Abstract
Xenograft models of human cancer play an important role in the screening and evaluation of candidates for new anticancer agents. The models, which are derived from human tumor cell lines and are classified according to the transplant site, such as ectopic xenograft and orthotopic xenograft, are still utilized to evaluate therapeutic efficacy and toxicity. The metastasis model is modified for the evaluation and prediction of cancer progression. Recently, animal models are made from patient-derived tumor tissue. The patient-derived tumor xenograft models with physiological characters similar to those of patients have been established for personalized medicine. In the discovery of anticancer drugs, standard animal models save time and money and provide evidence to support clinical trials. The current strategy for using xenograft models as an informative tool is introduced.
Keywords: Xenograft model, Mouse, In vivo, Anticancer drug development
INTRODUCTION
Animal models play an important role in drug development and studies of molecular biological mechanisms. Historically, the coal tar-induced skin cancer model in rabbit triggered the development of a carcinogen-induced mouse model. Various animal models have been established as an evaluation tool for the prediction of carcinogens and investigation of carcinogenic mechanisms (1). However, the approach of using chronic exposure to a carcinogen is timeintensive and expensive, thus limiting its application in drug development. Nevertheless, mouse models are still more attractive than big animal models because of the low cost, ease-of-handling and known genetic information (2). More recently, a syngenic mouse model injected with murine cell lines has been developed (3). The advantages of this model are reproducibility, ability to easily induce various tumor types, and immunocompetence. On the other hand, this model often shows a different response in comparison to the results from in vitro assays in human cancer cells. To overcome this disadvantage, the National Cancer Institute (NCI) used a method in which human cancer cells are injected into an immune-deficient mouse. A battery of xenograft models was developed from eight different NCI cancer cell lines (brain, colon, leukemia, lung, melanoma, ovarian, prostate and renal). In addition, various methods for generating mouse models have been established for the assessment of the efficacy and toxicity of new drugs. One model is the genetically engineered mouse model (GEMM), which is an advanced method for evaluating carcinogenesis mechanisms and drug resistance (4). Immunocompetent mice are used for the GEMM model, similar to a syngenic model. So, this model allows the application of immune adjuvant development for cancer. Moreover, this model is useful for elucidating biological processes and investigating tumor cells and their microenvironment, but it is very expensive, heterogeneous and complicated. Additionally, tumor frequency, development and growth do not coincide in the GEMM model (4-7). Many researchers have devised a strategy for preclinical evaluation to determine the therapeutic potential and to mimic the human tumor environment. In addition to the GEMM model, in vivo xenograft models use athymic nude mice and severe combined immune deficiency (SCID) mice for implantation of the human cancer cells or patient tumor tissue in translational research for clinical trials (8,9). In this review, the types and characteristics of the tumor xenograft models are focused towards use in the development of anticancer drugs.
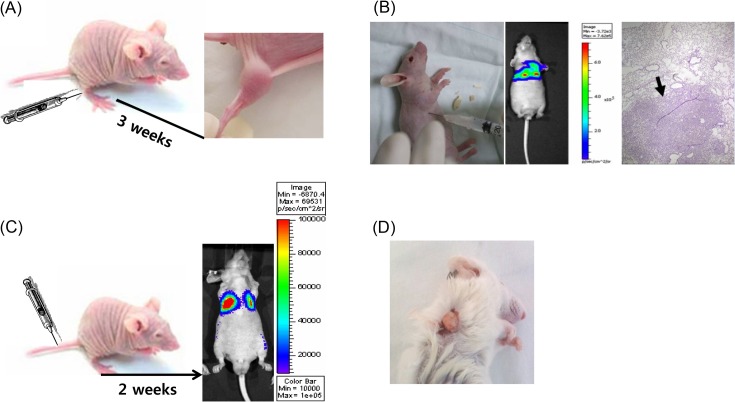
Ectopic tumor xenograft model. Generally, human cancer cells are subcutaneously injected into the hind leg or back of mice (Fig. 1A). In an ectopic tumor xenograft model (ectopic model), the transplanted site is different from the origin of the cultured cells. The ectopic model is the standard model of cancer used for validation and assessment in oncology studies. After establishment of cancer cell lines for anticancer screening in NCI, xenograft models derived from these cell lines were developed. Sixty cell lines derived from eight organs were used for establishment of xenograft models and information such as dou-bling time of tumor and tumorigenicity rate was reported (1). In Table 1, in human cancer cell lines, the reproducible take rate of the xenograft model was over 90%. For the evaluation of lead compounds obtained from an in vitro screening test, this model demonstrated that the same cancer cells can be useful and predictive, which is helpful for selection of an applicable cancer compound for translation to clinical trial.
Fig. 1. Various xenograft models. (A) Ectopic xenograft model. The cancer cells were subcutaneously injected into Balb/c nude mice. After approximately two weeks, the tumor was observed. (B) Orthotopic xenograft model. Human non-small cell lung cancer cells (A549 cells) were injected into the thoracic cavity of Balb/c nude mice. Tumor was observed by in vivo optical imaging. Isolated lung tissue was stained and observed by microscopy. (C) Metastasis model. Luciferase-expressing cancer cells were injected into the tail vein. Tumor was observed by in vivo optical imaging. (D) Patient-derived tumor xenograft model. Patient-derived tumor tissues were transplanted into the SCID mouse.
Table 1.
Human cell lines utilized for early-stage xenograft model
| Tumor origin | Good cell culture line | Acceptable cell culture line |
|---|---|---|
|
| ||
| Colon | SW-620, KM12, HCT-116, HCT-15 | HCC-2998, DLD-1, KM20L2, COLO 205, HT29 |
| CNS | SF-295, SNB-75, U251 | |
| Lung (Nonsmall cell) | NCI-H460, NCI-H522, NCI-H23 | NCI-H322M, EKVX, HOP-92 |
| Lung (Small cell) | DMS273 | DMS114 |
| Mammary | ZR-75-1, MX-1 | UISO-BCA-1, MDA-MB-231, MCF-7, MCF-7/ADR-res, MDA-MB-435, MDA-N |
| Melanoma | LOX-IMVI, SK-MEL-28 | UACC-257, M14, SK-MEL-5 |
| Ovarian | OVCAR-5, SK-OV-3 | OVCAR-3, OVCAR-4, IGROV1 |
| Prostate | PC-3 | DU-145 |
| Renal | CAKI-1, RXF393 | RXF631, A498,SN12C |
The data were modified from Ref. (1).
CNS, Central nervous system.
Because an ectopic model can be used to monitor tumorigenicity and tumor growth easily, many researchers have utilized this model for evaluation of anticancer efficacy (10-12). Tumor volume (V) is calculated from the largest length and the shortest length of the tumor (Equation1). From several parameters based on this data, anticancer activity can be evaluated. The ratio of treated group (T) to control group (C) (optimal % T/C), tumor growth delay and tumor regression were utilized (13-15). Drug-related deaths (DRD) and body weight change as parameters of toxicity were determined. DRD was presumed animal deaths within 15 days, and over 20% loss of treated mouse body weight compared to control was considered an adverse effect.
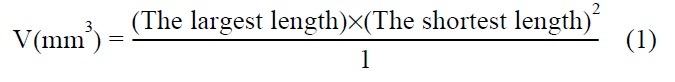
These parameters help to draw the lead compound from drug screening. Occasionally, drug response depending on cancer types could be compared without individual differences, because two types of cancer cells could be transplanted spontaneously into the same mouse and the two tumors can show differences in growth (16). Furthermore, the ectopic model is very reproducible, homogenous, and amenable to use.
However, not all tumors can be used as an assessment tool because some tumors show necrosis during tumorigenicity and some tumors are not solid (unsubstantial). The immunosuppressed mice used for making animal models represent a different microenvironment than that of human cancer. Therefore, assessment of invasion and metastasis is limited in this model.
Orthotopic tumor xenograft model. Alternative models for assessment of tumor sensitivity have been developed. The orthotopic tumor xenograft model (orthotopic model) is an advanced tool, but is based on a immunosuppressive murine microenvironment. In the orthotopic model, the human cancer cells are transplanted into the same origin site of the tumor. For instance, lung cancer cells were directly injected into the mouse lung for the orthotopic model (Fig. 1B). In this model, a well-trained expert with surgical skills is required to ensure reproducibility. The take rate of tumorigenicity is difficult to calculate because almost all tumors except melanoma are invisible to the naked eye. Moreover, orthotopic models are limited to measurement of tumor growth without sacrifice unlike subcutaneous ectopic models. To date, imaging is the chosen method to monitor the progression of growing tumors in orthotopic models. Currently, orthotopic models with cancer cell lines expressing fluorescence or luciferase are observed by optical imaging, computerized tomography (CT) or magnetic resonance imaging (MRI) (17). To assess carcinogenesis and determine the tumor growth without sacrifice, expensive equipment is required, so the availability of this model is limited. Nevertheless, this model is clinically relevant to the patientlike progression process (e.g., invasion). According to the report of Ho and his colleagues, orthotopic tumors show faster early-stage tumor growth, angiogenesis and hyperpermeability of blood vessels compared to ectopic tumors (18). In some cancer types, metastasis was also observed. For example, the orthotopic mammary fat pad tumor implant model is also a good model for breast cancer. In this model, spontaneous tumor metastases resemble the natural progression of human breast cancer (19). Therefore, anticancer activity and metastasis inhibition could be evaluated in the same model. The metastasis model is described below in detail.
Metastatic cancer model. Tumors that form locally by exposure to ultraviolet, ionizing radiation and carcinogens circulate within vessels and lymph nodes via invasion, causing metastasis (secondary cancer) at sites that are amenable to invasion. According to the seed and soil hypothesis of Paget, the primary cancer cell (seed) initiates metastasis in a suitable environment (soil) such as lung, liver, bone, lymph, and brain (20). Recent research has spurred the development of metastasis inhibitors and preventive drugs based on studies of the mechanisms of metastasis, but there has not been a preclinical evaluation tool to define guidelines for approving a clinical trial.
For establishment of a metastasis model, various methods have been developed and there are two types of human xenograft models. First, in orthotopic transplantation, transplanted tumor cells give rise to the primary tumor, the tumor is removed, and then metastasis is observed. For example, WM239 melanoma cells were transplanted into severe combined immune deficiency (SCID) mice and the primary tumor was isolated after 4 weeks. Then, lung metastasis was observed (21). The orthotopic model was made from prostate cancer cells (DU145), and the removed lymph node was cultured and isolated tumor cells were reinjected into mice to obtain a metastasis model (22). Second, cancer cells were intravenously injected into nude (Fig. 1C) or SCID mice, where they circulated like cancer stem cells and triggered metastasis (23). This model is generated faster than the former model. In the hybrid of the ectopic and orthotopic models, fluorescence-expressing HT- 29 cells (human colon cancer) are subcutaneously injected into the ectopic site and several pieces of HT-29 cellderived tumors are transplanted into the colon, and then metastasis is observed (24). Generally, a metastasis model is more easily obtained in SCID mice than in nude mice. Because metastasis, as in the orthotopic model, is difficult to observe in appearance except in the case of skin cancer (25), genetically engineered cell lines (fluorescence (24) or luciferase-expressing cells (26)) were utilized and monitored using in vivo optical imaging. Frequently, this model is applied for theragnosis, which involves imaging by MRI or positron emission tomography (PET) (27) to simultaneously diagnose and determine the appropriate anti-cancer therapy. To date, the guidelines of using metastasis as an assessment tool for drug development have not been established. Further studies on reproducibility, mechanisms underlying metastasis, and markers are needed.
Patient-derived tumor xenograft model. Xenograft models, despite their advantages, are limited in their ability to demonstrate how a cancer patient would respond to a particular treatment. The reliable prediction of drug response in a clinical trial is needed, and the current models are not sufficient. In an effort to address the shortcomings of these models, a patient-derived tumor xenograft (PDTX) was developed and utilized (28,29). Because PDTX involves transplanting cancer patient tissue directly into immunecompromised mice (Fig. 1D), genetic information and immunohistological markers are correlative to the patient and can be applied to evaluate new anticancer drugs (30) and personalized cancer therapies. The several advantages of PDTX can be summarized as follows: 1
However, the PDTX model has technical constraints, and is expensive and time-consuming. Most of all, freshly excised primary human tumors should be delivered from the operating room to the laboratory within several hours. Simultaneously, a sample of the primary human tumors should be examined by immunohistological analysis. Therefore, it is necessary to have cooperation among the surgeon, histologist and researcher. Then, the original primary human tumors can be compared with tumor tissues of the passaged tumorgraft. Moreover, approval of an institutional review board (IRB) is required because utilization of patientderived tumor tissue entails clinical and ethical considerations. Notwithstanding these efforts, the take rate of PDTX is about 25% (31-33), and establishment of PDTX takes about three months to the first passage (data not shown). As with xeno-organ transplantation, the first transplantation to SCID mice is required to escape acute immunorejection, and this is expensive. Additionally, the patient-derived tumor tissue volume is very limited, so the population number of PDTX should be increased via tumor tissue passage.Simultaneously, each passage of tumor tissues should be analyzed histopathologically and compared with the original tissue. From the second passage, nude mice can be utilized. The pieces of tumor tissues can be frozen and preserved in liquid nitrogen.
Despite these hurdles, the established PDTX model is available for validation of anticancer drug sensitivity and prediction of patient prognosis. PDTX is certainly an extremely promising model for personalized cancer therapy. Accordingly, global research centers are concerned with establishing a resource bank of PDTX. For the last decade, the PDTX model has been developing rapidly. This model is a promising tool for development of anticancer drugs and predictive biomarkers.
CONCLUSION
As shown in Fig. 1, various xenograft models have been developed and applied for preclinical assessment. Most in vivo animal models are imperfect in the extrapolation of human cancer. Species differences, tumor environment and immune responses are problems awaiting solutions. Nevertheless, these xenograft models are indispensable for validating the efficacy and toxicity of lead compounds for translation to clinical trials. In this review, we describe the characteristics, advantages, weaknesses and availability for the drug development process. Development and standardization of animal models can increase the predictability of the anticancer drug response and be utilized as a good tool for preclinical assessment of anticancer drugs.
Acknowledgments
This work was supported by the Duksung Women’s University Research Grants of 2013 (3000001910).
References
- 1.Teicher B.A., Andrews P.A. Anticancer drug development guide; preclinical screening, clinical trials, and approval. 2nd edition. Humana Press; New Jersey: (2004). pp. 99–123. [Google Scholar]
- 2.Cheon D.J., Orsulic S. Mouse models of cancer. Annu. Rev. Phytopathol. (2011);6:95–119. doi: 10.1146/annurev.pathol.3.121806.154244. [DOI] [PubMed] [Google Scholar]
- 3.Suggitt M., Bibby M.C. 50 years of preclinical anticancer drug screening: empirical to target-driven approaches. Clin. Cancer Res. (2005);11:971–981. [PubMed] [Google Scholar]
- 4.Frese K.K., Tuveson D.A. Maximizing mouse cancer models. Nat. Rev. Cancer. (2007);7:645–658. doi: 10.1038/nrc2192. [DOI] [PubMed] [Google Scholar]
- 5.Sharpless N.E., Depinho R.A. The mighty mouse: genetically engineered mouse models in cancer drug development. Nat. Rev. Drug Discovery. (2006);5:741–754. doi: 10.1038/nrd2110. [DOI] [PubMed] [Google Scholar]
- 6.Olive K.P., Tuveson D.A. The use of targeted mouse models for pre-clinical testing of novel cancer therapeutics. Clin. Cancer Res. (2006);12:5277–5287. doi: 10.1158/1078-0432.CCR-06-0436. [DOI] [PubMed] [Google Scholar]
- 7.Kucherlapati R. Genetically modified mouse models for biomarker discovery and preclinical drug testing. Clin. Cancer Res. (2012);18:625–630. doi: 10.1158/1078-0432.CCR-11-2021. [DOI] [PubMed] [Google Scholar]
- 8.Peterson J.K., Houghton P.J. Integrating pharmacology and in vivo cancer models in preclinical and clinical drug development. Eur. J. Cancer. (2004);40:837–844. doi: 10.1016/j.ejca.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Firestone B. The challenge of selecting the ‘right’ in vivo oncology pharmacology model. Curr. Opin. Pharmacol. (2010);10:391–396. doi: 10.1016/j.coph.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 10.Jung J., Park S.J., Chung H.K., Kang H.W., Lee S.W., Seo M.H., Park H.J., Song S.Y., Jeong S.Y., Choi E.K. Polymeric nanoparticles containing taxanes enhance chemoradiotherapeutic efficacy in non-small cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. (2012);84:e77–83. doi: 10.1016/j.ijrobp.2012.02.030. [DOI] [PubMed] [Google Scholar]
- 11.Choi J., Kim H.Y., Ju E.J., Jung J., Park J., Chung H.K., Lee J.S., Lee J.S., Park H.J., Song S.Y., Jeong S.Y., Choi E.K. Use of macrophages to deliver therapeutic and imaging contrast agents to tumors. Biomaterials. (2012);33:4195–4203. doi: 10.1016/j.biomaterials.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 12.Jung J., Kim M.S., Park S.J., Chung H.K., Choi J., Park J., Jin D.H., Song S.Y., Park H.J., Lee D.S., Jeong S.Y., Choi E.K. Enhancement of radiotherapeutic efficacy by paclitaxel-loaded pH-senstivie block copolymer micelles. J. Nanomater. 2;(2012):1–5. [Google Scholar]
- 13.Dorr R.T., Wisner L., Smulitis B.K., Landowski T.H., Remers W.A. Anti-tumor activity and mechanism of action for a cyanoaziridine-derivative, AMP423. Cancer Chemother. Pharmacol. (2012);69:1039–1049. doi: 10.1007/s00280-011-1784-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeong S.Y., Park S.J., Yoon S.M., Jung J., Woo H.N., Yi S.L., Song S.Y., Park H.J., Kim C., Lee J.S., Lee J.S., Choi E.K. Systemic delivery and preclinical evaluation of Au nanoparticle containing beta-lapachone for radiosensitization. J. Controlled Release. (2009);139:239–245. doi: 10.1016/j.jconrel.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Saxena R., Chandra V., Manohar M., Hajela K., Debnath U., Prabhakar Y.S., Saini K.S., Konwar R., Kumar S., Megu K., Roy B.G., Dwivede A. Chemotherapeutic potential of 2-[piperidinoethoxyphenyl]-3-phenyl-2H-benzo(b)pyran in estrogen receptor-negative breast cancer cells: Action via prevention of EGFR activation and combined inhibition of PI-3-K/Akt/FOXO and MEK/Erk/AP-1 pathways. PLoS One. (2013);8:e66246. doi: 10.1371/journal.pone.0066246. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Jung J., Matsuzaki T., Tatematsu K., Okajima T., Tanizawa K., Kuroda S. Bio-nanocapsule conjugated with liposomes for in vivo pinpoint delivery of various materials. J. Controlled Release. (2008);126:255–264. doi: 10.1016/j.jconrel.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Hsu A.R., Hou L.C., Veeravaqu A., Greve J.M., Vogel H., Tse V., Chen X. In vivo near-infrared fluorescence imaging of integrin alphaveta3 in an orthotopic glioblastoma model. Mol. Imaging Biol. (2006);8:315–323. doi: 10.1007/s11307-006-0059-y. [DOI] [PubMed] [Google Scholar]
- 18.Ho K.S., Poon P.C., Owen S.C., Shoichet M.S. Blood vessel hyperpermeability and pathophysiology in human tumour xenograft models of breast cancer; a comparison of ectopic and orthotopic tumours. BMC Cancer. (2012);12:579. doi: 10.1186/1471-2407-12-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nozaki S., Sissons S., Chien D.S., Sledge G.W. Jr. Activity of biphenyl matrix metalloproteinase inhibitor BAY 12-9566 in a human breast cancer orthotopic model. Clin. Exp. Metastasis. (2003);20:407–412. doi: 10.1023/a:1025473709656. [DOI] [PubMed] [Google Scholar]
- 20.Fidler I.J. The pathogenesis of cancer metastasis; the ‘seed and soil’ hypothesis revisited. Nat. Rev. Cancer. (2003);3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 21.Francia G., Cruz-Munoz W., Man S., Xu P., Kerbel R.S. Mouse models of advanced spontaneous metastasis for experimental therapeutics. Nat. Rev. Cancer. (2011);11:135–141. doi: 10.1038/nrc3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banyard J., Chung I., Wilson A.M., Vetter G., Le Bechec A., Bielenberg D.Z., Zetter B.R. Regulation of epithelial plasticity by miR-424 and miR-200 in a new prostate cancer metastasis model. Sci. Rep. (2013);3:3151. doi: 10.1038/srep03151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yano S., Muguruma H., Matsumori Y., Goto H., Nakataki E., Edakuni N., Tomimoto H., Kakiuchi S., Yamamoto A., Uehara H., Ryan A., Sone S. Antitumor vascular strategy for controlling experimental metastatic spread of human small-cell lung cancer cells with ZD6474 in natural killer cell-depleted severe combined immunedeficient mice. Clin. Cancer Res. (2005);11:8789–8798. doi: 10.1158/1078-0432.CCR-05-0674. [DOI] [PubMed] [Google Scholar]
- 24.Ma H., Li X., Yang Z., Okuno S., Kawaguchi T., Yagi S., Bouvet M., Hoffman R.M. High antimetastatic efficacy of MEN4901/T-0128, a novel camptothecin carboxymethyldextran conjugate. J. Surg. Res. (2011);171:684–690. doi: 10.1016/j.jss.2010.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong C.Y., Helm M.A., Kalb R.E., Helm T.N., Zeitouni N.C. The presentation, pathology, and current management strategies of cutaneous metastasis. N. Am. J. Med. Sci. (2013);5:499–504. doi: 10.4103/1947-2714.118918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Du L., Xu W.T., Fan Q.M., Tu B., Shen Y., Yan W., Tang T.T., Wang Y. Tumorigenesis and spontaneous metastasis by luciferase-labeled human xenograft osterosarcoma cells in nude mice. Chin. Med. J. (Engl.) (2012);125:4022–4030. [PubMed] [Google Scholar]
- 27.Kakhki V.R., Shahriari S., Treglia G., Hasanzadeh M., Zakavi S.R., Yousefi Z., Kadkhodayan S., Sadeghi R. Diagnostic performance of fluorine 18 fluorodeoxyglucose positron emission tomography imaging for detection of primary lesion and staging of endometrial cancer patients: Systemic review and meta-analysis of the literature. Int. J. Gynecol. Cancer. (2013);23:1536–1543. doi: 10.1097/IGC.0000000000000003. [DOI] [PubMed] [Google Scholar]
- 28.Cook N., Jodrell D.I., Tuveson D.A. Predictive in vivo animal models and translation to clinical trials. Drug Discovery Today. (2012);17:253–260. doi: 10.1016/j.drudis.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 29.Tentler J.J., Tan A.C., Weekers C.D., Jimeno A., Leong S., Pitts T.M., Arcaroli J.J., Messersmith W.A., Eckhardt S.G. Patient-derived tumour xenografts as models for oncology drug development. Nat. Rev. Clin. Oncol. (2012);9:338–350. doi: 10.1038/nrclinonc.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moro M., Bertolini G., Tortoreto M., Pastorino U., Sozzi G., Roz L. Patient-derived xenografts of non small cell lung cancer; resurgence of an old model for investigation of modern concepts of tailored therapy and cancer stem cells. J. Biomed. Biotechnol. (2012);2012:1–11. doi: 10.1155/2012/568567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fichtner I., Rolff J., Soong R., Hoffmann J., Hammer S., Sommer A., Becker M., Merk J. Establishment of patient-derived non-small cell lung cancer xenografts as models for the identification of predictive biomarkers. Clin. Cancer Res. (2008);14:6456–6468. doi: 10.1158/1078-0432.CCR-08-0138. [DOI] [PubMed] [Google Scholar]
- 32.Zhuo Y., Wu Y., Guo A., Chen S., Su J. [Establishment and its biological characteristics of patient-derived lung cancer xenograft models]. Zhongguo Feiai Zazhi. (2010);13:568–574. doi: 10.3779/j.issn.1009-3419.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin K., He K., Han N., Li G., Wang H., Xu Z., Jiang H., Zhang J., Teng L. Establishment of a PDTT xenograft model of gastric carcinoma and its application in personalized therapeutic regimen selection. Hepatogastroenterology. (2011);58:1814–1822. doi: 10.5754/hge11136. [DOI] [PubMed] [Google Scholar]