Summary
Molecules containing all-carbon quaternary stereocenters – carbon atoms bonded to four distinct carbon substituents – are prevalent in Nature. However, the construction of such compounds in an enantioselective fashion remains a long-standing challenge to synthetic organic chemists. In particular, methods for forging quaternary stereocenters that are remote from other functional groups are underdeveloped. Herein we report a catalytic and enantioselective intermolecular Heck-type reaction of trisubstituted-alkenyl alcohols with aryl boronic acids. The reported method allows direct access to quaternary all-carbon-substituted β-, γ-, δ-, ε- or ζ aryl carbonyl compounds, as the unsaturation of the alkene is relayed to the alcohol resulting in the formation of a carbonyl group. The scope of the process also includes incorporation of pre-existing stereocenters along the alkyl chain, which links the alkene and the alcohol, wherein the stereocenter is preserved. The described method is flexible, allowing access to diverse building blocks containing an enantiomerically enriched, quaternary center.
The quaternary stereocenter is a common structural motif in many natural products and pharmaceuticals1-3. However, the synthesis of these stereocenters in a catalytic and enantioselective manner represents a formidable challenge, especially in acyclic systems4. Typically, quaternary stereocenters are prepared from substrates with pre-existing functional groups adjacent to the site of reaction, whereas methods to access quaternary stereocenters distant from such groups present a significant, ongoing synthetic hurdle. The most common enantioselective and catalytic approaches utilize a carbonyl as a functional handle, wherein α-functionalization, via alkylation or aldol reactions4,5, can be accomplished through the reaction of enolate equivalents (I in Fig. 1a)6-11. Enantioselective β-functionalization of a carbonyl can be accomplished through 1,4-conjugate addition-type processes using various transition metals and coupling partners (II in Fig. 1a)12-16. A powerful alternative to the carbonyl as a pre-installed functional group is the allylic electrophile17-19 or nucleophile20, which yields a quaternary center adjacent to an alkene (III, Fig. 1a)21-23. However, in all of these approaches, the location of C–C bond formation relative to the functional group is strictly defined, which does not allow one to directly install a quaternary chiral center at more remote sites.
Figure 1. Approaches to constructing acyclic all-carbon quaternary stereocenters.
a, Conventional enantioselective, catalytic approaches. α-functionalization of carbonyls (I). β-functionalization of carbonyls (II). α-quaternary centers adjacent to alkene (III). b, Proposed modular strategy using a redox relay enantioselective Heck reaction of trisubstituted alkenes and resulting mechanistic analysis.
On the basis of our group’s recent success in developing asymmetric redox-relay Heck-type reactions of disubstituted alkenyl alcohols24,25, we surmised that a site- and enantioselective transformation of trisubstituted alkenes could address this synthetic limitation (Fig. 1b). Applying the proposed method, one could position the alcohol at different chain-lengths from the alkene to obtain a diverse range of functionalized carbonyl products. This is a mechanistic consequence of the process. Specifically, site-selective migratory insertion26,27 of an alkene into the organometallic intermediate produces a Pd-alkyl B, that can migrate toward the alcohol through a sequential β-hydride elimination/reinsertion process (Fig. 1b, D→E) to ultimately release the desired carbonyl product C28,29. Although venerable Heck cyclization reactions have been developed and extensively applied to the formation of quaternary centers by intramolecular reaction of trisubstituted alkenes30-32, no examples of catalytic, asymmetric, quaternary stereocenters synthesized via intermolecular Heck-type reactions of isolated (non-conjugated) trisubstituted alkenes are known2.
Several concerns were considered at the outset of this effort including questions regarding reactivity, site-selectivity, and enantioselectivity when using trisubstituted alkenes in intermolecular Heck-type reactions. Acyclic, non-conjugated trisubstituted alkenes are rare substrates in intermolecular Heck-type reactions likely due to either poor binding to the catalyst or slow migratory insertion33. If a reaction does occur, the question of site-selectivity is intriguing as the ability to forge a quaternary center relies on addition to the more substituted carbon. In our previous report, we found that subtle electronic variance of the alkenyl carbons, as determined by 13C chemical shift differences, correlates to site-selectivity, with the aryl nucleophile adding to the carbon that is more downfield shifted24. Additional support for electronically influenced site-selectivity was revealed by recent density functional theory calculations on the redox-relay Heck reactions of disubstituted alkenes34,35. These studies show that site-selectivity is controlled by remote dipole interactions of the attached alcohol. These observations suggested that, in the case of a trisubstituted alkene, insertion should occur preferentially at the more substituted carbon (the hindered and downfield shifted carbon). This also would likely relieve steric strain as the bulky Pd-catalyst is positioned at the less hindered carbon. As the final concern, it is not evident if this process would be highly enantioselective, as cis and trans disubstituted alkenes have previously yielded enantiomers as products24,25. Considering that trisubstituted alkenes contain both of these stereochemical relationships, the outcome is not simply predicted.
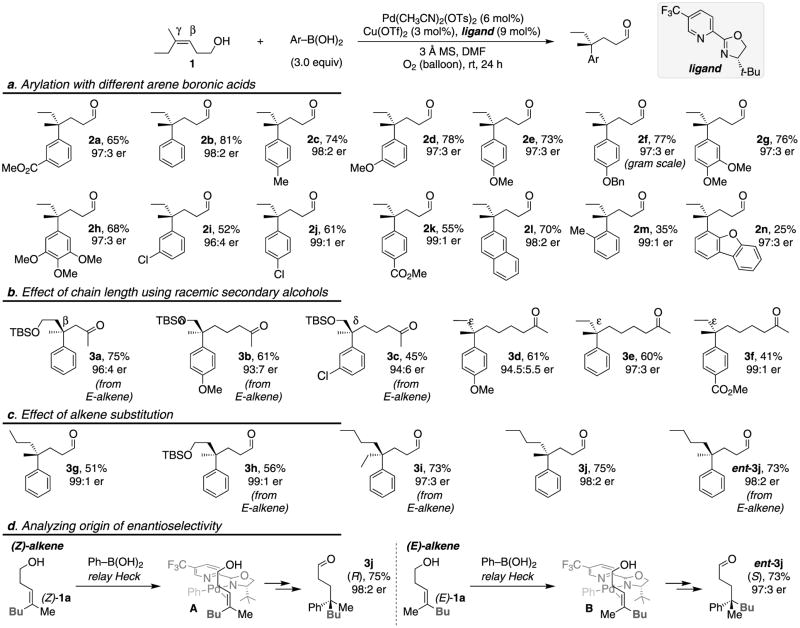
We began our investigation by revisiting our previously developed catalytic system24 for enantioselective oxidative Heck reactions36-40 of disubstituted alkenes. A trisubstituted homoallylic alcohol (1), which displays ethyl and methyl groups at the terminus of the alkene, was selected as a model substrate (Fig 2). Any success with this substrate would bode well for expanding the scope of the reaction to substrates containing other substituents on the alkene with more pronounced differences. Our initial efforts resulted in poor conversion to the desired product 2a (40% conversion, 23% yield). Nevertheless, migratory insertion occurred to exclusively install the aryl group at the γ-position (γ/β>15:1) and the product was generated in a high enantiomeric ratio (er) of 97:3 (Table S1, see SI). Encouraged by this initial result, we explored various changes to the reaction conditions, yet these afforded little noticeable improvements in yield. During our previous studies, we observed that the arylboronic acid coupling partner was consumed by various side reactions, such as decomposition of the boronic acid into a phenol and homocoupling of this reagent41-43. Indeed, we detected that the arylboronic acid was consumed after 24 h, with corresponding poor conversion of the alkene. We speculated that slow addition of the arylboronic acid would suppress the undesired pathways and favor product formation. Batch-wise addition of the arylboronic acid did improve the yield to 50%. Increasing the catalyst loading led to 65% yield (Fig 2, 2a), with >15:1 regioselectivity (γ:β) and excellent enantioselectivity (er: 97:3). A series of control experiments verified the importance of the various reaction components: removing either Cu(OTf)244 or 3 Å MS45 substantially reduced the yield, and when the palladium catalyst was excluded, no reaction was observed (Table S1). Both of these additives are frequently used in oxidative Pd-catalysis to facilitate reoxidation of Pd(0) although their precise role in this transformation is not currently understood.
Figure 2. Enantioselective construction of remote quaternary stereocenters.
Conditions for 2a, 2i–2n, 3c, 3f, 3i–3j: 10 mol% Pd(CH3CN)2(OTs)2, 4 mol% Cu(OTf)2, 14 mol% ligand, 3 equiv ArB(OH)2 (two batch addition, 12 h between additions). a, Exploration of scope using various arylboronic acids. b, Evaluation of various chain-lengths between the alkene and alcohol on the substrate. c, Exploration of the alkene substituents. d, Proposed origin of enantioselectivity as a function of alkene geometry. TBS is tert-butyldimethylsilyl.
The scope of arylboronic acid coupling partners was investigated with homoallylic alcohol 1 (Fig. 2a). A wide-array of arylboronic acids were found to be compatible, delivering the corresponding all-carbon quaternary γ-aryl aldehyde products with uniformly high enantioselectivity (er up to 99:1) and in moderate to good yields (2a–2n). High site-selectivity (γ/β ≥ 15:1) is observed with both electron-deficient and electron-rich arylboronic acids. This stands in contrast to our previous reports on enantioselective redox-relay Heck-type reactions of disubstituted alkenes, where only modest site-selectivity was achieved for electron-rich aryl boronic acids24. This observed difference suggests that the electronic nature of the alkene dictates site-selectivity. Higher yields are achieved with electron-rich arylboronic acids, as compared to their electron-poor counterparts (compare 2e with 2k), which is consistent with their greater nucleophilicity facilitating migratory insertion of the presumed alkene complex. In all cases, excellent enantioselectivity is observed and the reaction can be scaled to 10 mmol yielding >2 grams as demonstrated by example 2f. Not surprisingly, lower yields are observed when ortho-substituted arenes are utilized, as illustrated by 2m and 2n, although enantioselectivity remains high. The absolute configuration of a derivative of 2f was determined to be (R) via X-ray crystallography (see supplementary information for details).
The effect of chain length (the distance from the alcohol to alkene) using various racemic trisubstituted alkenyl secondary alcohols was evaluated (Fig. 2b). Of particular note, high site- and enantioselectivity is observed irrespective of the chain length, enabling access to β- (3a), δ- (3b, 3c), or ε- (3d–3f) quaternary functionalized ketone products. Alkenes bearing another oxygen substituent are well-tolerated (3a–3c) although styrene derived substrates are unreactive under the current reaction conditions. Substrates were also selected to probe the effect of differential size of the alkene aliphatic substituents (3g–3j, Fig. 2c). Gratifyingly, excellent site- and enantioselectivity were again observed in all cases. To highlight this, an alkene featuring an ethyl and a butyl group, which have negligible steric differences, performs well, yielding 3i in 97:3 er.
In analysis of the reaction scope, it appears that the enantioselectivity is essentially independent of the steric and electronic nature of both reaction partners, which is atypical in enantioselective reactions. To further explore this, the effect of alkene geometry on enantioselection was probed by comparing the reaction of (Z)-1a and (E)-1a (Fig. 2d). The magnitude of the enantioselectivity is the same for both substrates, again suggesting a robust enantioselective reaction. However, the major enantiomer produced in both cases is different. This is consistent with the binding orientation of the alkene not changing. Specifically, in comparing hypothesized intermediates A and B (Fig. 2d), the alkenyl carbon closer to the alcohol remains fixed leading to the observed stereochemical outcomes, which is supported by the relative insensitivity of the process to what is displayed on the terminal end of the trisubstituted alkenes. While the precise details of why this catalyst is exceptionally selective is under further investigation, these results support that few synthetic limitations should be encountered in variation of the alkenyl aliphatic substituents.
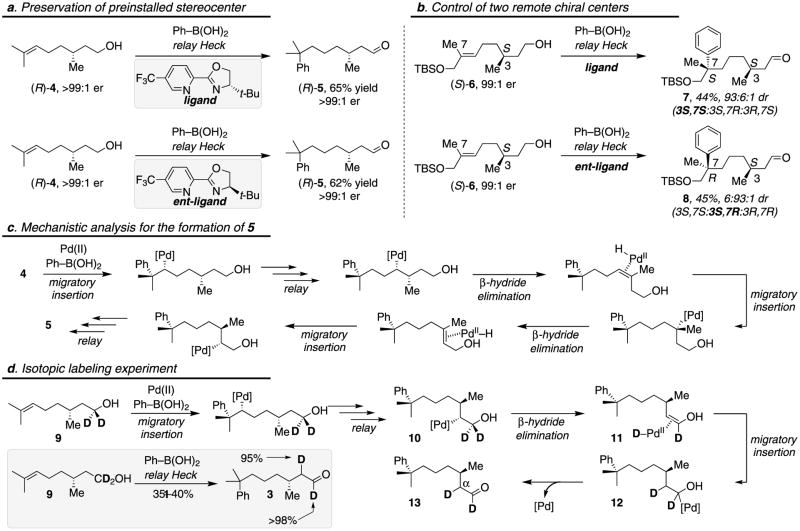
As hypothesized in the initial mechanistic proposal, the Pd-catalyst presumably migrates along the alkyl chain until the aldehyde is formed. Indeed, computational studies34,35 of the relay Heck reaction of disubstituted alkenes shows generally low energy barriers for the chain-walking events46-48. Therefore, a key question, with implications for the applicability of this method in more complex settings, is whether the catalyst disengages during the “chain-walking” process. To explore this possibility, a natural product derived substrate, (R)-4, containing a preinstalled stereogenic center in the alkyl chain was evaluated using both enantiomers of catalysts. Preservation of the enantiomeric composition was observed when treating this substrate with either catalyst enantiomer under redox relay Heck conditions to yield (R)-5 (Fig. 3a). This implies that as the catalyst proceeds through the iterative β-hydride elimination/reinsertion events depicted in Fig. 3c, the catalyst remains both ligated to the substrate and on the same face of the alkene throughout the relay process. As a more striking example, alkene (S)-6 was treated with both enantiomers of catalyst to yield the relay products 7 and 8 in high diastereoselectivity (Fig. 3b). Two distinct diasteresomers are produced by the use of different enantiomers of catalyst, since the initial migratory insertion is under catalyst-controlled face selection, but the preset stereogenic center is not altered during the relay process.
Figure 3. Evaluation of alkene substrates containing a branch point.
Conditions: 10 mol% Pd(CH3CN)2(OTs)2, 4 mol% Cu(OTf)2, 14 mol% ligand, 3 equiv PhB(OH)2. a, Independence of catalyst enantiomer on the conservation of the chiral center during the proposed chain-walking process. b, Accessing distinct diasteromers using a combination of catalyst and substrate controlled asymmetric synthesis. c, Proposed mechanistic origin for the observed formation of 5 from 4. d, Isotopic labeling experiment and analysis.
To further support the chain walking proposal, an isotopic labeling experiment was carried out (Fig. 3d). A deuterium labeled analog of 4, alkenol 9, bearing deuterium atoms at the carbon connecting to the alcohol, was synthesized and submitted to the redox relay Heck reaction. The experiment reveals clean repositioning of one deuterium atom at the site α to the carbonyl group in the product (13) (Fig. 3d). This result is consistent with a mechanism whereby the Pd-catalyst migrates through the chain to form intermediate 10, which undergoes β-D elimination followed by reinsertion into 11 to yield intermediate 12 (Fig. 3d).
In this work, we have described a catalytic and enantioselective addition of boronic acid derivatives to trisubstituted alkenes that is highly site-selective for the more hindered position. The method does not rely on a defined relationship between the site of addition and an adjacent functional group, thus providing a modular method to access quaternary stereocenters in high enantioselectivity. Furthermore, we anticipate that the mechanistic implications of both site-selective addition of an organometallic to a trisubstituted-alkene and the ability of the catalyst to migrate through existing chiral centers will inspire further studies in this area.
METHODS SUMMARY
General procedure for enantioselective Heck reaction
To a dry 100 mL Schlenk flask equipped with a stir bar was added Pd(CH3CN)2(OTs)2 (15.9 mg, 0.0300 mmol, 6.00 mol%), Cu(OTf)2 (5.43 mg, 0.0150 mmol, 3.0 mol%), ligand (12.3 mg, 0.0450 mmol, 9.0 mol%), 3 Å MS (75.0 mg, 150 mg/mmol), and DMF (8 mL). To this flask, a three-way adapter fitted with a balloon of O2 was added, and the flask was evacuated via house vacuum and refilled with O2 three times while stirring. The resulting mixture was stirred for 10 min. To this, a DMF solution (2 mL) of the alkenyl alcohol (0.5 mmol) and corresponding boronic acid (1.5 mmol, 3 equiv) was added via syringe. The resulting mixture was stirred for 24 h at room temperature. The mixture was diluted with diethyl ether (200 mL) and water (50 mL). The aqueous layer was extracted with diethyl ether (2 × 50 mL). The combined organic layers were washed with water (3 × 20 mL), brine (1 × 20 mL), and dried over sodium sulfate. The organic extracts were concentrated under reduced pressure, and the resulting residue was purified by silica gel flash chromatography using 2–10% EtOAc in hexanes containing 0.1% triethylamine to yield an aldehyde product. Full experimental details and characterization of new compounds can be found in the Supplementary Information.
Supplementary Material
Acknowledgments
We thank the National Institutes of Health (NIGMS GM063540) for their financial support.
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Author Contributions T.-S.M and H.H.P. performed the experiments and analysed the data. T.-S.M and M.S.S. designed the experiments. T.-S.M and M.S.S. prepared this manuscript with feedback from H.H.P..
Data for the crystalized product (a derivative of 2f) are deposited in the Cambridge Crystallographic Data Centre under accession number CCDC 988090.
The authors declare no competing financial interests.
References
- 1.Trost BM, Jiang C. Catalytic enantioselective construction of all-carbon quaternary stereocenters. Synthesis. 2006:369–396. [Google Scholar]
- 2.Douglas CJ, Overman LE. Catalytic asymmetric synthesis of all-carbon quaternary stereocenters. Proc Natl Acad Sci USA. 2004;101:5363–5367. doi: 10.1073/pnas.0307113101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christoffers J, Mann A. Enantioselective construction of quaternary stereocenters. Angew Chem Int Ed. 2001;40:4591–4597. doi: 10.1002/1521-3773(20011217)40:24<4591::aid-anie4591>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 4.Das JP, Marek I. Enantioselective synthesis of all-carbon quaternary stereogenic centers in acyclic systems. Chem Commun. 2011;47:4593–4623. doi: 10.1039/c0cc05222a. [DOI] [PubMed] [Google Scholar]
- 5.Marek I, et al. All-carbon quaternary stereogenic centers in acyclic systems through the creation of several C–C bonds per chemical step. J Am Chem Soc. 2014;136:2682–2694. doi: 10.1021/ja410424g. [DOI] [PubMed] [Google Scholar]
- 6.Masarwa A, et al. Merging allylic carbon-hydrogen and selective carbon-carbon bond activation. Nature. 2014;505:199–203. doi: 10.1038/nature12761. [DOI] [PubMed] [Google Scholar]
- 7.Liu W-B, Reeves CM, Stoltz BM. Enantio-, diastereo-, and regioselective iridium-catalyzed asymmetric allylic alkylation of acyclic β-ketoesters. J Am Chem Soc. 2013;135:17298–17301. doi: 10.1021/ja4097829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krautwald S, Sarlah D, Schafroth MA, Carreira EM. Enantio- and diastereodivergent dual catalysis: α-allylation of branched aldehydes. Science. 2013;340:1065–1068. doi: 10.1126/science.1237068. [DOI] [PubMed] [Google Scholar]
- 9.Taylor MS, Jacobsen EN. Enantioselective Michael additions to α,β-unsaturated imides catalyzed by a salen-Al complex. J Am Chem Soc. 2003;125:11204–11205. doi: 10.1021/ja037177o. [DOI] [PubMed] [Google Scholar]
- 10.Mermerian AH, Fu GC. Catalytic enantioselective synthesis of quaternary stereocenters via intermolecular C-acylation of silyl ketene acetals: dual activation of the electrophile and the nucleophile. J Am Chem Soc. 2003;125:4050–4051. doi: 10.1021/ja028554k. [DOI] [PubMed] [Google Scholar]
- 11.Sawamura M, Hamashima H, Ito Y. Catalytic asymmetric synthesis with trans-chelating chiral diphosphine ligand TRAP: rhodium-catalyzed asymmetric Michael addition of α-cyano carboxylates. J Am Chem Soc. 1992;114:8295–8296. [Google Scholar]
- 12.Mauleon P, Carretero JC. Enantioselective construction of stereogenic quaternary centers via Rh-catalyzed asymmetric addition of alkenylboronic acids to α,β-unsaturated pyridylsulfones. Chem Commun. 2005:4961–4963. doi: 10.1039/b508142d. [DOI] [PubMed] [Google Scholar]
- 13.Wu J, Mampreian DM, Hoveyda AH. Enantioselective synthesis of nitroalkanes bearing all-carbon quaternary stereogenic centers through Cu-catalyzed asymmetric conjugate additions. J Am Chem Soc. 2005;127:4584–4585. doi: 10.1021/ja050800f. [DOI] [PubMed] [Google Scholar]
- 14.Hawner C, et al. Rhodium-catalyzed asymmetric 1,4-addition of aryl alanes to trisubstituted enones: Binap as an effective ligand in the formation of quaternary stereocenters. Angew Chem Int Ed. 2010;49:7769–7772. doi: 10.1002/anie.201003300. [DOI] [PubMed] [Google Scholar]
- 15.Shintani R, Takeda M, Nishimura T, Hayashi T. Chiral tetrafluorobenzobarrelenes as effective ligands for Rhodium-catalyzed asymmetric 1,4-addition of arylboroxines to β,β-disubstituted α,β-unsaturated ketones. Angew Chem Int Ed. 2010;49:3969–3971. doi: 10.1002/anie.201000467. [DOI] [PubMed] [Google Scholar]
- 16.Dabrowski JA, Villaume MT, Hoveyda AH. Enantioselective synthesis of quaternary carbon stereogenic centers through Copper-catalyzed conjugate additions of aryl- and alkylaluminum reagents to acyclic trisubstituted enones. Angew Chem Int Ed. 2013;52:8156–8159. doi: 10.1002/anie.201304035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jung B, Hoveyda AH. Site- and enantioselective formation of allene-bearing tertiary or quaternary carbon stereogenic centers through NHC–Cu-catalyzed allylic substitution. J Am Chem Soc. 2012;134:1490–1493. doi: 10.1021/ja211269w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee Y, Hoveyda AH. Lewis base activation of grignard reagents with N-heterocyclic carbenes. Cu-free catalytic enantioselective additions to γ-chloro-α,β-unsaturated esters. J Am Chem Soc. 2006;128:15604–15605. doi: 10.1021/ja067456m. [DOI] [PubMed] [Google Scholar]
- 19.Luchaco-Cullis CA, Mizutani H, Murphy KE, Hoveyda AH. Modular pyridinyl peptide ligands in asymmetric catalysis: enantioselective synthesis of quaternary carbon atoms through copper-catalyzed allylic substitutions. Angew Chem Int Ed. 2001;40:1456–1460. doi: 10.1002/1521-3773(20010417)40:8<1456::AID-ANIE1456>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 20.Denmark SE, Fu J. Catalytic, Enantioselective Addition of substituted allylic trichlorosilanes using a rationally-designed 2,2‘-bispyrrolidine-based bisphosphoramide. J Am Chem Soc. 2001;123:9488–9489. doi: 10.1021/ja016552e. [DOI] [PubMed] [Google Scholar]
- 21.Shi W-J, et al. Highly enantioselective hydrovinylation of α-alkyl vinylarenes. An Approach to the Construction of all-carbon quaternary stereocenters. J Am Chem Soc. 2006;128:2780–2781. doi: 10.1021/ja057654y. [DOI] [PubMed] [Google Scholar]
- 22.Zhang A, RajanBabu TV. All-carbon quaternary centers via catalytic asymmetric hydrovinylation. New approaches to the exocyclic side chain stereochemistry problem. J Am Chem Soc. 2006;128:5620–5621. doi: 10.1021/ja060999b. [DOI] [PubMed] [Google Scholar]
- 23.Zhang P, Le H, Kyne RE, Morken JP. Enantioselective construction of all-carbon quaternary centers by branch-selective Pd-catalyzed allyl-allyl cross-coupling. J Am Chem Soc. 2011;133:9716–9719. doi: 10.1021/ja2039248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mei T-S, Werner EW, Burckle AJ, Sigman MS. Enantioselective redox-relay oxidative Heck arylations of acyclic alkenyl alcohols using boronic acids. J Am Chem Soc. 2013;135:6830–6833. doi: 10.1021/ja402916z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Werner EW, Mei T-S, Burckle AJ, Sigman MS. Enantioselective Heck arylations of acyclic alkenyl alcohols using a redox-relay strategy. Science. 2012;338:1455–1458. doi: 10.1126/science.1229208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sigman MS, Werner EW. Imparting catalyst control upon classical palladium-catalyzed alkenyl C–H bond functionalization reactions. Acc Chem Res. 2011;45:874–884. doi: 10.1021/ar200236v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beletskaya IP, Cheprakov AV. The Heck reaction as a sharpening stone of palladium catalysis. Chem Rev. 2000;100:3009–3066. doi: 10.1021/cr9903048. [DOI] [PubMed] [Google Scholar]
- 28.Calò V, Nacci A, Monopoli A, Ferola V. Palladium-catalyzed Heck arylations of allyl alcohols in ionic liquids: remarkable base effect on the selectivity. J Org Chem. 2007;72:2596–2601. doi: 10.1021/jo070005f. [DOI] [PubMed] [Google Scholar]
- 29.Bouquillon S, Ganchegui B, Estrine B, Hénin F, Muzart J. Heck arylation of allylic alcohols in molten salts. J Organomet Chem. 2001;634:153–156. [Google Scholar]
- 30.Dounay AB, Overman LE. The asymmetric intramolecular Heck reaction in natural product total synthesis. Chem Rev. 2003;103:2945–2964. doi: 10.1021/cr020039h. [DOI] [PubMed] [Google Scholar]
- 31.Shibasaki M, Vogl EM, Ohshima T. Asymmetric Heck reaction. Adv Synth Catal. 2004;346:1533–1552. [Google Scholar]
- 32.Mc Cartney D, Guiry PJ. The asymmetric Heck and related reactions. Chem Soc Rev. 2011;40:5122–5150. doi: 10.1039/c1cs15101k. [DOI] [PubMed] [Google Scholar]
- 33.Melpolder JB, Heck RF. Palladium-catalyzed arylation of allylic alcohols with aryl halides. J Org Chem. 1976;41:265–272. [Google Scholar]
- 34.Dang Y, Qu S, Wang Z-X, Wang X. A Computational mechanistic study of an unprecedented Heck-type relay reaction: insight into the origins of regio- and enantioselectivities. J Am Chem Soc. 2013;136:986–998. doi: 10.1021/ja410118m. [DOI] [PubMed] [Google Scholar]
- 35.Xu L, et al. Mechanism, reactivity, and selectivity in palladium-catalyzed redox-relay Heck arylations of alkenyl alcohols. J Am Chem Soc. 2014;137:1960–1967. doi: 10.1021/ja4109616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oliveira CC, Angnes RA, Correia CRD. Intermolecular enantioselective Heck–Matsuda arylations of acyclic olefins: application to the synthesis of β-aryl-γ-lactones and β-aryl aldehydes. J Org Chem. 2013;78:4373–4385. doi: 10.1021/jo400378g. [DOI] [PubMed] [Google Scholar]
- 37.Kikushima K, Holder JC, Gatti M, Stoltz BM. Palladium-catalyzed asymmetric conjugate addition of arylboronic acids to five-, six-, and seven-membered β-substituted cyclic enones: enantioselective construction of all-carbon quaternary stereocenters. J Am Chem Soc. 2011;133:6902–6905. doi: 10.1021/ja200664x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoo KS, et al. Asymmetric intermolecular boron Heck-type reactions via oxidative palladium(II) catalysis with chiral tridentate NHC-amidate-alkoxide ligands. J Org Chem. 2009;75:95–101. doi: 10.1021/jo901977n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoo KS, et al. Asymmetric intermolecular Heck-type reaction of acyclic alkenes via oxidative palladium(II) catalysis. Org Lett. 2007;9:3933–3935. doi: 10.1021/ol701584f. [DOI] [PubMed] [Google Scholar]
- 40.Yonehara K, et al. Palladium-catalyzed asymmetric intermolecular arylation of cyclic or acyclic alkenes using phosphinite-oxazoline ligands derived from d-glucosamine. J Organomet Chem. 2000;603:40–49. [Google Scholar]
- 41.Werner EW, Sigman MS. A highly selective and general palladium catalyst for the oxidative Heck reaction of electronically nonbiased olefins. J Am Chem Soc. 2010;132:13981–13983. doi: 10.1021/ja1060998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoo KS, Yoon CH, Jung KW. Oxidative palladium(II) catalysis: a highly efficient and chemoselective cross-coupling method for carbon–carbon bond formation under base-free and nitrogenous-ligand conditions. J Am Chem Soc. 2006;128:16384–16393. doi: 10.1021/ja063710z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Du X, et al. Mizoroki–Heck type reaction of organoboron reagents with alkenes and alkynes. A Pd(II)-catalyzed pathway with Cu(OAc)2 as an oxidant. Org Lett. 2001;3:3313–3316. doi: 10.1021/ol016529y. [DOI] [PubMed] [Google Scholar]
- 44.Gligorich KM, Sigman MS. Recent advancements and challenges of palladiumII-catalyzed oxidation reactions with molecular oxygen as the sole oxidant. Chem Commun. 2009:3854–3867. doi: 10.1039/b902868d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steinhoff BA, King AE, Stahl SS. Unexpected roles of molecular sieves in palladium-catalyzed aerobic alcohol oxidation. J Org Chem. 2006;71:1861–1868. doi: 10.1021/jo052192s. [DOI] [PubMed] [Google Scholar]
- 46.Kochi T, Hamasaki T, Aoyama Y, Kawasaki J, Kakiuchi F. Chain-walking strategy for organic synthesis: catalytic cycloisomerization of 1,n-dienes. J Am Chem Soc. 2012;134:16544–16547. doi: 10.1021/ja308377u. [DOI] [PubMed] [Google Scholar]
- 47.Johnson LK, Killian CM, Brookhart M. New Pd(II)- and Ni(II)-based catalysts for polymerization of ethylene and .alpha.-olefins. J Am Chem Soc. 1995;117:6414–6415. [Google Scholar]
- 48.Larock RC, Leung W-Y, Stolz-Dunn S. Synthesis of aryl-substituted aldehydes and ketones via palladium-catalyzed coupling of aryl halides and non-allylic unsaturated alcohols. Tetrahedron Lett. 1989;30:6629–6632. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.