Pseudoxanthoma elasticum (PXE; OMIM 264800) manifests with characteristic skin lesions of yellowish papules which coalesce into plaques of inelastic and leathery skin on the predilection sites (1). The ocular findings consist of angioid streaks, choroidal neovascularization and subretinal hemorrhages resulting in loss of visual acuity and occasional blindness. Cardiovascular problems include hypertension, intermittent claudication, and occasional myocardial infarcts and stroke. The prevalence of PXE is estimated to be in the range of 1:50,000–70,000 and to be more frequent in females than in males. The diagnosis can be challenging to clinicians due to late-onset of clinical manifestations and considerable heterogeneity.
PXE is associated with mutations in the ABCC6 gene which encodes a transmembrane efflux transporter expressed primarily in the liver and the kidneys (2). Consequently, PXE has been suggested to be a metabolic disorder with ectopic mineralization of the peripheral connective tissues. Recent studies have also suggested that cutaneous features of PXE can be found in patients with generalized arterial calcification of infancy due to mutations in the ENPP1 gene (3, 4).
Previous studies, which have documented close to 600 distinct mutations in the ABCC6 gene, have suggested the presence of unique mutations affecting certain ethnic groups with different ancestral backgrounds (5, 6). In this study, we asked the specific question whether Brazilian patients of mixed European, Native Indian and African ancestry with PXE harbor unique ABCC6 mutations, and whether such mutations might be correlated with the clinical phenotypes in this population with particular reference to heterozygous carriers.
METHODS
This study was approved by the Research Ethics Committee of Federal University of São Paulo (UNIFESP). Fifty-three members representing 4 Brazilian families with PXE participated in this study. DNA was isolated from saliva using an Oragene™ DNA collection kit (DNA Genotek Inc., Ottawa, Canada) and the DNA-SAL™ collection Kit (Oasio Diagnostic® Corp., Vancouver, WA, USA) or from peripheral blood using a Gentra Pure Gene Kit (Qiagen Sciences, Germantown, MD, USA). Multiplex ligation-dependent probe amplification (MLPA) analysis was performed using the P092 ABCC6 Probe Mix Kit (MRC-Holland, Amsterdam, The Netherlands). This kit contains 23 probes corresponding to ABCC6 gene exons 2, 4, 5, 7–15, 17, 18, 21–28 and 30, as well as 12 control probes for quality control. MLPA analysis was performed according to the Manufacturer’s recommendations (www.mlpa.com).
PCR amplification was performed on total genomic DNA with Taq DNA polymerase. The entire coding region and intron/exon boundaries of ABCC6 were amplified, and the PCR products were analyzed by direct nucleotide sequencing (Applied Biosystems 3730 Sequencer; Applied Biosystems, Foster City, CA) (5). The +1 in the ABCC6 gene corresponds to the A nucleotide in the ATG translation initiation codon (GenBank Accession number: AF076622).
FAMILY STUDIES
Four families from Brazil with established diagnosis of PXE were subjected to phenotypic evaluation and mutation analysis (Fig. S1; available from http://www.medicaljournals.se/acta/content/?doi=10.2340/00015555-1570). The proband of each family (arrows in Fig. S1) demonstrated characteristic skin lesions, angioid streaks with loss of visual acuity, and the diagnosis of PXE was confirmed by skin biopsy (Fig. 1). Clinical examination of the members of the nuclear family of the proband in Families 1 and 2 revealed no signs or symptoms of PXE. The proband in Family 3 had two older sisters and an older brother, who had died, with similar skin and eye findings, and the diagnosis was confirmed by skin biopsy. In Family 4, the proband had an older cousin with similar findings. In addition, two of her younger siblings (III-7 and III-8) had similar skin findings but they opted not to participate in this study.
Fig. 1.
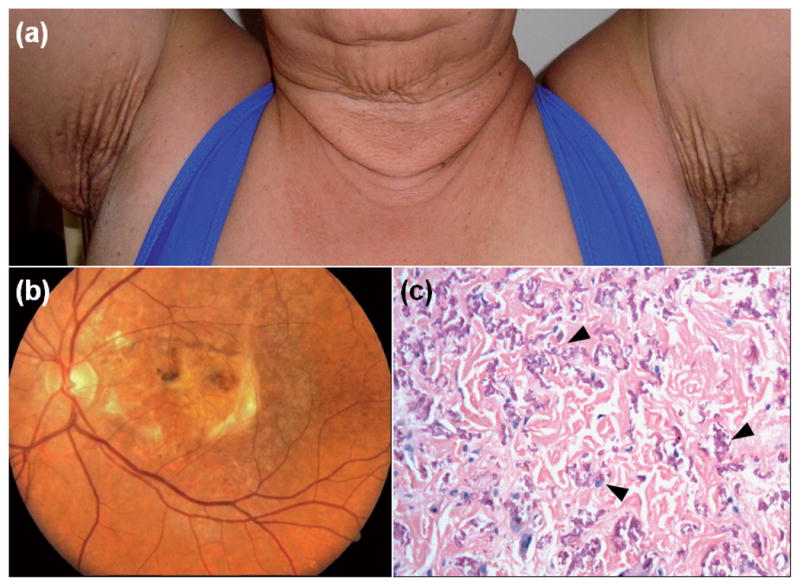
Diagnostic features of pseudoxanthoma elasticum in the probands, demonstrating characteristic skin lesions on the neck and axillary area, as well as angioid streaks and disciform scars in both eyes (a, b; Family 1). Skin biopsy (c, Family 4) revealed the presence of mineralized pleiomorphic elastic structures in the mid dermis (arrowheads) (H&E stain × 200).
Mutation analysis revealed the presence of allelic mutations in the ABCC6 gene in each affected individual (Figs S1 and S2; available from http://www.medicaljournals.se/acta/content/?doi=10.2340/00015555-1570; Table I), while unaffected members of these families showed only wild-type sequences or were heterozygous for one of the mutations identified in the proband. The proband’s mother in Family 3 (I-2), who is a heterozygous carrier of the p.R518Q mutation, presented with angioid streaks, but no skin findings. There was no skin biopsy from her.
Table I.
ABCC6 gene mutations in Brazilian patients with pseudoxanthoma elasticum
| Family | Age, years (sex) | Age at onset, years | Allele 1 | Allele 2 |
|---|---|---|---|---|
| 1 | 61 (F) | 16 | del23-29 | R1339C |
| 2 | 33 (F) | 28 | W1324X | not detected |
| 3 | 35 (F), 37 (F), 47 (F) | 5 | R518Q | G1296D |
| 4 | 40 (F) 27 (F) |
37 13 |
R1141X E1400K |
E1400K E1400K |
In Family 4, the proband’s mother (II-6), who was heterozygous for p.R1141X mutation, was noted to have lesions on her lower lip suggestive of PXE, however, no skin biopsy was available. The proband’s father (II-5), who was heterozygous carrier of the p.E1400K mutation, had loose skin of the neck, intermittent claudication and hypertension at the age of 65. He was suggested to have PXE, but no skin biopsy was available.
DISCUSSION
Our study is the first analysis of ABCC6 mutations in Brazilian patients with PXE. While previous studies have suggested the presence of unique mutations in ethnically restricted populations with distinct ancestral backgrounds, the 7 different mutations disclosed in our study have been encountered in other ethnic backgrounds previously (5). It should be noted that all 4 families had a history suggesting a mixture of European (Portugese and/or Italian), Native Indian and African ancestry, but contribution of these ethnic backgrounds to the genotype of each individual was not assessed in this study. Nevertheless, identification of these mutations in the families has been helpful in confirming the diagnosis as well as identification of heterozygous carriers and individuals with normal genotype.
One of the issues raised regarding the clinical presentation of PXE is the question whether heterozygous carriers depict mild or subclinical phenotypes (7, 8). In our study, members in the Family 3 (I-2) and Family 4 (II-5 and II-6) were suggested to have PXE due to limited skin involvement and intermittent claudication. However, it should be noted that these individuals were all in their 60’s, and no biopsy was performed to substantiate the diagnosis. Examination of all other heterozygous carriers, particularly in the two large Families 3 and 4, failed to reveal phenotypic presentation of PXE in heterozygote carriers. Thus, the inheritance of PXE is consistent with autosomal recessive, with no clear evidence of overt clinical phenotype in heterozygous carriers, in accordance with previous observations (9).
In conclusion, our study contributes to the growing database of ABCC6 mutations in PXE families from different ethnic backgrounds. Identification of specific mutations in these families is helpful towards confirmation of the diagnosis, with subsequent counseling for management of the disease with lifestyle modifications, such as dietary variation and avoidance of trauma (2, 8, 10).
Acknowledgments
We thank the families for their participation. Carol Kelly assisted in preparation of this manuscript. Supported by NIH/NIAMS R01AR28450-30 (JU) and Capes and Citogem Biotechnology (CSF). QL is the recipient of a Dermatology Foundation Research Career Development Award.
References
- 1.Neldner KH. Pseudoxanthoma elasticum. Clin Dermatol. 1988;6:1–159. doi: 10.1016/0738-081x(88)90003-x. [DOI] [PubMed] [Google Scholar]
- 2.Uitto J, Li Q, Jiang Q. Pseudoxanthoma elasticum: molecular genetics and putative pathomechanisms. J Invest Dermatol. 2010;130:661–670. doi: 10.1038/jid.2009.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nitschke Y, Baujat G, Botschen U, Wittkampf T, du Moulin M, Stella J, et al. Generalized arterial calcification of infancy and pseudoxanthoma elasticum can be caused by mutations in either ENPP1 or ABCC6. Am J Hum Genet. 2012;90:25–39. doi: 10.1016/j.ajhg.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Q, Schumacher W, Siegel D, Jablonski D, Uitto J. Cutaneous features of pseudoxanthoma elasticum in a patient with generalized arterial calcification of infancy due to a homozygous missense mutation in the ENPP1 gene. Br J Dermatol. 2012;166:1107–1111. doi: 10.1111/j.1365-2133.2012.10811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pfendner EG, Vanakker OM, Terry SF, Vourthis S, McAndrew PE, McClain MR, et al. Mutation detection in the ABCC6 gene and genotype-phenotype analysis in a large international case series affected by pseudoxanthoma elasticum. J Med Genet. 2007;44:621–628. doi: 10.1136/jmg.2007.051094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larusso J, Ringpfeil F, Uitto J. Pseudoxanthoma elasticum: a streamlined, ethnicity-based mutation detection strategy. Clin Transl Sci. 2010;3:295–298. doi: 10.1111/j.1752-8062.2010.00243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin L, Maître F, Bonicel P, Daudon P, Verny C, Bonneau D, et al. Heterozygosity for a single mutation in the ABCC6 gene may closely mimic PXE: consequences of this phenotype overlap for the definition of PXE. Arch Dermatol. 2008;144:301–306. doi: 10.1001/archderm.144.3.301. [DOI] [PubMed] [Google Scholar]
- 8.Uitto J, Bercovitch L, Terry SF, Terry PF. Pseudoxanthoma elasticum: progress in diagnostics and research towards treatment: Summary of the 2010 PXE International Research Meeting. Am J Med Genet A. 2011;155A:1517–1526. doi: 10.1002/ajmg.a.34067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christen-Zäch S, Huber M, Struk B, Lindpaintner K, Munier F, Panizzon RG, et al. Pseudoxanthoma elasticum: evaluation of diagnostic criteria based on molecular data. Br J Dermatol. 2006;155:89–93. doi: 10.1111/j.1365-2133.2006.07278.x. [DOI] [PubMed] [Google Scholar]
- 10.Li Q, Larusso J, Grand-Pierre AE, Uitto J. Magnesium carbonate-containing phosphate binder prevents connective tissue mineralization in Abcc6(−/−) mice-potential for treatment of pseudoxanthoma elasticum. Clin Transl Sci. 2009;2:398–404. doi: 10.1111/j.1752-8062.2009.00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


