Abstract
The functional coupling of residues that are far apart in space is the quintessential property of allosteric receptors. Data from functional studies of allosteric receptors, such as whole-cell dose-response relations, can be used to determine if mutation to a receptor significantly impacts agonist potency. However, the classification of perturbations as primarily impacting binding or allosteric function is more challenging, often requiring detailed kinetic studies. This protocol describes a simple strategy, derived from mutant cycle analysis, for elucidating long-range functional coupling in allosteric receptors (ELFCAR). Introduction of a gain-of-function reporter mutation, followed by a mutant cycle analysis of the readily-measured macroscopic EC50 values can provide insight into the role of many physically distant targets. This new method should find broad application in determining the functional roles of residues in allosteric receptors.
Keywords: Allostery, signal transduction, conformational change, coupling, structure-function study, ion channel, nicotinic receptor, amino acids
1. Introduction
In allosteric receptors, high affinity binding of a specific small molecule(s) or ion(s) to a particular binding site initiates a functional response, termed activation(1, 2). Typically this functional response is most prominent in a region that is spatially distinct from the binding site, and the binding event conveys changes to structural and/or electronic properties of the protein over distances of tens of angstroms or more. This is a hallmark of allosteric receptors—the ability to propagate specific conformational changes over long distances across a macromolecule. A natural consequence of this effect is the categorization of functional domains or specific amino acids of the protein into two classes: those that are primarily involved in binding the small molecule and those that contribute to the functional response. In a typical characterization of such a receptor, a dose-response curve based on a readout of receptor function produces, for example, an EC50. When a specific perturbation is introduced – often a side chain mutation – any significant shift in EC50 could reflect changes in either agonist binding or functional coupling, as EC50 is a composite number that can be influenced by both these properties of an allosteric receptor(3). Which property the perturbation affects, however, cannot be resolved from the dose-response curve alone. Here we describe a strategy based on mutant cycle analysis(4, 5) that, in favorable cases, provides a simple way to make such a classification.
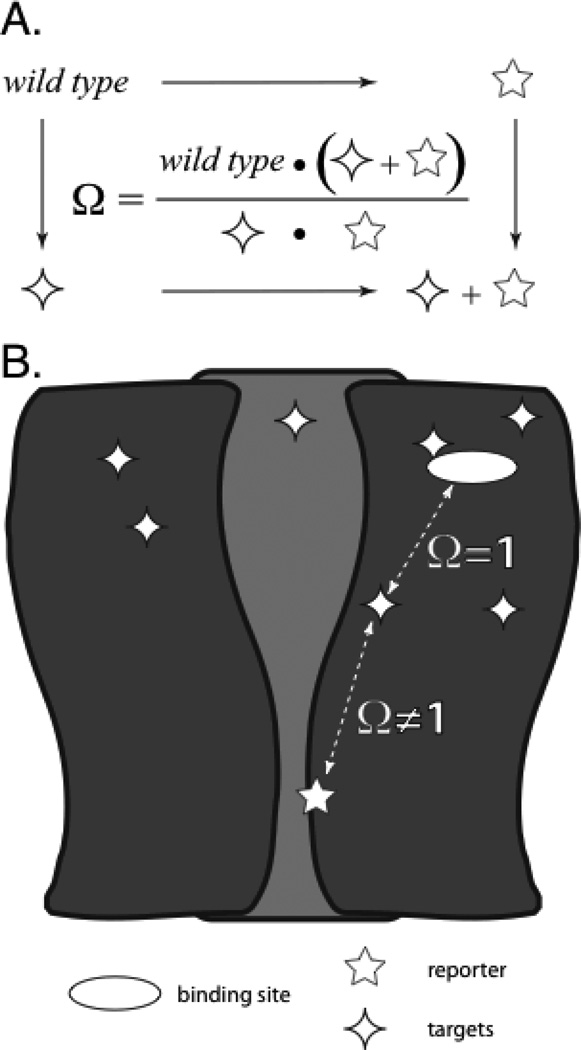
In mutant cycle analysis two perturbations are made to the system, first in isolation and then in tandem. The key metric is Ω, the coupling parameter, here given in terms of the functional metric EC50 (Fig. 1A). If the two perturbations are independent of one another, Ω should be ~1. That is, for independence the individual mutations’ effects on functional metrics should be multiplicative (additive when placed on a relative energy scale). If Ω deviates significantly from 1, an interaction between the perturbed sites is established. Typically the method has been used to establish proximity between a pair of amino acids, often implicating a stabilizing interaction such as a hydrogen bond or an ion pair(6–8). However, in an allosteric protein two residues can be functionally coupled even if they are quite far apart, and such behavior has been commonly seen(9).
Figure 1.
Schematic representation of an allosteric receptor and mutant cycle analysis. The 5-point star is the gain-of-function reporter mutation and the 4-point stars are the distant targets being altered throughout the allosteric receptor. In these studies, the specific functional metric used is EC50. (A) Definition of Ω, the coupling parameter, in terms of a generic functional metric for receptors with altered target, reporter, and both. (B) ELFCAR enables the assignment of functional coupling of physically distant regions of allosteric receptors (Ω ≠ 1) versus binding mutations (Ω = ~1) when mutant cycle analysis is performed on wild type receptors with high Kact and using reporter(s) that further significantly increase Kact.
In the present system, we define one perturbation as the “reporter”. We confine this discussion to cases where the reporter is a well-characterized, gain-of-function mutation that is remote from the binding site of the agonist or allosteric modulator. The reporter mutation is then used to evaluate a target (Fig. 1B). Typically the target is a particular amino acid whose role in receptor function is unknown, although in principle the method could also be used to probe the consequences of chemical changes to an agonist or allosteric modulator. In the present discussion we will focus on amino acid targets. A mutation to the target is then paired with the reporter mutation, with the goal of determining whether the target can be classified as primarily contributing to agonist binding or to the functional response. Our reporter-based application of mutant cycle analysis is termed elucidating long-range functional coupling in allosteric receptors (ELFCAR)(10).
The key to ELFCAR analysis is the composite nature of the functional metric, EC50. Consider a minimal kinetic scheme for an allosteric receptor such as this 3 state model.
For this scheme, R is an unactivated allosteric receptor, A is agonist, and R* is an activated allosteric receptor. This gives rise to the EC50 value defined by Eq. 1, where KD = k−/k+ and Kact = kact/kdeact.
| (Eq. 1) |
Note that more complex kinetic models retain the essential features of this scheme(11). As noted above, the reporter mutation is chosen to be one that impacts only Kact. A mutation that is physically quite remote to the binding site would be appropriate, although detailed analysis of its impact on function by, for example, single channel analysis, is desirable(12–14). When plotted on a log scale, the relationship between EC50 and functional coupling, as judged by the equilibrium constant for receptor activation, Kact, is linear with high slope over a considerable range. However, as shown in Fig. 2, EC50 plateaus at small values of Kact. Since most allosteric receptors are efficiently activated by one or more agonists, the activation of the wild type receptor will typically lie in the high slope region of the plot. By design, the gain-of-function reporter mutation solely impacts Kact, causing a consistent rightward shift along the x-axis.
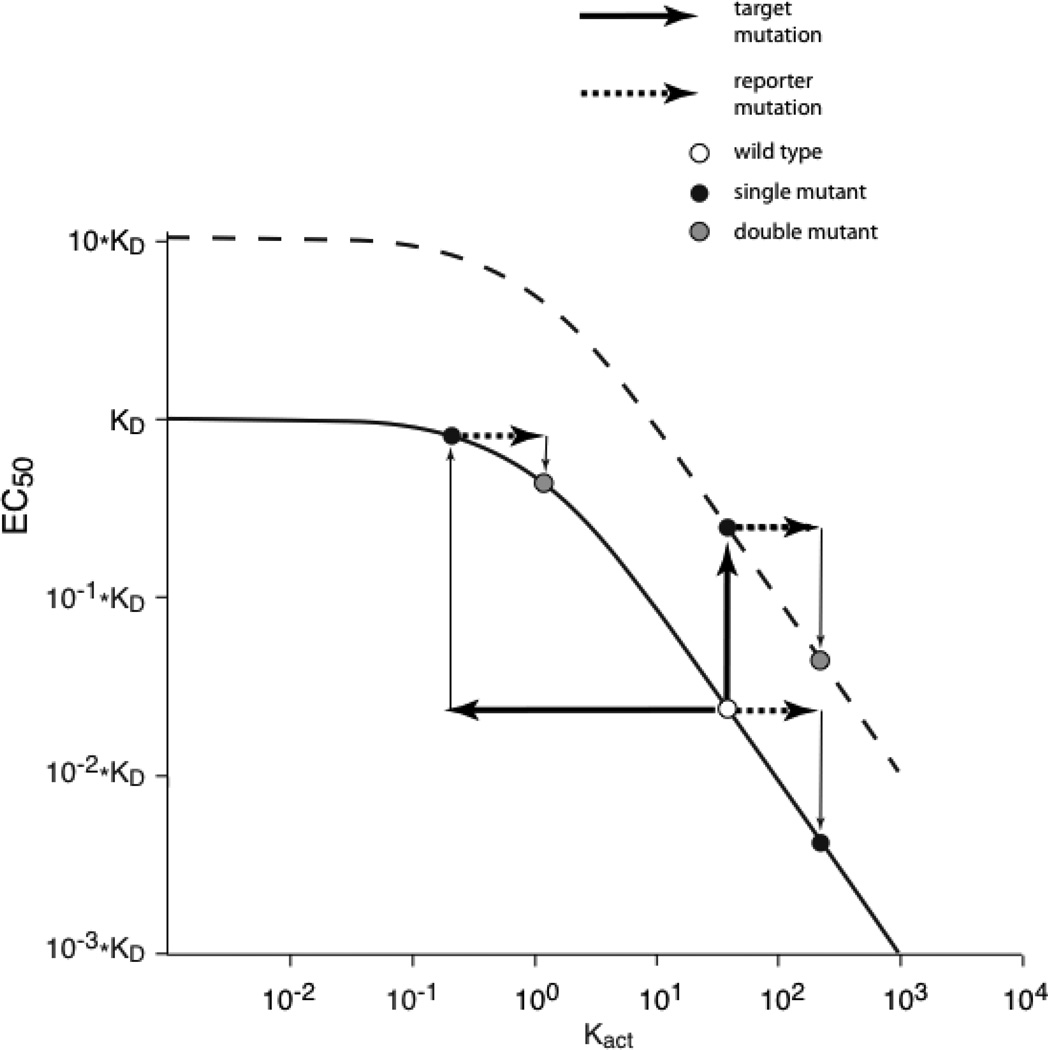
Figure 2.
Plot of EC50 versus Kact. (Solid line) Relationship between EC50 and Kact for a simple allosteric model (Eq. 1). (Dotted line) Plot of the same model, but with KD 10-fold larger. For both plots, in the high slope region changes to Kact produce significant changes in EC50 (thin arrows), as shown when the reporter mutation is made to the wild type receptor. However, for allosteric receptors with Kact in the plateau region, such as wild type receptors with high Kact into which a significant loss-of-function target mutation, a much smaller shift in EC50 is seen for equivalent shifts in Kact.
Now consider a target mutation that produces a significant loss-of-function. This can impact the curve of Fig. 2 two ways. If the increase in EC50 is a consequence of an increase in KD, the entire curve shifts upward (dashed line). This keeps EC50 in the high slope region, and so the reporter has the same fold shift in EC50 (thin arrows) for both the wild type and altered target receptors. Necessarily, mutant cycle analysis will produce an Ω = ~1. Alternatively, a loss-of-function target mutation could act by shifting Kact to the left (thick arrow). If the target mutation substantially inhibits allosteric function, it will produce a significant shift that will move EC50 into the plateau region (thin arrow). Now the effect of the reporter mutation on EC50 will be diminished relative to wild type and Ω ≠ 1. Thus, in favorable circumstances, a simple mutant cycle analysis using macroscopic measurements can provide insights into the detailed mechanism of receptor function.
Our discussion, and the protocols described below, have emphasized receptors that are activated by binding a small molecule. However, the essential feature of allosteric receptors is embedded in Eq. 1 and Fig. 2, and so any system with appropriate wild type activation kinetics and for which a significant gain-of-function reporter can be identified will be amenable to ELFCAR analysis.
2. Materials
Unless otherwise stated, water is Millipore-filtered water with resistivity ≥18 MΩ-cm. 0.2 μm filters are Nalgene (Thermo Fisher Scientific, Inc., Wilmington, DE).
2.1. PCR-based Site-directed Mutagenesis, DNA Amplification, and mRNA Transcription
DNA Amplification: Super Competent Top 10 Escherichia coli cells.
DNA isolation: QIAprep Miniprep kit (Quiagen, Valencia, CA).
DNA linearization: NotI enzyme and 10x buffer (New England Biolabs, Ipsich, MA).
Transcription: mMESSAGE mMACHINE T7 kit (Applied Biosystems, Foster City, CA).
RNA cleanup: RNeasy Mini kit (Quiagen, Valencia, CA).
Gel running buffer: dilute 1 part 50x Tris/acetic acid/EDTA (TAE; Bio-Rad Laboratories, Hercules, CA) with 49 parts water.
1% agarose gel: dissolve 0.60 g agarose (Invitrogen, Carlsbad, CA) in 60 mL of 1x TAE with heat (e.g. microwave for 45–60 s). Prepare as needed; pour gel into mold with desired number of lanes and store at 4° C for 1 – 12 h until use.
2.2. Expression of ligand-gated ion channels in Xenopus laevis oocytes
ND96 solution: 96 mM NaCl, 2 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, 5 mM HEPES, pH to 7.5 with NaOH. Filter with 0.2 μm filter and store at 4° C (see Note 1).
Oocyte incubation media: Add 138 mg sodium pyruvate (to 2.5 mM) and 60 mg theophylline (to 0.67 mM) to 500 ml of ND96 solution; filter with 0.2 μm filter. Add 500 μl of 50 mg/ml gentamicin solution (to ~0.11 mM). Add 1–2% horse serum, and store for <10 d at 4° C (see Note 2).
Oocyte viewing station: Use a standard dissecting microscope with ~20 x eyepieces and adjustable ~0.5x-4 x objective (such as Leica S6E, Spectra Services, Ontario, NY) with a flexible light source that has multiple lighting levels (such as model 3100 Dolan-Jenner Industries, Inc., Lawrence MA).
Injection needles: Pull injection needles from 8 inch with outer diameter = 1.14 mm, inner diameter = 0.53 mm (Drummond Scientific, Broomall, PA) in groups of ~10 on Sutter P-97 horizontal pipette puller (Sutter Instrument Co., Novato, CA).
Oocyte injection: In addition to the oocyte viewing station, install an x, y, z manipulator on a stable base (Nanoject Support Base, Drummond Scientific, Broomall, PA). Use a 10 μl microdispenser (Drummond Digital Microdispenser) to control volume of injected solutions.
2.3. Whole-cell recording with OpusXpress
1.00 M stock solutions of agonists: E.g. acetylcholine (ACh) chloride (Sigma-Aldrich/RBI) in water and immediately store at −80° C in aliquots of 100–500 μl (see Note 3).
Ca2+-free ND96 solution: 96 mM NaCl, 2 mM KCl, 1 mM MgCl2, 5 mM HEPES, pH to 7.5 with NaOH. Filter with 0.2 μm filter and store at 4° C (see Note 2).
Recording drug solutions: thaw then dilute previously made aliquots of 1.00 M agonist into Ca2+-free ND96 solution within 12 h prior to recording. ~10 doses over ~3 orders of magnitude of concentration centered on the anticipated EC50 value is a good starting point (see Note 4).
Recording electrodes: Use borosilicate glass with length 15 cm, outer diameter = 1.5 mm, and inner diameter = 1.17 mm (Sutter Instrument Co., Novato, CA). Pull >30 electrodes on horizontal pipette puller to a resistance of 0.3 – 3 MΩ for current headstages and 0.3 – 10 MΩ for voltage headstages.
Electrophysiological recording: OpusXpress 6000A (Molecular Devices Axon Instruments, Sunnyvale, CA).
2.4. Data Analysis
Clampex software (Axon Instruments, Union City, CA).
Software for graphing and fitting dose-response curves: E.g. KaleidaGraph, Origin, or SigmaPlot).
3. Methods
The purpose of this protocol is to use a reporter mutation to determine the functional role of distant targets by mutant cycle analysis. The first step is to identify a gain-of-function reporter. The technique then proceeds as is outlined in Fig. 3. While ELFCAR is broadly applicable to allosteric receptors, the protocol detailed here is specific for elucidating the functional role of a number of amino acids in the nicotinic acetylcholine receptor (nAChR), the prototypical ligand-gated ion channel (LGIC) of the Cys-loop receptor superfamily(15–17). The fetal mouse muscle receptor studied here is a heteropentamer comprised of 2 α, 1 β, 1 γ, and 1 δ subunits. Activation of the nAChR is initiated by the binding of ligand, such as ACh, in the extracellular domain. This induces a conformational change in which the channel pore gates from a closed, non-conducting form, to an open, conducting, form. Receptor activation is readily measured in a whole-cell voltage clamp assay over a range of concentrations and a dose-response relation is plotted and fit to the Hill equation, Eq. 2, where nH is the Hill coefficient, Imax is maximal response, and EC50 is the concentration of agonist needed to elicit half maximal response.
| (Eq. 2) |
(12–14) For ELFCAR, an EC50 value is obtained for each of 4 receptor sets — wild type, target, reporter, and target + reporter — in order to perform the mutant cycle analysis calculation of the Ω value. Details of the general ELFCAR procedure including (3.1) making the mutated allosteric receptors, (3.2) expressing them in a heterologous system, (3.3) obtaining functional data for the allosteric receptors, and (3.4) analyzing this data to calculate Ω, are described below. Once all necessary mutants are made, steps 3.2–3.4 can be performed rapidly (<1 week) and in parallel.
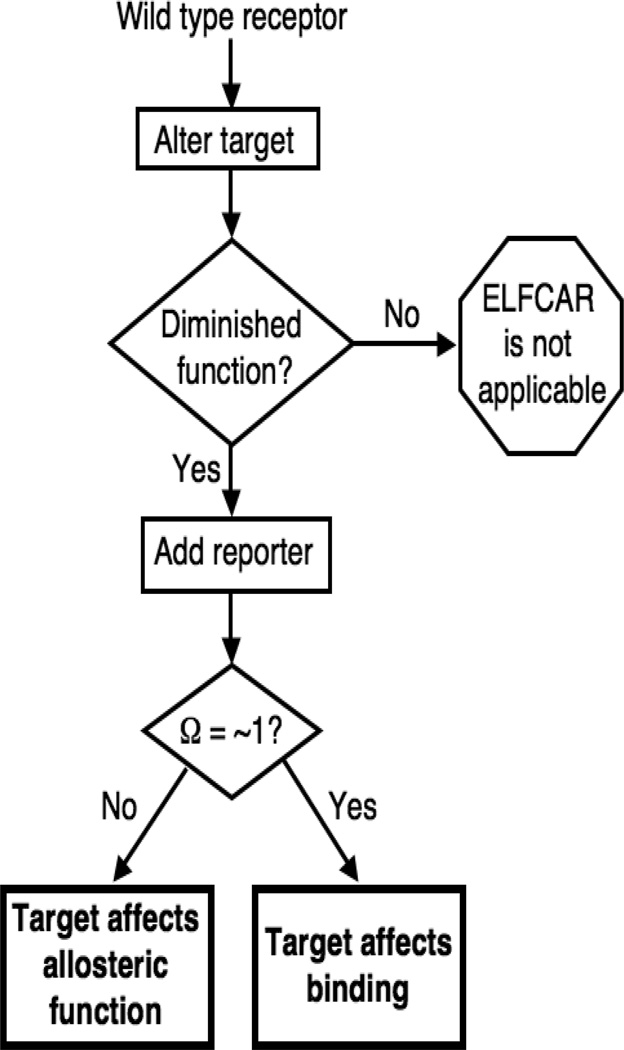
Figure 3.
Overview of the generic ELFCAR protocol described here. ELFCAR begins by altering targets and identifying those that are significantly deleterious to function. These targets are then combined with a well-characterized gain-of-function reporter mutation. Mutant cycle analysis is performed on 4 total allosteric receptors: by obtaining functional data for wild type as well as mutated receptors (separately and in tandem) and a value for Ω is calculated (Fig. 1A). For altered targets that produce a loss of function, if Ω ≠ 1, the altered target primarily impacts allosteric function. If Ω = ~1, the altered target presumably primarily affects binding.
3.1. PCR-based Site-directed Mutagenesis, DNA Amplification, and mRNA Transcription
Using the following protocol, perform site-directed mutagenesis for each of the mutations needed—reporter and target(s), if applicable. For all molecular biology reactions combine reagents in appropriate sized Eppendorf tube (>3x reaction volume), then mix by pipetting for 30 s, vortexing for 3 s, then microcentrifuging for 1–3 s.
Design mutagenic primers of 25–45 bases with 50–55% GC content and Tm ≥78° C; terminate with one or more C/G at 3’ end and check for primer dimers. For each mutation of interest, order forward and reverse (complementary sequence) primers.
Perform polymerase chain reaction (PCR) using Stratagene QuickChange method. For each mutation, a 50 µl reaction mix contains: 5 µl 10x polymerase buffer, 1 µl circular dsDNA template (5–50 ng), 1.25 µl of both mutagenic primers (~125 ng each), 1 µl 25 mM dNTP mix, 39.5 µl RNAse-free H2O, and 1 µl Pfu Turbo Hotstart DNA polymerase. Use a 10 min extension time in each thermocycle and modify annealing temperatures as required for successful incorporation of the mutation.
Once PCR is complete, remove 10 µl of the PCR reaction mixture for gel screen.
Perform 1.5 – 3 h of digestion by DpnI of the remaining PCR reaction to eliminate methylated template DNA from the PCR product, leaving the desired mutated DNA.
Perform a gel screen (1% agarose gel) of the PCR reaction by loading 10 µl of each PCR reaction mixture (from step 3) to each well and the corresponding circular DNA (control) in neighboring lanes; run for ~1 h. Develop gel to visualize with ethidium bromide (0.1% in gel running buffer) for 15 minutes and image gel.
Amplify the PCR products by electroporation of Super Competent Top 10 Escherichia coli cells at 1800 V followed by ~12 h of growth on agar/Luria broth/ampicillin plates in an incubator at 37° C.
Select single colonies and amplify each separately in 4 ml liquid Luria broth + 4 µl ampicillin with orbital shaking at 37° C for ~12 h.
Using a QIAPrep Miniprep kit, isolate the DNA from each bacterial sample separately as follows. Pellet DNA from step 7 and resuspend in 250 µl chilled buffer P1 in a 1.5 ml Eppendorf tube. Add 250 µl buffer P2 and mix by inverting 6 times, then add 350 µl buffer N3 and mix immediately by inverting 6 times. Microcentrifuge for 10 min at 13,0000 rpm, pipet supernatant to QIAprep spin column, centrifuge 1 min and discard flow-through. Wash QIAprep spin column with 0.5 ml buffer PB, centrifuge for 1 min and discard flow-through; repeat rinse with 0.75 ml Buffer PE. Microcentrifuge an additional 1 min to remove residual buffer. Elute DNA into a clean 1.5 ml Eppendorf tube by placing 30–50 µl water on the center of the column, letting stand for 1 min, and centrifuging for 1 min (see Note 5).
Determine DNA concentration.
Sequence DNA to verify the successful incorporation of the mutation at the selected site.
Linearize nAChR subunit DNA in expression vector pAMV by incubating 50 µg (~50–100 µl) with 20 µl 10x buffer, 20 µl NotI, and RNAse-free water to a final reaction volume of 200 µl for 8–16 h at 37° C. Verify complete linearization by running a 1% agarose gel in running buffer of ~2 µl reaction mixture against 2 µl of circular DNA.
Transcribe linearized DNA(s) using the T7 mMessage mMachine system individually for each subunit required for the receptor to be expressed; for the fetal mouse muscle nAChR these are α, β or βL9’S—where L9’S is the reporter mutation—γ, and δ (see Note 6). Perform a typical transcription by combining the following (in this order): 6 µl 10x transcription buffer, 30 µl 2x NTP mix, 8 µl RNAse-free water, 10 µl (~10 ug) linearized plasmid DNA, 6 µl T7 enzyme mix and incubating for 3–4 h at 37° C.
Stop transcription by adding 3 µl DNAse and incubating at 37° C for 0.5 – 1 h.
To cleanup mRNA using the RNeasy Mini kit, start by adjusting sample volume to 100 µl by adding 37 µl RNase free water. Add 350 µl buffer RLT (with 1% β-mercaptoethanol previously added to buffer RLT), vortex 5 s and microcentrifuge 10 s. Add 250 µl 100% ethanol, mix with gentle pipetting, then apply the 700 µl sample to an RNeasy column placed in a 2 ml collection tube and microcentrifuge for 15 s at 14,000 rpm. Discard flow-through and place column in a new 2 ml collection tube. Wash column with 500 µl diluted buffer RPE (4:1, 100% ethanol: RPE concentrate), with 15 s 12 microcentrifuging. Discard flow-through and repeat with 500 µl diluted buffer RPE and 2 min microcentrifuging, then place column in a new 2 ml collection tube and microcentrifuge for 1 min to ensure dryness and minimize ethanol carryover. Transfer column to a new 1.5 ml Eppendorf tube, pipet 30–60 µl RNase-free water directly onto the silica-gel membrane of the column and elute with 1 min microcentrifuging. Reapply eluent to silica-gel of column and re-elute to increase concentration (see Note 5)
Determine mRNA concentration.
3.2. Expression of ligand-gated ion channels in Xenopus laevis oocytes(18)
For the wild type or mutant receptors, prepare mRNA solution for injection by combining subunits in a 2:1:1:1 ratio (α:β:δ:γ, by mass) with a total mRNA concentration of ~1 – 2 ng/nl (see Note 7).
With freshly flame-sterilized Inox forceps break an injection needle to ~10–20 µm tip diameter (at injection station). Back fill completely with mineral oil, load onto a microdispenser and place in injection manipulator. Place mRNA solution, typically 1–3 µl, on a clean coverslip and draw mRNA solution into injection needle (see Note 8).
Inject defolliculated stage V–VI Xenopus laevis oocytes by pressing the injection needle through the cell membrane until the membrane bounces back, then expelling ~50 nl of the combined mRNA solution into the cell.
Change the oocyte incubation media 30–60 minutes after injection, and incubate injected oocytes at 16±2° C for 12–36 h with orbital shaking in 35 mm dishes (see Note 9).
3.3. Whole-cell recording with OpusXpress
Perform semi-automated whole-cell voltage clamp recording of Xenopus laevis oocytes expressing LGICs using the OpusXpress 6000A (see Note 10).
Fill previously pulled electrodes with 3.0 M KCl and place in electrode holders, then place one holder with electrode on each of the 16 headstages. Lower electrodes into Ca2+-free ND96 solution in chambers and check resistances: 0.3 – 10 MΩ for voltage electrodes, 0.3 – 3 MΩ for current electrodes (see Note 11).
Fill 96-well drug plates with increasing concentrations of drug solutions, starting with 0.
Place 1 oocyte in each chamber and impale oocytes individually. Common recording parameters are: Capacitance neutralization (0.0), Output gain (1), Ultra-high DC gain (on), and initial recording gain (1500). Optimize recording gain by increasing or decreasing gain individually on each impaled oocyte to achieve maximally square responses to test pulse with minimal ringing (usually 3000–6000 for healthy oocytes).
Voltage clamp each oocyte at a holding potential between −40 mV and −80 mV (see Note 12).
Perform a typical run by applying each concentration of drug solution for 15 s followed by a 130 s wash with Ca2+-free ND96 solution between each applied drug concentration. During drug application, superfuse oocytes with a flow rate of 4 ml/min; during wash, use a flow rate of 3 ml/min. Sample data at 125 Hz and filter at 50 Hz (see Note 13).
Start run and obtain dose-response data for ≥ 6 concentrations of agonist and for ≥ 5 oocytes with sufficient expression for each mutant (see Note 14). It is advisable to apply at least two control doses (Ca2+-free ND96 with no added agonist) during each run to verify non-response as well as to differentiate from leak current and fluidics effects. For each mutant, recordings should be performed on oocytes from ≥ 3 different donor Xenopus laevis frogs.
3.4. Data Analysis
Open each recorded data file in Clampex and determine the response at each concentration as the largest current deflection from average baseline.
Plot each oocyte’s normalized current response and corresponding drug concentration (see Note 15).
For remaining cells, average each concentration’s normalized response and plot the average dose-response relation for all oocytes with error bars (standard error).
Fit whole-cell dose-response relations to Hill equation (Eq. 2); record results.
Using the EC50 values for each of the 4 components of ELFCAR, calculate Ω (Fig. 1A). An example of the results produced for 27 different target mutations is shown in Fig. 4.
As shown in Fig. 3., targets that significantly diminish receptor function and with calculated Ω ≠ 1 are assigned as playing a role in the allosteric function of the receptor. Targets with Ω = ~1 are assigned as primarily affecting binding. For further studies, see Notes 16 and 17.
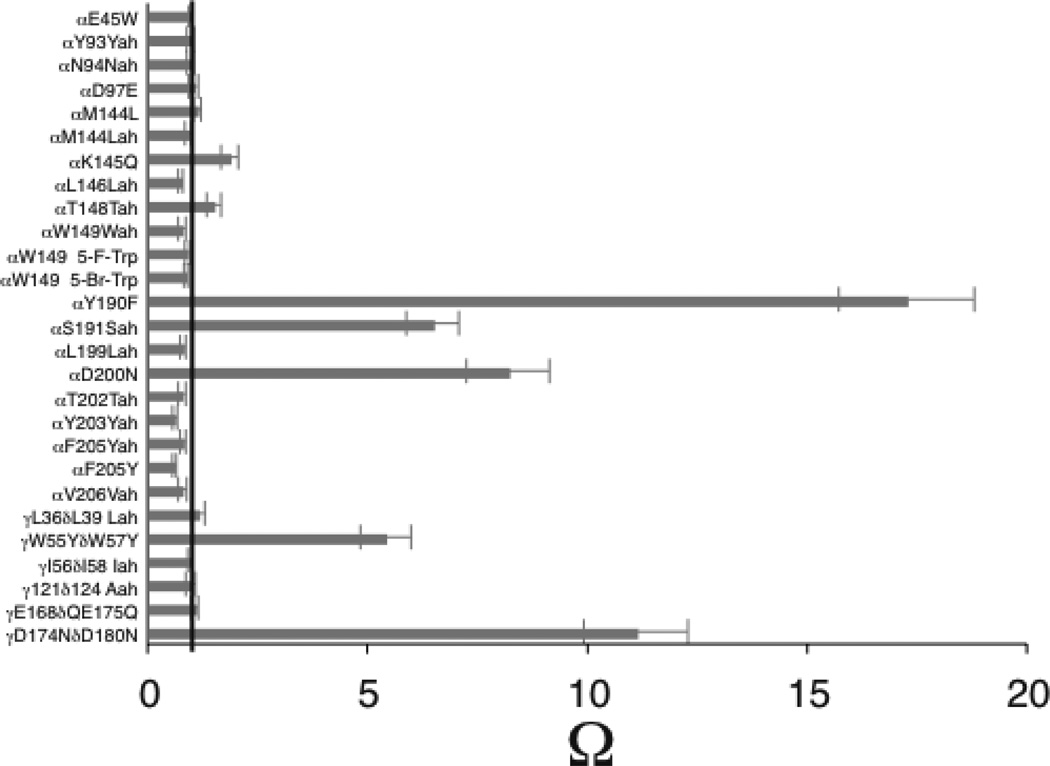
Figure 4.
Bar chart of omega values that ELFCAR produces. Gray bars represent Ω for 27 altered targets in the nAChR extracellular domain with βL9’S reporter. Note, βL9’S is a well-characterized gain-of-function mutation in the channel pore known primarily to impact channel activation (Kact)(12–14). Five altered targets show an Ω value that deviates significantly from 1 (black line) and are thereby assigned by ELFCAR to primarily impact allosteric function. Of the other 22, those altered targets that produce a significant loss-of-function (data not shown) are assigned as primarily impacting binding. For example, 5-F-Trp (5-fluorotryptophan) at αW149 produces an EC50 that is four times higher than wild type, but with Ω = 0.9, so ELFCAR assigns this altered target to impact binding. 5-Br-Trp is 5-bromotryptophan. For the α–hydroxy acids (Yah, Nah, Lah, Wah, Vah, Iah, and Aah), a three-letter abbreviation is used: the one-letter code for the parent amino acid is followed by -ah; thus Yah is the α–hydroxy acid of tyrosine.
Acknowledgements
We thank Kristin Rule Gleitsman for essential discussions throughout the development of ELFCAR. This work was supported by the National Institutes of Health (NS 34407; NS 11756). J.A.P.S. and S.F. were partially supported by National Research Service Awards from the NIH.
Footnotes
It is often convenient to prepare a concentrated (20x) stock solution of ND96 and Ca2+-free ND96 in order to quickly prepare the large volume of buffers used in semi-automated electrophysiology. Both the 1x and 20x stock ND96 and Ca2+-free ND96 should be stored with Parafilm around the cap.
Osmolarity of the incubation media should be 190–230 osmol/L. It is especially important that Parafilm be used to seal the cap of the bottle containing the oocyte incubation media to reduce solvent evaporation and prevent contamination.
Aliquot size should be chosen to ensure ≤3 freeze-thaw cycles. For agonists that are less stable, use individual stock solution aliquots for each fresh batch of drug solutions.
Use 4 doses per order of magnitude if there is reason to suspect multiple populations of receptors in order to more definitively detect and characterize a possible biphasic dose-response behavior. To determine whether or not a dose-response relation has multiple components, as opposed to having a low nH, use the F-test to determine whether 1 component (monophasic) or 2 components (biphasic) fits the dose-response data better.
Ensure that all water with DNA or RNA is recovered from the spin column by drawing a line on the Eppendorf tube at a level corresponding to volume of water used in the final DNA or RNA elution step. Spin additional times if necessary to recover this total volume from the column.
Our α-subunit cDNAs encode an HA epitope in the M3-M4 cytoplasmic loop for Western blot studies (not described). The HA epitope provides an independent way of determining if a mutation(s) giving no current is not functional or is not being assembled, transported, and inserted into the cell membrane. Control experiments show that this epitope does not significantly alter EC50.
Set aside bench space that is dedicated to mRNA work only. Mix the mRNA in this area.
Once the mRNA solution is placed on the coverslip, and especially under the light source, work quickly since these small volumes evaporate, and therefore change concentration, quickly.
We find that the 35 mm dishes from CELLSTAR (Grenier Bio-One) extend oocyte viability and reduce oocyte adhesion to the dish. After injection check oocytes at 30 – 60 min, then continue every 12–24 h. Store 10–40 oocytes per 35 mm dish. Discard oocytes that are damaged or have begun the maturation process (expanding circle of degenerating membrane); also change media. Do this as often as is necessary to keep the media clear. If oocytes aren’t living as long as your experiments require, change the dishes each time oocyte media is changed.
Due to heavy use (>80 h/week), we have implemented weekly and monthly maintenance procedures of the OpusXpress.
Weekly: Clean electrode holders by completely disassembling and rinsing in water with stirring for 30–60 min (don’t rinse the gold pin, threaded Teflon collar, or electrode wire with nylon sleeve and silicone seal); dry each piece thoroughly and reassemble. Remove debris from solution filters by sonicating them in water for 10 min. Inspect headstages for corrosion and inspect chambers and rinse individually by pipetting water to remove debris. Wipe the liquid handler nozzles and headstages with a moistened Kimwipe and dry.
Monthly: In addition to the weekly maintenance, replace all peristaltic tubing on pumps A, B, and the aspirator pump. Prime the new peristaltic tubing by rinsing with 70% isopropanol for 10 min, followed by water for 10 min. In each case use a flow rate of ≥1 ml/min for pump A and B and ≥ 4 ml/min for the aspirator pump. Spray the headstage tracks with Inox lubricant and apply fluorocarbon gel to the driving assembly shafts.
Implement similar maintenance for electrophysiology rigs used to record on one oocyte at a time. Especially important are rinsing perfusion lines and chamber after each use with water, regular maintenance of electronics, and frequent checking of the wiring/cables for salt buildup, fraying, and grounding.
Use these electrodes for repeated experiments as long as their resistances are still in range, they do not show drift, and they are free of significant oocyte membrane; usually replace electrodes every 1–3 d of heavy use (≥15 h/d).
Larger holding potentials increase Imax. This can be used to increase the signal-to-noise ratio if it is too low. However, higher holding potentials often decrease oocyte viability through an entire run. Make Imax comparisons (see Note 17a) at the same holding potentials, and after similar incubation times with parallel treatment of oocytes.
As in most experiments on LGICs, information from macroscopic experiments is usually limited by the speed of the solution change. We’ve estimated solution exchange times of ~1–2 s for these flow application rates on the OpusXpress. These are much slower than nAChR kinetics, but faster than some receptors (i.e. ρ1 GABAA)(19). In the rare cases where activation/deactivation kinetics can be resolved by the OpusXpress, one must first optimize electronic filter characteristics. Use an analog-to-digital sample rate > 2.5 times the analog filter’s cutoff frequency, to avoid aliasing.
For the wild type nAChR, define functional mutants (sufficient expression) as having Imax of ≥ ~100 nA. This produces a signal-to-noise ratio of > ~3–5. For these channels with single-channel conductance (gchannel) of ~40 pS (with Na+ as the permeant ion), 100 nA at a potential of −60 mV corresponds to ~40,000 receptors on the ooycte’s surface (goocyte = I/V = 100 nA/0.06 V = 1.7*10−6 S; goocyte/gchannel = ~40,000). Define receptors with a lower gchannel as functional for Imax values proportionally lower than 100 nA, provided that Imax can be determined, given the signal-to-noise ratio of the current responses.
Verify that there was no response to control doses (no drug). It is common to have a larger standard error for the regions of the dose-response curve that are near EC50.
If the target significantly diminishes function, but Ω = ~1, more detailed information regarding binding can be obtained by systematic structural and/or electronic perturbations of an amino acid side chain using site-directed and unnatural amino acid mutagenesis(20, 21).
If significant coupling is observed (Ω ≠ 1), several experimental strategies that complement, but are redundant to, ELFCAR can be pursued. Many of these can also be used in other allosteric receptors.
- Increase of Imax (maximal function) by the reporter in the background of the target (versus target alone)(10).
- Block of recovery of Imax (maximal function) of a partial agonist. In our studies the relative efficacy of succinylcholine (SuCh) to ACh (Imax(SuCh)/Imax(ACh)) generally recovered from ~4% to 70–100% from the wild type receptor to non-coupled perturbations (Ω = ~1). Such block of recovery of Imax was not seen for coupled mutations (Ω ≠ 1)(10).
- Other reporter mutations that couple to the target. In the case of the nAChR, we found that several reporter mutations gave similar coupling results to the βL9’S reporter. All of the alternative reporter mutations that we tested (γL9’S, δL9’S, αL9’S, αL13’S, and αL16’S) were in the channel pore and also exhibited gain-of-function behavior on their own(10).
In particular, we have shown that steps a, b, and d are readily implemented as coarse-grained verification of the ELFCAR result. Note that combining ELFCAR with steps a, b, and d can theoretically allow for identification of functional coupling in an allosteric receptor when a traditional functional metric, such as whole-cell dose-response relation, may have concluded that no functional change occurred when the target was altered based on an observed small shift or no shift in EC50. However, if the gain-of-function reporter introduces significant increases in Imax (a) or Popen (b) in the background of the altered target, the target may still be play a significant role in allosteric function. This is especially true if the reporter’s gain-of-function is larger than the target’s loss-of-function and/or if the wild type allosteric receptor has a Kact value that places the system between the negative slope and plateau regions.
References
- 1.Changeux JP, Edelstein SJ. Allosteric mechanisms of signal transduction. Science. 2005;308:1424–1428. doi: 10.1126/science.1108595. [DOI] [PubMed] [Google Scholar]
- 2.Fenton AW. Allostery: an illustrated definition for the 'second secret of life'. Trends Biochem Sci. 2008;33:420–425. doi: 10.1016/j.tibs.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colquhoun D. Binding, gating, affinity and efficacy: the interpretation of structure-activity relationships for agonists and of the effects of mutating receptors. Br J Pharmacol. 1998;125:924–947. doi: 10.1038/sj.bjp.0702164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carter PJ, Winter G, Wilkinson AJ, Fersht AR. The use of double mutants to detect structural changes in the active site of the tyrosyl-tRNA synthetase (Bacillus stearothermophilus) Cell. 1984;38:835–840. doi: 10.1016/0092-8674(84)90278-2. [DOI] [PubMed] [Google Scholar]
- 5.Kash TL, Jenkins A, Kelley JC, Trudell JR, Harrison NL. Coupling of agonist binding to channel gating in the GABA(A) receptor. Nature. 2003;421:272–275. doi: 10.1038/nature01280. [DOI] [PubMed] [Google Scholar]
- 6.Gleitsman KR, Kedrowski SM, Lester HA, Dougherty DA. An intersubunit hydrogen bond in the nicotinic acetylcholine receptor that contributes to channel gating. J Biol Chem. 2008;283:35638–35643. doi: 10.1074/jbc.M807226200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Price KL, Millen KS, Lummis SC. Transducing agonist binding to channel gating involves different interactions in 5-HT3 and GABAC receptors. J Biol Chem. 2007;282:25623–25630. doi: 10.1074/jbc.M702524200. [DOI] [PubMed] [Google Scholar]
- 8.Venkatachalan SP, Czajkowski C. A conserved salt bridge critical for GABA(A) receptor function and loop C dynamics. Proc Natl Acad Sci U S A. 2008;105:13604–13609. doi: 10.1073/pnas.0801854105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alexiev U, Mollaaghababa R, Khorana HG, Heyn MP. Evidence for long range allosteric interactions between the extracellular and 21 cytoplasmic parts of bacteriorhodopsin from the mutant R82A and its second site revertant R82A/G231C. J Biol Chem. 2000;275:13431–13440. doi: 10.1074/jbc.275.18.13431. [DOI] [PubMed] [Google Scholar]
- 10.Gleitsman KR, Shanata JA, Frazier SJ, Lester HA, Dougherty DA. Long-range coupling in an allosteric receptor revealed by mutant cycle analysis. Biophys J. 2009;96:3168–3178. doi: 10.1016/j.bpj.2008.12.3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalbaugh TL, VanDongen HM, VanDongen AM. Ligand-binding residues integrate affinity and efficacy in the NMDA receptor. Mol Pharmacol. 2004;66:209–219. doi: 10.1124/mol.66.2.209. [DOI] [PubMed] [Google Scholar]
- 12.Filatov GN, White MM. The role of conserved leucines in the M2 domain of the acetylcholine receptor in channel gating. Mol Pharmacol. 1995;48:379–384. [PubMed] [Google Scholar]
- 13.Kearney PC, Zhang H, Zhong W, Dougherty DA, Lester HA. Determinants of nicotinic receptor gating in natural and unnatural side chain structures at the M2 9' position. Neuron. 1996;17:1221–1229. doi: 10.1016/s0896-6273(00)80252-4. [DOI] [PubMed] [Google Scholar]
- 14.Labarca C, Nowak MW, Zhang H, Tang L, Deshpande P, Lester HA. Channel gating governed symmetrically by conserved leucine residues in the M2 domain of nicotinic receptors. Nature. 1995;376:514–516. doi: 10.1038/376514a0. [DOI] [PubMed] [Google Scholar]
- 15.Connolly CN, Wafford KA. The Cys-loop superfamily of ligand-gated ion channels: the impact of receptor structure on function. Biochemical Society Transactions. 2004;32:529–534. doi: 10.1042/BST0320529. [DOI] [PubMed] [Google Scholar]
- 16.Dougherty DA. Cys-loop neuroreceptors: structure to the rescue? Chem Rev. 2008;108:1642–1653. doi: 10.1021/cr078207z. [DOI] [PubMed] [Google Scholar]
- 17.Sine SM, Engel AG. Recent advances in Cys-loop receptor structure and function. Nature. 2006;440:448–455. doi: 10.1038/nature04708. [DOI] [PubMed] [Google Scholar]
- 18.Dascal N, Lotan I. Expression of exogenous ion channels and neurotransmitter receptors in RNA-injected Xenopus oocytes. In: Longstaff A, Revest P, editors. Methods in Molecular Biology. Totowa, NJ: Humana Press; 1992. pp. 205–225. [Google Scholar]
- 19.Wang J, Lester HA, Dougherty DA. Establishing an ion pair interaction in the homomeric rho1 gamma-aminobutyric acid type A receptor that contributes to the gating pathway. J Biol Chem. 2007;282:26210–26216. doi: 10.1074/jbc.M702314200. [DOI] [PubMed] [Google Scholar]
- 20.Nowak MW, Gallivan JP, Silverman SK, Labarca CG, Dougherty DA, Lester HA. In vivo incorporation of unnatural amino acids into ion channels in Xenopus oocyte expression system. Methods in enzymology. 1998;293:504–529. doi: 10.1016/s0076-6879(98)93031-2. [DOI] [PubMed] [Google Scholar]
- 21.Dougherty DA. Physical organic chemistry on the brain. J Org Chem. 2008;73:3667–3673. doi: 10.1021/jo8001722. [DOI] [PubMed] [Google Scholar]
- 22.Mortensen M, Smart TG. Single-channel recording of ligand-gated ion channels. Nat Protoc. 2007;2:2826–2841. doi: 10.1038/nprot.2007.403. [DOI] [PubMed] [Google Scholar]
- 23.Chakrapani S, Bailey TD, Auerbach A. Gating dynamics of the acetylcholine receptor extracellular domain. J Gen Physiol. 2004;123:341–356. doi: 10.1085/jgp.200309004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grosman C, Zhou M, Auerbach A. Mapping the conformational wave of acetylcholine receptor channel gating. Nature. 2000;403:773–776. doi: 10.1038/35001586. [DOI] [PubMed] [Google Scholar]