates after pancreatic resection.
Another striking finding is that some high-volume hospitals are able to achieve more than 80% of surgeries within the first 24 hours of admission. This finding suggests that these institutions have a streamlined mechanism for completing the workup and preoperative assessment of these patients in the outpatient setting. As a result, they can admit patients the night before or the day of admission and control costs by decreasing inpatient hospital time. Whereas no studies specifically evaluate the ability to operate on patients within 24 hours of admission, the variability demonstrated here suggests significant differences in practice patterns among high-volume providers. Although these data do not prove this hypothesis, it is intuitive that decreasing unnecessary hospital stays during the workup of patients would decrease the cost of taking care of these patients and would allow these patients to spend time at home in their natural environments. By looking at the positive deviants in this study (those hospitals that operated on nearly all patients within 24 hours of admission), we may be able to develop guidelines for the workup of these patients and minimize long preoperative hospital stays.
Differences in total median duration of stay among the high-volume hospitals can be explained in part by the difference in ability to operate within the first 24 hours of admission; however, even postoperative duration of stay was different among the different hospitals, suggesting differences in postoperative care. It has been demonstrated that critical pathways decrease variability in care, the duration of stay, and total hospital charges after complex hepatobiliary and pancreatic procedures.24,27-29 Critical pathways are best-described as structured multidisciplinary care plans that detail essential steps (process measures) in the care of patients with specific clinical problems.27 The outcomes from studies of these critical pathways should be used to develop guidelines for standards of care and outcomes for pancreatic resection. Hospitals should be required to use established and proven critical pathways to be considered referral centers.
There were a wide range of total hospital charges among the high-volume hospitals. All charges were recorded for the hospital admission during which the pancreatic surgery was performed only and did not include any charges from preoperative workup or other admissions/readmissions. Therefore, parts of the workup performed as an outpatient or at a different admission were not included. This finding likely explains some of the differences in charges among hospitals. We did not attempt an in-depth cost analysis in this study.
Although this administrative dataset is good for measuring the endpoints listed here, it is poor for measuring some of the complications specific to pancreatic surgery. These complications include pancreatic fistula formation,30-32 delayed gastric emptying,33 intraabdominal abscess formation, wound infections, bleeding, and others.25 As with other datasets collected for administrative purposes, the identification of these complications is dependent on appropriate coding and is not accurate. We tried to evaluate these complications with the Texas State Discharge data, but most of the complications examined were seen at much lower rates than those observed in large single-institution series in the literature, suggesting undercoding. In addition, this study evaluates only the hospital admission during which pancreatic surgery was performed. Patients did not have a unique identifier, and so we were unable to identify readmission and any complications that occurred after the initial discharge. This dataset also did not allow us to examine the effect of individual surgeon volume on outcomes, which has been shown to be important as well.
Based on the variability in outcomes among high-volume providers in Texas, the data suggest that the structure measure of volume alone is insufficient to designate centers as referral centers for pancreatic resection. Some of the high-volume centers do not have ideal outcomes, and it is likely that some lower volume centers are achieving acceptable outcomes. As pay-for-performance becomes increasingly important, hospitals, and surgeons will be under increased pressure to provide evidence of the quality of care that they deliver. It is critical that pancreatic surgeons work together to form a network of surgeons, hospitals, and medical systems that have a standardized process of recording and appropriately risk-adjusting outcome measures, both general and specific to pancreatic surgery. It will be critical for surgeons to evaluate themselves. Such comparative data will allow us to examine the positive deviants, learn why their results are so good, and begin to work toward duplicating them. Likewise, it will help us understand when and why we might be negative outliers in a particular area.
Acknowledgments
Work Supported by the Society of University Surgeons-Wyeth Clinical Scholars Award.
Footnotes
Presented at the 3rd Annual Academic Surgical Congress, Huntington Beach, California, February, 2008.
REFERENCES
- 1.Donabedian A. The quality of care. How can it be assessed? JAMA. 1988;260:1743–8. doi: 10.1001/jama.260.12.1743. [DOI] [PubMed] [Google Scholar]
- 2.Donabedian A. Quality assurance. Structure, process and outcome. Nurs Stand. 1992;7(11 Suppl QA):4–5. [PubMed] [Google Scholar]
- 3.Fong Y, Gonen N, Rubin D, Radzyner M, Brennan MF. Long-term survival is superior after resection for cancer in high-volume centers. Ann Surg. 2005;242:540–7. doi: 10.1097/01.sla.0000184190.20289.4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gordon TA, Burleyson GP, Tielsch JM, Cameron JL. The effects of regionalization on cost and outcome for one general high-risk surgical procedure. Ann Surg. 1995;221:43–9. doi: 10.1097/00000658-199501000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lieberman MD, Kilburn H, Lindsey M, Brennan MF. Relation of perioperative deaths to hospital volume among patients undergoing pancreatic resection for malignancy. Ann Surg. 1995;222:638–45. doi: 10.1097/00000658-199511000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ho V, Heslin MJ. Effect of hospital volume and experience on in-hospital mortality for pancreaticoduodenectomy. Ann Surg. 2003;237:509–14. doi: 10.1097/01.SLA.0000059981.13160.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Heek NT, Kuhlmann KFD, Scholten RJ, et al. Hospital volume and mortality after pancreatic resection. A systematic review and an evaluation of intervention in the Netherlands. Ann Surg. 2005;242:781–90. doi: 10.1097/01.sla.0000188462.00249.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birkmeyer JD, Sun Y, Goldfaden A, Birkmeyer NJO, Stukel TA. Volume and process of care in high-risk cancer surgery. Cancer. 2006;106:2476–81. doi: 10.1002/cncr.21888. [DOI] [PubMed] [Google Scholar]
- 9.Kotwall CA, Maxwell JG, Brinker CC, Koch GG, Covington DL. National estimates of mortality rates for radical pancreaticoduodenectomy in 25,000 patients. Ann Surg Oncol. 2002;9:847–54. doi: 10.1007/BF02557520. [DOI] [PubMed] [Google Scholar]
- 10.Gouma DJ, van Geenen RCI, van Gulik TM, et al. Rates of complications and death after pancreaticoduodenectomy: risk factors and the impact of hospital volume. Ann Surg. 2000;6:786–95. doi: 10.1097/00000658-200012000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gordon TA, Bowman HM, Tielsch JM, Bass EB, Burleyson GP, Cameron JL. Statewide regionalization of pancreaticoduodenectomy and its effect on in-hospital mortality. Ann Surg. 1998;228:71–8. doi: 10.1097/00000658-199807000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sosa JA, Bowman HM, Bass EB, et al. Importance of hospital volume in the overall management of pancreatic cancer. Ann Surg. 1998;228:429–38. doi: 10.1097/00000658-199809000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Birkmeyer JD, Warshaw AL, Finlayson SRG, Grove MR, Tosteson ANA. Relationship between hospital volume and late survival after pancreaticoduodenectomy. Surgery. 1999;126:178–83. [PubMed] [Google Scholar]
- 14.Riall TS, Eschbach KA, Townsend CM, Nealon WH, Freeman JL, Goodwin JS. Trends and disparities in regionalization of pancreatic resection. J Gastrointest Surg. 2007;11:1242–51. doi: 10.1007/s11605-007-0245-5. [DOI] [PubMed] [Google Scholar]
- 15.Meguid RA, Ahuja N, Chang DC. What constitutes a high-volume hospital for pancreatic resection? J Am Coll Surg. 2008;206:622–e1-9. doi: 10.1016/j.jamcollsurg.2007.11.011. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 16.Birkmeyer JD, Dimick JB. Potential benefits of the new Leapfrog standards: effect of process and outcomes measures. Surgery. 2004;135:569–75. doi: 10.1016/j.surg.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Bunker JP, Luft HS, Enthoven A. Should surgery be regionalized? Surg Clin N Am. 1982;62:657–68. doi: 10.1016/s0039-6109(16)42785-4. [DOI] [PubMed] [Google Scholar]
- 18.Birkmeyer JD. Raising the bar for pancreaticoduodenectomy. Ann Surg Oncol. 2002;9:826–7. doi: 10.1007/BF02557516. [DOI] [PubMed] [Google Scholar]
- 19.Afsari A, Zhandoug Z, Young S, Ferguson L, Silapaswan S, Mittal V. Outcome analysis of pancreaticoduodenectomy at a community hospital. Am Surg. 2002;68:281–4. [PubMed] [Google Scholar]
- 20.Chew DK, Attiyeh FF. Experience with the Whipple procedure (pancreaticoduodenectomy) in a university-affiliated community hospital. Am J Surg. 1997;174:312–5. doi: 10.1016/s0002-9610(97)00110-4. [DOI] [PubMed] [Google Scholar]
- 21.Metreveli RE, Sahm K, Abdel-Misih R, Petrelli NJ. Major pancreatic resections for suspected cancer in a community-based teaching hospital: lessons learned. J Surg Oncol. 2007;95:201–6. doi: 10.1002/jso.20662. [DOI] [PubMed] [Google Scholar]
- 22.Maa J, Gosnell JE, Gibbs VC, Harris HW. Exporting excellence for Whipple resection to refine the Leapfrog Initiative. J Surg. Res. 2007;138:189–97. doi: 10.1016/j.jss.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 23.Hospital Data: Texas Health Care Information Collection Center for Health Statistics [Internet] Texas Department of State Health Services; Austin (TX): 1999-[cited 2007 Mar 17]. Available from: http://www.dshs.state.tx.us/thcic/Hospitals/HospitalData.shtm. [Google Scholar]
- 24.Kennedy EP, Rosato EL, Sauter PK, Rosenberg LM, Doria C, Marino IR, et al. Initiation of a critical pathway for pancreaticoduodenectomy at an academic institution—the first step in multidisciplinary team building. J Am Coll Surg. 2007;204:917–23. doi: 10.1016/j.jamcollsurg.2007.01.057. [DOI] [PubMed] [Google Scholar]
- 25.Winter JM, Cameron JL, Campbell KA, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg. 2006;10:1199–210. doi: 10.1016/j.gassur.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 26.Hutter MM, Glasgow RE, Mulvihill SJ. Does the participation of a surgical trainee impact patient outcomes? A study of major pancreatic resections in California. Surgery. 2000;128:286–92. doi: 10.1067/msy.2000.107416. [DOI] [PubMed] [Google Scholar]
- 27.Pitt HA, Murray KP, Bowman HM, Coleman J, Gordon TA, Yeo CJ, et al. Clinical pathway implementation improves outcomes for complex biliary surgery. Surgery. 1999;156:751–6. [PubMed] [Google Scholar]
- 28.Porter GA, Pisters PW, Mansyur C, Bisanz A, Reyna K, Stanford P, et al. Cost and utilization impact of a clinical pathway for patients undergoing pancreaticoduodenectomy. Ann Surg Oncol. 2000;7:484–9. doi: 10.1007/s10434-000-0484-0. [DOI] [PubMed] [Google Scholar]
- 29.Campbell H, Hotchkiss R, Bradshaw N, Porteous M. Integrated care pathways. BMJ. 1998;316:133–7. doi: 10.1136/bmj.316.7125.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lillemoe KD, Cameron JL, Kim MP, Campbell KA, Sauter PK, Coleman J, et al. Does fibrin glue sealant decrease the rate of pancreatic fistula after pancreaticoduodenectomy? Results of a prospective randomized trial. J Gastrointest Surg. 2004;8:766–72. doi: 10.1016/j.gassur.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 31.Büchler MW, Bassi C, Fingerhut A, Klempa I. Does prophylactic octreotide decrease the rates of pancreatic fistula and other complications after pancreaticoduodenectomy? Ann Surg. 2001;234:262–3. doi: 10.1097/00000658-200108000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeo CJ, Lillemoe KD, Sauter PK, Coleman J, Sohn TA, Campbell KA, et al. Does prophylactic octreotide really decrease the rates of pancreatic fistula and other complications following pancreaticoduodenectomy? Ann Surg. 2000;232:419–29. doi: 10.1097/00000658-200009000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yeo CJ, Barry MK, Sauter PK, Sostre S, Lillemoe KD, Pitt HA, et al. Erythromycin accelerates gastric emptying following pancreaticoduodenectomy: a prospective, randomized placebo-controlled trial. Ann Surg. 1993;218:229–38. doi: 10.1097/00000658-199309000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]