Summary
Cellular reprogramming from somatic cells to induced pluripotent stem cells (iPSCs) can be achieved through forced expression of the transcription factors Oct4, Klf4, Sox2 and c-Myc (OKSM) [1-4]. These factors, in combination with environmental cues, induce a stable intrinsic pluripotency network that confers indefinite self-renewal capacity on iPSCs. In addition to Oct4 and Sox2, the homeodomain-containing transcription factor Nanog is an integral part of the pluripotency network [5-11]. Although Nanog expression is not required for the maintenance of pluripotent stem cells, it has been reported to be essential for the establishment of both embryonic stem cells (ESCs) from blastocysts and iPSCs from somatic cells [10, 12]. Here we revisit the role of Nanog in direct reprogramming. Surprisingly, we find that Nanog is dispensable for iPSC formation under optimized culture conditions. We further document that Nanog-deficient iPSCs are transcriptionally highly similar to wild-type iPSCs and support the generation of teratomas and chimeric mice. Lastly, we provide evidence that the presence of ascorbic acid in the culture media is critical for overcoming the previously observed reprogramming block of Nanog knockout cells.
Results
Endogenous Nanog is Not Required for Induced Pluripotency
In order to test whether Nanog is required for direct reprogramming, we derived Nanog−/− mouse embryonic fibroblasts (MEFs) from chimeric embryos [13] since complete deletion of Nanog is embryonic lethal [10, 12]. Nanog−/− MEFs could be distinguished from host blastocyst-derived wild-type cells based on constitutive CAG-GFP expression as well as Nanog promoter-driven neomycin resistance. Fluorescence activated cell sorting (FACS) of GFP+ cells yielded a starting population of 89% purity. The remaining GFP- cells were expected to be wild type MEFs or Nanog-/- MEFs that had silenced the GFP transgene. The GFP-enriched MEFs were transduced with lentiviral vectors expressing OKSM from a doxycycline (dox)-inducible polycistronic construct (also referred to as STEMCCA) and rtTA (reverse tetracycline transactivator)[14]. After 12 days of dox induction, we recovered GFP+ and GFP– iPSC-like colonies at a ratio similar to that in the starting MEF population. Moreover, GFP+ and GFP– colonies could be maintained in the absence of dox, indicating autonomous self-renewal capacity without the continuous need for exogenous factor expression (Fig. 1a, b).
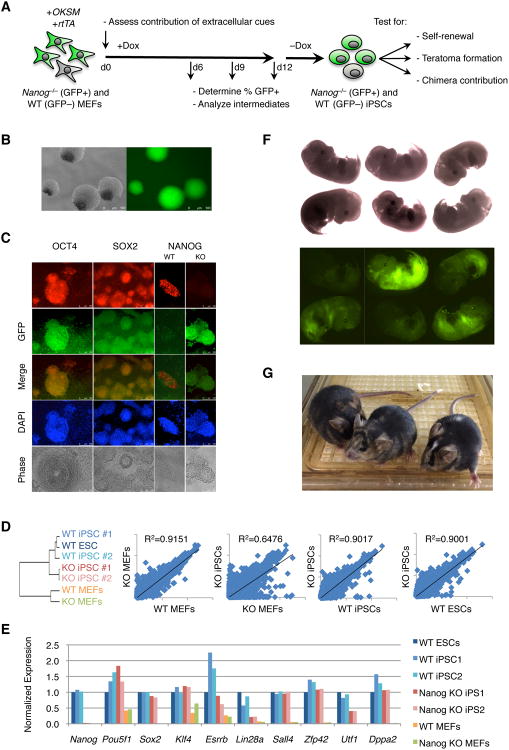
Figure 1. Nanog−/− MEFs Can be Reprogrammed to iPSCs.
(A) Experimental outline. (B) Fluorescent image of Nanog−/− iPSCs maintained in 2i/LIF and neomycin (left, phase; right, GFP). (C) Immunofluorescence for OCT4, SOX2, and NANOG on a mixed culture of GFP+ Nanog−/− (KO) and GFP– wild-type (WT) iPSCs. (D) Global gene expression microarrays were performed on RNA purified from the indicated cell lines. Shown is the hierarchical clustering of 1 WT ESC line, 2 WT iPSC lines, 2 Nanog−/− iPSC lines, and WT and KO MEFs (left panel), as well as scatter plot analyses comparing the indicated populations. (E) Expression data from the microarray for selected pluripotency markers. Results are shown normalized to WT ESC expression levels. (F) Fluorescent images of E13.5 chimeras generated from injecting Nanog−/− iPSCs into wild-type blastocysts (left, phase; right, GFP). (G) Adult chimeric mice generated from Nanog−/− iPSCs. Agouti coat color indicates cells derived from the Nanog−/− donor cells, whereas the black coat color indicates cells derived from the recipient wild-type blastocyst cells.
To determine whether iPSC-like colonies exhibit molecular hallmarks of authentic iPSCs, we evaluated endogenous pluripotency factor expression by immunostaining for OCT4, SOX2, and NANOG (Fig. 1c). We found that GFP+ colonies expressed both OCT4 and SOX2 after dox withdrawal, indicating that they had induced the endogenous pluripotency network. GFP+ iPSC-like colonies also expressed PECAM1, a marker of undifferentiated ESCs and iPSCs that is absent from more mature epiblast stem cells [15, 16](Fig. S1a). Importantly, NANOG expression was absent from GFP+ colonies whereas it was detectable in GFP– (wild-type) colonies, confirming that GFP expression indeed identifies Nanog-deficient cells.
We next performed global gene expression analysis using microarrays to determine how similar Nanog−/− iPSC-like cells are to wild-type ESCs and iPSCs. Unsupervised clustering of these samples revealed that Nanog−/− iPSCs are highly similar to wild-type pluripotent cells but different from the MEFs from which they were derived (Fig. 1d). Importantly, Nanog−/− MEFs clustered closely with independently derived wild-type MEFs, indicating that the starting cell populations for reprogramming were differentiated fibroblasts. Of note, the two Nanog−/− iPSC lines were more similar to each other than they were to wild-type iPSC and ESC lines, suggesting that the loss of Nanog results in mild gene expression differences as has been reported previously for Nanog−/− ESCs [10, 17]. Alternatively, differences in genetic background between Nanog-deficient iPSCs and wild-type ESCs and iPSCs might account for the differential clustering [18]. The microarray data also confirmed that Nanog−/− colonies express endogenous pluripotency genes [5-9] at ESC-like levels including Oct4 (Pou5f1), Sox2, Klf4, Sall4, Rex1 (Zfp42), and Dppa2 (Fig. 1e). However, Esrrb levels were reduced in Nanog−/− cells, which is in agreement with the previous finding that Esrrb is a direct NANOG target [17]. Lin28a and Utf1 levels were also reduced whereas Nanog transcripts were undetectable in Nanog-deficient iPSC-like cells. Bisulfite sequencing of the Nanog and Oct4 promoter regions showed extensive demethylation relative to fibroblasts (Fig. S1b), indicating that both loci are in an accessible ESC-like epigenetic state. Together, these results show that Nanog−/− MEFs can generate iPSC-like cells that are phenotypically and molecularly highly similar to bona fide iPSCs.
Nanog-Deficient iPSCs Give Rise to Teratomas and Chimeras
At a functional level, iPSCs are defined by the capacity to self-renew indefinitely in culture and pluripotency, the ability to give rise to cell types of all three germ layers. Indeed, we were able to maintain GFP+ iPSC-like cells in culture for multiple passages, regardless of culture conditions (ESC media supplemented with serum/LIF or serum-free 2i/LIF conditions)[19]. However, we noticed that GFP+ cells had a propensity to differentiate in culture, in accord with the reported phenotype of Nanog−/− ESCs [10]. Of note, exposure of Nanog-deficient iPSC-like cells to neomycin eliminated differentiated cells and maintained phenotypically undifferentiated colonies (Fig. 1b). To assess the differentiation potential of Nanog−/− colonies, we sorted GFP+ and GFP– cells and injected them separately into the flanks of SCID mice. Both Nanog−/− and wild-type cells gave rise to well-differentiated teratomas, characterized by ectodermal, endodermal and mesodermal derivatives, hence meeting one criteria of pluripotency (Fig. S1c).
A more stringent assay of pluripotency is the ability of cells to contribute to chimeras. We therefore injected GFP+ Nanog−/− iPSC-like cells into E3.5 wild-type blastocysts, transplanted them into the uterus of pseudo-pregnant recipient females, and isolated resultant fetuses at mid-gestation. We obtained 14 viable E13.5 embryos from 53 implanted blastocyts, of which 11 embryos had variable contributions of GFP chimerism (Fig. 1f). These embryos gave rise to GFP+ MEFs and GFP+ neural progenitor cells (NPCs) in vitro, corroborating that the reprogrammed Nanog−/− cells had the potential to differentiate into mesodermal and a defined ectodermal lineage, respectively (Fig. S1d). Additionally, immunohistochemistry of the chimeric embryos for GFP demonstrated that the Nanog−/− iPSC-like cells contributed to all three germ layers including the neuroectoderm of the brain, the endoderm-derived lining of the gastrointestinal tract, and the mesoderm-derived smooth muscle layers of the gastrointestinal tract (Fig S1e). We also found that Nanog−/− iPSC-like cells could contribute to adult chimeric mice (Fig. 2g), indicating that these progenitors have the capacity to fully mature and contribute to adult tissues. Collectively, these data demonstrate that the reprogrammed Nanog−/− cells are pluripotent iPSCs and thus functionally equivalent to Nanog−/− ESCs.
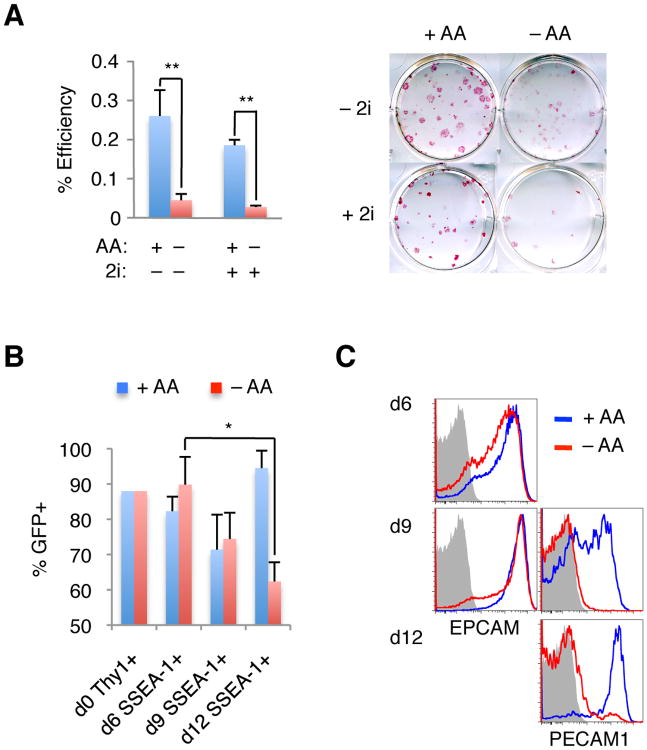
Figure 2. Ascorbic Acid Rescues the Nanog−/− Reprogramming Defect.
(A) Nanog−/− MEFs were reprogrammed in serum/LIF with or without 2i and/or AA as indicated. Resulting dox-independent iPSC colonies were stained for alkaline phosphatase and counted. Results are shown as the average % reprogramming efficiency (iPSC colonies / starting number of MEFs), based on 4 separate replicates +/- 1 S.D. (left panel). Alkaline phosphatase staining of a representative plate is shown (right panel). (B) MEFs (d0 Thy1+) or reprogramming intermediates (Thy1− SSEA-1+) where analyzed by flow cytometry for average % GFP positivity +/- 1 S.D., based on 3 to 5 replicates per time-point. (C) Reprogramming intermediates at the indicated times post dox induction were analyzed by flow cytometry for Thy1, SSEA-1, and EpCAM (left panels) or PECAM1 expression (right panels). Plots are gated on Thy−1SSEA-1+ cells (gray shaded histogram, isotype-matched control antibody). Statistical significance was determined by the Student's T test (* p<0.05; ** p<0.005).
Ascorbic Acid Rescues the Reprogramming Potential of Nanog-Deficient Cells
Our results differ from a previous report, which documented that Nanog is required for the generation of iPSCs [12]. A number of experimental differences between our studies may account for this discrepancy, including the selection of starting cell type (NPCs versus MEFs used here) and iPSC derivation conditions. We found that Nanog−/− NPCs derived from our chimeras could be reprogrammed into iPSCs (data not shown), thus excluding the possibility that the use of distinct cell types can explain the observed difference in reprogramming potential. We therefore focused on the possible effect of environmental cues on the reprogramming potential of Nanog−/− cells (Fig 1a). Our reprogramming media contained serum/LIF and ascorbic acid (AA) [20], whereas the previous study initiated reprogramming experiments in serum/LIF and then switched to 2i (2 inhibitors; a combination of GSK3β and MEK inhibitors)/LIF media [19]. Given these differences in reprogramming conditions, we tested the individual effects of 2i and AA on the reprogramming ability of Nanog−/− MEFs. Whereas the addition of 2i had only a minor effect on reprogramming efficiency, the removal of AA significantly impaired the reprogramming potential of Nanog−/− MEFs (Fig. 2a). Together, these data suggest that a lack of AA impedes the formation of iPSCs in serum/LIF or serum/2i/LIF conditions and thus may account for the previous failure to derive or detect Nanog-deficient iPSCs.
To gain mechanistic insights into the effect Nanog and AA may have on reprogramming, we analyzed nascent iPSCs based on surface markers that distinguish refractory (THY1+SSEA-1−) from progressing (THY1−SSEA-1+) intermediates [21-23]. Nanog deficiency appears to impact only mid-to-late stages of reprogramming, as suggested by the relative decrease of GFP+SSEA1+ intermediates by d12 of reprogramming in the absence of AA (Fig. 2b). This finding is consistent with the late activation of a Nanog-GFP reporter during iPSC formation (Fig. S2). Remarkably, exposure of reprogramming cultures to AA entirely rescued this defect.
We next analyzed Nanog−/− reprogramming intermediates for EPCAM and PECAM1 surface expression, which identify mid and late stages of reprogramming, respectively [21], in order to delineate the precise step at which Nanog is required (Fig. 2c). In wild-type cells undergoing reprogramming, EPCAM expression becomes detectable by d6 of OKSM expression, and correlates with Nanog transcription. Furthermore, the Epcam locus is bound by NANOG in ESCs, suggesting a direct regulation of Epcam expression by NANOG [21]. In contrast, PECAM1 expression is activated late (d9) in iPSC formation and coincides with Oct4 expression in wild-type cells. Surprisingly, EPCAM was expressed normally in Nanog−/− cultures at d6, indicating that Nanog deficiency neither affects Epcam transcription nor mid stages of reprogramming. However, PECAM1 expression was absent from Nanog−/− intermediates at d9 under serum/LIF conditions. Importantly, continuous AA treatment of Nanog−/− reprogramming cultures restored normal PECAM expression at d9. Whereas nearly all SSEA1+ cells had turned on PECAM1 by d12 of reprogramming in the presence of AA, a minor population of PECAM1+ cells was also detectable in the absence of AA and these cultures gave rise to rare iPSC-like cells. Altogether, these results are consistent with the interpretation that Nanog is important during late stages of reprogramming by facilitating the transition to a stable self-sustaining pluripotency network (as indicated by PECAM1 and hence Oct4 positivity). AA treatment facilitates this step but may not be absolutely required (Fig. 2a).
Discussion
Our results show that Nanog is dispensable for iPSC induction when directly reprogramming fibroblasts in serum/LIF in the presence of AA. More generally, these results demonstrate that subtle changes in culture conditions can profoundly influence the genetic requirements for induced pluripotency. We surmise that the previous failure to derive iPSCs from Nanog-deficient cells was due to alternative derivation conditions, which involved the generation of a pre-iPSC intermediate and a switch from serum/LIF to 2i/LIF in the absence of AA [12]. A recent study demonstrated that overexpression of Nanog's target Esrrb can substitute for Nanog during induced pluripotency, suggesting functional redundancy [17]. However, iPSC formation in that study also required addition of the global demethylating agent 5-aza-cytidine, whereas we obtained iPSC colonies in conventional culture conditions without the need for 5-aza-cytidine or ectopic expression of Esrrb. Given the enhancing effect of AA on iPSC formation from Nanog-null cells, it will be interesting to further dissect the mechanism by which AA compensates for the lack of Nanog expression. One attractive model is that AA acts as a cofactor for TET enzymes, which have been shown to bind to NANOG and induce demethylation of pluripotency targets including Esrrb and Oct4, thus promoting induced pluripotency [24, 25].
Supplementary Material
Highlights.
Endogenous Nanog is not required for induced pluripotency.
Nanog-deficient iPSCs support teratoma and chimera formation.
Ascorbic acid overcomes reprogramming block of Nanog-deficient cells.
Acknowledgments
We thank members of the Hochedlinger lab for their help and support, as well as the MGH CRM/HSCI flow cytometry core, the Harvard University Genome Modification Facility, and the Partners Center for Personalized Genetic Medicine core microarray facility. BAS was supported through an MGH Pathology Department grant (NIH 5-T32-CA-9216-33), OB was supported by the Gruss Lipper postdoctoral fellowship, and KH was supported by the NIH (R01-HD-058013).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 3.Maherali N, Sridharan R, Xie W, Utikal J, Eminli S, Arnold K, Stadtfeld M, Yachechko R, Tchieu J, Jaenisch R, et al. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell stem cell. 2007;1:55–70. doi: 10.1016/j.stem.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 4.Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, Hochedlinger K, Bernstein B, Jaenisch R. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–324. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- 5.Ramalho-Santos M, Yoon S, Matsuzaki Y, Mulligan R, Melton D. Science. Vol. 298. New York, N.Y.: 2002. “Stemness”: transcriptional profiling of embryonic and adult stem cells; pp. 597–600. [DOI] [PubMed] [Google Scholar]
- 6.Chen X, Xu H, Yuan P, Fang F, Huss M, Vega V, Wong E, Orlov Y, Zhang W, Jiang J, et al. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell. 2008;133:1106–1117. doi: 10.1016/j.cell.2008.04.043. [DOI] [PubMed] [Google Scholar]
- 7.Kim J, Chu J, Shen X, Wang J, Orkin S. An extended transcriptional network for pluripotency of embryonic stem cells. Cell. 2008;132:1049–1061. doi: 10.1016/j.cell.2008.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ng HH, Surani M. The transcriptional and signalling networks of pluripotency. Nature cell biology. 2011;13:490–496. doi: 10.1038/ncb0511-490. [DOI] [PubMed] [Google Scholar]
- 9.Buganim Y, Faddah D, Cheng A, Itskovich E, Markoulaki S, Ganz K, Klemm S, van Oudenaarden A, Jaenisch R. Single-cell expression analyses during cellular reprogramming reveal an early stochastic and a late hierarchic phase. Cell. 2012;150:1209–1222. doi: 10.1016/j.cell.2012.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, Maruyama M, Maeda M, Yamanaka S. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113:631–642. doi: 10.1016/s0092-8674(03)00393-3. [DOI] [PubMed] [Google Scholar]
- 11.Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–655. doi: 10.1016/s0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- 12.Silva J, Nichols J, Theunissen T, Guo G, van Oosten A, Barrandon O, Wray J, Yamanaka S, Chambers I, Smith A. Nanog is the gateway to the pluripotent ground state. Cell. 2009;138:722–737. doi: 10.1016/j.cell.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Theunissen T, Costa Y, Radzisheuskaya A, van Oosten A, Lavial F, Pain B, Castro L, Silva J. Development. Vol. 138. Cambridge, England: 2011. Reprogramming capacity of Nanog is functionally conserved in vertebrates and resides in a unique homeodomain; pp. 4853–4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sommer C, Stadtfeld M, Murphy G, Hochedlinger K, Kotton D, Mostoslavsky G. Stem cells. Vol. 27. Dayton, Ohio: 2009. Induced pluripotent stem cell generation using a single lentiviral stem cell cassette; pp. 543–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brons I, Smithers L, Trotter M, Rugg-Gunn P, Sun B, Chuva de Sousa Lopes S, Howlett S, Clarkson A, Ahrlund-Richter L, Pedersen R, et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448:191–195. doi: 10.1038/nature05950. [DOI] [PubMed] [Google Scholar]
- 16.Tesar P, Chenoweth J, Brook F, Davies T, Evans E, Mack D, Gardner R, McKay R. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2007;448:196–199. doi: 10.1038/nature05972. [DOI] [PubMed] [Google Scholar]
- 17.Festuccia N, Osorno R, Halbritter F, Karwacki-Neisius V, Navarro P, Colby D, Wong F, Yates A, Tomlinson S, Chambers I. Esrrb is a direct Nanog target gene that can substitute for Nanog function in pluripotent cells. Cell stem cell. 2012;11:477–490. doi: 10.1016/j.stem.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stadtfeld M, Apostolou E, Akutsu H, Fukuda A, Follett P, Natesan S, Kono T, Shioda T, Hochedlinger K. Aberrant silencing of imprinted genes on chromosome 12qF1 in mouse induced pluripotent stem cells. Nature. 2010;465:175–181. doi: 10.1038/nature09017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ying QL, Wray J, Nichols J, Batlle-Morera L, Doble B, Woodgett J, Cohen P, Smith A. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stadtfeld M, Apostolou E, Ferrari F, Choi J, Walsh R, Chen T, Ooi S, Kim S, Bestor T, Shioda T, et al. Ascorbic acid prevents loss of Dlk1-Dio3 imprinting and facilitates generation of all-iPS cell mice from terminally differentiated B cells. Nature genetics. 2012;44:398. doi: 10.1038/ng.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polo J, Anderssen E, Walsh R, Schwarz B, Nefzger C, Lim S, Borkent M, Apostolou E, Alaei S, Cloutier J, et al. A molecular roadmap of reprogramming somatic cells into iPS cells. Cell. 2012;151:1617–1632. doi: 10.1016/j.cell.2012.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stadtfeld M, Maherali N, Breault D, Hochedlinger K. Defining molecular cornerstones during fibroblast to iPS cell reprogramming in mouse. Cell stem cell. 2008;2:230–240. doi: 10.1016/j.stem.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brambrink T, Foreman R, Welstead G, Lengner C, Wernig M, Suh H, Jaenisch R. Sequential expression of pluripotency markers during direct reprogramming of mouse somatic cells. Cell stem cell. 2008;2:151–159. doi: 10.1016/j.stem.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Costa Y, Ding J, Theunissen T, Faiola F, Hore T, Shliaha P, Fidalgo M, Saunders A, Lawrence M, Dietmann S, et al. NANOG-dependent function of TET1 and TET2 in establishment of pluripotency. Nature. 2013;495:370–374. doi: 10.1038/nature11925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blaschke K, Ebata K, Karimi M, Zepeda-Martínez J, Goyal P, Mahapatra S, Tam A, Laird D, Hirst M, Rao A, et al. Vitamin C induces Tet-dependent DNA demethylation and a blastocyst-like state in ES cells. Nature. 2013;500:222–226. doi: 10.1038/nature12362. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.