Abstract
A 64-year-old Filipino man presented to a Baltimore hospital with a 4-month history of worsening midback pain, progressive leg weakness, and intermittent bladder and bowel incontinence. He had no fever or pulmonary symptoms. Magnetic resonance imaging (MRI) of the thoracic spine revealed hypointense T1-weighted and hyperintense T2-weighted bone marrow signal involving vertebral bodies T2, T3, and T4 (findings that were consistent with osteomyelitis); vertebral compression fractures; an epidural fluid collection; and spinal cord compression (Fig. 1). Multiple blood cultures were negative. Because the spine was considered unstable, he underwent T2, T3, and T4 vertebrectomy with fusion from C3 to T8. Pathological studies of the operative specimen revealed granulation and chronic inflammation. No organisms were identified with the use of routine or special stains, including an auramine– phenol stain for acid-fast bacilli. Vertebral bone and material from the fluid collection were sent for fungal, mycobacterial, and routine bacterial cultures before the initiation of treatment with antimicrobial agents.
In the developed world, vertebral osteomyelitis is most commonly acquired hematogenously. Staphylococcus aureus is the most frequent isolate, followed by streptococcus and gram-negative organisms. The lumbar spine is most commonly involved, but the thoracic spine may also be infected. The patient’s progressive pain over a period of months is typical of vertebral osteomyelitis. Involvement of contiguous vertebral bodies and intervertebral disks is more suggestive of vertebral osteomyelitis than of malignant infiltration. Spinal epidural abscesses are caused by the same organisms and may result from hematogenous seeding of the epidural space or extension from vertebral osteomyelitis. For most patients who have vertebral osteomyelitis without epidural abscess, empirical antibiotic treatment can be delayed (but not by more than 24 hours) until biopsy is performed to obtain a microbiologic diagnosis; operative management is not required. In this case, however, the MRI and clinical findings are consistent with compression of the spinal cord by an epidural abscess; these findings mandate immediate neurosurgical consultation and mechanical decompression in an attempt to preserve neurologic function.
Less common causes of vertebral osteomyelitis and epidural abscess include Mycobacterium tuberculosis, fungi (e.g., Cryptococcus neoformans, candida species, Histoplasma capsulatum, and Coccidioides immitis), and brucella species. Spinal tuberculosis tends to involve the lower thoracolumbar spine and typically involves the anterior vertebral body. Arthritis, sacroiliitis, and spondylitis are the most common skeletal complications of brucella infection. Occasionally, spondylitis progresses to osteomyelitis and even abscess formation that may be difficult to distinguish from skeletal tuberculosis. The absence of granulomas on examination of the biopsy specimens and the absence of organisms identified with the use of special stains make these causes less likely but do not rule them out.
A purified protein derivative (PPD) test was positive, with 12 cm of induration. A chest radiograph did not show cavitary disease or other findings suggestive of pulmonary tuberculosis. On the basis of the clinical presentation and the results of the PPD test, a presumptive diagnosis of vertebral tuberculosis was made, and a regimen of ethambutol, isoniazid, pyrazinamide, and rifampin was started. Smears of three induced sputum specimens, obtained before therapy, were negative for acid-fast bacilli.
The patient then returned to the Philippines. There, despite reporting adherence to his antituberculosis regimen, he began to have high temperatures and drenching night sweats, and he lost 30 lb (14 kg). Seven months after his return to the Philippines, his relatives brought him back to the Baltimore hospital because of his difficulty with ambulation and new-onset fecal and urinary incontinence. He also had had a cough productive of bloody sputum for the previous 3 months.
Diagnosing skeletal tuberculosis is challenging. Cultures are positive in only half the cases; thus, empirical antituberculosis treatment is reasonable in patients with a positive PPD test, a compatible clinical presentation, and no other identified etiologic factors. However, granulomas are present in the majority of cases (particularly in surgical specimens), and their absence decreases the likelihood of tuberculosis. Possible reasons for failure of therapy in this patient include progression of drug-sensitive tuberculosis despite treatment, multidrug-resistant tuberculosis, poor adherence to the medication regimen, superinfection of the surgical site, or the presence of a disorder other than skeletal tuberculosis. His current symptoms suggest a process involving both the spine and lung. In a patient from Southeast Asia, infection with Burkholderia pseudomallei (i.e., melioidosis) should be considered. Endocarditis (with septic emboli) is also possible in a patient with vertebral osteomyelitis.
The patient had a history of poorly controlled type 2 diabetes mellitus, coronary artery disease with stent placement, and hypertension. His medications were aspirin, clopidogrel, lisinopril, and glyburide. He was born in the Philippines but had immigrated to the United States at 21 years of age. He had served in Vietnam for 4 years during the Vietnam War. Since his retirement 3 years ago, he had spent 10 months a year in the Philippines. He reported no use of tobacco, alcohol, or illicit drugs and no risk factors for human immunodeficiency virus (HIV) infection.
Diabetes is a risk factor for osteomyelitis caused by less common agents, including aspergilli, brucellae, and B. pseudomallei. Reactivation of B. pseudomallei has been reported to develop in veterans of the Vietnam War, which may cause pulmonary and bone lesions. Alternatively, the patient could have acquired an infection endemic to Southeast Asia more recently, given the amount of time spent in the Philippines.
On examination, the patient’s temperature was 39.5°C; pulse, 110 beats per minute; blood pressure, 130/70 mm Hg; respiratory rate, 22 breaths per minute; and arterial oxygen saturation, 94% while he was breathing ambient air. He appeared emaciated. There was no palpable lymphadenopathy. Examination of his chest revealed dullness on percussion, bronchial breath sounds, and coarse crackles over the right upper lobe. The cardiovascular and abdominal evaluations were unremarkable. Neurologically, he was oriented only to person. He had muscle wasting in the legs, with 4/5 strength proximally and 3/5 strength distally. The patellar reflexes were brisk, and the plantar responses were flexor. There was no sensory deficit. On his back, there was a large, fluctuant area of induration and erythema extending from the midcervical to the lower thoracic region, corresponding to the incision made during his previous surgery.
The patient’s upper-motor-neuron signs and leg weakness (combined with findings on his back) suggest that a recurrent abscess may be compressing his spinal cord. Inadequately treated tuberculosis remains possible, but I am increasingly concerned about an alternative chronic infection. If present initially, common pathogens such as S. aureus would have been expected to grow in multiple cultures obtained before the use of antibiotics, although a more recent superinfection with this or another bacterium remains possible. Serologic testing should be performed for brucella and B. pseudomallei. Specimens should also be obtained from both the lung and the paraspinal abscess for culture and histopathological examination, and the microbiology laboratory should be informed of the possibility of infection with fastidious organisms (e.g., brucella).
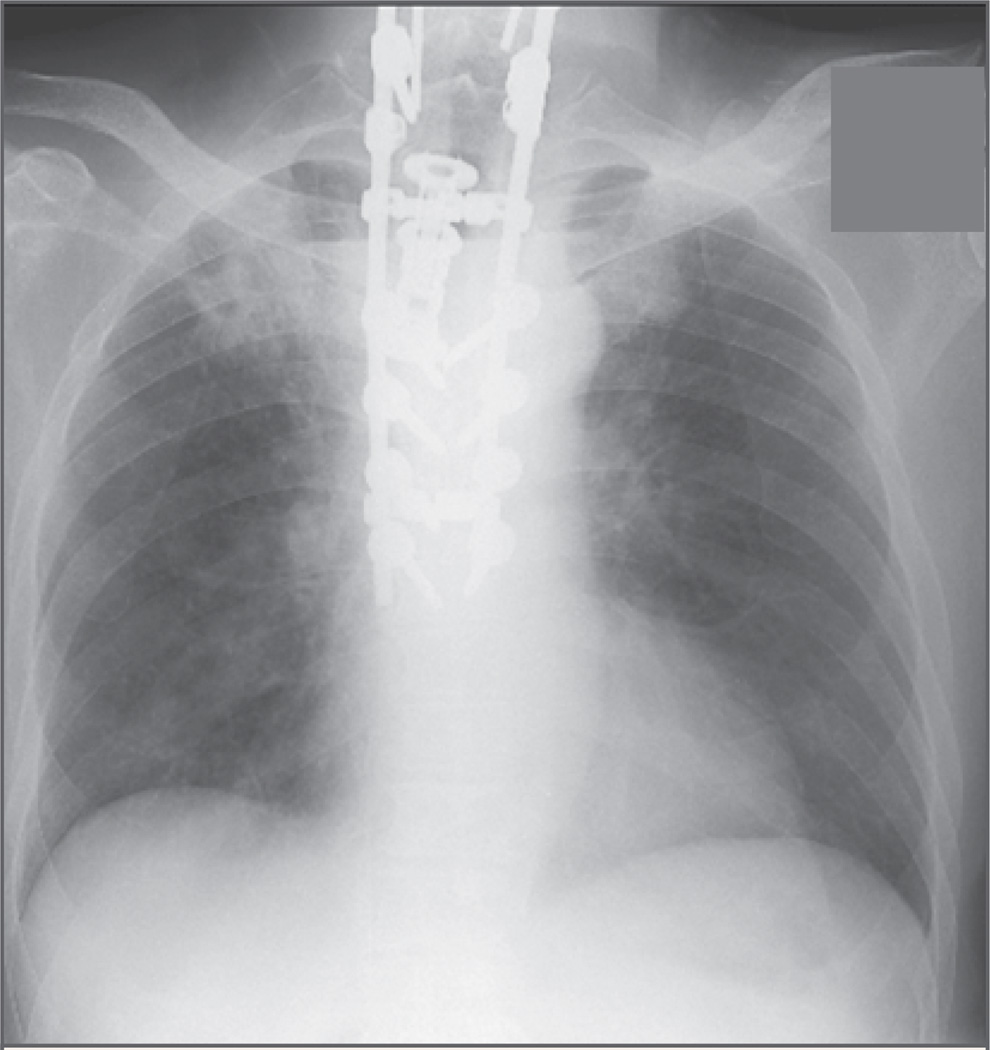
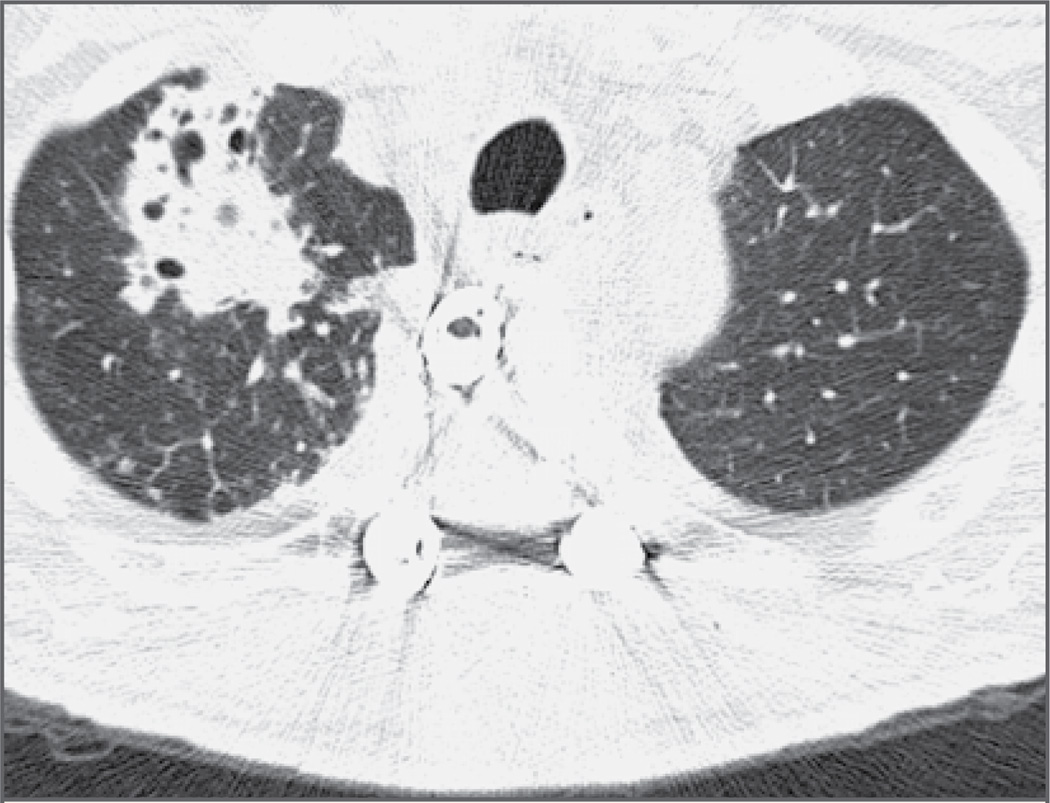
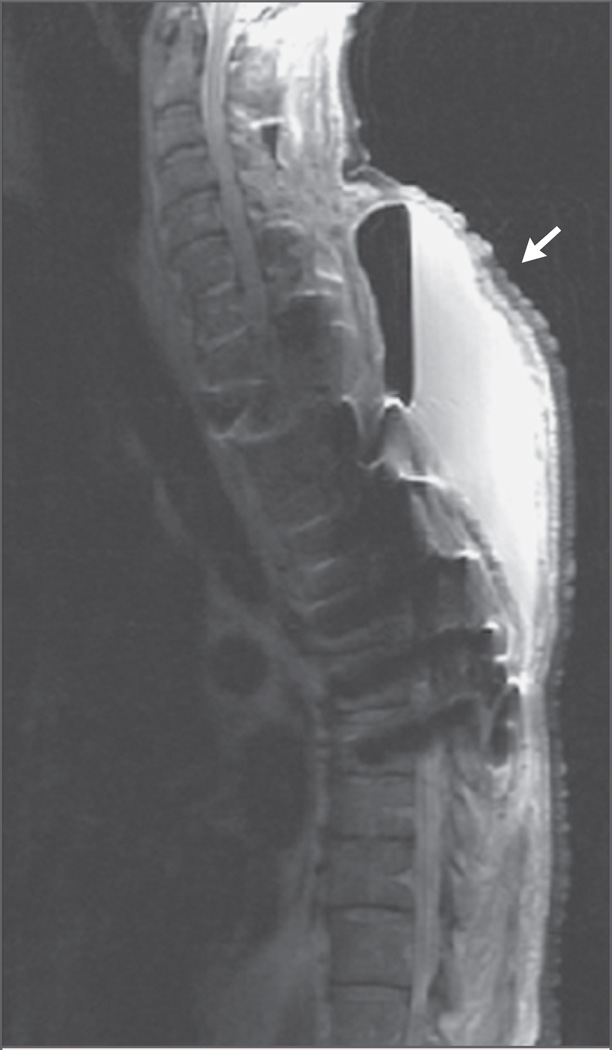
Laboratory studies on admission revealed a white-cell count of 11,150 per cubic millimeter, with 83% polymorphonuclear leukocytes, 10% lymphocytes, 5% mononuclear cells, 1% eosinophils, and 1% basophils. The hematocrit was 30.1%, and the platelet count was 339,000 per cubic millimeter. The erythrocyte sedimentation rate was 107 mm per hour, and the C-reactive protein value was 25.4 mg per liter (normal range, 0 to 0.5). Serum chemical profiles were notable for a potassium level of 2.9 mmol per liter (normal range, 3.5 to 5.0), a glucose level of 287 mg per deciliter (15.9 mmol per liter; normal range, 60 to 99 mg per deciliter [3.3 to 5.5 mmol per liter]), an albumin level of 1.9 g per deciliter (normal range, 3.5 to 5.5), and a total protein level of 5.0 g per deciliter (normal range, 6.0 to 8.2). Chest radiography revealed patchy infiltrates and a cavitary lesion in the right upper lobe that was not seen on the previous radiograph (Fig. 2). Computed tomography (CT) of the chest confirmed this finding (Fig. 3) and also showed multiple smaller infiltrates throughout the right lung. CT and MRI of the spine revealed a large fluid collection behind the vertebral column, which was consistent with a paraspinal abscess (Fig. 4). Analysis of the cerebrospinal fluid (CSF) showed 4 white cells per cubic millimeter, with normal glucose and protein levels. CSF was sent for Gram’s staining and staining for acid-fast bacilli and fungi.
Figure 2. Chest Radiograph Showing Patchy Consolidation in the Right Upper Lobe of the Lung.
Figure 3. Axial CT Scan of the Chest Obtained without the Use of Contrast Material, Showing an Irregular Multicavitary Infiltrate in the Anterior Right Upper Lobe.
Figure 4. MRI Scan of the Cervical and Thoracic Spine Obtained after the Administration of Gadolinium, Showing a Large Posterior Subcutaneous Abscess (Arrow) with a Visible Air–Liquid Level, Extending from C5 to T7.
As suggested by the clinical findings, the patient has a large paraspinal abscess. Inflammatory markers are unhelpful in distinguishing one infectious cause from another. It is likely that the cavitary lung lesion was produced by the same organism that caused the paraspinal abscess. Tuberculosis usually involves the lung apex posteriorly; the anterior location in this case makes tuberculosis somewhat less likely. The patient requires surgical drainage, and specimens from both the lung lesion and the paraspinal abscess should be sent for bacterial, mycobacterial, and fungal cultures (with attention to culture of fastidious organisms). Assessment of specimens from both sources by polymerase chain reaction (PCR) for the presence of M. tuberculosis may be helpful.
The patient was admitted to the surgical service. A liter of purulent material was drained from his back and sent for Gram’s staining, staining for acid-fast bacilli and fungi, and culture. The spinal hardware appeared to be stable, so it was left in place. His antituberculosis regimen was broadened to include moxifloxacin and amikacin in order to cover multidrug-resistant tuberculosis. Empirical ceftriaxone therapy was started for a possible bacterial superinfection, and the patient was transferred to the medicine service.
Pending further culture data, the addition of two drugs for possible multidrug-resistant tuberculosis is reasonable; however, successful management is likely to require a definitive diagnosis, given the many possible etiologic factors. Bacterial pathogens that are difficult to grow on routine mediums remain a particular concern.
Multiple bacterial cultures from blood, induced sputum, CSF, and urine were negative. Staining of induced sputum, blood, and CSF for acid-fast bacilli and fungi was also negative. HIV testing was negative. In addition, none of the bacterial, mycobacterial, and fungal cultures obtained during the patient’s first admission (including those from operative specimens) showed growth of any organisms. Gram’s staining of material from the paraspinal abscess, however, revealed gram-negative bacilli, which later grew and were identified as oxidase-positive.
The gram-negative rods are likely to be either B. pseudomallei (causing melioidosis) or a brucella species. The patient may have had exposure to B. pseudomallei in Vietnam (since this bacterium can have a prolonged latency) or in the Philippines. A history of exposure to animals (e.g., cattle, sheep, or swine) or ingestion of unpasteurized milk products would increase the suspicion that the patient has brucellosis. B. pseudomallei is most commonly acquired by inhalation or percutaneous inoculation from moist soil in areas where it is endemic.
The medical team informed the microbiology laboratory of the clinical suspicion of melioidosis. Speciation of the gram-negative rod remained difficult, however, since it could not be identified by standard methods. Meanwhile, the condition of the patient (who continued to receive maximal antituberculosis therapy and ceftriaxone) began to improve. Fever, cough, and hemoptysis all resolved during the first several days of hospitalization. His mental status also cleared, and his leg strength increased. By the seventh hospital day, he was no longer incontinent of urine. The surgical site began to heal, without signs of erythema or exudate.
The clinical manifestations of melioidosis range from localized cutaneous abscesses to multiorgan involvement to septic shock. Symptoms develop in most patients within several weeks after exposure, but disease reactivation many years later has also been reported. Diabetes is a known risk factor, and this patient’s vertebral osteomyelitis and cavitary pulmonary disease fit this diagnosis. If the organism cannot be definitively identified in the local microbiology laboratory, it should be sent to a reference facility. Serologic testing is available, but identification from culture provides the most definitive diagnosis. Ceftriaxone, although not standard therapy, is active against some strains of B. pseudomallei and may explain the clinical improvement in the patient’s condition. Drainage of his abscess presumably also contributed to his improving neurologic status.
On the ninth hospital day, the organism was speciated by a reference laboratory as burkholderia. Antimicrobial-susceptibility studies showed sensitivity to carbapenems, trimethoprim–sulfamethoxazole, tetracyclines, ceftriaxone, and piperacillin– tazobactam. Antituberculosis therapy was discontinued, and treatment with intravenous meropenem and high-dose oral trimethoprim– sulfamethoxazole was started. The organism was later identified by PCR at the Centers for Disease Control and Prevention (CDC) as B. pseudomallei, confirming the diagnosis of melioidosis. A chest CT obtained before the patient was discharged showed a reduction in the size of his lung cavity. After 2 weeks of intravenous antibiotics, long-term eradication therapy with oral doxycycline and trimethoprim–sulfamethoxazole was started. During an outpatient follow-up visit 3 months later, he reported feeling markedly improved; the erythrocyte sedimentation rate and the C-reactive protein had normalized.
Commentary
Melioidosis, caused by the gram-negative aerobic bacillus B. pseudomallei, is endemic to northern Australia and parts of Southeast Asia, including Vietnam and the Philippines.1 Almost all clinical cases of melioidosis reported in the United States are acquired during travel to or habitation in regions in which the disease is endemic.2 Our patient grew up in the Philippines and returned there periodically as an adult; he also served in Vietnam as a young man. He may have acquired the infection in either place. B. pseudomallei is a freeliving natural inhabitant of soil and water, ubiquitous in rice-farming areas. Transmission occurs largely by means of percutaneous inoculation through exposed or abraded skin and also by inhalation or ingestion. It may also occur by means of aerosol in laboratory technicians who handle clinical specimens.3 B. pseudomallei is listed by the CDC as a potential agent of biologic warfare.4
The clinical presentation of melioidosis is diverse and includes acute and chronic, localized and systemic, subclinical and clinical disease. It is most commonly manifested as cavitary pneumonia, skin or soft-tissue infection, genitourinary infection, or sepsis. The pneumonia is often acquired by hematogenous spread but sometimes by inhalation. Cases of prostatic abscess, encephalitis, cerebral abscess, parotitis, hepatic abscess, splenic abscess, osteomyelitis, septic arthritis, and pericarditis have also been reported.5 Risk factors for melioidosis include diabetes, renal disease, chronic lung disease, alcoholism, and other causes of immunosuppression.6 HIV infection does not appear to be a risk factor for melioidosis.7 Once acquired, B. pseudomallei can remain dormant for months or years, only to be reactivated when the immunity of the host is compromised. A latent period as long as 62 years has been reported.8
The standard in diagnosing melioidosis is isolation of B. pseudomallei by culture from clinical specimens. Our patient had not been treated with antibiotics at the time of initial cultures, yet multiple specimens, including operative material from his first surgery, were negative despite the use of mediums that would have been expected to grow B. pseudomallei; the reason for this is unclear. PCR has been used increasingly to detect B. pseudomallei DNA directly from clinical specimens.9 However, immunofluorescence microscopy is a more sensitive diagnostic tool and can be performed more rapidly.10 In all cases, the laboratory should be specifically alerted if there is a clinical suspicion of melioidosis. This will also help protect microbiology staff from inadvertent exposure.
Melioidosis can be challenging to distinguish from tuberculosis both clinically and histologically. Chronic melioidosis may produce necrotizing granulomas that are indistinguishable from those of tuberculosis and should be considered especially when the presentation or course of a chronic infection is atypical for tuberculosis. In acute melioidosis, necrosis and abscess formation can serve as distinguishing features. Treatment of melioidosis requires a short course of “initiation” therapy followed by prolonged “eradication” therapy. According to a Cochrane meta-analysis,11 intravenous ceftazidime, imipenem, or meropenem is an appropriate choice in the acute phase, and the selected antibiotic should be given for 14 days. Resistance to ceftazidime can arise during the course of treatment.12 For eradication therapy, high-dose oral trimethoprim–sulfamethoxazole (8 mg of trimethoprim per kilogram of body weight per day and 40 mg of sulfamethoxazole per kilogram per day), given together with doxycycline (4 mg per kilogram per day; maximum dose, 100 mg twice daily) for at least 20 weeks, is recommended.13 Comprehensive therapy also includes management of septic shock, abscess drainage, débridement of infected bone, tight glycemic control in patients with diabetes, and supportive care. Even with adequate treatment, however, mortality rates can be high (e.g., 20% in Australia14 and 40% in northeast Thailand5). Ten percent of patients have a relapse despite recommended durations of maintenance therapy.15
As in the present case, tuberculosis is often the initial diagnosis that is considered in patients who present with signs of chronic infection involving the lungs and other systems, but melioidosis should also be routinely considered in patients with even a remote history of travel to regions in which the disease is endemic. A high clinical suspicion of B. pseudomallei in the appropriate circumstances will facilitate the identification of this organism and the protection of laboratory workers handling it.
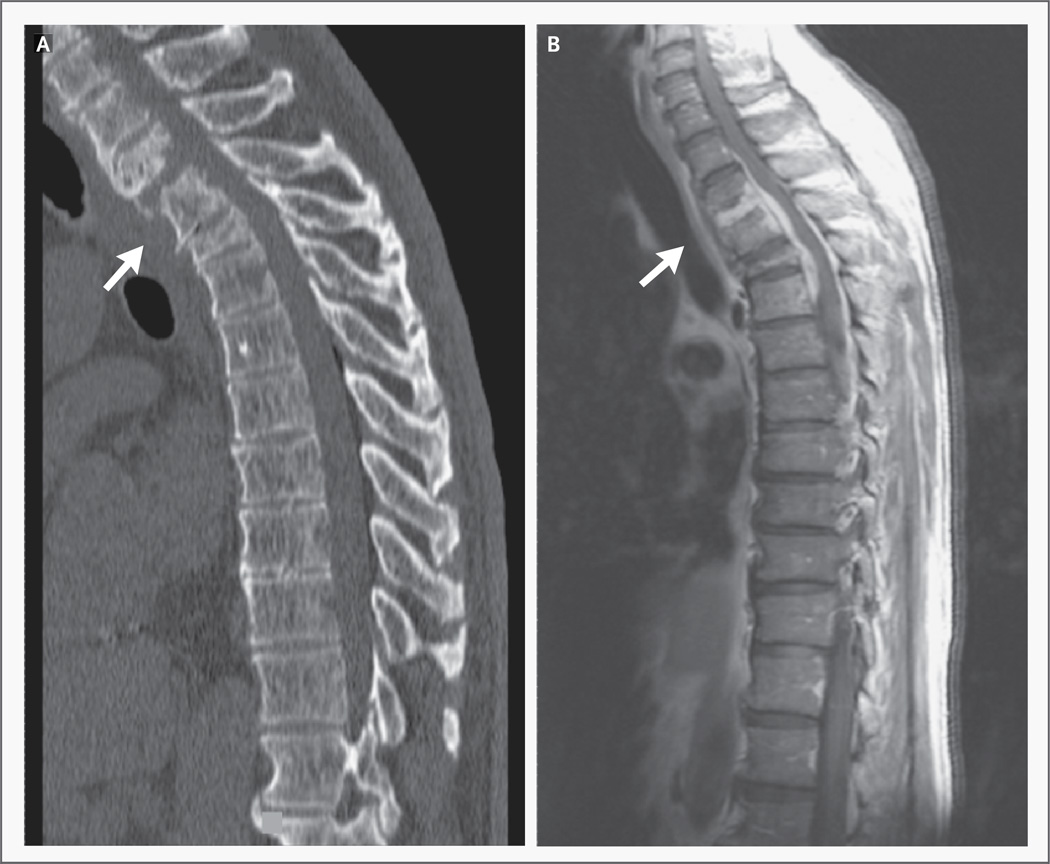
Figure 1. CT and MRI Scans Showing Osteomyelitis, Vertebral Compression Fractures, and Spinal Cord Compression.
A CT scan of the thoracic spine obtained without the use of contrast material (Panel A) shows vertebral osteomyelitis of T2, T3, and T4, with bony destruction and compression fractures (arrow). An MRI scan of the thoracic spine obtained after the administration of gadolinium (Panel B) further demonstrates diskitis of the T2–T3 space (arrow), an epidural abscess extending from T1 to T5, and thoracic cord compression.
Acknowledgments
Supported by an Advanced Career Development Award from the Health Services Research and Development Program of the Department of Veterans Affairs (to Dr. Saint).
We thank Drs. Lisa Mills, Emily Erbelding, Violette Renard, Jean-Paul Wolinsky, and Timothy Witham for their help with the treatment of the patient.
Footnotes
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Cheng AC, Currie BJ. Melioidosis: epidemiology, pathophysiology, and management. Clin Microbiol Rev. 2005;18:383–416. doi: 10.1128/CMR.18.2.383-416.2005. [Erratum, Clin Microbiol Rev 2007;20:533.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ip M, Osterberg LG, Chau PY, Raffin TA. Pulmonary melioidosis. Chest. 1995;108:1420–1424. doi: 10.1378/chest.108.5.1420. [DOI] [PubMed] [Google Scholar]
- 3.Laboratory exposure to Burkholderia pseudomallei — Los Angeles, California, 2003. MMWR Morb Mortal Wkly Rep. 2004;53:988–990. [PubMed] [Google Scholar]
- 4.Bioterrorism agents/diseases. Atlanta: Centers for Disease Control and Prevention; [Accessed July 14, 2008]. at http://www.bt.cdc.gov/agent/agentlist-category. asp. [Google Scholar]
- 5.White NJ. Melioidosis. Lancet. 2003;361:1715–1722. doi: 10.1016/s0140-6736(03)13374-0. [DOI] [PubMed] [Google Scholar]
- 6.Suputtamongkol Y, Chaowagul W, Chetchotisakd P, et al. Risk factors for melioidosis and bacteremic melioidosis. Clin Infect Dis. 1999;29:408–413. doi: 10.1086/520223. [DOI] [PubMed] [Google Scholar]
- 7.Chierakul W, Wuthiekanun V, Chaowagul W, et al. Disease severity and outcome of melioidosis in HIV coinfected individuals. Am J Trop Med Hyg. 2005;73:1165–1166. [PubMed] [Google Scholar]
- 8.Ngauy V, Lemeshev Y, Sadkowski L, Crawford G. Cutaneous melioidosis in a man who was taken as a prisoner of war by the Japanese during World War II. J Clin Microbiol. 2005;43:970–972. doi: 10.1128/JCM.43.2.970-972.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sura T, Smith MD, Cowan GM, Walsh AL, White NJ, Krishna S. Polymerase chain reaction for the detection of Burk-holderia pseudomallei. Diagn Microbiol Infect Dis. 1997;29:121–127. doi: 10.1016/s0732-8893(97)81800-7. [DOI] [PubMed] [Google Scholar]
- 10.Walsh AL, Smith MD, Wuthiekanun V, et al. Immunofluorescence microscopy for the rapid diagnosis of melioidosis. J Clin Pathol. 1994;47:377–379. doi: 10.1136/jcp.47.4.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Samuel M, Ti TY. Interventions for treating melioidosis. Cochrane Database Syst Rev. 2002;4:CD001263. doi: 10.1002/14651858.CD001263. [DOI] [PubMed] [Google Scholar]
- 12.Tribuddharat C, Moore RA, Baker P, Woods DE. Burkholderia pseudomallei class A beta-lactamase mutations that confer selective resistance against ceftazidime or clavulanic acid inhibition. Antimicrob Agents Chemother. 2003;47:2082–2087. doi: 10.1128/AAC.47.7.2082-2087.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaowagul W, Chierakul W, Simpson AJ, et al. Open-label randomized trial of oral trimethoprim-sulfamethoxazole, doxycycline and chloramphenicol compared with trimethoprim-sulfamethoxazole and doxycycline for maintenance therapy of melioidosis. Antimicrob Agents Chemother. 2005;49:4020–4025. doi: 10.1128/AAC.49.10.4020-4025.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Currie BJ, Fisher DA, Howard DM, et al. Endemic melioidosis in tropical northern Australia: a 10-year prospective study and review of the literature. Clin Infect Dis. 2000:31981–31986. doi: 10.1086/318116. [DOI] [PubMed] [Google Scholar]
- 15.Limmathurotsakul D, Chaowagul W, Chierakul W, et al. Risk factors for recurrent melioidosis in northeast Thailand. Clin Infect Dis. 2006;43:979–986. doi: 10.1086/507632. [DOI] [PubMed] [Google Scholar]